Abstract
Background
Creatinine is a commonly used measure of kidney function, but serum levels are also influenced by muscle mass. We hypothesized that higher serum creatinine would be associated with self-reported functional limitation in community-dwelling elderly.
Methods
Subjects (n = 1,553) were participants in the Study of Physical Performance and Age-Related Changes in Sonomans, a cohort to study aging and physical function. We explored three strategies to account for the effects of muscle mass on serum creatinine.
Results
We observed a J-shaped association of creatinine with functional limitation. Above the study-specific mean creatinine (0.97 mg/dL in women and 1.15 mg/dL in men), the unadjusted odds ratio of functional limitation per standard deviation (0.20 mg/dL in women and 0.23 mg/dL in men) higher creatinine was 2.27 (95% confidence interval [CI] 1.75–2.94, p < .001) in women and 1.42 (95% CI 1.12–1.80, p = .003) in men. This association was inverted in persons with creatinine levels below the mean. Adjustment for muscle mass did not have an important effect on the association between creatinine and functional limitation. These associations remained after multivariable adjustment for demographics and health conditions but were statistically significant only in women.
Conclusions
In elderly adults, higher creatinine levels are associated with functional limitation, consistent with prior literature that has demonstrated reduced physical performance in persons with kidney disease. However, the association of low creatinine levels with functional limitation suggests that creatinine levels are influenced by factors other than kidney function and muscle mass in the elderly.
Keywords: Aging, Creatinine, Kidney disease, Mobility limiation
THE association between severe kidney disease and poor physical performance is well established (1–4); however, less research has focused on physical function in persons with mild reductions in kidney function. Mild to moderate kidney disease is associated with an increased likelihood of frailty, functional limitation, decreased exercise capacity, and decreased strength (5–8). Together, these studies suggest that impaired kidney function is associated with reduced physical function across a spectrum of outcomes. Understanding characteristics of this association may be of particular importance in elderly populations where kidney disease and functional limitation are common conditions.
One reason for the paucity of literature on this association may be the difficulty in accurate assessment of kidney function in the elderly. Most epidemiological and clinical studies have used creatinine-based measures of kidney function. Creatinine is filtered by the glomerulus; therefore, the serum creatinine level is used as an indirect measure of glomerular filtration. However, creatinine is derived largely from skeletal muscle, which limits the ability of creatinine to assess kidney function accurately among persons with different levels of muscle mass (9,10). This is an important limitation in elderly persons because of the high prevalence of frailty and other chronic diseases that are associated with loss of muscle mass. Creatinine-based equations have been developed to overcome this limitation and to estimate the glomerular filtration rate (GFR); however, several recent reports have suggested that these equations are less accurate among persons with early stages of chronic kidney disease (CKD) or in the elderly (6,11–13 ).
In the present study, we hypothesized that lower levels of kidney function, estimated by serum creatinine, would be associated independently with self-reported functional limitation in elderly adults. Because kidney function and muscle mass are believed to be the dominant determinants of the level of serum creatinine, we also hypothesized that the association of creatinine with functional limitation would be J shaped—low creatinine levels, indicative of lower muscle mass, would also be associated with a higher likelihood of functional limitation. To account for differences in muscle mass, we explored alternative strategies to adjust for the effects of muscle mass on serum creatinine.
METHODS
Study Sample
The Study of Physical Performance and Age-Related Changes in Sonomans (SPPARCS) is a prospective cohort to study the effect of aging on physical functioning in the elderly. The cohort included 2,092 women and men aged 55 years and older who resided in and around Sonoma, California (14,15). The present cross-sectional study includes 1,553 individuals with blood samples and self-reported physical performance measures at baseline (May 1993 to December 1994). The protocols were approved by the Committee for the Protection of Human Subjects, University of California, Berkeley, and by the Committee for Human Research at the University of California, San Francisco.
Functional Limitation
Self-reported functional limitation was determined from 10 questions that assessed the degree of difficulty that a participant reported in various domains of physical functioning (16). Questions were derived from a published assessment instrument (17–19). Functional limitation was defined as a lot of difficulty or inability to do one or more functions or avoidance of at least one function because a physician advised the participant not to do the activity; other response categories included some difficulty, a little difficulty, no difficulty, and never do activity.
Kidney Function
Creatinine was measured from serum samples by the Jaffe method. We assessed kidney function using three methods: (i) creatinine, (ii) standardized creatinine residuals from a model that included lean mass, and (iii) estimated glomerular filtration rate (eGFR).
To calculate the standardized creatinine residuals, we regressed creatinine on lean mass and then calculated the standardized residual values from the model. This is a well-described method for removing the confounding effects of nuisance factors on a measure of interest and has been used extensively in nutritional epidemiology (20,21).
We calculated eGFR based on the abbreviated Modification of Diet in Renal Disease (MDRD) equation as recommended by the National Kidney Foundation (22).
Lean Mass
Estimates of lean mass were derived from reactance and resistance measured with bioelectric impedance (BIA) using the BIA101Q Quantum Body Composition Analyzer System (RJL Systems, Clinton Township, MI). With the participant lying supine, bipolar electrodes were placed on the middle finger of the right hand and the lateral aspect of the right ankle. As recommended by Roubenoff and colleagues (23), lean mass was estimated from study-specific regression equations predicting lean mass as measured with dual energy x-ray absorptiometry (DEXA). The equations were developed in a validation substudy with 99 men and 101 women randomly selected from the cohort members with no chronic conditions in 10 age-specific (above and below the median) and gender-specific body mass index strata (<10th, 10th to <50th, 50th to <75th, 75th to <90th, and ≥90th percentiles). These participants had duplicate BIA measurements and a whole-body DEXA scan using a LUNAR DPQIX machine (LUNAR, Madison, WI). Gender-specific multivariable linear regressions were conducted with lean mass measured by DEXA as the dependent variable. The analyses produced the following prediction equations:
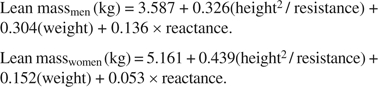 |
The total variances in lean mass accounted for by these regressions were 0.85 for men and 0.80 for women (16).
Other Variables
Weight and height were measured by standard protocol. Systolic and diastolic blood pressures were recorded as the mean of three measurements made in the sitting position using a random-zero sphygmomanometer. Hypertension, diabetes mellitus, cardiovascular disease, cerebrovascular disease, smoking, income, and education were assessed by self-report. Walking speed (ft/s) was measured by the number of feet walked in 60 seconds (15). Handgrip strength was measured with a handheld isometric dynamometer; results are reported for the dominant hand.
Statistical Analysis
We compared baseline characteristics in the 1,553 SPPARCS participants with creatinine and self-reported functional limitation measures and the 2,092 participants in the total SPPARCS study population at baseline, based on a t test for continuous variables and chi-square test for categorical variables. Baseline characteristics also were compared in persons with and without a reported functional limitation at baseline. All analyses were sex stratified because the prevalence of functional limitation was much higher in women compared with men (34% vs 20%, p < .001). To examine the unadjusted association between creatinine and functional limitation, we plotted the sex-specific prevalence of functional limitation by level of creatinine and overlaid a smoothed regression plot.
Logistic regression models were used to examine the association between creatinine and prevalent functional limitation. Because of the J-shaped association between creatinine and functional limitation, we used piecewise regression models, with a cut-point at the mean for each measure. This cut-point was chosen based on visual inspection of the plots. To explore potential confounders and mediators of the association between creatinine and functional limitation, we used a series of logistic regression models. Model 1 included only age and lean mass, and Model 2 added systolic and diastolic blood pressure, chronic health conditions (hypertension, diabetes, atherosclerotic heart disease, cerebrovascular disease), smoking, and socioeconomic status (income <$30,000 per year and education <12 years). An exploratory analysis was performed (Model 3) to see if adjustment for physical function measures (grip strength and walking speed) and physical activity attenuated the association between creatinine and functional limitation.
We examined the potential interactions of chronic health conditions (hypertension, diabetes, cardiovascular disease, and cerebrovascular disease) with creatinine in sex-specific, age- and lean mass–adjusted piecewise logistic regression models.
All analyses were conducted with Stata 8.0 (Stata Corp., College Station, TX).
RESULTS
There were 1,553 SPPARCS participants with adequate blood specimens for creatinine measurement and self-reported functional limitation. On average, participants in the present study were younger (70 [SD 8] vs 73 [SD 9] years, p < .001), more often men (42% vs 36%, p = .01), and had less functional limitation (25% vs 38%, p < .001) compared with those in the entire SPPARCS study population. Those included in the present study had less history of chronic health conditions compared with those in the larger SPPARCS sample; they were less likely to report a history of hypertension (41% vs 47%, p = .03), atherosclerotic heart disease (16% vs 22%, p = .003), and cerebrovascular disease (7% vs 12%, p < .001).
Persons with functional limitation were on average 5 years older, more often women, and less likely to smoke compared with those without functional limitation (Table 1). Lower income and education were also associated with functional limitation. Participants with functional limitation had lower lean mass and higher systolic blood pressure compared with those without functional limitation, and were more likely to report a history of hypertension, diabetes, atherosclerotic heart disease, and cerebrovascular disease. Lower grip strength and slower average walking speed were also associated with functional limitation.
Table 1.
Characteristics of Participants With and Without Functional Limitation at Baseline
| Characteristics | Functional Limitation, n = 384 | No Functional Limitation, n = 1,169 | p Value* | Total Population |
| Sociodemographic factors | ||||
| Age, mean ± SD y | 73 ± 9 | 68 ± 8 | <.001 | 70 ± 8 |
| Female, n (%) | 275 (72) | 624 (53) | <.001 | 654 (58) |
| Smoking, n (%) | .01 | |||
| Never | 140 (36) | 533 (46) | 673 (43) | |
| Former | 213 (55) | 554 (47) | 767 (49) | |
| Current | 31 (8) | 82 (7) | 113 (7) | |
| Income <$30K/y, n (%) | 185 (53) | 352 (33) | <.001 | 537 (38) |
| Education <12 y, n (%) | 165 (43) | 346 (30) | <.001 | 511 (33) |
| Physiological measurements | ||||
| Lean mass, mean ± SD kg | 45 ± 10 | 47 ± 11 | <.001 | 47 ± 11 |
| Systolic blood pressure, mean ± SD mm Hg | 144 ± 20 | 137 ± 20 | <.001 | 139 ± 20 |
| Diastolic blood pressure, mean ± SD mm Hg | 77 ± 11 | 77 ± 10 | .58 | 77 ± 10 |
| Chronic health conditions | ||||
| Hypertension, n (%) | 220 (57) | 421 (36) | <.001 | 641 (41) |
| Diabetes, n (%) | 32 (8) | 50 (4) | .002 | 82 (5) |
| Atherosclerotic heart disease, n (%) | 82 (21) | 163 (14) | .001 | 245 (16) |
| Cerebrovascular disease, n (%) | 49 (13) | 52 (4) | <.001 | 101 (7) |
| Physical function measurements | ||||
| Grip strength, mean ± SD kg | 25.6 ± 10.6 | 33.2 ± 12.2 | <.001 | 31.4 ± 12.3 |
| Walking speed, mean ± SD ft/s | 1.9 ± 0.5 | 2.4 ± 0.5 | <.001 | 2.3 ± 0.5 |
| Physical activity (METs/wk) | 34.1 ± 33.6 | 48.3 ± 34.7 | <.001 | 45.7 ± 35 |
Notes: MET = metabolic equivalents; SD = standard deviation.
Using a t test for continuous variables and a chi-square test for categorical variables.
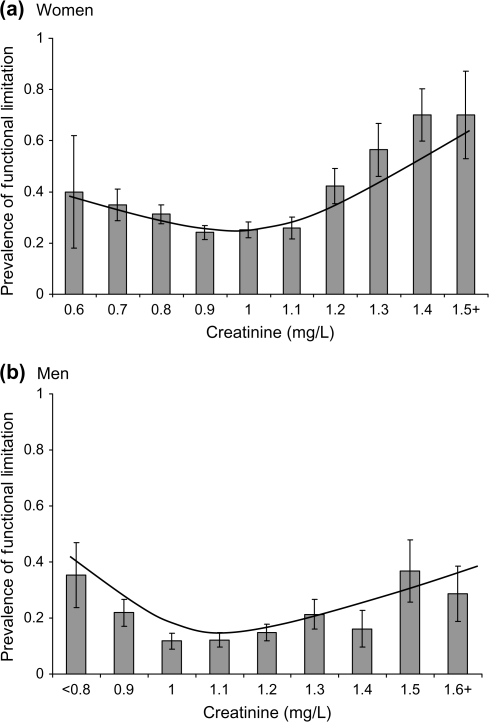
On average, study participants had near-normal kidney function; mean creatinine was 0.97 (SD 0.20) mg/dL in women and 1.15 (SD 0.23) mg/dL in men, which corresponds to a mean eGFR of 63 (SD 13) mL/min/1.73 m2 in women and 70 (SD 13) mL/min/1.73 m2 in men. There were 501 participants (382 women and 119 men) with an eGFR <60 mL/min/1.73 m2 (Stage 3 CKD) and only eight participants (three women and five men) with an eGFR <30 mL/min/1.73 m2 (Stage 4 CKD). There were J-shaped associations between creatinine and functional limitation in both women and men (Figure 1A and B). In participants with creatinine levels greater than the mean, higher creatinine was associated with a higher odds of functional limitation, although the association appeared weaker in men (Table 2). In contrast, in participants with a creatinine below the mean, lower creatinine levels were associated with a higher odds of functional limitation.
Figure 1.
Prevalence (± standard error) of functional limitation by level of serum creatinine and overlaid smoothing plot in (a) women and (b) men.
Table 2.
Association Between Creatinine-Based Measures of Kidney Function and Functional Limitation Based on Several Methods to Adjust for Muscle Mass
| Creatinine*, OR (95% CI) per SD | Multivariable Adjustment, OR (95% CI) per SD | Standardized Residuals, OR (95% CI) per SD | eGFR†, OR (95% CI) per SD | |
| Women | ||||
| Kidney function > mean (n = 419) | 2.27 (1.75–2.94) | 2.56 (1.89–3.47) | 2.78 (1.97–3.92) | 2.41 (1.83–3.18) |
| Kidney function < mean (n = 481) | 0.57 (0.39–0.82) | 0.47 (0.31–0.71) | 0.62 (0.45–0.86) | 0.71 (0.55–0.92) |
| Men | ||||
| Kidney function > mean (n = 273) | 1.42 (1.12–1.80) | 1.42 (1.09–1.83) | 1.33 (1.07–1.65) | 1.79 (1.28–2.51) |
| Kidney function < mean (n = 381) | 0.62 (0.37–1.03) | 0.57 (0.33–1.01) | 0.30 (0.13–0.73) | 0.68 (0.48–0.98) |
Notes: CI = confidence interval; eGFR = estimated glomerular filtration rate; OR = odds ratio; SD = standard deviation.
Mean ± standard deviation creatinine is 0.97 ± 0.20 mg/dL in women and 1.15 ± 0.23 mg/dL in men.
Based on the Modification of Diet in Renal Disease equation, mean ± standard deviation eGFR is 63 ± 13 mL/min/1.73 m2 in women and 70 ± 13 mL/min/1.73 m2 in men.
Adjustment for muscle mass did not have an important effect on the association between creatinine and functional limitation. Table 2 shows the three methods in which we accounted for differences in muscle mass across participants: (i) multivariable adjustment, (ii) standardized residuals from a model that includes lean mass, and (iii) use of the MDRD eGFR formula. None of these methods altered the J-shaped association between creatinine and functional limitation.
In women, the inverted associations above and below the mean remained significant after adjustment for age, lean mass, health conditions, smoking, income, and education (Table 3). Further adjustment for physical function and physical activity measures had little effect on either association.
Table 3.
The Association Between Higher Creatinine and Functional Limitation in Women and Men, Stratified by Serum Creatinine and Below the Mean
| Model | Unadjusted | Model 1† | Model 2‡ | Model 3§ |
| OR (95% CI) per SD* Higher Creatinine | ||||
| Women | ||||
| Creatinine > mean* (n = 419) | 2.27 (1.75 – 2.94) | 2.14 (1.55 – 2.95) | 1.96 (1.40 – 2.75) | 1.96 (1.38 – 2.77) |
| Creatinine < mean* (n = 481) | 0.57 (0.39 – 0.82) | 0.36 (0.24 – 0.57) | 0.36 (0.22 – 0.59) | 0.42 (0.25 – 0.69) |
| Men | ||||
| Creatinine > mean* (n = 273) | 1.42 (1.12 – 1.80) | 1.30 (0.99 – 1.70) | 1.23 (0.92 – 1.64) | 1.21 (0.87 – 1.67) |
| Creatinine < mean* (n = 381) | 0.62 (0.37 – 1.03) | 0.46 (0.25 – 0.83) | 0.42 (0.22 – 0.81) | 0.66 (0.32 – 1.36) |
Notes: Mean ± SD (standard deviation) creatinine is 0.97 ± 0.20 mg/dL in women and 1.15 ± 0.23 mg/dL in men.
Model 1 includes age & lean mass.
Model 2 includes model 1 plus systolic and diastolic blood pressure, hypertension, diabetes, atherosclerotic heart disease, cerebrovascular disease, smoking, income and education.
Model 3 includes model 2 plus grip strength, walking speed, and physical activity.
In men, the associations between creatinine and functional limitation were weaker than in women, both above and below the mean (Table 3). In men with creatinine above the mean, the association between creatinine and functional limitation no longer reached statistical significance after adjustment for age and lean mass (p = .06). Similarly, in men with a creatinine less than the mean, the association remained significant after adjustment for age, lean mass, health conditions, smoking, income, and education, but not after adjustment for physical function and physical activity.
We found no statistically significant interactions of creatinine with any of the chronic health conditions in men or women. We found a suggestive interaction between diabetes and creatinine for predicting functional limitation in men with creatinine levels below the mean, although there were only 24 diabetic men with creatinine below the mean. In diabetic men with creatinine below the mean, the odds ratio for functional limitation was 2.63 (95% confidence interval [CI] 0.28–24.6) in diabetics compared with 0.45 (95% CI 0.25–0.81) in nondiabetics (p value for interaction .06). Thus, in male diabetics, higher creatinine levels appear to be associated with higher odds for functional limitation, across the range of creatinine levels. All other interaction estimates were estimated with very low precision and are not presented.
DISCUSSION
We observed a J-shaped association of creatinine with functional limitation in elderly adults, even after adjusting for muscle mass. In participants with creatinine levels greater than the study population mean (0.97 mg/dL in women and 1.15 mg/dL in men), higher creatinine was associated with a higher odds of functional limitation. An association in the opposite direction was observed in persons with creatinine levels below the mean. These associations were stronger in women and were present even after multivariable adjustment for age, lean mass, chronic health conditions, physical function, and physical activity. The association of higher creatinine with functional limitation is consistent with the hypothesis that worse kidney function is associated with poor physical performance. The inversed association of creatinine and functional limitation in participants with low creatinine suggests that creatinine levels may be affected by factors other than glomerular filtration and muscle mass in elderly adults.
Our finding is consistent with other studies of measures that have reported a J-shaped association of creatinine-based eGFR and poor outcomes (6,12,13,24). At near-normal levels, serum creatinine is an insensitive measure of kidney function; this is often thought to be due to low creatinine production in individuals with low muscle mass (6,9,12,13). Surprisingly, the J-shaped relation between creatinine and functional limitation was nearly unchanged after accounting for differences in muscle mass. Creatinine generation may be not simply a product of muscle mass but influenced by muscle composition, muscle function, activity, diet, health status, and other factors (25,26). Another potential explanation may be the increased tubular secretion of creatinine in some patients with kidney dysfunction (9,27). If the tubular secretion of creatinine is sufficient, it could reduce the creatinine concentration in the blood, resulting in a false-negative report of kidney dysfunction by creatinine-based methods. We believe it to be unlikely that kidney function truly has a J-shaped association with functional limitation.
The exception to the J-shaped association was in male diabetics, in whom the association of creatinine with functional limitation was positive across the range of serum creatinine. We are not aware of a physiologic explanation for this finding, and due to the small sample size, we recommend this interaction be confirmed in future studies.
The direct association of creatinine and functional limitation is consistent with prior studies of physical performance in persons with severe kidney disease. Numerous studies have shown that patients with end-stage renal disease or advanced CKD have reduced physical performance (1–4,28,29). Less research has been conducted on persons with mild to moderate CKD. One study reported that 24-hour creatinine clearance was associated with reduced exercise capacity and self-assessed physical limitation in adults with coronary artery disease (7). In another cohort of well-functioning elderly adults, cystatin C, a marker of kidney function independent of muscle mass, was associated with worse performance on four measures of physical function and incident functional limitation. In this study, investigators reported a J-shaped association of creatinine-based eGFR with each of the four measures of physical function (5,6).
Although the reason for poor physical function in persons with kidney disease has been explored extensively, the mechanisms remain unclear. We have demonstrated that the association between high creatinine and functional limitation was independent of commonly proposed explanatory factors: age, lean mass, health conditions, physical function, and physical activity. Another possibility is that kidney disease represents a biological marker of aging, and low kidney function identifies persons with limited functional reserve. The kidney is sensitive to vascular disturbances, and kidney dysfunction may be an indicator of cumulative damage from hypertension, diabetes, inflammation, or other chronic health conditions. Although we adjusted for several of these chronic health conditions in the present study, these one-time assessments of a risk factor cannot encompass the entire life-course of the exposure. Future studies of kidney function and other potential confounders across the life-course may better elucidate this relationship.
In the present study, the association between creatinine and functional limitation was stronger and more robust in women than in men. Women were more likely to report a functional limitation compared with men in the study population. The sex difference implies that the men in our study population were more resilient, perhaps due to greater functional reserve or because they represent a survivor population.
This study has several limitations that should be considered. On average, participants in the present study were healthier compared with those in the entire SPPARCS sample, which may have limited variability in creatinine levels and could reduce the generalizability of the findings. Estimates of muscle mass were derived from BIA measures, based on study-specific validation equations. Measurement error in muscle mass could result in incomplete adjustment in the models of creatinine and functional limitation. However, if muscle mass were the primary explanation for the inverse association between lower creatinine and functional limitation, some attenuation of the J-shaped relation would be expected after adjustment for muscle mass. We did not measure GFR or cystatin C levels, and creatinine levels were not calibrated to the Cleveland clinic. This is a cross-sectional study, which limits our ability to assess the temporal sequence of events. In addition, we cannot exclude the possibility that some residual confounding remains. Finally, we did not have a direct measure of GFR in our study population.
In conclusion, higher creatinine was associated with increased odds for functional limitation in patients with creatinine levels above 0.97 mg/dL in women and 1.15 mg/dL in men, but reduced odds for functional limitation in patients with creatinine levels below these study-specific means. These associations were stronger in women compared with men and persisted after adjustment for age, lean mass, health conditions, smoking, socioeconomic status, physical function, and physical activity. This study illustrates that in elderly persons, serum creatinine levels appear to be influenced by factors other than kidney function and muscle mass. Longitudinal studies of kidney function, physical performance, and other physiological factors will be needed to elucidate the mechanisms underlying the association of creatinine with functional limitation.
Acknowledgments
This study was supported by grant R01-AG09389 from the National Institute on Aging. Dr M.G.S. is supported by grant R01-AG027002 from the National Institute on Aging.
References
- 1.Clyne N, Jogestrand T, Lins LE, Pehrsson SK. Progressive decline in renal function induces a gradual decrease in total hemoglobin and exercise capacity. Nephron. 1994;67:322–326. doi: 10.1159/000187987. [DOI] [PubMed] [Google Scholar]
- 2.Painter P, Hanson P, Messer-Rehak D, Zimmerman SW, Glass NR. Exercise tolerance changes following renal transplantation. Am J Kidney Dis. 1987;10:452–456. doi: 10.1016/s0272-6386(87)80192-0. [DOI] [PubMed] [Google Scholar]
- 3.Painter P, Johansen K. Physical functioning in end-stage renal disease. Introduction: a call to activity. Adv Ren Replace Ther. 1999;6:107–109. doi: 10.1016/s1073-4449(99)70029-4. [DOI] [PubMed] [Google Scholar]
- 4.Painter P, Messer-Rehak D, Hanson P, Zimmerman SW, Glass NR. Exercise capacity in hemodialysis, CAPD, and renal transplant patients. Nephron. 1986;42:47–51. doi: 10.1159/000183632. [DOI] [PubMed] [Google Scholar]
- 5.Fried LF, Lee JS, Shlipak M, et al. Chronic kidney disease and functional limitation in older people: Health, Aging and Body Composition Study. J Am Geriatr Soc. 2006;54:750–756. doi: 10.1111/j.1532-5415.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 6.Odden MC, Chertow GM, Fried LF, et al. Cystatin C and measures of physical function in elderly adults: the Health, Aging, and Body Composition (HABC) Study. Am J Epidemiol. 2006;164:1180–1189. doi: 10.1093/aje/kwj333. [DOI] [PubMed] [Google Scholar]
- 7.Odden MC, Whooley MA, Shlipak MG. Association of chronic kidney disease and anemia with physical capacity: the Heart and Soul Study. J Am Soc Nephrol. 2004;15:2908–2915. doi: 10.1097/01.ASN.0000143743.78092.E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shlipak MG, Stehman-Breen C, Fried LF, et al. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis. 2004;43:861–867. doi: 10.1053/j.ajkd.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 9.Lamb EJ, O’Riordan SE, Delaney MP. Kidney function in older people: pathology, assessment and management. Clin Chim Acta. 2003;334:25–40. doi: 10.1016/s0009-8981(03)00246-8. [DOI] [PubMed] [Google Scholar]
- 10.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933–1953. [PubMed] [Google Scholar]
- 11.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from kidney disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 12.Sarnak MJ, Katz R, Stehman-Breen CO, et al. Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med. 2005;142:497–505. doi: 10.7326/0003-4819-142-7-200504050-00008. [DOI] [PubMed] [Google Scholar]
- 13.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 14.Satariano WA, Smith J, Swanson A, Tager IB. A census-based design for the recruitment of a community sample of older adults: efficacy and costs. Ann Epidemiol. 1998;8:278–282. doi: 10.1016/s1047-2797(97)00235-4. [DOI] [PubMed] [Google Scholar]
- 15.Tager IB, Hollenberg M, Satariano WA. Association between self-reported leisure-time physical activity and measures of cardiorespiratory fitness in an elderly population. Am J Epidemiol. 1998;147:921–931. doi: 10.1093/oxfordjournals.aje.a009382. [DOI] [PubMed] [Google Scholar]
- 16.Sternfeld B, Ngo L, Satariano WA, Tager IB. Associations of body composition with physical performance and self-reported functional limitation in elderly men and women. Am J Epidemiol. 2002;156:110–121. doi: 10.1093/aje/kwf023. [DOI] [PubMed] [Google Scholar]
- 17.Comoni-Huntley J, Brock DB, Ostfeld AM. Established Populations for Epidemiologic Studies of the Elderly: Resource Data Book. Bethesda, MD: National Institute on Aging; 1986. Vol. NIH Publication No. 86-2443. (Aging NIo, ed) [Google Scholar]
- 18.Jette AM, Branch LG. The Framingham Disability Study: II. Physical disability among the aging. Am J Public Health. 1981;71:1211–1216. doi: 10.2105/ajph.71.11.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagi SZ. An epidemiology of disability among adults in the United States. Milbank Mem Fund Q Health Soc. 1976;54:439–467. [PubMed] [Google Scholar]
- 20.Willett W. Nutritional Epidemiology. New York, NY: Oxford University Press:; 1990. [Google Scholar]
- 21.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 22.K/DOQI. Clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002;39(suppl):S1–S266. [PubMed] [Google Scholar]
- 23.Roubenoff R, Baumgartner RN, Harris TB, et al. Application of bioelectrical impedance analysis to elderly populations. J Gerontol A Biol Sci Med Sci. 1997;52:M129–M136. doi: 10.1093/gerona/52a.3.m129. [DOI] [PubMed] [Google Scholar]
- 24.Shlipak MG, Fyr CL, Chertow GM, et al. Cystatin C and mortality risk in the elderly: the Health, Aging, and Body Composition Study. J Am Soc Nephrol. 2006;17:254–261. doi: 10.1681/ASN.2005050545. [DOI] [PubMed] [Google Scholar]
- 25.Banfi G, Del Fabbro M. Serum creatinine values in elite athletes competing in 8 different sports: comparison with sedentary people. Clin Chem. 2006;52:330–331. doi: 10.1373/clinchem.2005.061390. [DOI] [PubMed] [Google Scholar]
- 26.Preiss DJ, Godber IM, Lamb EJ, Dalton RN, Gunn IR. The influence a cooked-meat meal on estimated glomerular filtration rate. Ann Clin Biochem. 2007;44:35–42. doi: 10.1258/000456307779595995. [DOI] [PubMed] [Google Scholar]
- 27.Branten AJ, Vervoort G, Wetzels JF. Serum creatinine is a poor marker of GFR in nephrotic syndrome. Nephrol Dial Transplant. 2005;20:707–711. doi: 10.1093/ndt/gfh719. [DOI] [PubMed] [Google Scholar]
- 28.Johansen KL. Physical functioning and exercise capacity in patients on dialysis. Adv Ren Replace Ther. 1999;6((2)):141–148. doi: 10.1016/s1073-4449(99)70032-4. [DOI] [PubMed] [Google Scholar]
- 29.Moore GE, Brinker KR, Stray-Gundersen J, Mitchell JH. Determinants of VO2peak in patients with end-stage renal disease: on and off dialysis. Med Sci Sports Exerc. 1993;25:18–23. doi: 10.1249/00005768-199301000-00004. [DOI] [PubMed] [Google Scholar]