Abstract
Background
We examined age differences in levels of biological risk factors in the U.S. population by poverty status. It is not clear how socioeconomic status differentials in biological risk change with age because of mortality.
Methods
We used two nationally representative samples (National Health and Nutrition Examination Survey [NHANES] III, 1988–1994, and NHANES, 1999–2004) with data for more than 12,000 people aged 20 and older in each data set to examine biological risk for persons in families with incomes below and close to poverty level and those with higher income. We examined how mortality and life expectancy in the earlier sample are related to poverty status and biological risk. We examined life table survivorship to clarify how mortality differentially removes those who are poor and those with high biological risk from the population.
Results
Differences in biological risk by poverty status are larger before old age and become insignificant at older ages. Life expectancy at age 20 differs markedly by biological risk and poverty status.
Conclusions
Population differentials in health at older ages result from a lifetime of differences. Socioeconomic differences in health in old age disappear because of health and mortality differentials at earlier ages. Poorer people “age” earlier and this affects the age pattern of social differentials.
Keywords: Poverty, Biological risk, Mortality, Gender, Life expectancy, NHAHES, SES differentials
PEOPLE who have less education and who are poorer are more likely to experience earlier disease onset, loss of functioning, and physical impairment (1–4). Hayward and his colleagues have reported onset of diseases and death 5–10 years earlier for persons with lower socioeconomic status (SES) (1,2). The average number of biological risk factors indicating physiological dysregulation is also higher for poorer people and people with less education (5,6). Many scholars report that SES differentials in health and mortality are reduced at older ages (2,7–10). It is also true that race or ethnic differences in mortality are relatively small at the oldest ages (11,12). Population levels of biological risk also level off at older ages (7).
This article investigates socioeconomic differences by age in an indicator of biological risk or physiological dysregulation and how both poverty and biological risk are related to morality. Our hypothesis is that socioeconomic differences in health are reduced at older ages because persons who are poor and who have higher biological risk die at younger ages and those with less risk are more likely to survive to old age. Our intent is to indicate the way in which differentials over the life cycle affect the composition of the surviving population at older ages to show that we are comparing very different groups late in the life cycle than at younger ages. We examine differences in the age patterning of risk factors that characterize physiological changes associated with aging by poverty status in samples representative of the U.S. community-dwelling population. These biological risk factors have been related to the major health outcomes in old age: mortality, loss of cognitive and physical function, and cardiovascular disease (13). Clearly, physiological aging involves changes in a variety of physiological systems including the cardiovascular, metabolic, and immune systems. Often these changes are not independent; as changes in one system can be accompanied by changes in other systems. We believe that higher levels of biological risk at younger ages among those in poverty indicate earlier onset of the physiological changes, distinct from, but commonly associated with aging. In turn, the convergence of biological risk at the oldest ages results from survival of the fittest or those without high levels of biological risk (14). Our population-based results have important implications for how we interpret population-level SES differences across the age range in risk factors and other health indicators.
METHODS
Levels of biological risk are indicated using data from two National Health and Nutrition Examination Survey (NHANES) samples that include survey data, laboratory analysis, and clinical exams for a representative sample of the U.S. noninstitutionalized population of all ages although we limit our analysis to those aged 20 and older, which is near the bottom range of ages when these biological risks start to appear. While the samples are weighted to represent the community-dwelling populations in the United States, they are ethnically diverse. There are substantial numbers of African Americans and Latino Americans, most of whom are Mexican Americans (Table 1).
Table 1.
Characteristics of the Samples
| NHANES III (N = 14,912) | NHANES 1999–2004 (N = 12,752) | |
| Mean age, y | 44.43 (SD = 18.47) | 45.59 (SD = 15.32) |
| Died (before 2000), % | 9.14 | |
| Female, % | 52.14 | 51.92 |
| White (and others), % | 80.55 | 75.93 |
| Black, % | 10.62 | 10.37 |
| Hispanic, % | 8.83 | 13.69 |
| Poor (poverty ratio ≤1.25), % | 17.54 | 21.60 |
| Current smoking, % | 28.46 | |
| Heavy drinking, % | 10.75 | |
| No exercise, % | 61.12 | |
| Low BMI (<18.5), % | 2.45 | |
| High BMI(> = 30), % | 22.22 |
Note: NHANES = National Health and Nutrition Examination Survey; SD = standard deviation; BMI = body mass index.
The NHANES data from 1999–2004 are used to indicate current differentials in biological risk. The NHANES III data collected from 1988 to 1994 are used both to indicate the consistency of age differentials by poverty status in biological risk over time and to link poverty and biological risk to mortality. The NHANES III sample has been linked to the National Death Index from the time of the survey up through 2000, so we can examine the proportions dying by age, poverty status, and biological risk to clarify how biological risk is linked to mortality and how differentials in biological risk change with age in the population if the cross-sectional relationships were to occur in real cohorts. Some people are followed for death for up to 12 years after examination; on average, the length of time from interview to the end of the follow-up period is about 8.3 years. In our sample, 9% or 2,134 persons are reported dead.
The NHANES 1999–2004 sample included 12,953 people aged 20 and older who participated in the exam and laboratory parts of the study and for whom poverty status is known, and the NHANES III sample included 14,912 persons. To be retained in the NHANES III sample, mortality status needed to be known and those who died from external causes (eg, accident or violence) were eliminated from the sample. Our analysis is based on those who have information on at least five of the nine indicators of biological risk included in our analysis (N = 12,752 in NHANES 1999–2004; N = 14,912 in NHANES III). In NHANES 1999–2004, those missing are more likely to be younger (p = .0245) and black (p < .0001), but they did not differ in gender. About 5.7% of those whose household income was in the low category and 3.8% of those with higher income were excluded because of insufficient data on biological markers (p = <.0001). In NHANES III, those missing are not significantly different from those included in our analysis in age.
We examined nine indicators of physiological status all of which are recognized as risk factors for disease and mortality: systolic blood pressure, diastolic blood pressure, pulse, total cholesterol, high-density lipoprotein-cholesterol, body mass index, glycated hemoglobin, C-reactive protein, and albumin. For each of these individual indicators, values in the range regarded as clinically significant (or empirically defined through other studies if no clinical cut point exists) are coded as at high risk. Table 2 indicates the definitions of high risk and sources of the cut points used to divide each person's measured value into high risk or not. For each individual, our index of biological risk is the number of factors an individual has measured as in the high-risk range. Although more complex approaches to creating an index of biological risk such as unequal weighting of factors and recursive partitioning techniques may marginally increase predictive ability (21), there are no major differences in predicting health outcomes with the simple count of high-risk markers and the more complex approaches. The simple summed measure is more easily interpreted across populations and subgroups (22).
Table 2.
High-Risk Cut Point for Risk Factors in NHANES III and 1999–2004
| Indicator | High-risk cut point (with source for cut point) |
| Systolic blood pressurea | ≥140 mm Hg (15) |
| Diastolic blood pressurea | ≥90 mm Hg (15) |
| Pulse rate at 60 s | ≥90 |
| Total cholesterolb | ≥240 mg/dL (16) |
| High-density lipoprotein-cholesterolb | <40 mg/dL (16) |
| Body mass indexc | ≥30 kg/m (17) |
| Glycated hemoglobind | ≥6.4% (18) |
| C-reactive proteine | >3.0 mg/L (19) |
| Albumin | <3.8 g/dL (20) |
Notes: NHANES = National Health and Nutrition Examination Survey.
Average of one to three sets of blood pressure measurements.
Hitachi 737 Analyzer/Boehringer-Mannheim Diagnostics (Roche, Indianapolis, IN).
Exam weight, standard anthropometry.
Boronate affinity chromotagraphy.
Enzyme-linked immunosorbent assay: low sensitivity in NHANES III and high sensitivity in NHANES 1999–2004.
For those who are missing on any of the nine biomarkers examined in our analysis, summary scores were calculated assuming that the ratio of high-risk scores was the same for the missing markers as for those available. Results were examined with and without these imputations and none of the conclusions is changed by the imputation procedures.
We examine differences in biological risk for those living in families with incomes below or near poverty (within 125%) and those living above poverty. Although there are many indicators of SES, not all are appropriate for examining differences across a wide range of ages. For instance, a specific educational level may indicate a different relative place in the educational hierarchy for a 20-year-old and an 80-year-old. The meaning of family income may also be very different depending on the number of family members dependent on it. In contrast, poverty status has a relatively similar meaning at different ages. NHANES provides the ratio of reported current family income to the poverty level income for a family of the specified size and composition. Reported total family income is combined income for respondents and the other members of their family in dollars. We divide the sample into those who live within 125% of poverty status (and we call these poverty and near poverty) and those with higher incomes. We use 125% rather than the poverty line because more older people live above, but close to, the poverty line and the 1.25 definition appears to be the best way to compare groups across ages (23,24).
We examine current smoking status, heavy drinking, and no exercise as covariates that might mediate the effect of poverty on death. Heavy drinking is defined by reporting consuming five or more drinks on an average drinking day during the past 12 months. No exercise is defined as reporting not engaging in any vigorous or moderate exercise for at least 10 minutes during the past 30 days. Examples of exercise include running, lap swimming, aerobics classes, fast bicycling, brisk walking, bicycling for pleasure, golf, and dancing.
Sample data are weighted in our analysis to reflect the national noninstitutionalized population. Our analysis consists of examination of differences in the mean number of risk factors by age, the proportion dying by age in the two income categories, and the level of biological risk among those who survive and those who die. The significance of the differences in means and percentages is examined controlling for the design effect arising from the cluster sample design of NHANES. We then test effect of both poverty and biological risk on mortality using a series of hazard models. The models introduce a series of controls to clarify the stability of the relationships between poverty, poverty and age, and biological risk and death. We then use the results of the hazard models to calculate mortality rates and life tables and clarify how strongly life table cohort survival is related to poverty and biological risk.
RESULTS
Age Patterns of Biological Risk
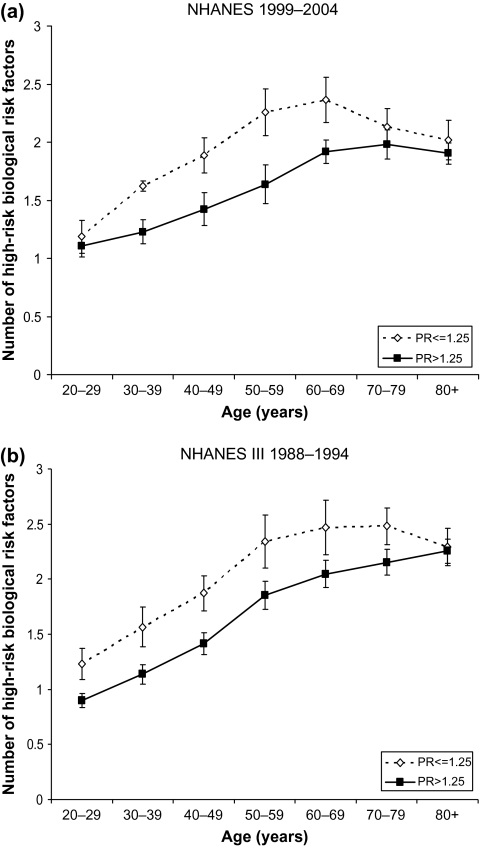
Examination of age patterns in average biological risk by poverty status provides an example of the narrowing of health differentials that occurs with age. The number of indicators of biological high risk increases with age among those who are poor and those who have incomes above poverty (Figure 1), but the increase with age is greater at younger ages for those living in or near poverty. People who are poor have higher levels of biological risk than those who are not poor during their 20s (only in NHANES III), and 30s, 40s, 50s, 60s, and 70s (only in NHANES III). A person in his or her 40s who lives in or near poverty has a level of biological risk similar to that of a person about 60 in a better-off family. The differences maximize in middle age and then decline so that at older ages, 70s and older in NHANES 1999–2004 and 80s and older in NHANES III, population levels of biological risk are similar for both income categories. Age patterns of biological risk are fairly similar in the two studies except that there was a significantly higher level of biological risk among those in their 20s and 70s in the earlier study. The pattern of age differences is also shown in the ratio of the number of biological risk factors at the high-risk level among the poor and the better off (Table 3).
Figure 1.
Mean number of high-risk biological risk factors by poverty status and age. (a) National Health and Nutrition Examination Survey (NHANES) 1999–2004; (b) NHANES III 1988–1994.
Table 3.
Ratio of Measured Number of High-Risk Biological Risk Factors for the Poor to the Nonpoor
| Ages (y) | NHANES 1999–2004 | NHANES III 1988–1994 |
| 20–29 | 1.07 | 1.37a |
| 30–39 | 1.32a | 1.38a |
| 40–49 | 1.32a | 1.32a |
| 50–59 | 1.38a | 1.26a |
| 60–69 | 1.23a | 1.20a |
| 70–79 | 1.08 | 1.15a |
| 80+ | 1.06 | 1.02 |
| N | 12,752 | 14,912 |
Notes: NHANES = National Health and Nutrition Examination Survey.
Significant difference at the .05 level in the numbers used to compute the ratio.
Mortality by Poverty Status and Biological Risk
We now examine the links between poverty, biological risk, and mortality, first descriptively and then in a model-based analysis. The percent dying before the year 2000 by income group and age for those in the NHANES III sample is shown in Table 4. We find significant differences in mortality by income for those at all ages below 70 but not above. The percent dying in an age group is two to four times higher among the poor.
Table 4.
Percent in Age Group Dying by 2000 by Poverty Status: NHANES III
| Ages (y) | Poor | Nonpoor | Ratio poor/nonpoor | p for difference |
| 20–29 | 1.34 | 0.52 | 2.58a | .0097 |
| 30–39 | 2.26 | 0.57 | 3.96a | <.0001 |
| 40–49 | 5.42 | 3.08 | 1.76a | .0170 |
| 50–59 | 20.19 | 7.07 | 2.86a | <.0001 |
| 60–69 | 31.42 | 17.73 | 1.77a | <.0001 |
| 70–79 | 41.00 | 35.06 | 1.17 | .0772 |
| 80+ | 65.37 | 67.23 | 0.97 | .6758 |
Notes: NHANES = National Health and Nutrition Examination Survey.
Significant difference at the .05 level in the numbers used to compute the ratio.
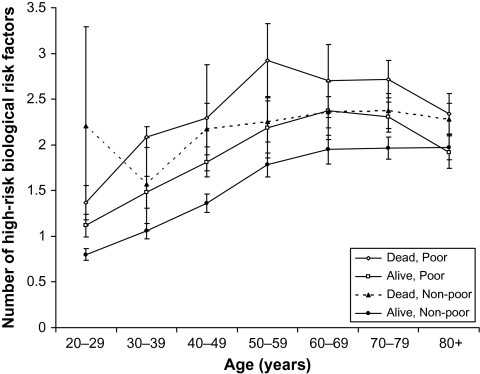
Figure 2 indicates the higher biological risk among those who die relative to those who survive. The highest levels of biological risk as indicated by these nine factors occur among those who die before old age. Although the top line indicating the average number of risk factors for the poor who die in the years after their exam appears to be higher than the line indicating the average number of risk factors for those non- poor who die, the differences are not statistically significant. Among those who survive through 2000, the poor have higher risk than the nonpoor survivors at most ages before 80.
Figure 2.
Biological risk for those who die before 2000 and those still alive in 2000 by poverty status.
Results from hazard models predicting death are shown in Table 5. Model 1 includes sex, age, poverty status, age-poverty interaction, and the number of biological risk factors. We also included an interaction between poverty level and biological risk but eliminated it because it was not significant. Models 2 and 3 include controls for health behaviors and race/ethnicity to see how the inclusion of these variables affects the odds ratios in model 1. If poverty and the number of biological risk factors were no longer significant in models 2 and 3, health behaviors or race/ethnicity, or both, would be the determinants of the differential mortality and they would be the reason that we have observed mortality differentials by both biological risk and poverty.
Table 5.
Odds Ratios (ORs) Predicting Death: NHANES III
| Model 1 |
Model 2 |
Model 3 |
||||
| OR | p | OR | p | OR | p | |
| Age (y) | 1.07 | .0000 | 1.08 | .0000 | 1.08 | .0000 |
| Female | 0.64 | .0000 | 0.65 | .0000 | 0.65 | .0000 |
| Poor | 6.99 | .0000 | 5.56 | .0002 | 5.92 | .0002 |
| Age-poor interaction | 1.02 | .0001 | 1.02 | .0008 | 1.02 | .0006 |
| Number of biological risk factors | 1.22 | .0001 | 1.26 | .0000 | 1.27 | .0000 |
| Current smoking | 1.78 | .0000 | 1.75 | .0000 | ||
| Heavy drinking | 1.34 | .1327 | 1.37 | .1013 | ||
| No exercise | 1.08 | .4421 | 1.08 | .4280 | ||
| Normal BMI is reference | ||||||
| Low BMI (<18.5) | 1.86 | .0034 | 1.93 | .0019 | ||
| High BMI (≥30) | 0.80 | .0075 | 0.76 | .0020 | ||
| White is referencea | ||||||
| Black | 1.20 | .0277 | ||||
| Hispanic | 0.69 | .0018 | ||||
| N | 14,912 | 14,443 | 14,443 | |||
| Approximate chi square (−2 log L ratio) (df) | 3,204.50 (5) | 3,203.07 (10) | 3,223.78 (12) | |||
Notes: NHANES = National Health and Nutrition Examination Survey; BMI = body mass index.
White includes race or ethnic groups other than black and Hispanic.
The effects in model 1 are as expected from the earlier analysis; all variables are significant. People with more biological risk, who live in or near poverty, or who are older are more likely to die but age attenuates the effect of poverty. Model 2 controls for smoking, heavy drinking, exercise, overweight, and underweight. Again, the results are generally as expected, although heavy drinking and exercise are not significantly related to mortality. The odds ratios on the variables of major interest—poverty, age by poverty interaction, and biological risk—are little affected by the inclusion of health behaviors. There is also little effect on these variables of adding race and Hispanic ethnicity to the equation, although black people have higher mortality and Hispanics lower mortality even with controls for poverty status and biological risk.
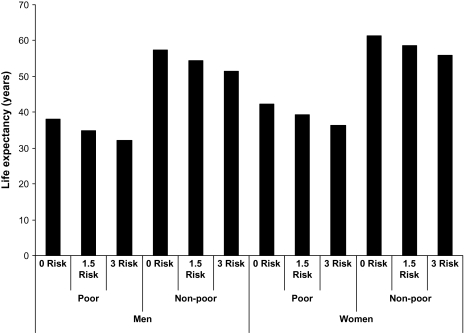
Using the coefficients from a hazard model containing variables included in model 1 in Table 5, we estimate age-specific mortality schedules for subgroups of the population: men and women who are poor and not poor, and who have three levels of biological risk factors: 0, 1.5 (which is close to the population average), and 3 (which is the average among people who are poor and die in their 50s). These mortality rates are then used to build a set of life tables to see the effects of sex, poverty, and biological risk on average life expectancy at age 20 and to determine the expected survivors out of a group of 100,000 at each age above 20. These calculations are done assuming that the cross-sectional age-specific mortality schedules characterize a hypothetical cohort that experiences all the age-specific rates; thus, they may not characterize the actual life experience of any one cohort. This method, however, is an efficient way to clarify the size of the life cycle effects implied by the differences observed in the cross-section.
Being poor has the largest effect on life expectancy. The poor have life expectancy about 20 years shorter than the nonpoor in any of the subgroups similar in biological risk and sex (Figure 3). This is five times greater than the effect of sex—4 years. Those at high biological risk have a life expectancy that is 6 years shorter than those at low biological risk with similar poverty status and sex.
Figure 3.
Estimated life expectancy at age 20 for poor and nonpoor, men and women, by number of biological risk factors.
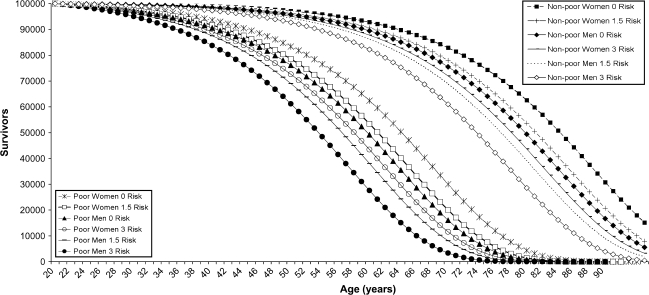
The number of survivors in the life table is computed assuming that each group begins at age 20 with 100,000 people (Figure 4). The survival curves for poor men and women are on the left and the curves for the nonpoor are on the right. The age at which only 50% of the cohort reaching 20 is still alive ranges from 53 for men who are poor and have a high number of biological risk factors to 84 for women who are not poor and have no risk factors. If a group of men experienced the current mortality of the poor throughout their adult lives, almost none would make it to age 80, and this is true no matter what level of biological risk they have. For poor women, the results are almost as bad; with no biological risk factors, about 4% (4,379) would make it to age 80 and with high levels of risk, less than 1% (287) would live to 80. If men are not poor and have no high-risk factors, almost half (49,406) will make it to age 80; this is true for 63% of women (62,592). This exercise demonstrates the effect of experiencing large differentials in mortality across the life span and clarifies how the population is increasingly selected for higher SES and lower biological risk as age increases. We have long recognized how the population becomes increasingly female, but survivors are also increasingly better off socioeconomically as well as biologically.
Figure 4.
Number of survivors (out of 100,000 alive at age 20) for poor and nonpoor, men and women, by number of biological risk factors.
DISCUSSION
This study demonstrates that socially patterned heterogeneity in population health relates to social patterning in mortality. Biological risk increases with age but increases more rapidly among those who are poor, yielding significantly higher levels for the poor from ages 30 through 60, with maximum differences seen in the 50s and 60s. We test the hypothesis that reduced health differentials by social status at older ages result from the elimination of persons with high levels of biological risk through death. People with high levels of risk die at younger ages, and this results in a greater similarity in biological risk by social status among those who reach old age. Early-life differentials in biological risk are the cause of late-life similarity (7). We also observe mortality differences by poverty status that maximize at ages in the 50s and become similar across socioeconomic groups at the oldest ages. One of the explanations for reductions in socioeconomic differentials in heath at older ages is survival of the fittest (ie, those who are not poor and those with the least biological risk even among the poor).
People have speculated about the lack of differentials in older age, attributing the selection of healthy people to differences in “frailty” usually implying that frailty is a randomly distributed characteristic (25). We would suggest that poverty and biological risk factors are an indicator of frailty or vulnerability of population members and that they are anything but randomly distributed but that their distribution is highly socially determined (6). We should note that our indicator of biological risk includes indicators relevant to the onset of chronic conditions more commonly associated with old age. We do not include indicators of diseases such as human immunodeficiency virus and other infectious conditions that are more common among younger people.
We should also clarify that our analysis of differentials in measured biological risk and mortality within a survey eliminates some ways in which data quality could affect changing age differentials by SES. There should not be a problem with age misstatement of older people as the age of the person is the age reported by the person while alive at exam, not an age that is reported on a death certificate. In addition, biological risk is a measured indicator of health not a self-reported indicator that could be affected by social and cultural factors.
We note that our measurement has limitations. Income is current income, rather than permanent or lifetime income. As such there may be differences across age in the link between current and permanent income. It is also possible that income is affected by living arrangements so that poverty status could be affected. For instance, people could move in with other family members in order to escape poverty. It is also true that our measures of smoking, drinking, and exercise reflect current rather than lifetime conditions. However, when we included lifetime smoking, calculated as years of smoking, rather than current smoking, the effect of smoking on death was not meaningfully different in our models. Better indicators of lifetime conditions of health behaviors may need to be examined in further studies.
Our results make clear why social differentials need to be analyzed in a life course perspective. Studying only the older population will result in very different conclusions about health differentials than studying the population from younger ages. One should not interpret the lack of social differentials in old age as resulting from equality of treatment at older ages. Although we have demonstrated the magnitude of the lifetime effects that could be generated by observed differentials, we need to be somewhat cautious in our suggested interpretation of these data as we are examining age differences in different cohorts rather than age differences in the life cycle of one cohort.
Acknowledgments
This work was supported by the National Institutes of Health grants R01 AG023347 and P30 AG17265.
References
- 1.Hayward MD, Crimmins EM, Miles TP, Yu Y. The significance of socioeconomic status in explaining the racial gap in chronic health conditions. Am Sociol Rev. 2000;65:910–930. [Google Scholar]
- 2.Crimmins EM, Hayward MD, Seeman TE. Race/ethnicity, socioeconomic status and health. In: Anderson N, Bulato R, Cohen B, editors. Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. Washington, DC: The National Academies Press; 2004. pp. 310–352. [PubMed] [Google Scholar]
- 3.Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96:826–833. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geronimus AT, Bound J, Keene D, Hicken M. Black-white differences in age trajectories of hypertension prevalence among adult women and men, 1999–2002. Ethn Dis. 2007;17:40–49. [PubMed] [Google Scholar]
- 5.Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostasis, homeostasis, and the cost of physiological adaptation. In: Schulkin J, editor. Allostatic Load: Operationalizing Allostatic Load. Cambridge, UK: Cambridge University Press; 2001. pp. 113–149. [Google Scholar]
- 6.Seeman T, Stein-Merkin S, Crimmins E, Koretz B, Carette S, Karlamangla A. Education, income and ethnic differences in cumulative biological risk profiles in a national sample of US adults: NHANES III (1988–1994) Soc Sci Med. 2008;66:72–87. doi: 10.1016/j.socscimed.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crimmins E. Socioeconomic differentials in mortality and health at the older ages. Genus. 2005;61:163–176. [Google Scholar]
- 8.House JS, Lepkowski JM, Kinney AM, Mero RP, Kessler RC, Herzog AR. The social stratification of aging and health. J Health Soc Behav. 1994;35:213–234. [PubMed] [Google Scholar]
- 9.Marmot MG, Shipley MJ. Do socioeconomic differences in mortality persist after retirement? 25 year follow-up of civil servants from the first Whitehall study. BMJ. 1996;313:1177–1180. doi: 10.1136/bmj.313.7066.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robert S, House J. SES differentials in health by age and alternative indicators of SES. J Aging Health. 1996;8:359–388. doi: 10.1177/089826439600800304. [DOI] [PubMed] [Google Scholar]
- 11.Hummer RA, Benjamins MR, Rogers RG. Race/ethnic disparities in health and mortality among the elderly: a documentation and examination of social factors. In: Anderson N, Bulato R, Cohen B, editors. Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. Washington, DC: The National Academies Press; 2004. pp. 53–94. [PubMed] [Google Scholar]
- 12.Liao Y, Cooper RS, Cao G, et al. Mortality patterns among older Hispanics: findings from the National Health Interview Survey (1986 to 1994) J Am Coll Cardiol. 1998;30:1200–1205. doi: 10.1016/s0735-1097(97)00278-7. [DOI] [PubMed] [Google Scholar]
- 13.Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci U S A. 2001;98:4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manton KG, Stallard E. Methods for evaluating the heterogeneity of aging processes in human populations using vital statistics data: explaining the black/white mortality crossover by a model of mortality selection. Hum Biol. 1981;53:47–67. [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 16.National Cholesterol Education Program. 2004. Detection, evaluation, and treatment of high blood cholesterol in adults. Available at: http://www.nhlbi.nih.gov/guidelines/cholesterol/atp3xsum.pdf. Accessed March 4. [Google Scholar]
- 17.World Health Organization Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Preventive Services Task Force. 2004. Use of glycated hemoglobin and microalbuminuria in the monitoring of diabetes mellitus. Available at: http://www.ahrq.gov/clinic/epcsums/glycasum.pdf. Accessed March 4. [Google Scholar]
- 19.Ridker PM. C-reactive protein: a simple test to help predict risk of heart attack and stroke. Circulation. 2003;108:e81–e85. doi: 10.1161/01.CIR.0000093381.57779.67. [DOI] [PubMed] [Google Scholar]
- 20.Corti M, Guralnik JM, Salive ME, Sorkin JD. Serum albumin level and physical disability as predictors of mortality in older persons. JAMA. l994;272:1036–1042. [PubMed] [Google Scholar]
- 21.Gruenewald T, Seeman TE, Ryff C, Karlamangla A, Singer H. Combinations of biomarkers predictive of later life mortality. Proc Natl Acad Sci U S A. 2006;103:14158–14163. doi: 10.1073/pnas.0606215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crimmins EM, Seeman TE. Integrating biology into the study of health disparities. Popul Dev Rev. 2004;30:89–107. [Google Scholar]
- 23.Citro CF, Michael RT, editors. Measuring Poverty: A New Approach. Washington, DC: National Academy Press; 1995. [Google Scholar]
- 24.Rendall M. Aggregating poor and near-poor elderly under different resource definitions. J Gerontol. 1996;51B:S209–S216. doi: 10.1093/geronb/51b.4.s209. [DOI] [PubMed] [Google Scholar]
- 25.Vaupel J, Manton K, Stallard E. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography. 1979;16:439–454. [PubMed] [Google Scholar]