Abstract
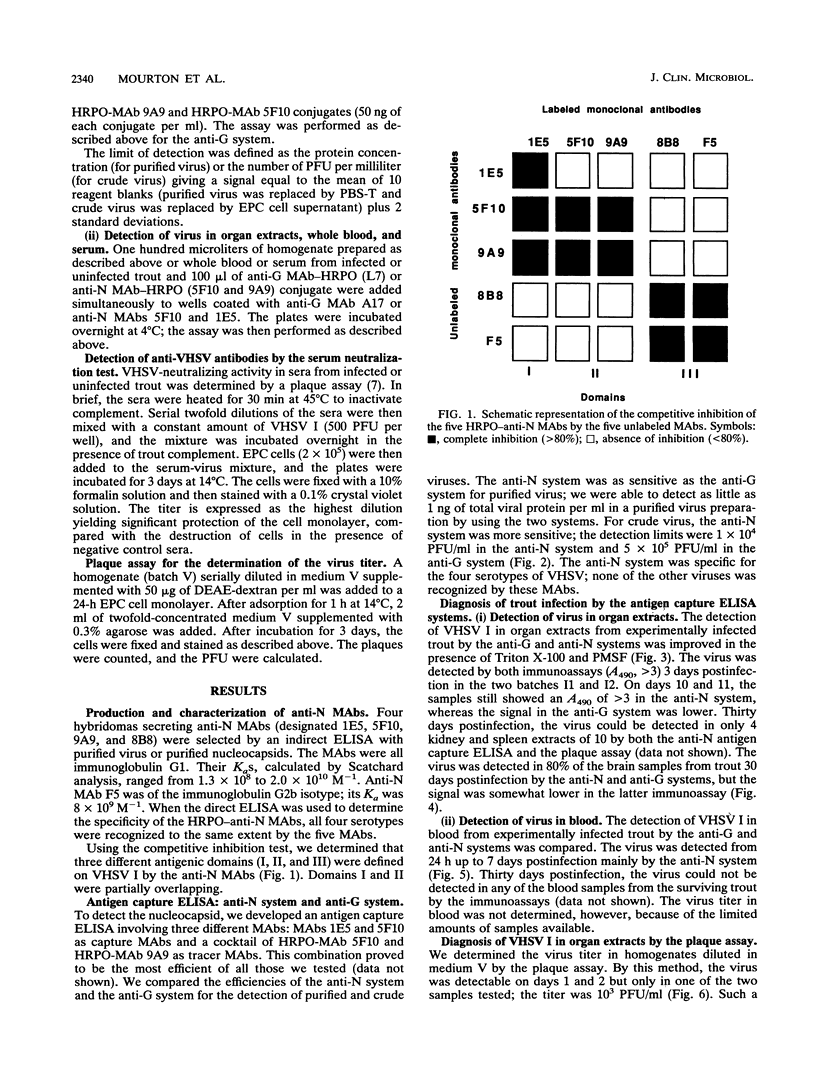
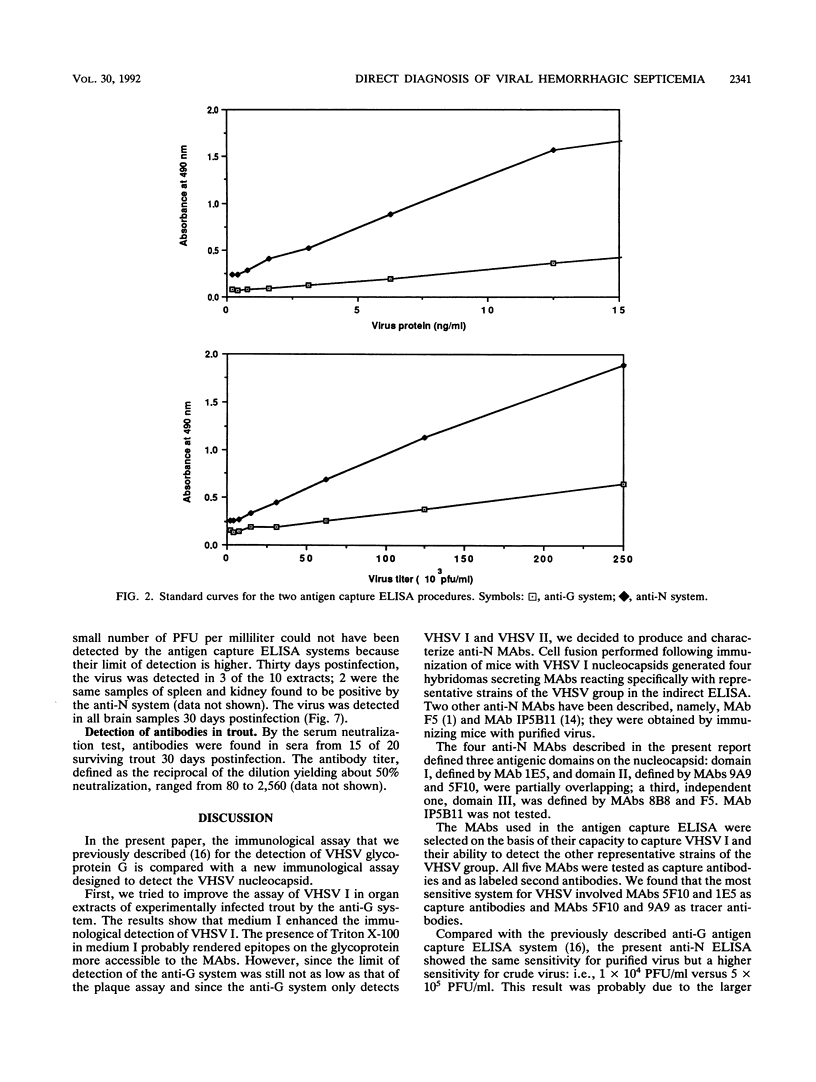
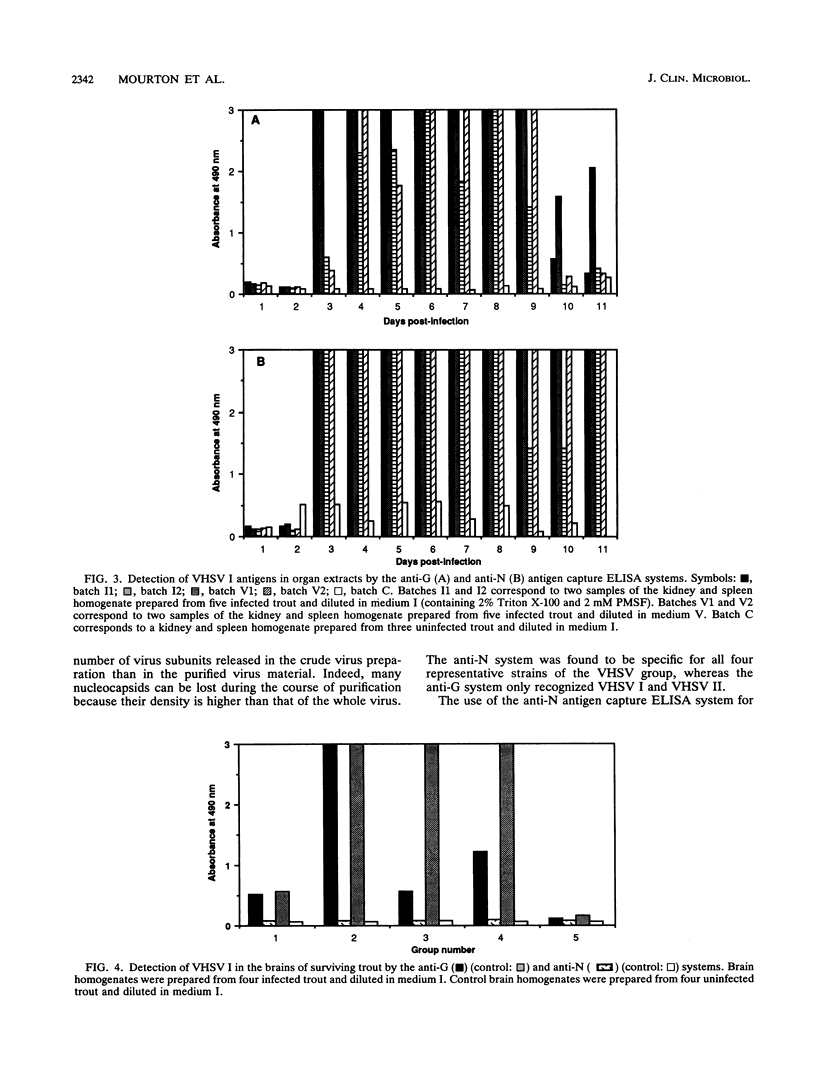
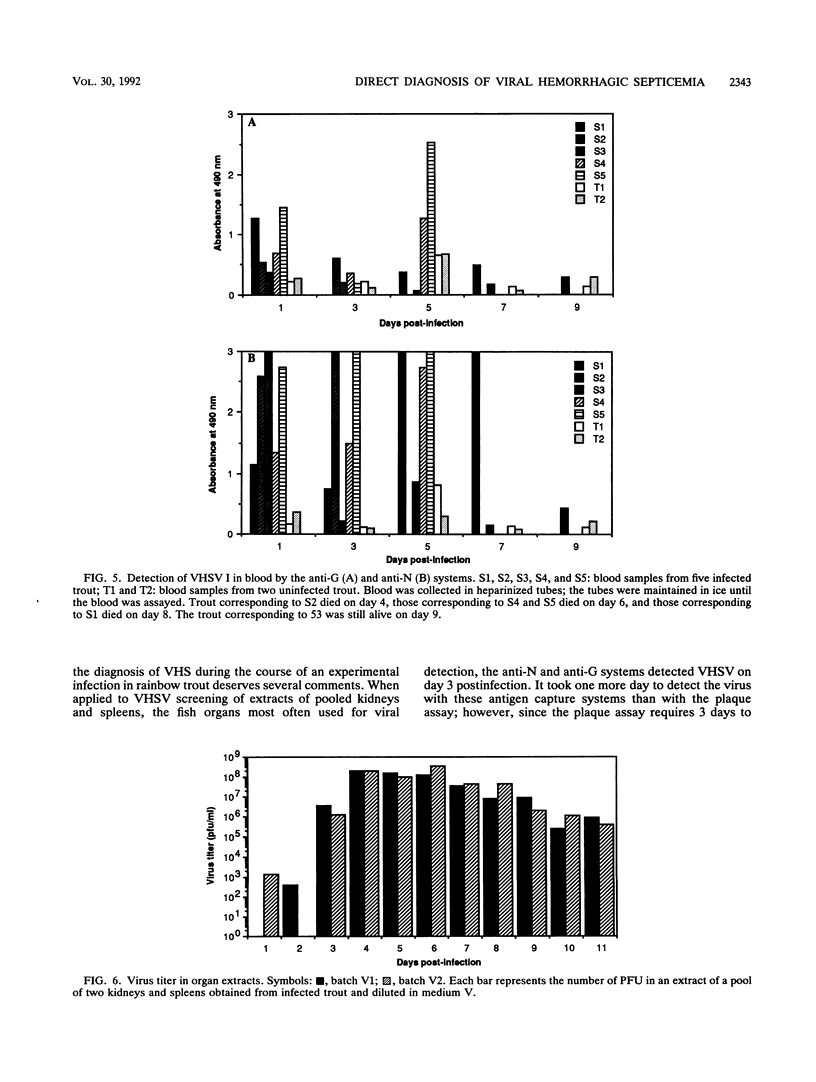
An antigen capture enzyme-linked immunosorbent assay (ELISA) based on the detection of the viral nucleocapsid (anti-N system) was developed for the diagnosis of viral hemorrhagic septicemia. Four monoclonal antibodies directed against the viral nucleocapsid were produced; they all recognized the four viral hemorrhagic septicemia virus (VHSV) serotypes. Three of these monoclonal antibodies were used in a new antigen capture ELISA. The efficiency of the anti-N system in detecting purified and crude viruses as well as the virus in infected-organ extracts and infected blood was compared with that of a recently described antigen capture ELISA based on the detection of viral envelope glycoprotein Gp (anti-G system). For the detection of purified virus, the anti-N system was found to be as sensitive as the anti-G system (detection limit, 1 ng of total viral protein per ml), but the anti-N system was much more sensitive than the anti-G system for the detection of crude VHSV I (detection limits, 1 x 10(4) PFU/ml versus 5 x 10(5) PFU/ml). In organ extracts, VHSV I could be detected by both systems 3 days postinfection. The signal for the assay of VHSV I in blood 24 h postinfection was higher with the anti-N system than the anti-G system. Furthermore, VHSV I could be detected in 80% of the brain samples of surviving trout by the anti-N system and also by the anti-G system, but with a lower signal. In conclusion, we have developed a highly sensitive immunoassay for VHSV I that is more rapid and easier to perform than the currently used plaque assay.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Jensen M. H. Research on the virus of Egtved disease. Ann N Y Acad Sci. 1965 Aug 10;126(1):422–426. doi: 10.1111/j.1749-6632.1965.tb14292.x. [DOI] [PubMed] [Google Scholar]
- Mourton C., Bearzotti M., Piechaczyk M., Paolucci F., Pau B., Bastide J. M., de Kinkelin P. Antigen-capture ELISA for viral haemorrhagic septicaemia virus serotype I. J Virol Methods. 1990 Sep;29(3):325–333. doi: 10.1016/0166-0934(90)90059-o. [DOI] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Pal R., Grinnell B. W., Snyder R. M., Wagner R. R. Regulation of viral transcription by the matrix protein of vesicular stomatitis virus probed by monoclonal antibodies and temperature-sensitive mutants. J Virol. 1985 Nov;56(2):386–394. doi: 10.1128/jvi.56.2.386-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechaczyk M., Chardes T., Cot M. C., Pau B., Bastide J. M. Production and characterization of monoclonal antibodies against human thyroglobulin. Hybridoma. 1985 Winter;4(4):361–367. doi: 10.1089/hyb.1985.4.361. [DOI] [PubMed] [Google Scholar]
- WOLF K., QUIMBY M. C. Established eurythermic line of fish cells in vitro. Science. 1962 Mar 23;135(3508):1065–1066. doi: 10.1126/science.135.3508.1065. [DOI] [PubMed] [Google Scholar]
- de Kinkelin P., Le Berre M. Mass virus production in fish cell system. Dev Biol Stand. 1979;42:99–104. [PubMed] [Google Scholar]


