Abstract
An adverse environment restricted to the time of conception and implantation has been shown to influence later fetal growth and development to term in humans and sheep. In one rat study, protein undernutrition during the pre-implantation period only elevated the systolic blood pressure of the resulting adult offspring. No study has yet followed up adult cardiovascular function in a slower growing, non-litter bearing species after exposure to peri-implantation undernutrition. In the present study, eight ewes were fed to 50% equivalent food intake of twelve control ewes from 1-30 days (term ∼147 days) only. Following consumption of an adequate diet to term, natural lambing and then weaning, basal cardiovascular status and baroreflex function were examined in the resultant young adult offspring. We show that peri-implantation undernutrition has no effect on birth weight or postnatal growth rate but reduces brain weight in the offspring at one year of age i.e. as young adults. Early nutrient restricted sheep also show increased pulse pressure, a reduced rate pressure product and a leftward shift in their baroreflex function curve. However, baroreflex sensitivity in early nutrient restricted sheep is significant blunted during angiotensin II infusion, although not after phenylephrine and sodium nitroprusside treatment. In addition, peri-implantation undernutrition appears to potentiate the tachycardia produced after an experimentally controlled reduction in central blood pressure, relative to controls. The data suggest that peri-implantation undernutrition may program long-term cardiovascular dysfunction that ultimately increases the risk of hypertension in later life. An increase in regional angiotensin II activity during this critical early phase of development is a likely candidate mechanism for the effects observed. The data have broad implications for the health outcome of those offspring from mothers who were poorly nourished during early, often unknown pregnancy and for embryos artificially manipulated because of infertility treatment.
Keywords: Implantation, Undernutrition, Angiotensin II, Blood pressure
INTRODUCTION
Hypertension is a major risk factor associated with coronary heart disease (CHD) and represents a common cause of death in the population over the age of 50 1. Hypertension and coronary heart disease are both multifactorial in their etiology but epidemiological studies in a number of different human populations have shown that the prenatal environment has an important role in determining the incidence of these diseases 2. Consequently, there has been renewed interest in the effect of poor gestational nutrition upon pre- and postnatal growth after the early studies of McCance & Widdowson 3;4. These original hypotheses have been advanced to include changes in physiological function leading to adult pathology- the “developmental origins of adult disease hypothesis” 5;6. The methodologies and inferences of the hypothesis have criticised 7;8 but meta-analyses have illustrated the strength of the epidemiological findings 1;9. Work from the many animal models that now exist strongly supports the hypothesis and suggests that programming of disease risk is both biologically plausible and of major importance in terms of public health 5;10.
The majority of studies investigating the developmental origins of adult disease hypothesis have concentrated on exposing the developing fetus to a poor diet throughout gestation. However, transient undernutrition during defined critical periods of fetal development has been shown to have an equal ‘programming’ influence 11-16. A major restriction of nutrient availability may be expected to influence the development of fetal form and function over late gestation, when the stoicheiometry of nutrient supply and demand are close 12. However, while it is known that manipulation of embryos as a result of infertility treatments may alter fetal growth leading to ‘large offspring syndrome’ and perhaps influence later health outcomes 17, a number of studies have now shown that the periconceptual 18-21 or even pre-implantation 15;16;22 nutritional environment may also programme later physiological function - a time at which overall fetal demands for energy are remarkably low. In only two of these studies, both of which studied protein restriction in the rat 21;22 - a species with a remarkable rate of protein accretion and thus sensitivity to protein restriction, were the exposed fetuses followed-up into adult life. In both, periconceptual 21 or pre-implantation 22 protein restriction elevated the systolic blood pressure of the young adult offspring. The mechanism underlying this effect remains elusive. While the follow-up evidence from the ‘Dutch Hunger Winter Famine’ suggests that undernutrition confined to early development has the greatest effects on the cardiovascular system 23;24; no study to date has yet followed up adult cardiovascular function, after exposure to undernutrition during the peri-implantation period only, in a non-litter bearing species whose pre- and postnatal growth rate is more comparable to human infants. Hence, the present study investigated the hypothesis that global energy restriction during early gestation programmes alters cardiovascular control and increases blood pressure in the resultant offspring as young adults i.e. at one year of age. Resting cardiovascular status was assessed by measuring systolic, diastolic and mean arterial blood pressure and heart rate. Short-term cardiovascular control was assessed through an analysis of the range, sensitivity and set-point of the baroreceptor reflex using phenylephrine (PE) and sodium nitroprusside (SNP) infusion, to raise and lower arterial blood pressure, respectively. In addition, a cursory examination of baroreflex function was made through similar changes in pressure and heart rate during angiotensin II infusion - a protocol known to produce a different baroreflex response to the physiologically purer stimuli of PE and SNP 25. Operation of the cardiovascular baroreflex is key to maintaining central pressure during ambulatory changes in blood pressure: if inadequate, then risk of later hypertension is increased 26-28. We show that global undernutrition during this period does indeed programme cardiovascular dysfunction in the young adult offspring, and suggest that the primary triggering mechanism may be related to regional increases in angiotensin II activity, especially in centrally-located areas involved in baroreflex function.
MATERIALS AND METHODS
Animals
All procedures were performed under the UK Animals (Scientific Procedures) Act, 1986. Twenty Blue-faced Leicester x Swaledale ewes were used in the study. All ewes were of similar age (7-8 months), body weight and fat distribution as assessed from physical characteristics in the lumbar spine and loin. Ewes were mated with a single ram and within 12hrs of mating individually housed and randomly assigned to one of two groups; Group I, ewes were fed to adequately meet 100% metabolisable energy (ME) requirements as defined by the AFRC 29 throughout gestation to term (Controls; term ∼ 147dGA); Group II, ewes were fed to 50% AFRC ME requirement from day 1-30 gestation and thereafter to 100% ME requirement to term (nutrient restricted; NR). All ewes were bedded on wood shavings and fed a diet consisting of chopped hay (ME content of 8.9 MJ/kg dry matter (DM) and a digestible crude protein (DCP) content of 45 g/kg DM) and a barley-based concentrate (ME, 8.9 MJ/kg DM; DCP, 218 g/kg DM) in a ratio of 3:1, respectively. All ewes were weighed and body condition scored at fortnightly internals and the nutritional regimen for each individual ewe was adjusted accordingly to allow for any weight gain and growth of the conceptus. At term, lambs were delivered naturally and birth weights recorded. The twin lambs that were delivered were reared as singletons with the ewe, the other twin being sacrificed and tissues collected. Of the control group n,6 were twins and n,6 were singletons; for the NR group n,3 were twins and n,5 were singletons. The offspring were ewe reared until weaning at 3 months of age and thereafter grass-fed until one year of age. During this time, the weights and growth of individual lambs were recorded monthly.
At one year of age, all sheep were group housed indoors for one week prior to surgery. For 24 h prior to surgery all food, but not water, was withdrawn from the animals. Anaesthesia was induced with sodium thiopentone (20 mg.kg-1 I.V. Intraval Sodium; Rhone Mérieux, Dublin, Ireland) and maintained with 1-2% halothane in 50:50 O2/N2O. Carotid and jugular catheters were inserted into each sheep and the incision closed. All sheep received a dose of long-acting antibiotic (15 mg.kg-1 I.M. amoxycillin, ‘Duphamox’; Fort Dodge Animal Health Ltd, Southampton, UK) and analgesia (1 mg.kg-1 flunixin meglumine; ‘Finadyne’; Shering-Plough, Kenilworth, UK) post-operatively. Catheter patency was maintained by daily flushing with heparinized saline (50 I.U. heparin.ml-1). All sheep had established normal feeding patterns within 1-2 hrs after surgery and showed no visible signs of discomfort for the duration of the experimental period.
Experimental protocols
A period of 2-4 days post-operative recovery was allowed with the investigator blinded to the dietary origin of the sheep prior to any experiment being performed. Over a 10-day period and on 5-6 separate occasions and days all sheep were placed in metabolism crates with ad libitum hay and water available. After at least an hour catheters were connected to pre-calibrated pressure transducers (SensorNor 840; S 4925) attached at heart level linked to a data acquisition system (Po-Ne-Mah; Version 3, Gould Instrument Systems Inc) and a baseline recording taken over a 30min period. Analogue signals for real-time systolic, diastolic, mean arterial pressure and heart rate were recorded at one-second intervals, digitized and stored on an Excel spreadsheet for further analysis. From these data, pulse pressure (systolic-diastolic) and the rate pressure product (mean arterial blood pressure (mmHg) x heart rate (beats min-1); an index of myocardial work and thus oxygen consumption) were derived.
Angiotensin II
Step-wise I.V. increases in angiotensin II (0, 2.5,5,10,20,40 & 60 ng.kg-1.min-1) were administered every 10mins, followed by a 10min recovery period in which cardiovascular variables returned to baseline.
Phenylephrine
Sheep were administered a bolus dose (75 μg.kg-1; I.V. given over 2 mins) of the sympathomimetic (α-adrenergic agonist), phenylephrine hydrochloride (PE; Sigma, Poole, UK) and cardiovascular variables followed for a 1-hour period.
Sodium nitroprusside
After return of cardiovascular variables to baseline the sheep were infused I.V. (2.5 μg.kg-1.min-1) with the endothelium-dependent vasodilator sodium nitroprusside (SNP; Abbott Laboratories, Maidenhead, UK) for 5 mins after a 5 min baseline recording period.
Statistical analyses
All data are expressed as Means ± S.E.M. unless otherwise stated. The data for maternal feed intake, birth weight, current weight, postnatal growth rates and cardiovascular variables (blood pressures, heart rate, rate pressure product) were continuous and analysed by one-way analysis of variance with repeated measures (SPSS Inc, Chicago, USA). Baroreceptor reflex curves describing the relationship between mean arterial blood pressure and heart rate were analysed by a four-parameter logistical sigmoid function (Sigmaplot; SPSS Inc, U.S.A.) using equation 1 30;31:
| Equation 1 |
where a is the heart rate (HR) range (i.e. max HR − min HR; beats.min-1), b is a measure of the slope over the linear portion of the curve (1/mmHg), xo is the mid- or setpoint (mmHg) for equal pressor and depressor responses and yo is the minimum HR. The sigmoidal parameters generated for each individual animal were compared by Students’ t-test. The maximal gain or baroreflex sensitivity was derived by calculus from the first derivative of equation 1.
| Equation 2 |
Areas under the curve (AUC) were calculated using a custom designed Excel spreadsheet according to Equation 3.
| Equation 3 |
Where a is the first data point, z is the last data point and b-y are the data points enclosed by the curve. Average values were then compared by one-way analysis of variance. For a comparison between the slopes of linear regression curves obtained during angiotensin II infusion the data were analysed by ANCOVA 32. For all comparisons, statistical significance was accepted when P<0.05.
RESULTS
Nutrition and Pregnancy
Ewe body weight and condition score at the time of conception was not different between dietary groups (Controls, 46.0±5.5 kg and 2.0 ± 0.5 units; NR, 45.9±5.1 kg and 2.5±0.5 units, means±1 S.D). Control ewes consumed between 7.68±0.24 to 17.7±0.59 MJ/day during weeks 1 and 21 of pregnancy, respectively and gained 11.6±1.5 kg over the duration of gestation. NR ewes consumed 3.61±0.11 MJ/day for the first 30 days of pregnancy i.e. ∼50% control intake and, after one week of realimentation, dietary intake was equivalent to control intake (Figure 1). Consequently, NR ewes lost more weight than controls over the first 4 weeks of pregnancy (4.1±0.4 vs. 1.5±0.4 kg, respectively; P<0.05) and their overall weight gain tended to be lower but not statistically different (8.2±1.2 kg).
Figure 1. Maternal metabolisable energy (ME) intake during gestation.
Values are means ± S.E.M. for control (-O-, n = 12) and nutrient restricted (NR; -●-, n = 8) ewes for weekly ME intake (MJ/day) from conception to term (∼147 days). Statistical differences are: *, P<0.05 control vs. NR intake. For dietary details see Materials and Methods.
Birth weights and postnatal growth rates
There were no differences between singles and twins of either group for any data and therefore subsequent values represent the combined data. Lamb weights were similar between maternal dietary groups (Control, 3.9±0.3; NR, 4.2±0.3 kg) and were appropriate given the size of the ewe and plane of nutrition during pregnancy, according to recommended guidelines for the nutrition of housed, pregnant, lowland ewes 29. There was no difference in the growth rate (current weight-birth weight/time; kg/week) of control or NR lambs during the postnatal period (Controls: 1.93±0.15, 1.43±0.09, 0.92±0.07; NR: 1.80±0.14, 1.43±0.07, 0.92±0.06 kg/week for growth over 0-3, 4-6 and 7-12 months, respectively) but the rate of growth slowed over time in both groups (P<0.001). At one year of age there was no difference in body weight between the two groups of sheep (control, 51.5±3.5; NR, 51.6±3.0 kg).
Cardiovascular responses to angiotensin II infusion
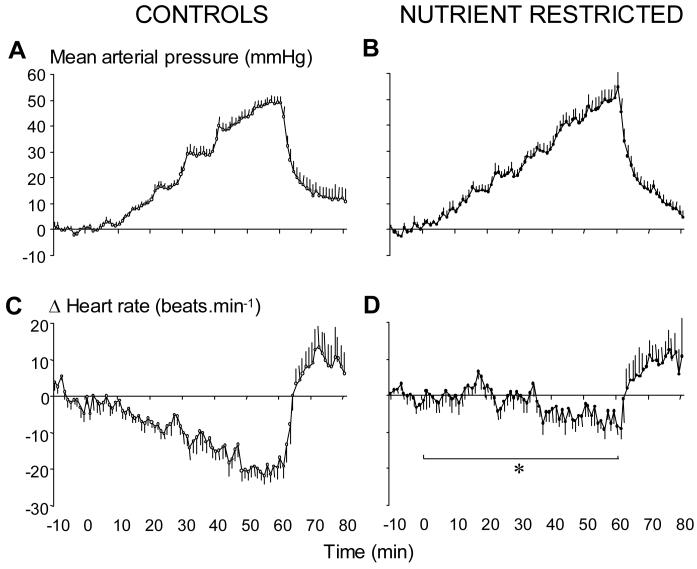
Baseline cardiovascular status was assessed on different days between 9:00-10:00am on 5-6 occasions for each individual and data averaged to give a mean basal value for each sheep. Values for systolic (100±4 vs. 98±3 mmHg), diastolic (77±4 vs. 70±3 mmHg), mean arterial pressure (87±4 vs. 81±3 mmHg) and heart rate (107±5 vs. 100±4 beats.min-1) were similar in control and NR sheep, respectively. However values for pulse pressure (28±1 vs. 23±1 mmHg, P=0.06) and the rate pressure product (7.91±0.46 vs. 9.36±0.72 (beats.min-1).mmHg/103; P=0.005) tended to be higher and lower in NR relative to control sheep, respectively. In control sheep, angiotensin II infusion resulted in dose-dependent increments in arterial blood pressure and decrements in heart rate (Figure 2A & 2C). In NR sheep, however, the increment in arterial blood pressure was similar but heart rate failed to decline significantly (Figure 2B & 2D). Consequently, the area under the curve for delta heart rate during the challenge was significantly (four-fold) lower in NR relative to control sheep (−125±120 vs. −841±107 units respectively; P=0.004). When plotted as a linear relationship between individual data points for mean arterial blood pressure and heart rate (baroreflex sensitivity) the slope was significantly blunted in NR (y=−0.15x + 109; r2=0.49, n=8) sheep relative to controls (y=−0.41x + 138; r2=0.92, t= 10.96; P<0.0001, n=12), as illustrated in Figure 3.
Figure 2. The change in mean arterial blood pressure and heart rate during angiotensin II infusion in control and periconceptually nutrient-restricted sheep.
Values are minute means ± S.E.M. for control (left panel, n = 12) and nutrient restricted (NR; right panel, n = 8) fetuses for a baseline period (10 min), 1 h of angiotensin II infusion (stepwise dose increments from 2.5-60 ng.kg-1.min-1 every 10 min) and 10 min of recovery. Statistical differences are: *, P<0.01 control vs. NR as analysed by area under the curve for the period between the start and end of angiotensin II infusion (time zero to 1 h: control, −125±120; NR, −841±107 units).
Figure 3. Regression of mean arterial blood pressure with heart rate during angiotensin II infusion.
Data points are the minute means for control (-O-, n = 12) and nutrient restricted (NR; -●-, n = 8) for paired mean arterial blood pressure and heart rate values obtained during the 1 h of angiotensin II infusion. For clarity, S.E.M. has been included with means every 9 min only. There was a significant change in the slope of the relationship between mean arterial blood pressure and heart rate in NR sheep (y=−0.15x + 109; r2=0.49, n=8) relative to controls (y=−0.41x + 138; r2=0.92, t= 10.96; P<0.0001, n=12).
Cardiovascular responses to phenylephrine (PE) and sodium nitroprusside (SNP) infusion
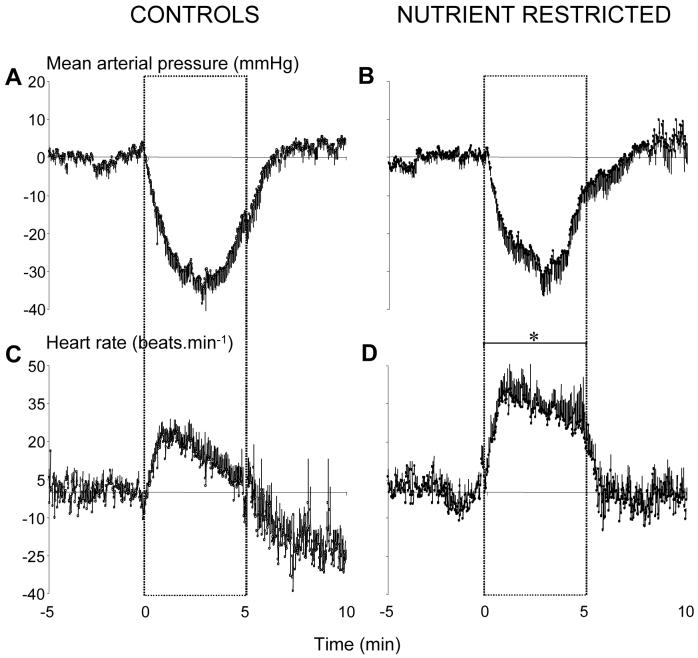
Resting mean arterial blood pressure and heart rate was similar prior to PE and SNP challenges in both groups of sheep (Control: PE, 92±3 mmHg and 115±4 beats.min-1; SNP, 92±4 mmHg and 107±5 beats.min-1; NR: PE, 89±5 mmHg and 99±5 beats.min-1; SNP, 82±4 mmHg and 93±4 beats.min-1). Injection of a bolus dose of PE significantly increased mean arterial blood pressure (by 120±11 and 100±13 mmHg) and decreased heart rate (by 88±4 and 80±4 beats.min-1) in control and NR sheep, respectively (Figure 4). The slope of the linear relationship between individual data points for mean arterial blood pressure and heart rate during PE (i.e. parasympathetic arm of the baroreflex) was similar in control and NR sheep (Control, y=−1.00x + 208; r2=0.91; NR, y=−1.02x + 194; r2=0.88). Infusion of SNP significantly lowered arterial pressure; the reduction in diastolic pressure being significantly greater than the reduction in systolic pressure in both dietary groups (diastolic by 29±5 and 24±4; systolic by 19±3 and 15±4 mmHg, in control and NR sheep, respectively; P<0.05). Consequently, pulse pressure significantly increased in control and NR sheep (by 11±3 and 11±4 mmHg, respectively; P<0.05). The reduction in pressure was compensated for by significant increases in heart rate in control and NR sheep (Figure 5), however the mean increment from baseline was greater in NR sheep (by 12±6 and 30±6 beats.min-1, respectively; P=0.04). Figure 6 shows the mean individual data points for the relationship between mean arterial pressure and heart rate, during the initial (2 min) phases of parasympathetic and sympathetic activation induced by PE and SNP, respectively. Statistical analysis of the curve indicated no significant difference in either the operating range of the baroreflex (a; 70±14 and 65±5 beats.min-1), estimated slope (b; −6.5±2.1 and −6.6±1.5 mmHg) or derived maximal gain (b; −5.9±2.4 and −3.1±0.5 bpm/mmHg) and the minimum heart rate achieved (yo; 58±4 and 62±5 beats.min-1) in control and NR sheep, respectively. However, the set point (xo) was significantly shifted to the left i.e. toward a lower mean pressure, in NR relative to control sheep (86±7 vs. 100±3 mmHg, respectively; P=0.05, Figure 6).
Figure 4. The change in mean arterial blood pressure and heart rate during phenyleprine (PE) infusion in control and periconceptually nutrient-restricted sheep.
Values are minute means ± S.E.M. for control (left panel, n = 11) and nutrient restricted (NR; right panel, n = 7) sheep for a baseline period (10 min) and for 30 min after a bolus dose of PE (75 μg.kg-1).
Figure 5. The change in mean arterial blood pressure and heart rate during sodium nitroprusside (SNP) infusion in control and periconceptually nutrient-restricted sheep.
Values are minute means ± S.E.M. for control (left panel, n = 11) and nutrient restricted (NR; right panel, n = 7) sheep for a baseline period (5 min), 5 min of SNP infusion (2.5 μg.kg-1.min-1) and 5 min of recovery. Statistical differences are: *, P<0.05 control vs. NR for the mean increment in heart rate during SNP infusion.
Figure 6. The range and function of the baroreflex in control and nutrient restricted sheep.
Data points are (A) all individual points for a representative control (-O-) and nutrient restricted (NR; -●-) sheep and (B) for means ± S.E.M. (included every 20 sec for clarity) for control (-O-, n = 11) and NR (-●-, n = 7) for mean arterial blood pressure and heart rate values obtained during phenylephrine and sodium nitroprusside infusion. The slope, range, minimum heart rate value and sensitivity of the relationship between mean arterial blood pressure and heart rate were similar in control and NR sheep. However the set-point was significantly (P<0.05) shifted to the left in NR sheep relative to controls.
Sheep biometry at one year of age
There were no differences between the two dietary groups in the weights of any organ measured with the exception of the brain, which was significantly (P=0.03) smaller in NR relative to control sheep (Table 1). When organ weights were expressed relative to body weight there were, again, no significant differences between groups. The significance of the difference in the weight of the brain was, however, weakened when expressed relative to body weight (P=0.08; data not shown).
Table 1. Sheep biometrical measurements at post mortem.
Sheep were humanely put down using a lethal dose of sodium pentobarbitone (200mg.kg-1) and body and all major organ weights measured.
Sheep biometry at one year of age
| CONTROLS | NR | P | |
|---|---|---|---|
| Body weight (kg) | 51.5 ± 3.5 | 51.6 ± 3.0 | NS |
| Brain weight (g) | 100 ± 2 | 94 ± 1 | 0.03 |
| Heart weight (g) | 214 ± 15 | 207 ± 8 | NS |
| Left ventricular wall thickness (mm) | 10.6 ± 0.6 | 9.8 ± 0.3 | NS |
| Right ventricular wall thickness (mm) | 5.6 ± 0.5 | 4.5 ± 0.2 | NS |
| Septum thickness (mm) | 12.9 ± 0.4 | 12.8 ± 0.6 | NS |
| Liver weight (g) | 634 ± 69 | 708 ± 58 | NS |
| Kidney weight (g) | 121 ± 5 | 121 ± 5 | NS |
| Adrenal weight (g) | 3.1 ± 0.2 | 3.3 ± 0.3 | NS |
| Spleen weight (g) | 125 ± 12 | 120 ± 19 | NS |
| Lung weight (g) | 516 ± 21 | 478 ± 29 | NS |
| Pancreas weight (g) | 47.6 ± 5.2 | 43.0 ± 5 | NS |
| Perirenal adipose tissue (g) | 154 ± 21 | 145 ± 20 | NS |
| Cardiac adipose tissue (g) | 43 ± 5 | 44 ± 4 | NS |
| Omental adipose tissue (g) | 278 ± 42 | 252 ± 31 | NS |
DISCUSSION
The present study provides the first documented evidence of programming influences operating prior to and during embryo attachment in a species that in terms of fetal number, pre- and postnatal growth is comparable to human infants. Furthermore, the peri-implantation nutritional environment of the embryo is clearly able to programme cardiovascular dysfunction in the young adult independently of reductions in birth weight or accelerated postnatal ‘catch-up’ growth - key processes thought to contribute to the developmental origins of adult cardiovascular disease 33. It is known that baroreceptor sensitivity per se is blunted when analysed under conditions of high, but physiological infusion doses of angiotensin II, when compared to the purer stimulus produced with the synthetic α-adrenergic agonist, phenylephrine 25 and this is reproduced in the current manuscript (witness the differing curve gradients produced by angiotensin II and PE). This may be due, in part, to the differing methodologies used to assess baroreflex function in the current study i.e. acute (PE & SNP) vs. a more prolonged stimulus with angiotensin II infusion, or a centrally-orientated mechanism e.g. angiotensin II-mediated inhibition of efferent parasympathetic outflow from the area postrema, rather than a direct effect on the heart or baroreceptors themselves (for review see Reid (1992) 25. Nevertheless, in this respect, there is no difference between dietary groups. Importantly, while the full range and sensitivity of the baroreflex is similar between dietary groups when constructed with PE and SNP treatment, the baroreflex set-point is significantly shifted to the left in NR sheep. We speculate that this data, together with the observation of no greater pressor responses to peripherally infused angiotensin II infusion (Figure 2), but increased blunting of baroreflex sensitivity, suggests a central origin for the re-setting of baroreflex function in NR sheep. Possible mechanisms for these data are 1) increased angiotensin II action within the cardiovascular control centers of the brain i.e. the area postrema and/or nucleus of the solitary tract, or 2) a direct chronotrophic action on the heart. The present study cannot differentiate between these two possible mechanisms, but nutritional regulation of regional angiotensin II receptor populations (AT1 & AT2) has been demonstrated in term ovine neonates34 and adult rat offspring 35;36. Interestingly, increased densities of central AT1 in mice has been shown to influence baroreflex setting and sensitivity 37.
The current study has shown that peri-implantation undernutrition has no major effect on resting systolic or diastolic pressures 25-27 in the resulting young adult sheep. However, at this age, we show evidence of altered baroreflex function and renin-angiotensin system activity. At three years of age, male sheep that have undergone a similar nutritional paradigm also have altered baroreflex function but, importantly, have elevated pre-feeding blood pressure 38. We therefore speculate that the results of the current study may provide an early indication of compromised cardiovascular control that eventually leads to a pathophysiological outcome as the individual ages 39.
Perspectives
Global undernutrition during peri-implantation, embryonic development only programmes cardiovascular dysfunction in the young adult offspring. The data suggest two, possibly interrelated, mechanisms for this phenomena: 1) altered baroreflex control and 2) regional increases in angiotensin II activity. The results of this study have wide-ranging implications with regard to the health outcomes of those offspring from mothers who were poorly nourished during early, often unknown pregnancy and from embryos artificially manipulated because of infertility treatment.
ACKNOWLEDGEMENTS
The authors wish to acknowledge Mick Baker and Robert Greenhalgh for the routine care of the animals used in this study and Professor Fiona Broughton-Pipkin for a critical appraisal of the manuscript. This work was supported by the British Heart Foundation.
REFERENCES
- 1.Law CM, Shiell AW. Is blood pressure inversely related to birth weight? The strength of evidence from a systematic review of the literature. J Hypertens. 1996;14:935–941. [PubMed] [Google Scholar]
- 2.Barker DJP. Mothers, Babies, and Disease in Later life. BMJ; London: 1994. [Google Scholar]
- 3.McCance RA, Widdowson EM. The determinants of growth and form. Proc R Soc Lond B Biol Sci. 1974;185:1–17. doi: 10.1098/rspb.1974.0001. [DOI] [PubMed] [Google Scholar]
- 4.Widdowson EM, McCance RA. A review: new thoughts on growth. Pediatr Res. 1975;9:154–156. doi: 10.1203/00006450-197503000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Hoet JJ, Hanson MA. Intrauterine nutrition: its importance during critical periods for cardiovascular and endocrine development. J Physiol. 1999;514(Pt 3):617–627. doi: 10.1111/j.1469-7793.1999.617ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 7.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18:815–831. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- 8.Lucas A, Fewtrell MS, Cole TJ. Fetal origins of adult disease-the hypothesis revisited. BMJ. 1999;319:245–249. doi: 10.1136/bmj.319.7204.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huxley R, Neil A, Collins R. Unravelling the fetal origins hypothesis: is there really an inverse association between birthweight and subsequent blood pressure? Lancet. 2002;360:659–665. doi: 10.1016/S0140-6736(02)09834-3. [DOI] [PubMed] [Google Scholar]
- 10.Langley-Evans SC. Fetal programming of cardiovascular function through exposure to maternal undernutrition. Proc Nutr Soc. 2001;60:505–513. doi: 10.1079/pns2001111. [DOI] [PubMed] [Google Scholar]
- 11.Langley-Evans SC, Clamp AG, Grimble RF, Jackson AA. Influence of dietary fats upon systolic blood pressure in the rat. Int J Food Sci Nutr. 1996;47:417–425. doi: 10.3109/09637489609006955. [DOI] [PubMed] [Google Scholar]
- 12.Edwards LJ, McMillen IC. Maternal undernutrition increases arterial blood pressure in the sheep fetus during late gestation. J Physiol. 2001;533:561–570. doi: 10.1111/j.1469-7793.2001.0561a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards LJ, McMillen IC. Impact of maternal undernutrition during the periconceptional period, fetal number, and fetal sex on the development of the hypothalamo-pituitary adrenal axis in sheep during late gestation. Biol Reprod. 2002;66:1562–1569. doi: 10.1095/biolreprod66.5.1562. [DOI] [PubMed] [Google Scholar]
- 14.Edwards LJ, McMillen IC. Periconceptional nutrition programs development of the cardiovascular system in the fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2002;283:R669–R679. doi: 10.1152/ajpregu.00736.2001. [DOI] [PubMed] [Google Scholar]
- 15.Bloomfield FH, Oliver MH, Giannoulias CD, Gluckman PD, Harding JE, Challis JR. Brief undernutrition in late-gestation sheep programs the hypothalamic-pituitary-adrenal axis in adult offspring. Endocrinology. 2003;144:2933–2940. doi: 10.1210/en.2003-0189. [DOI] [PubMed] [Google Scholar]
- 16.Bloomfield FH, Oliver MH, Hawkins P, Campbell M, Phillips DJ, Gluckman PD, Challis JR, Harding JE. A periconceptional nutritional origin for noninfectious preterm birth. Science. 2003;300:606. doi: 10.1126/science.1080803. [DOI] [PubMed] [Google Scholar]
- 17.Sinclair KD, Young LE, Wilmut I, McEvoy TG. In-utero overgrowth in ruminants following embryo culture: lessons from mice and a warning to men. Hum Reprod. 2000;15(Suppl 5):68–86. doi: 10.1093/humrep/15.suppl_5.68. [DOI] [PubMed] [Google Scholar]
- 18.Hawkins P, Steyn C, Ozaki T, Saito T, Noakes DE, Hanson MA. Effect of maternal undernutrition in early gestation on ovine fetal blood pressure and cardiovascular reflexes. Am J Physiol Regul Integr Comp Physiol. 2000;279:R340–R348. doi: 10.1152/ajpregu.2000.279.1.R340. [DOI] [PubMed] [Google Scholar]
- 19.Hawkins P, Steyn C, McGarrigle HH, Saito T, Ozaki T, Stratford LL, Noakes DE, Hanson MA. Effect of maternal nutrient restriction in early gestation on responses of the hypothalamic-pituitary-adrenal axis to acute isocapnic hypoxaemia in late gestation fetal sheep. Exp Physiol. 2000;85:85–96. doi: 10.1111/j.1469-445x.2000.01914.x. [DOI] [PubMed] [Google Scholar]
- 20.Hawkins P, Hanson MA, Matthews SG. Maternal undernutrition in early gestation alters molecular regulation of the hypothalamic-pituitary-adrenal axis in the ovine fetus. J Neuroendocrinol. 2001;13:855–861. doi: 10.1046/j.1365-2826.2001.00709.x. [DOI] [PubMed] [Google Scholar]
- 21.Langley-Evans SC, Welham SJ, Sherman RC, Jackson AA. Weanling rats exposed to maternal low-protein diets during discrete periods of gestation exhibit differing severity of hypertension. Clin Sci (Lond) 1996;91:607–615. doi: 10.1042/cs0910607. [DOI] [PubMed] [Google Scholar]
- 22.Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127:4195–4202. doi: 10.1242/dev.127.19.4195. [DOI] [PubMed] [Google Scholar]
- 23.Roseboom TJ, van der Meulen JH, van Montfrans GA, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Maternal nutrition during gestation and blood pressure in later life. J Hypertens. 2001;19:29–34. doi: 10.1097/00004872-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Schroeder-Tanka JM, van Montfrans GA, Michels RP, Bleker OP. Coronary heart disease after prenatal exposure to the Dutch famine, 1944-45. Heart. 2000;84:595–598. doi: 10.1136/heart.84.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reid IA. Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Am J Physiol. 1992;262:E763–E778. doi: 10.1152/ajpendo.1992.262.6.E763. [DOI] [PubMed] [Google Scholar]
- 26.Eckberg DL. Carotid baroreflex function in young men with borderline blood pressure elevation. Circulation. 1979;59:632–636. doi: 10.1161/01.cir.59.4.632. [DOI] [PubMed] [Google Scholar]
- 27.Ookuwa H, Takata S, Ogawa J, Iwase N, Ikeda T, Hattori N. Abnormal cardiopulmonary baroreflexes in normotensive young subjects with a family history of essential hypertension. J Clin Hypertens. 1987;3:596–604. [PubMed] [Google Scholar]
- 28.Gordon FJ, Matsuguchi H, Mark AL. Abnormal baroreflex control of heart rate in prehypertensive and hypertensive Dahl genetically salt-sensitive rats. Hypertension. 1981;3:I135–I141. doi: 10.1161/01.hyp.3.3_pt_2.i135. [DOI] [PubMed] [Google Scholar]
- 29.AFRC . Energy and protein requirements of ruminants. An advisory manual prepared by the AFRC technical committee on responses to nutrients. CAB International; 1993. [Google Scholar]
- 30.Kent BB, Drane JW, Blumenstein B, Manning JW. A mathematical model to assess changes in the baroreceptor reflex. Cardiology. 1972;57:295–310. doi: 10.1159/000169528. [DOI] [PubMed] [Google Scholar]
- 31.Korner PI, Shaw J, West MJ, Oliver JR. Central nervous system control of baroreceptor reflexes in the rabbit. Circ Res. 1972;31:637–652. doi: 10.1161/01.res.31.5.637. [DOI] [PubMed] [Google Scholar]
- 32.Armitage P, Berry G, Matthews JNS. Statistical Methods in Medical Research. Blackwell science; Oxford: 2002. Modelling continuous data. [Google Scholar]
- 33.Eriksson J, Forsen T, Tuomilehto J, Osmond C, Barker D. Fetal and childhood growth and hypertension in adult life. Hypertension. 2000;36:790–794. doi: 10.1161/01.hyp.36.5.790. [DOI] [PubMed] [Google Scholar]
- 34.Whorwood CB, Firth KM, Budge H, Symonds ME. Maternal undernutrition during early to midgestation programs tissue-specific alterations in the expression of the glucocorticoid receptor, 11β-hydroxysteroid dehydrogenase isoforms, and type 1 angiotensin II receptor in neonatal sheep. Endocrinology. 2001;142:2854–2864. doi: 10.1210/endo.142.7.8264. [DOI] [PubMed] [Google Scholar]
- 35.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res. 2001;49:460–467. doi: 10.1203/00006450-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 36.McMullen S, Gardner DS, Langley-Evans SC. Prenatal programming of angiotensin II type 2 receptor expression in the rat. British Journal of Nutrition. 2004;91:133–140. doi: 10.1079/bjn20031029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lazartigues E, Dunlay SM, Loihl AK, Sinnayah P, Lang JA, Espelund JJ, Sigmund CD, Davisson RL. Brain-selective overexpression of angiotensin (AT1) receptors causes enhanced cardiovascular sensitivity in transgenic mice. Circ Res. 2002;90:617–624. doi: 10.1161/01.res.0000012460.85923.f0. [DOI] [PubMed] [Google Scholar]
- 38.Gopalakrishnan G, Gardner DS, Rhind SM, Rae MT, Kyle CE, Walker RM, Ramsay MM, Stephenson T, Symonds ME. Programming of adult cardiovascular function after early maternal undernutrition in sheep. Am J Physiol Regul Integr Comp Physiol. 2004 doi: 10.1152/ajpregu.00687.2003. In the press. [DOI] [PubMed] [Google Scholar]
- 39.Jackson AA. Nutrients, growth, and the development of programmed metabolic function. Adv Exp Med Biol. 2000;478:41–55. doi: 10.1007/0-306-46830-1_4. [DOI] [PubMed] [Google Scholar]