Abstract
Background
To examine the association between sleep-disordered breathing (SDB) and 24-hour blood pressure (BP) pattern among community-dwelling older adults.
Methods
A convenience sample of 70 community-dwelling older adults, recruited from senior housing, community centers, and learning centers, were admitted to General Clinical Research Center, Emory University Hospital, Atlanta, Ga. Information regarding demographic and clinical history was obtained using questionnaires. Twenty-four–hour BP monitoring in supine position was performed using Spacelabs model 20207. Breathing during sleep was monitored with the use of a modified sleep recording system (Embletta, PDS), which monitors nasal and oral airflow, chest and abdominal movements, and pulse oximetry. Night time–daytime (night-day) BP ratio (average night-time BP divided by daytime BP) was calculated both for systolic and diastolic BPs.
Results
Sixty-nine participants, mean age 74.9 ± 6.4 years (41 [57%] women), completed the study. The mean apnea-hypopnea index (AHI) was 13 ± 13 per hour of sleep, and 20 participants (29%) had AHI ≥15 per hour of sleep, indicating moderate to severe SDB. Moderate to severe SDB (AHI ≥15 per hour of sleep) was significantly associated with nocturnal hypertension, whereas there was no statistically significant difference in wake-time BP between those with and without moderate to severe SDB. Stepwise multiple regressions showed that AHI independently predicted increased night-day systolic and night-day diastolic BP ratio, even after controlling for nocturia frequency.
Conclusions
The results indicate increased BP load associated with increased AHI in this group of older adults. This increased BP load may contribute to increased hypertension-related morbidity and disease burden.
Keywords: Sleep-Disordered breathing, 24-hour BP pattern
SLEEP-DISORDERED BREATHING (SDB) is a common condition among community-dwelling older adults, with a prevalence of about 20% reported for moderate to severe SDB (apnea-hypopnea index [AHI] ≥15 per hour of sleep) (1). Previous studies have shown a statistically significant association between SDB, hypertension, and cardiovascular morbidity (2– 6), suggesting that SDB may play a significant part in the pathogenesis of these disorders. Interestingly, these associations were seen only in those younger than 60 years, and no statistically significant association was described in older adults (1–3,7,8). Because of these reports, the role of SDB in the pathogenesis of hypertension and hypertension-related morbidity among older adults remains unclear. The possibility that older adults may be subject to survival effects may explain the lack of association between SDB and increased cardiovascular and cerebrovascular morbidity in this group of the population has been forwarded (9,10). On the other hand, more recent studies have described significant associations between SDB and cardiovascular and cerebrovascular morbidity among older adults (11,12).
Because previous studies that examined the relationship between SDB and hypertension relied on the average of a few daytime blood pressure (BP) readings to define hypertension, the BP values may not accurately reflect the 24-hour BP pattern and, specifically, the status of BP during sleep. Twenty-four–hour BP pattern and BP during sleep have shown higher correlations with target organ damage and cardiovascular outcomes compared with office BP (13– 15). This implies that relying on office BP values may be imprecise for examining the relationship between SDB and hypertension or hypertension-related morbidity.
Previous studies have reported a significant association between SDB and nondipping of BP (16– 19), but in almost all of these studies the impact of conditions that are known to influence 24-hour BP pattern, such as posture, daytime, and night-time activities, was not accounted for (20–24). In this study, we report the 24-hour BP pattern in older adults with and without SDB, obtained in a constant environment in which specific measures are employed to control for the effects of posture and daytime and night-time activities. We hypothesized that SDB is independently associated with blunting of the nocturnal decline in BP typically observed during sleep in this community-dwelling older adults, regardless of the BP while awake.
METHODS
Data for this analysis were obtained from a study that examines the association between SDB, 24-hour BP pattern, and 24-hour urine output. A convenience sample of study participants was recruited from senior housing and community and learning centers in Atlanta, Ga. Individuals who were aged 65 years and older and independent in performing all activities of daily living were eligible for inclusion in the study. Exclusion criteria included history of sleep apnea and use of continuous positive airway pressure treatment, symptomatic heart failure (New York Heart Association class 3 or 4), administration of diuretics except for hydrochlorothiazide ≤25 mg/d, uncontrolled diabetes mellitus (Hgb A1C >7.5), and history of diabetes insipidus. Individuals with history of urinary incontinence who use more than one pad in 24 hours were also excluded because it was felt that 24-hour urine collection would be unreliable in these individuals. The study was approved by the Emory University Institutional Review Board.
Demographic and clinical information were collected using questionnaires prepared for the study. Information about use of prescription medication was obtained from study participants and was verified by the hospital pharmacist. The number of prescription medications was used as a proxy for burden of illness (comorbidity) (25,26). In addition, the effect of hypertension medications on 24-hour BP pattern was examined.
Study participants were admitted to the General Clinical Research Center (GCRC) of Emory University for 48 hours. Participants continued to take their routine medications while they were in GCRC, following verification by the hospital pharmacist. During the first day, 24-hour BP monitoring was performed with the use of Spacelabs model 20207 (Redmond, Wash) using the nondominant arm. The ambulatory blood pressure monitor (ABPM) was programed to take readings every 30 minutes during the 24-hour period. Because previous studies have indicated changes in BP associated with daytime activities (20–22), we opted to keep the position and activities of study participants uniform during the daytime (6:01 AM–10:00 PM). Hence, study participants remained at bed rest, with the head of the bed elevated to 45 degrees inclination, except at meal times and for bathroom visits. But while in bed, they were allowed to engage in different activities that included reading, watching TV, doing puzzles, or sewing and encouraged to stay awake until bedtime. In addition, the unit nurses made frequent visits into the rooms of study participants during the daytime to make sure they were not asleep. Night time, corresponding to sleep time (10:00 PM–6:00 AM), and daytime, corresponding to wake time, were determined based on lights off (10:00 PM) and lights on (6:00 AM) time.
To be considered a successful ambulatory BP study, it was required that at least 80% of attempted BPs measured met the qualifications of the recorder algorithm (27). The ABPM software output calculates the 24-hour mean daytime and night-time systolic and diastolic BPs. The following definitions were used to identify differences between night-time and daytime BP values.
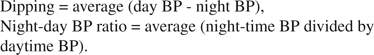 |
Based on the suggested operational thresholds for ambulatory BP monitoring, daytime hypertension was defined as average daytime BP values ≥140 mm Hg systolic and ≥90 mm Hg diastolic, whereas nocturnal hypertension was defined as average night-time BP values ≥125 mm Hg systolic and ≥75 mm Hg diastolic (28).
In addition to the ambulatory BP recordings, 3 supine BP readings were taken by the GCRC nursing staff, while the patient was awake, between the hours of 8:00 AM and 9:00 AM, 12:00 PM and 1:00 PM, and 4:00 PM and 5:00 PM with the use of Dinamap Procare 420 (GE Healthcare, Waukesha, Wis) during the same 24-hour period.
During the second 24-hour period, 24-hour urine collections were obtained and a modified sleep study was performed using overnight ambulatory sleep recording (Embletta, PDS; Medcare, Reykjavik, Iceland). The device recorded nasal and oral airflow, chest and abdominal movement, and pulse oximetry. The sleep recordings were downloaded to a computer and scored by the principal investigator. A minimum of 5 hours of recording was accepted to be adequate for scoring. The number of apneas and hypopneas per hour of sleep (AHI) was used to determine the severity of SDB. Apnea was defined as the cessation of airflow through the nose and mouth lasting 10 seconds or more. Hypopnea was defined as decrease in airflow of 50% or more accompanied by a decline in oxygen saturation of 4% or greater (29,30). The AHI recorded using the Embletta system has been shown to have a high correlation with AHI derived from polysomnography (31). Total sleep time was estimated based on the light off and light on time.
Data Analysis
Data analysis was performed using SPSS for windows version 15 software (SPSS Inc., Chicago, Ill). The outcomes of interest were night-day BP ratio and BP level during sleep. The night-day BP ratio was used to determine the status of nocturnal BP decline, with values <0.9 indicating a normal BP decrease during sleep, and values ≥0.9 showing loss of the normal decline in nocturnal BP. Study participants were divided into two groups based on their AHI values (AHI ≥15 per hour [SDB] vs AHI <15 per hour of sleep [no SDB]). Demographic, clinical, and BP characteristics were compared in those with and without SDB using the χ2 test for categorical variables and Mann-Whitney U, Kruskal-Wallis, or Spearman rank correlation tests for continuous variables. Stepwise multiple linear regression analysis was performed to determine the independent association between night-day BP ratio and SDB.
RESULTS
A total of 70 participants were enrolled in the study with a mean age 74.9 ± 6.4 years. There were 41 (57%) women, 52 (74%) whites, and 15 (21%) African Americans. Thirty-four participants (49%) lived with their spouses, 31 (44%) lived alone, and 5 (7%) lived with a friend or roommate. Twenty participants (29%) were taking 0–1 prescription medications, whereas 35 (50%) and 15 (21%) were taking 2–4 and 5 or more prescription medications, respectively.
Breathing Pattern During Sleep
There were 69 participants who completed the sleep study. The mean AHI was 13 ± 13 events per hour of sleep, and these were predominantly obstructive events (83%). The mean and lowest oxygen saturation levels were 93% ± 3% and 83% ± 6%, respectively. Eighteen participants (26%) had normal results (AHI <5 per hour), whereas 31 (45%), 14 (20%), and 6 (9%) participants had mild (AHI 5–14 per hour), moderate (AHI 15–29 per hour), and severe (AHI ≥30 per hour) SDB, respectively (29). Table 1 shows the demographic characteristics of those with and without moderate to severe SDB (AHI ≥ or < 15 per hour, respectively).
Table 1.
Demographic Characteristics of Study Participants by Apnea-Hypopnea Index Status
| Demographic Characteristic | AHI <15 per hour* (N = 49) | AHI ≥15 per hour* (N = 20) | Total |
| Age (mean ± SD),† years | 74.4 ± 6.6 | 76.1 ± 6.0 | 75.0 ± 6.4 |
| Gender: woman† | 30 (61%) | 10 (50%) | 40 (58%) |
| Body mass index† (mean ± SD), kg/m2 | 27.1 ± 4.4 | 29.6 ± 6.5 | 27.6 ± 4.8 |
| Marital status (N = 68)† | |||
| Married | 24 (50%) | 8 (40%) | 32 (47%) |
| Not married (single, divorced, widowed) | 24 (50%) | 12 (60%) | 36 (53%) |
| Living place (N = 68)†,‡ | |||
| Home | 34 (71%) | 17 (85%) | 51 (74%) |
| Independent living facility | 14 (29%) | 3 (15%) | 17 (26%) |
Notes: SD = standard deviation.
Apnea-Hypopnea Index (AHI) ≥15 per hour indicates moderate to severe sleep-disordered breathing.
There was no statistically significant difference between the two groups.
One person did not respond to this question.
Twenty-four–Hour BP Pattern
The 24-hour systolic and diastolic BP values showed normal distribution (Kolmogorov-Smirnov test for normality p = .980), and the average values were 129 ± 14 and 71 ± 7 mm Hg, respectively. Systolic and diastolic BP values during daytime ranged from 104 to 168 mm Hg and 54 to 87 mm Hg, with a mean of 132 ± 15 and 72 ± 7 mm Hg, respectively; night-time systolic and diastolic BPs ranged from 91 to 161 and 53 to 84 mm Hg with a mean of 124 ± 16 and 67 ± 8 mm Hg, respectively. The night-day systolic and diastolic BP ratio for women and men were .95 ± .06 and .94 ± .07 (p = .66) and .93 ± .07 and .92 ± .09 (p = .50), respectively. The distribution of night-day BP ratio by gender is shown in Table 2. There was no statistically significant correlation between day-night systolic or diastolic BP ratio and age.
Table 2.
Distribution of Sleep-Wake BP Ratio by Gender
| Night-Day BP Ratio* | Women | Men | Total |
| <.8 | 0 | 3 (10%) | 3 (4%) |
| 0.8–.89 | 9 (22%) | 4 (14%) | 13 (19%) |
| .9–.99 | 25 (61%) | 15 (52%) | 40 (57%) |
| ≥1 | 7 (17%) | 7 (24%) | 14 (20%) |
| Total | 41 | 29 | 70 |
Notes: BP = blood pressure.
Night-day BP ratio values (35, 36): Normal ratio is between .8 and .9, indicating a blood pressure decrease during sleep by 10%–20%. A ratio ≥.9 suggests loss of the normal decline in nocturnal BP whereas a ratio ≥1 depicts sleep BP equal or greater than wake-time BP. A ratio of <.8 indicates excessive drop in BP during sleep.
Hypertension during the daytime (BP ≥140/90 mm Hg) and night time (BP≥ 125/75 mm Hg) was observed in 27% and 54% of the participants, respectively (28). There was a significant positive association between daytime hypertension and body mass index, with daytime hypertension occurring in 11 of the 21 obese (52%), 5 of the 24 overweight (21%), and 3 of the 25 normal weight (12%) participants (χ2 = 10.15, df = 2, p = .006). There was no statistically significant association between night-time BP and body mass index.
The mean of three supine BP values obtained by the GCRC staff were 133 ± 16 and 71 ± 8 mm Hg for systolic and diastolic BP, respectively, and these values were highly correlated with daytime average 24-hour BP values (Spearman ρ = 0.67 [p < .001] and 0.66 [p < .001] for systolic and diastolic BPs, respectively).
Relationship Between AHI and BP Pattern
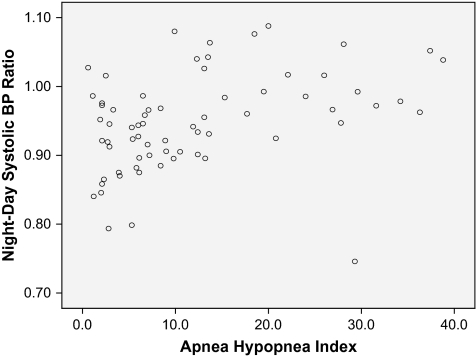
Table 3 depicts daytime and night-time BP values in those with and without moderate to severe SDB. Individuals with moderate to severe SDB showed significantly lower BP dipping during sleep and higher night-day BP ratio. AHI was significantly correlated with increased night-day systolic and diastolic BP ratio after controlling for age, gender, and BMI (partial correlation = .358, p = .003, and .305, p = .013, respectively). Figure 1 depicts a scatterplot that shows the approximate linear relationship between AHI and night-day systolic BP ratio. The night-day systolic BP ratio for those with AHI <5, AHI = 5–14, AHI = 15–29, and AHI ≥30 were .92 ± .07, .94 ± .06, .98 ± .08, and .99 ± .04, respectively (Kruskal-Wallis test p = .002).
Table 3.
Wake and Sleep BP Values by Apnea-Hypopnea Index Status
| BP and AHI* | AHI <15 Per Hour* (N = 49) | AHI ≥15 Per Hour* (N = 20) | Mann-Whitney U test, p value |
| Average daytime systolic BP (mean ± SD) | 132 ± 15 mm Hg | 131 ± 14 mm Hg | .81 |
| Average daytime diastolic BP (mean ± SD) | 73 ± 7 mm Hg | 71 ± 7 mm Hg | .37 |
| Average night-time systolic BP (mean ± SD) | 123 ± 16 mm Hg | 129 ± 16 mm Hg | .08 |
| Average night-time diastolic BP (mean ± SD) | 67 ± 8 mm Hg | 69 ± 7 mm Hg | .29 |
| Average systolic BP dipping (mean ± SD) | 9 ± 8 mm Hg | 2 ± 9 mm Hg | <.001 |
| Average diastolic BP dipping (mean ± SD) | 6 ± 6 mm Hg | 3 ± 5 mm Hg | .02 |
| Night-day BP ratio | |||
| Systolic BP | .93 ± .06 | .98 ± .07 | <.001 |
| Diastolic BP | .92 ± .07 | .97 ± .08 | .02 |
| AHI (mean ± SD) | 7 ± 4 per hour | 30 ± 7 per hour | <.001 |
| Mean oxygen saturation (mean ± SD) | 93.6 ± 1.9 | 92.0 ± 2.9 | .03 |
Notes: BP = blood pressure; SD = standard deviation.
Apnea-Hypopnea Index (AHI) ≥15 per hour indicated moderate to severe sleep-disordered breathing.
Figure 1.
Scatterplot of apnea-hypopnea index and night-day systolic blood pressure ratio.
Moderate to severe SDB (AHI ≥15 per hour) was significantly associated with nocturnal hypertension (χ2 = 4.52, p = .03), whereas there was no statistically significant difference in daytime BP between those with and without moderate to severe SDB. Stepwise multiple linear regression analysis was performed with night-day systolic BP ratio as the dependent variable and AHI as the independent variable with age, gender, BMI, 24-hour mean systolic BP, number of prescription drugs, percent time spent in oxygen saturation <90%, and nocturia frequency included as covariates. AHI significantly predicted increased night-day systolic BP ratio (β = .002, t = 2.959, p = .004), and the model explained a significant proportion of the variance in sleep-wake BP ratio (R2 = .20). The significance pattern did not change when use of BP medications was entered into the model. Because diabetes mellitus has been reported to be independently associated and possibly causally related with nondipping of BP during sleep (32,33), the linear regression analysis was repeated after excluding individuals with diabetes mellitus (n = 8). The results of the regression analysis showed that AHI was an independent predictor of increased night-day systolic BP ratio (β = .003, p = .005, R2 = .14).
DISCUSSION
The results of this study show that SDB is associated with increased night-day BP ratio (nondipping of BP during sleep) among older adults, independent of daytime and night-time activities. This is an important finding because previous studies have shown that both daytime and night-time activities can influence 24-hour BP pattern (20– 24), suggesting that these activities may confound the effect of SDB on 24-hour BP pattern. Especially relevant to older adults is to establish if AHI predicts nondipping of BP during sleep independent of the impact of nocturia on 24-hour BP pattern. Nocturia is a common complaint among older adults (34,35) and has been reported to show a significant association with nondipping of BP during sleep (23,24). To our knowledge, this is the first study to show the significant association of SDB and nondipping of night-time BP after controlling for posture, daytime activities, and nocturia frequency.
Previous studies have reported significant association between nondipping status of BP during sleep and cardiovascular morbidity and mortality both in patients with known hypertension (36,37) and normal daytime BP (38). The significant relationship between sleep-time hypertension and moderate to severe SDB reported in this study implies that SDB contributes to increased BP load and associated related morbidity in this population. This might be one of the mechanisms to explain the increased risk of cardiovascular and cerebrovascular morbidity in older adults with SDB reported previously (11,12).
The results of this study denote the significance of SDB among older adults, as well as the importance of 24-hour BP monitoring in assessing risk related to increased BP in this population. Although this study has several strengths, it also has limitations. Study participants were a convenience sample and may not be representative of the population of older adults. In addition, because of small sample size, the study may not have had adequate power to detect differences between those with and without SDB (type II error). For example, the average sleep-time BP in those with AHI ≥15 per hour was higher than those with AHI <15 per hour (129 mm Hg vs 123 mm Hg), and this clinically significant difference in BP (39) was not statistically significant (Table 3; p = .08). A study with larger sample size may be needed to confirm and advance our findings. Another limitation in this study is the absence of objective measurement of sleep both during daytime and night time. Because the participants stayed in bed during most of the ABPM monitoring period, the possibility that the participants may be asleep during the daytime cannot be ruled out. As a consequence one can argue that the average daytime BP may be inappropriately lower than normal. We have tried to avoid sleep during the daytime by advising the participants to stay awake and by promoting frequent visits by the nurses to their rooms. Although these measures cannot prevent these individuals from falling asleep, we think the effect of this is minimal as the average BP value taken by the GCRC staff while the participants were awake in supine position showed a large correlation (0.7 for both systolic and diastolic BPs) with the wake-time ABPM value.
In summary, the results of this study have demonstrated independent association between increased AHI and nondipping of BP during sleep, and a significant association between moderate to severe SDB and sleep-time hypertension in this group of older adults, indicating increased BP load during sleep. This increased BP load may be a risk factor for hypertension-related morbidity and increased disease burden in the elderly adults.
FUNDING
This work was supported by grants from the National Institute of Health—K23 AG 025963, NCRR K12 RR017643, and M01-RR00039.
Acknowledgments
The authors would like to thank the clinical staff of GCRC, Emory University School of Medicine, for the excellent care provided to the study participants.
References
- 1.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 2.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 3.Haas DC, Foster GL, Nieto FJ, et al. Age-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study. Circulation. 2005;111:614–621. doi: 10.1161/01.CIR.0000154540.62381.CF. [DOI] [PubMed] [Google Scholar]
- 4.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746–1752. [PubMed] [Google Scholar]
- 5.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 6.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 7.Phillips BA, Berry DT. Lipke-Molby TC. Sleep-disordered breathing in healthy, aged persons. Fifth and final year follow-up. Chest. 1996;110:654–658. doi: 10.1378/chest.110.3.654. [DOI] [PubMed] [Google Scholar]
- 8.Enright PL, Newman AB, Wahl PW, Manolio TA, Haponik EF, Boyle PJ. Prevalence and correlates of snoring and observed apneas in 5,201 older adults. Sleep. 1996;19:531–538. doi: 10.1093/sleep/19.7.531. [DOI] [PubMed] [Google Scholar]
- 9.Lanuois SH, Pepin JL, Levy P. Sleep apnea in the elderly: a specific entity? Sleep Med Rev. 2007;11:87–97. doi: 10.1016/j.smrv.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Lavie L, Lavie P. Ischemic preconditioning as a possible explanation for the age decline relative mortality in sleep apnea. Med Hypotheses. 2006;66:1069–1073. doi: 10.1016/j.mehy.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 11.Mehra R, Stone KL, Balckwell T, et al. Prevalence and correlates of sleep-disordered breathing in older men: osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2007;55:1356–1364. doi: 10.1111/j.1532-5415.2007.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munoz R, Duran-Cantolla J, Martinez-Vila E, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37:2317–2321. doi: 10.1161/01.STR.0000236560.15735.0f. [DOI] [PubMed] [Google Scholar]
- 13.Shimada K, Kawamoto A, Matsubayashi K, Ozawa T. Silent cerebrovascular disease in the elderly. Correlation with ambulatory pressure. Hypertension. 1990;16:692–699. doi: 10.1161/01.hyp.16.6.692. [DOI] [PubMed] [Google Scholar]
- 14.O’Sullivan C, Duggan J, Lyons S, Thornton J, Lee M, O’Brien E. Hypertensive target-organ damage in the very elderly. Hypertension. 2003;42:130–135. doi: 10.1161/01.HYP.0000084050.73533.C5. [DOI] [PubMed] [Google Scholar]
- 15.Hoshide S, Kario K, Hoshide Y, et al. Associations between nondipping of nocturnal blood pressure decrease and cardiovascular target organ damage in strictly selected community-dwelling normotensives. Am J Hypertens. 2003;16:434–438. doi: 10.1016/s0895-7061(03)00567-3. [DOI] [PubMed] [Google Scholar]
- 16.Stradling JR, Partlett J, Davies RJ, Siegwart D, Tarassenko L. Effect of short term graded withdrawal of nasal continuous positive airway pressure on systemic blood pressure in patients with obstructive sleep apnoea. Blood Press. 1996;5:234–240. doi: 10.3109/08037059609079677. [DOI] [PubMed] [Google Scholar]
- 17.Pankow W, Nabe B, Lies A, et al. Influence of sleep apnea on 24-hour blood pressure. Chest. 1997;112:1253–1258. doi: 10.1378/chest.112.5.1253. [DOI] [PubMed] [Google Scholar]
- 18.Loredo JS, Ancoli-Israel S, Dimsdale JE. Sleep quality and blood pressure dipping in obstructive sleep apnea. Am J Hypertens. 2001;14:887–892. doi: 10.1016/s0895-7061(01)02143-4. [DOI] [PubMed] [Google Scholar]
- 19.Ancoli-Israel S, Stepnowsky C, Dimsdale J, Marler M, Cohen-Zion M, Johnson S. The effect of race and sleep-disordered breathing on nocturnal BP “dipping”: analysis in an older population. Chest. 2002;122:1148–1155. doi: 10.1378/chest.122.4.1148. [DOI] [PubMed] [Google Scholar]
- 20.Cavelaars M, Tulen JHM, van Bemmel JH, van den Meiracker AH. Physical activity, dipping and haemodynamics. J Hypertens. 2004;22:2303–2309. doi: 10.1097/00004872-200412000-00012. [DOI] [PubMed] [Google Scholar]
- 21.O’Shea JC, Murphy MB. Nocturnal blood pressure dipping: a consequence of diurnal physical activity blipping? Am J Hypertens. 2000;13:601–606. doi: 10.1016/s0895-7061(99)00263-0. [DOI] [PubMed] [Google Scholar]
- 22.Kario K, Schwartz JE, Pickering TG. Ambulatory physical activity as a determinant of diurnal blood pressure variation. Hypertension. 1999;34:685–691. doi: 10.1161/01.hyp.34.4.685. [DOI] [PubMed] [Google Scholar]
- 23.Turgut F, Bayrak O, Kanbay M, et al. Circadian rhythm of blood pressure in patients with benign prostatic hyperplasia. Scand J Urol Nephrol. 2008;42:47–52. doi: 10.1080/00365590701520008. [DOI] [PubMed] [Google Scholar]
- 24.Graugaard-Jensen C, Rittig S, Djurhuus JC. Nocturia and circadian blood pressure profile in healthy elderly male volunteers. J Urol. 2006;176:1034–1039. doi: 10.1016/j.juro.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 25.Kjelsberg E, Hartvig P. Can morbidity be inferred from prescription drug use? Results from a nation-wide prison population study. Eur J Epidemiol. 2005;20:587–592. doi: 10.1007/s10654-005-8156-9. [DOI] [PubMed] [Google Scholar]
- 26.Moxey ED, O’Connor JP, Novielli KD, Teutsch S, Nash DB. Prescription drug use in the elderly: a descriptive analysis. Health Care Financ Rev. 2003;24:127–141. [PMC free article] [PubMed] [Google Scholar]
- 27.Baumgart P, Kamp J. Accuracy of the SpaceLabs Medical 90217 ambulatory blood pressure monitor. Blood Press Monit. 1998;3:303–307. [PubMed] [Google Scholar]
- 28.Staessen JA, Bieniaszewski L, O’Brien ET, Fagard R. What is normal blood pressure on ambulatory monitoring. Nephrol Dial Transplant. 1996;11:241–245. doi: 10.1093/oxfordjournals.ndt.a027247. [DOI] [PubMed] [Google Scholar]
- 29.Anonymous. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 30.Redline S, Min NI, Shahar E, Rapoport D, O’Connor G. Polysomnographic predictors of blood pressure and hypertension: is one index best? Sleep. 2005;28:1122–1130. doi: 10.1093/sleep/28.9.1122. [DOI] [PubMed] [Google Scholar]
- 31.Dingli K, Coleman EL, Vennelle M, et al. Evaluation of a portable device for diagnosing the sleep apnoea/hypopnoea syndrome. Eur Respir J. 2003;21:253–259. doi: 10.1183/09031936.03.00298103. [DOI] [PubMed] [Google Scholar]
- 32.Perk G, Mekler J, Ben Ishay D, Bursztyn M. Non-dipping in diabetic patients: insights from the siesta. J Hum Hypertens. 2002;16:435–438. doi: 10.1038/sj.jhh.1001412. [DOI] [PubMed] [Google Scholar]
- 33.Kondo K, Matsubara T, Nakamura J, Hotta N. Characteristic patterns of circadian variation in plasma catecholamine levels, blood pressure and heart rate variability in Type 2 diabetic patients. Diabet Med. 2002;19:359–365. doi: 10.1046/j.1464-5491.2002.00720.x. [DOI] [PubMed] [Google Scholar]
- 34.Middelkoop HA, Smilde-van den Doel DA, Neven AK, Kamphuisen HA, Springer CP. Subjective sleep characteristics of 1,485 males and females aged 50–93: effects of sex and age, and factors related to self-evaluated quality of sleep. J Gerontol A Biol Sci Med Sci. 1996;51:M108–M115. doi: 10.1093/gerona/51a.3.m108. [DOI] [PubMed] [Google Scholar]
- 35.Jackson S. Lower urinary tract symptoms and nocturia in men and women: prevalence, aetiology and diagnosis. BJU Int. 1999;84((suppl 1)):5–8. doi: 10.1046/j.1464-410x.84.s1.6.x. [DOI] [PubMed] [Google Scholar]
- 36.Staessen JA, Thijs L, Fagard R, et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators. JAMA. 1999;282:539–546. doi: 10.1001/jama.282.6.539. [DOI] [PubMed] [Google Scholar]
- 37.Verdecchia P, Porcellati C, Schillaci G, et al. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793–801. doi: 10.1161/01.hyp.24.6.793. [DOI] [PubMed] [Google Scholar]
- 38.Ohkubo T, Imai Y, Tsuji I, et al. Relation between nocturnal decline in blood pressure and mortality. The Ohasama Study. Am J Hypertens. 1997;10:1201–1207. doi: 10.1016/s0895-7061(97)00274-4. [DOI] [PubMed] [Google Scholar]
- 39.Turnbull F. Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 40.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 41.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]