Abstract
Background
The pathophysiological mechanisms that underlie health disparities by socioeconomic status and race/ethnicity are poorly understood. Promising new research suggests that the burden of persistent infection may influence adult disease risk and mortality. This article examines how multiple persistent infections cluster within individuals and how this clustering varies by socioeconomic position and race/ethnicity in U.S. adults.
Methods
We analyze data from the National Health and Nutrition Examination Survey III (N = 19,275) for adults aged 17–90 years. The clustering of infections within individuals is studied using tetrachoric correlations. Multiple indicator multiple cause models are used to analyze the infection burden construct as measured by seropositivity to Helicobacter pylori, cytomegalovirus, herpes simplex virus-1, and hepatitis B, focusing on the burden's distribution by socioeconomic position and race/ethnicity. The results are corroborated using ordered logistic regression for a commonly used count index of individual infections.
Results
Seroprevalence of individual persistent infections is positively correlated, suggesting common factors related to exposure or susceptibility. Education, income, and race/ethnicity are strong and significant independent predictors of infection burden in U.S. adults in all models.
Conclusion
The disproportionate burden of persistent infections among disadvantaged groups across all ages may be one biologic pathway by which low socioeconomic position is related to increased rates of morbidity and mortality in the United States.
Keywords: Socioeconomic, Race, Ethnic, United states, Adults, Infection, Biomarkers
HEALTH of U.S. adults is strongly associated with their socioeconomic position (1). Adults with more education and income have a lower burden of disease, fewer physical limitations, less disability, and longer life expectancy (2–4). The pathophysiological mechanisms linking lower socioeconomic position (SEP) to worse health, however, are poorly understood. Exposure to infections and inflammation has been proposed as a potentially important determinant of chronic disease and mortality risk (5), but current knowledge of the socioeconomic patterning of infection burden in the U.S. population is limited.
Increasing evidence points to links between chronic disease and persistent infections including herpesviruses, Helicobacter pylori, periodontal pathogens, and hepatitis viruses. For example, the herpesviruses cytomegalovirus (CMV) and herpes simplex virus type 1 (HSV-1) have been linked to cardiovascular disease, frailty, cognitive outcomes, and Alzheimer's disease (6–9). Infection with CMV and HSV-1 is common in childhood (10), with the viruses remaining latent in the host throughout the life course. The bacterium H. pylori can also lie dormant in the body for decades until the bacteria-host equilibrium is disturbed. Besides its role in peptic ulcer disease, H. pylori has been implicated in the development of stroke and ischemic heart disease through inflammatory pathways, lipid alterations, and homocysteine-induced endothelial dysfunction (11). Hepatitis B virus (HBV), known for its role in chronic liver disease, has been hypothesized to contribute to atherogenic diseases via systemic effects on immune response and colonization of vascular tissues (12,13). Common periodontal pathogens such as Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis have also been implicated in coronary heart disease and stroke and have been found in atherosclerotic plaques (14,15). Persistent infection has also been implicated as a factor in the development of functional limitations (1,5). For example, seropositivity to CMV was associated with frailty in one study, and in another study, higher CMV IgG antibody levels were associated with functional impairment (9,16).
The presence of multiple persistent infections may contribute to morbidity beyond the impact of individual infections (17–20). Presence of one infection might raise the risk of other infections or increase the deleterious effects of other pathogens, or both. For example, work by Vilkuna-Rautiainen and colleagues suggests that combined infection with HSV and periodontal bacteria may increase the risk for cardiovascular disease through decreasing levels of high-density lipoprotein cholesterol more than each pathogen alone (21). From a population perspective, burden of infection and inflammation has been hypothesized as an explanation for cohort changes in life expectancy as well as black or white differences in artherosclerosis in the early 20th century (5,22,23).
Recent research has begun addressing socioeconomic and race/ethnic differences in individual infection status in the United States. Adults with less education and income, as well as minorities, have been shown to have a higher prevalence of hepatitis viruses, Toxoplasma gondii, H. pylori, HSV-2, and CMV (10,24–26). Data from the Whitehall study in the UK found that employment grade was related to burden of infection as measured by the summed infection serostatus for three pathogens (CMV, HSV-1, and Chlamydia pneumonia) (27). However, there is little research on the relationship between social factors and coinfection with multiple persistent infections in the United States. Therefore, the purpose of this study was to investigate whether education, income, and race/ethnicity are related to infection burden in a representative sample of U.S. adults. In addition, we attempt to improve upon earlier approaches to studying burden of infection by constructing a novel latent variable representing multiple persistent infections within individuals.
METHODS
Sample
The analyses used data from the National Health and Nutrition Examination Survey (NHANES) III survey conducted by the National Center for Health Statistics between 1988 and 1994. NHANES III was a cross-sectional, stratified, multistage probability sample of the civilian noninstitutionalized U.S. population aged 2 months to 90 years, with an oversample of Mexican American and non-Hispanic black respondents. The survey was collected in two phases (1988–1991 and 1991–1994), each individually designed to give nationally representative estimates. Some variables were not collected during both phases; specifically, H. pylori was only measured only during phase I and dental pathogens only in phase II. Data were collected in household interviews, clinical examinations, and laboratory tests. Details of the sampling design and protocol have been previously reported (28,29). We analyzed data for non-Hispanic white, non-Hispanic black, and Mexican adults aged 17–90 years (N = 19,275). There were no missing observations on most demographic variables, including race/ethnicity. Education and income variables were missing in 1% and 10% of cases, respectively. Among the infection variables, the proportion missing ranged from 14% for CMV to 21% for H. pylori (out of phase I respondents). Respondents with missing information on any given pathogen were excluded from the corresponding pathogen-specific analyses including the count index models, but they were included in the multiple indicator multiple cause (MIMIC) models unless all four pathogen data points were missing. Participants missing infection data tended to be older than those with valid data but otherwise not systematically different in key sociodemographic variables.
Measures
Laboratory assays.—
NHANES III conducted the seropositivity coding. The laboratory procedures, analytical methods, and quality control are described in detail in the NHANES III laboratory manual (30). The cut points for seropositivity to each infection are based on commercially available immunoassay clinical diagnostics that provide information on IgG antibody seropositivity (HSV, H. pylori, CMV) and the presence of HBV core antigen as described in the NHANES lab manuals (30). For P. gingivalis and A. actinomycetemcomitans, continuous IgG antibody measured in enzyme-linked immunosorbent assay units (EU) values were provided by NHANES for each of these pathogens. We used the standard cutoffs for P. gingivalis (≥168 EU) and A. actinomycetemcomitans (≥156 EU).
Burden of infection.—
Helicobacter pylori, CMV, HSV-1, and HBV were used for the infection burden analyses. Dental pathogens were not included because they were measured only during phase II of the survey, whereas H. pylori was measured only in phase I. Due to the lack of overlap, we could only include one of these. We chose H. pylori because the number of participants who had values for H. pylori contemporaneous with other pathogens (HSV, CMV, HBV) was more than double compared with those with dental pathogen values measured at phase II. The burden variable was constructed in two ways: (a) using confirmatory factor analysis incorporated into MIMIC models and (b) as a count index (range 0–4) where we added the seropositive status for the four infections.
Socioeconomic position.—
Education was trichotomized: less than high school (<12 years), high school completion (reference), and more than high school (>12 years). Income categories were based on poverty-income ratio, the ratio of the family's income to the federal poverty line for a specific household composition. The cut points were 125% and 250% of the poverty threshold, with middle category as reference.
Demographic variables.—
Race/ethnicity was classified as non-Hispanic white, non-Hispanic black, and Mexican American. We adjusted for age in years, gender, census region, rural or urban residence, marital status, foreign born, and household size.
Statistical Analysis
First, prevalence of individual infections by race/ethnicity was compared using chi-square tests. Second, associations among individual infections were measured using tetrachoric correlations with pairwise deletion and exact two-sided significance tests.
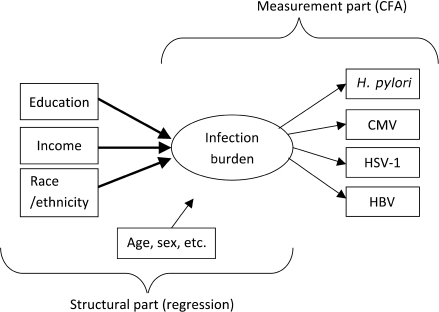
Third, infection burden was modeled using MIMIC models, a special case of structural equation models (31), to capture the underlying clustering of these infections within individuals. The MIMIC model had two components: (a) measurement part, where a latent variable (infection burden), was modeled as influencing observed indicators (individual infections), and (b) structural part where the latent variable was a function of observed predictors (SEP and race/ethnicity). The model is shown schematically in Figure 3. We used a weighted least squares mean and variance-adjusted estimator, optimal for data with missing values and ordinal dependent variables (27). This estimator allowed using data from both phases of the survey. The fit of the model to data was evaluated by comparative fit index (CFI) and root mean square error of approximation (RMSEA). These two measures indicate a satisfactory fit to the data if their values are above .9 and below .05, respectively. Results from the structural component are presented as standardized coefficients.
Finally, we estimated ordered logit models to test the association of SEP and race/ethnicity with the count infection burden index. These models were estimated using only data from phase I of the survey because H. pylori was not measured during phase II. The descriptive and multivariate analyses were adjusted for sampling weights and clustering. Analyses were conducted using Stata 10.0 (2007; StataCorp, College Station, TX) and Mplus 4.21 (2007; Muthén and Muthén, Los Angeles, CA).
RESULTS
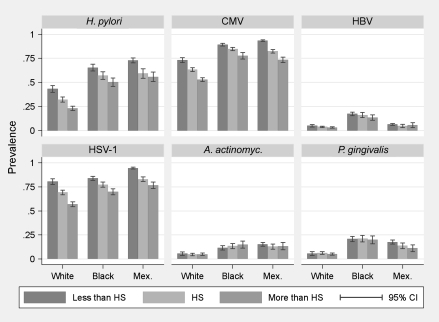
Descriptive statistics are presented in Table 1. Non-Hispanic white adults had a lower prevalence of each persistent infection, as well as burden of infection, compared with non-Hispanic black or Mexican American adults. Figure 1 shows the age- and sex-adjusted prevalence of each infection by race/ethnicity and education (patterns by income were similar—not shown). Black and Mexican American adults and those with less education evidenced higher infection prevalence.
Table 1.
Descriptive Statistics by Race/Ethnicity, National Health and Nutrition Examination Survey III
| Total (N = 19,275), % | Race/ethnicity |
Race/ethnic Differencea | |||
| White (n = 8,483), % | Black (n = 5,486), % | Mexican (n = 5,306), % | |||
| Age (y) | p < .001 | ||||
| 17–34 | 37.7 | 35.3 | 45.3 | 55.4 | |
| 35–59 | 40.3 | 40.8 | 38.8 | 35.2 | |
| 60–90 | 22.1 | 23.9 | 15.9 | 9.3 | |
| Female | 52.2 | 52.1 | 55.5 | 47.8 | p < .001 |
| Race/ethnicity | — | ||||
| White | 82.2 | — | — | — | |
| Black | 12.2 | — | — | — | |
| Mexican | 5.7 | — | — | — | |
| Education | p < .001 | ||||
| Less than high school | 25.8 | 22.2 | 34.2 | 59.8 | |
| High school (12 y) | 34.3 | 34.6 | 37.2 | 23.6 | |
| More than high school | 39.9 | 43.2 | 28.6 | 16.6 | |
| Household incomeb | p < .001 | ||||
| Low income | 17.4 | 13.2 | 35.1 | 46.2 | |
| Mid-income | 26.2 | 25.2 | 32.0 | 30.6 | |
| High income | 56.4 | 61.7 | 32.9 | 23.1 | |
| Infections | |||||
| H. pylori | 32.7 | 27.9 | 53.7 | 62.1 | p < .001 |
| CMV | 61.6 | 56.8 | 82.5 | 85.3 | p < .001 |
| HSV-1 | 67.6 | 64.8 | 76.4 | 87.8 | p < .001 |
| HBV | 4.9 | 3.5 | 14.6 | 4.6 | p < .001 |
| P. gingivalis | 7.2 | 5.2 | 19.0 | 12.2 | p < .001 |
| A. actinomyc. | 6.6 | 5.0 | 13.2 | 15.2 | p < .001 |
| Infection burden index | p < .001 | ||||
| 0 | 16.3 | 18.5 | 5.7 | 3.2 | |
| 1 | 27.0 | 29.6 | 16.1 | 10.4 | |
| 2 | 31.7 | 31.5 | 33.1 | 32.2 | |
| 3 | 23.0 | 19.3 | 36.4 | 51.4 | |
| 4 | 2.0 | 1.0 | 8.7 | 2.8 | |
Notes: H. pylori = Helicobacter pylori; CMV = cytomegalovirus; HBV = hepatitis B virus; HSV-1 = herpes simplex virus type 1; A. actinomyc. = Actinobacillus actinomycetemcomitans; P. gingivalis = Porphyromonas gingivalis.
p values of chi-square tests for the difference in proportions by race/ethnicity.
Income was divided into three categories as low income, <125% of poverty threshold; mid-income = 125%–250% of poverty threshold; and high income, >250% of poverty threshold.
Figure 1.
Age- and sex-standardized prevalence of individual infections.
HS = high school; CI = confidence interval; H. pylori = Helicobacter pylori; CMV = cytomegalovirus; HBV = hepatitis B virus; HSV-1 = herpes simplex virus type 1; A. actinomyc. = Actinobacillus actinomycetemcomitans; P. gingivalis = Porphyromonas gingivalis.
Correlations among individual infections are shown in Table 2. The strongest correlations were among H. pylori, CMV, and HSV-1, between .42 and .46. The associations between HBV, P. gingivalis, and A. actinomycetemcomitans were weaker but significant for most pairs.
Table 2.
Tetrachoric Correlations of Seroprevalence Status for Six Persistent Infections
| H. pylori | CMV | HSV-1 | HBV | P. gingivalis | A. actinomyc. | |
| H. pylori | 1 | |||||
| CMV | .46* | 1 | ||||
| HSV-1 | .42* | .45* | 1 | |||
| HBV | .23* | .29* | .15* | 1 | ||
| P. gingivalis | —a | .17* | .12† | .16* | 1 | |
| A. actinomyc. | —a | .17* | .02‡ | .03‡ | .31* | 1 |
Notes: H. pylori = Helicobacter pylori; CMV = cytomegalovirus; HBV = hepatitis B virus; HSV-1 = herpes simplex virus type 1; A. actinomyc. = Actinobacillus actinomycetemcomitans; P. gingivalis = Porphyromonas gingivalis.
Data not available because H. pylori was measured only during phase I and P. gingivalis and A. actinomycetemcomitans only during phase II of the National Health and Nutrition Examination Survey III.
p < .0001;
p < .002;
p > .1.
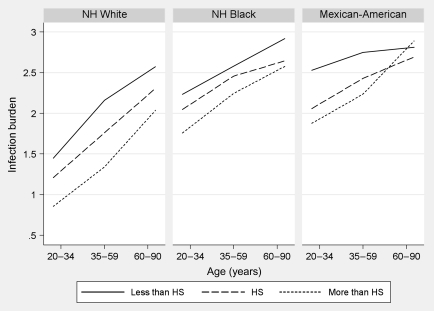
Figure 2 shows the mean level of the count infection burden ranging from 0 to 4 across age, by race/ethnicity and education (results by income were similar—not shown). Infection burden increased monotonically with age. In all age groups, the burden was higher for those with less education across all race/ethnic groups, except for the crossover among elderly Mexican Americans.
Figure 2.
Mean infection burden (0–4) by Age, Race, and Education.
The y-axis shows the mean infection count and the x-axis the age range. NH = non-Hispanic; HS = high school.
Figure 3.
Diagram of the Full Multiple Indicator Multiple Cause (MIMIC) Model.
The MIMIC model has two components: (a) a measurement component, where one latent variable (ie, infection burden), is modeled as underlying a structure of observed indicators (ie, individual infections), and (b) a structural component where the latent variable is modeled as a function of observed predictors (socioeconomic position and race/ethnicity). For parsimony, the figure does not show correlations among the predictors or the residual errors for the burden indicators and their covariance. H. pylori = Helicobacter pylori; CMV = cytomegalovirus; HBV = hepatitis B virus; HSV-1 = herpes simplex virus type 1; CFA = confirmatory factor analysis.
Results from the MIMIC models are summarized in Table 3. All three models had an acceptable fit to the data (CFI ≥ .90 and RMSEA ≤ .02). The measurement component indicated that the latent burden construct explained a significant proportion of variance in the observed infections. The R2 values were between .33 and .58 for H. pylori, CMV, and HSV-1, and .14 to .15 for HBV. All unconstrained factor loadings were reasonably high and had p values less than .01, suggesting a strong relationship with the underlying burden variable. These results corroborated the correlation findings and supported the conceptualization of infection burden as a valid latent construct. The standardized coefficients in the model's structural component indicate change in the infection burden, measured in standard deviations, for one standard deviation change in the predictor. Lower education and income were associated with a significantly higher burden of infection. Independent of education and income, non-Hispanic black adults and Mexican Americans had a significantly higher burden of infection compared with non-Hispanic white adults. The predictors explained 59%–63% of variance in the burden variable.
Table 3.
Multiple Indicator Multiple Cause Models of Latent Infection Burden on SEP and Race/Ethnicitya
| Model 1 (n = 15,342) |
Model 2 (n = 14,878) |
Model 3 (n = 13,941) |
||||
| Measurement Part | Loading | R2 | Loading | R2 | Loading | R2 |
| H. pylori | .70 | .49 | .66 | .43 | .69 | .48 |
| CMV | .76 | .58 | .75 | .57 | .76 | .58 |
| HSV-1 | .58 | .33 | .59 | .35 | .58 | .34 |
| HBV | .39 | .15 | .38 | .15 | .38 | .14 |
| Structural part | Coef. | s.e. | Coef. | s.e. | Coef. | s.e. |
| Education | ||||||
| Less than HS | .13 | .04 | — | — | .10 | .04 |
| HS | 0 | — | — | — | 0 | — |
| More than HS | −.18 | .04 | — | — | −.16 | .04 |
| Income | ||||||
| Low income | — | — | .07 | .04 | .05 | .04 |
| Mid-income | — | — | 0 | — | 0 | — |
| High income | — | — | −.16 | .03 | −.11 | .03 |
| Race/ethnicity | ||||||
| White | 0 | — | 0 | — | 0 | — |
| Black | .30 | .04 | .28 | .04 | .27 | .05 |
| Mexican | .18 | .05 | .19 | .05 | .16 | .05 |
| Structural part R2 | .61 | .59 | .63 | |||
| Overall model fit | ||||||
| CFI | .90 | .92 | .90 | |||
| RMSEA | .02 | .02 | .02 | |||
Notes: HS = high school; H. pylori = Helicobacter pylori; CMV = cytomegalovirus; HBV = hepatitis B virus; HSV = herpes simplex virus type 1; SEP = socioeconomic position; CFI = comparative fit index; RMSEA = root mean square error of approximation.
For the measurement part of the model, standardized factor loadings (indicator R2) are presented. All unconstrained indicators have p values <.001. In the structural part of the model, the latent infection burden is regressed on SEP and race/ethnicity, adjusting for age, gender, census region, rural residence, marital status, foreign-born status, and household size. Shown are standardized coefficients (standard errors). Models with education restrict age to 20 and above. All p values in the table were <.01 for nonreferent coefficients.
Results from the ordered logit models (Table 4) corroborated the MIMIC model findings. Both SEP and race/ethnicity were strongly associated with the count burden index. Individuals with less than a high school education had roughly 50% higher odds of having an additional infection compared with high school graduates, whereas those with postsecondary education had 50% lower odds. Income had similar effects, whereby low income was associated with 33% higher odds of an additional infection, and high income with 45% lower odds, compared with the middle-income group. Education and income had independent effects on the infection burden (model 3). Net of socioeconomic position, Mexican Americans had three to four times the odds of an additional infection, and non-Hispanic black adults had four to five times the odds, compared with non-Hispanic white adults. Interactions of race/ethnicity and SEP were not statistically significant, suggesting that the effect of SEP was similar within the three race/ethnic groups.
Table 4.
Ordered Logistic Models of Count Infection Burden Index (0–4) on SEP and Race/Ethnicitya
| Model 1 (n = 7,020) |
Model 2 (n = 6,741) |
Model 3 (n = 6,279) |
||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Education | ||||||
| Less than HS | 1.57 | 1.29–1.91 | — | — | 1.44 | 1.19–1.74 |
| HS | 1.00 | — | — | — | 1.00 | — |
| More than HS | 0.52 | 0.43–0.63 | — | — | 0.55 | 0.45–0.67 |
| Income | ||||||
| Low income | — | — | 1.33 | 1.07–1.64 | 1.20 | 0.99–1.46* |
| Mid-income | — | — | 1.00 | — | 1.00 | — |
| High income | — | — | 0.55 | 0.46–0.66 | 0.66 | 0.54–0.80 |
| Race/ethnicity | ||||||
| White | 1.00 | — | 1.00 | — | 1.00 | — |
| Black | 5.07 | 4.14–6.19 | 4.05 | 3.17–5.17 | 4.42 | 3.54–5.51 |
| Mexican | 3.44 | 2.57–4.60 | 3.85 | 2.79–5.31 | 3.10 | 2.27–4.23 |
Notes: HS = high school; OR = odds ratio; CI = confidence interval.
Model 1 includes education, model 2 includes income, and model 3 includes both. All models adjust for age, gender, census region, rural residence, marital status, foreign-born status, and household size. Models with education restrict age to 20 and above.
p = .07. All other p values ≤.01.
DISCUSSION
This study identified socioeconomic and racial or ethnic disparities in the burden of multiple persistent infections in a nationally representative sample of U.S. adults. The individual infections are positively correlated with one another, suggesting a nonrandom clustering of infections within individuals. Moreover, this clustering is systematically associated with socioeconomic characteristics. Adults with more education and income have a lower burden of persistent infections throughout adulthood, and non-Hispanic white adults have a lower burden than minority adults. These findings suggest that the burden of persistent infection may be one pathway through which lower socioeconomic position “gets under the skin” and leads to an earlier onset of disease and mortality.
Our results are consistent with recent work from the UK that found occupational grade associated with pathogen burden in 451 middle-aged adults, as measured by the summed serostatus of CMV, HSV-1 and Chlamydia pneumoniae (27). Our study adds to this earlier research by investigating a broader set of infections thought to be associated with chronic disease, a wider age range, more flexible analytic methods, and two measures of socioeconomic position in a large, nationally representative sample of U.S. adults.
The results of this study suggest that race/ethnic differences in the burden of infection are not due solely to differences in income or education. One reason for the race/ethnic differentials may be transmission dynamics, whereby groups that live and work together may retain their historically high infection rates even as socioeconomic conditions change. Foreign-born Mexican Americans have higher infection rates than native-born Mexican Americans, and these higher rates may increase transmission within the Mexican American community, compared with the non-Hispanic white adults. Another potential explanation is that even at a given level of education or income, racial and ethnic minorities may be subject to increased social stress (32). This stress may downregulate immunity, making non-Hispanic black adults and Mexican Americans more susceptible to infections (33). Although the current data do not allow investigation of these hypotheses, it is a promising direction for future research.
Growing evidence supports the notion of infection burden as an important risk factor for chronic diseases. There are several ways in which burden of infection may affect chronic health outcomes. Depending on the infection and where it resides within the hosts cells, persistent infection may lead to (a) direct damage to infected host tissue, (b) chronic immune reactivity and promotion of a systemic proinflammatory environment characterized by an increase in cytokines and acute phase proteins (19), and (c) changes in normal cellular processes through pathogen-host genomic integration (eg, alterations in tumor suppressor gene function) (34).
The relationship between SEP and inflammatory markers related to both infectious and chronic diseases have been established recently (35). In the United States, those with more education were found to have significantly lower levels of inflammatory markers including C-reactive protein, interleukin-6, and soluble intercellular adhesion molecule-1. The biologic inducers of inflammation, however, are rarely studied. Little is known regarding the role of persistent infections as mediators of the relationship between SEP and inflammation. An important next step is to test whether clustering of persistent infections is a source of the increased inflammation levels found among low-SEP adults.
Because timing of exposure to each infection is not available in the NHANES data set, it is not possible to distinguish whether the observed SEP and race/ethnicity patterns are a result of differentials in initial exposure or heightened susceptibility to infections, or an interaction of the two. Second, it is likely that there are other pathogens that could be added to our burden index, including those not measured in NHANES III and those yet to be discovered. Nevertheless, pathogens included in this study have been implicated in chronic age-related diseases and therefore likely represent key infections of interest for disease disparity research (6–9). In addition, it is not possible to distinguish whether the observed age patterns for infection burden reflect age or cohort effects in this cross-sectional study. However, the availability of a wide range of persistent infections in a very large nationally representative sample of U.S. adults has allowed us to move beyond traditional methods of simply summing the number of infections and allowed us to test the degree to which infection with one pathogen is associated with other infections.
In conclusion, this is the first published study to our knowledge that has examined the influence of socioeconomic position on infection burden in a nationally representative sample of the U.S. population. Future studies are needed to study infection burden as a potential mediator of the SEP-health gradient. Given the growing research implicating persistent infections in an array of chronic disease processes, reducing social disparities in the burden of infection may be an important target for reducing health disparities and enhancing health of the population.
References
- 1.Adler NE, Boyce T, Chesney MA, et al. Socioeconomic status and health: the challenge of the gradient. Am Psychol. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Elo IT, Martikainen PT, Smith KP. Socioeconomic differentials in mortality in Finland and the United States: the role of education and income. Eur J Popul. 2006;22:179–203. [Google Scholar]
- 3.Molla MT, Madans JH, Wagener DK. Differentials in adult mortality and activity limitations by years of education in the United States at the end of the 1990s. Popul Dev Rev. 2004;30:624–646. [Google Scholar]
- 4.Schoeni RF, Martin LG, Andreski PM, et al. Persistent and growing socioeconomic disparities in disability among the elderly: 1982–2002. Am J Public Health. 2005;95:2065–2070. doi: 10.2105/AJPH.2004.048744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crimmins EM, Finch CE. Infection, inflammation, height, and longevity. Proc Natl Acad Sci USA. 2006;103:498–503. doi: 10.1073/pnas.0501470103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorlie PD, Nieto FJ, Adam E, et al. A prospective study of cytomegalovirus, herpes simplex virus 1, and coronary heart disease: the atherosclerosis risk in communities (ARIC) study. Arch Intern Med. 2000;160:2027–2032. doi: 10.1001/archinte.160.13.2027. [DOI] [PubMed] [Google Scholar]
- 7.Itzhaki RF, Wozniak MA, Appelt DM, et al. Infiltration of the brain by pathogens causes Alzheimer's disease. Neurobiol Aging. 2004;25:619–627. doi: 10.1016/j.neurobiolaging.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 8.Aiello AE, Haan MN, Blythe L, et al. The influence of latent viral infection on rate of cognitive decline over 4 years. J Am Geriatr Soc. 2006;54:1046–1054. doi: 10.1111/j.1532-5415.2006.00796.x. [DOI] [PubMed] [Google Scholar]
- 9.Schmaltz HN, Fried LP, Xue QL, et al. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53:747–754. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- 10.Staras SAS, Dollard SC, Radford KW, et al. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis. 2006;43:1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 11.Manolakis A, Kapsoritakis AN, Potamianos SP. A review of the postulated mechanisms concerning the association of Helicobacter pylori with ischemic heart disease. Helicobacter. 2007;12:287–297. doi: 10.1111/j.1523-5378.2007.00511.x. [DOI] [PubMed] [Google Scholar]
- 12.Sung J, Song YM, Choi YH, et al. Hepatitis B virus seropositivity and the risk of stroke and myocardial infarction. Stroke. 2007;38:1436–1441. doi: 10.1161/STROKEAHA.106.466268. [DOI] [PubMed] [Google Scholar]
- 13.Ishizaka N, Ishizaka Y, Takahashi E, et al. Increased prevalence of carotid atherosclerosis in hepatitis B virus carriers. Circulation. 2002;105:1028–1030. doi: 10.1161/hc0902.105718. [DOI] [PubMed] [Google Scholar]
- 14.Pussinen PJ, Jousilahti P, Alfthan G, et al. Antibodies to periodontal pathogens are associated with coronary heart disease. Arterioscler Thromb Vasc Biol. 2003;23:1250–1254. doi: 10.1161/01.ATV.0000072969.71452.87. [DOI] [PubMed] [Google Scholar]
- 15.Pussinen PJ, Alfthan G, Jousilahti P, et al. Systemic exposure to Porphyromonas gingivalis predicts incident stroke. J Atherosclerosis. 2007;193:222–228. doi: 10.1016/j.atherosclerosis.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 16.Aiello AE, Haan MN, Pierce CM, Simanek AM, Liang J. Persistent infection, inflammation and functional impairment in older Latinos. J Gerontol Med Sci. 2008;63:610–618. doi: 10.1093/gerona/63.6.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elkind MSV, Cole JW. Do common infections cause stroke? Semin Neurol. 2006;26:88–99. doi: 10.1055/s-2006-933312. [DOI] [PubMed] [Google Scholar]
- 18.Espinola-Klein C, Rupprecht HJ, Blankenberg S, et al. Impact of infectious burden on extent and long-term prognosis of atherosclerosis. Circulation. 2002;105:15–21. doi: 10.1161/hc0102.101362. [DOI] [PubMed] [Google Scholar]
- 19.Zhu J, Quyyumi AA, Norman JE, et al. Effects of total pathogen burden on coronary artery disease risk and C-reactive protein levels. Am J Cardiol. 2000;85:140–146. doi: 10.1016/s0002-9149(99)00653-0. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Real J-M, Lopez-Bermejo A, Vendrell J, et al. Burden of infection and insulin resistance in healthy middle-aged men. Diabetes Care. 2006;29:1058–1064. doi: 10.2337/diacare.2951058. [DOI] [PubMed] [Google Scholar]
- 21.Vilkuna-Rautiainen T, Pussinen PJ, Roivainen M, et al. Serum antibody response to periodontal pathogens and herpes simplex virus in relation to classic risk factors of cardiovascular disease. Int J Epidemiol. 2006;35:1486–1494. doi: 10.1093/ije/dyl166. [DOI] [PubMed] [Google Scholar]
- 22.Costa DL, Helmchen LA, Wilson S. Race, infection, and arteriosclerosis in the past. Proc Natl Acad Sci USA. 2007;104:13219–13224. doi: 10.1073/pnas.0611077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrucci L, Ble A, Bandinelli S, et al. A flame burning within. Aging Clin Exp Res. 2004;16:240–243. doi: 10.1007/BF03327390. [DOI] [PubMed] [Google Scholar]
- 24.McQuillan GM, Kruszon-Moran D, Kottiri BJ, et al. Racial and ethnic differences in the seroprevalence of 6 infectious diseases in the United States: data from NHANES III, 1988–1994. Am J Public Health. 2004;94:1952–1958. doi: 10.2105/ajph.94.11.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Everhart JE, Kruszon-Moran D, Perez-Perez GI, et al. Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. J Infect Dis. 2000;181:1359–1363. doi: 10.1086/315384. [DOI] [PubMed] [Google Scholar]
- 26.Dowd JB, Aiello AE, Alley DE. Socioeconomic gradients in cytomegalovirus seropositivity in the U.S.: NHANES III. Epidemiol Infect. 2009;137:58–65. doi: 10.1017/S0950268808000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steptoe A, Shamaei-Tousi A, Gylfe A, et al. Socioeconomic status, pathogen burden, and cardiovascular disease risk. Heart. 2007;93:1567–1570. doi: 10.1136/hrt.2006.113993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Center for Health Statistics. Laboratory Procedures Used for the Third National Health and Nutrition Survey (NHANES III), 1988–1994. Hyattsville, MD: Department of Health and Human Services, National Center for Health Statistics, Centers for Disease Control and Prevention. 1996; 2001. [Google Scholar]
- 29.National Center for Health Statistics. Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988–1994. 1994 U.S.D.o.H.a.H. Services. [Google Scholar]
- 30.NHANESIII. Sample Design: NHANES III Laboratory Reference Manuals and Reports. Hyattsville, MD: Department of Health and Human Services, National Center for Health Statistics, Centers for Disease Control and Prevention; 1996. [Google Scholar]
- 31.Muthén LK, Muthén BO. Mplus User's Guide. 4th ed. Los Angeles, CA: Muthén and Muthén; 2007. [Google Scholar]
- 32.Williams DR, Yan Y, Jackson JS, et al. Racial differences in physical and mental health: socio-economic status, stress and discrimination. J Health Psychol. 1997;2:335–351. doi: 10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]
- 33.Cohen S. Social status and susceptibility to respiratory infections. Ann NY Acad Sci. 1999;896:246–253. doi: 10.1111/j.1749-6632.1999.tb08119.x. [DOI] [PubMed] [Google Scholar]
- 34.O’Connor SM, Taylor CE, Hughes JM. Emerging infectious determinants of chronic diseases. (PERSPECTIVE) Emerg Infect Dis. 2006;12:1051. doi: 10.3201/eid1207.060037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loucks EB, Sullivan LM, Hayes LJ, et al. Association of educational level with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol. 2006;163:622–628. doi: 10.1093/aje/kwj076. [DOI] [PubMed] [Google Scholar]