Abstract
Ménière’s disease and related disease of the vestibular system are common and debilitating. Current therapy is multi-modal and includes drug therapy and lifestyle adaptations. Unfortunately many of the drugs used in treatment (particularly those used to control nausea) are sedative and hamper the process of vestibular compensation. Although betahistine (Serc®, BetaSerc®; Solvay Pharmaceuticals) is the mainstay of drug treatment in these illnesses, its efficacy has not, until recently, been evaluated to modern standards. Betahistine is an analog of histamine with weak agonist properties at histamine H1 receptors and more potent anatgonistic effects at histamine H3 receptors. Growing evidence suggests that the mechanism of action of betahistine lies in the central nervous system and in particularly in the neuronal systems involved in the recovery from process after vestibular loss. The histaminergic neurones of the tuberomamillary and vestibular nuclei are implicated. In recent years the clinical efficacy of betahistine has been demonstrated in double-blind, randomized, placebo, and active controlled studies in adequate numbers of patients. Although the results of comparative studies between betahistine and other drugs (flunarizine, cinnarizine, and cinnarizine + dimenhydrate) are equivocal, the efficacy of betahistine is now clear.
Keywords: Ménière’s disease, vestibular disorders, betahistine
Introduction
Ménière’s disease is an illness in which multi-modal treatment is the norm. Lifestyle changes, physical therapy, vestibular adaptation, drug therapy, and ablative and non-ablative surgery are all options in its treatment and are frequently used in combination. Among all of the drug therapies used, betahistine (Serc®, BetaSerc®; Solvay Pharmaceuticals) is the most frequently chosen in Europe; in a recent survey, 94% of physicians in the UK prescribe betahistine for Ménière’s disease (Smith et al 2005) and the drug has been employed in the treatment of Ménière’s disease and vertigo of peripheral vestibular origin for more than 40 years. However, until recently, the efficacy of this drug had not been evaluated to modern standards and its mechanism of action remained obscure. The objective of this review is to evaluate the evidence for the efficacy of betahistine in Ménière’s disease and examine the latest evidence on its mechanism of action.
Ménière’s disease
Definition
Ménière’s disease comprises recurrent spontaneous episodes of rotatory vertigo spells that patients describe as a spinning or whirling feeling and sensorineural hearing loss (SNHL) accompanied by recruitment and tinnitus. An unpleasant sensation of aural fullness on the affected side may also occur (Van de Heyning et al 2005). Ménière’s disease is also called idiopathic endolymphatic hydrops (ELH), a term which describes a disorder of the inner ear in which there is a build-up of endolymph (AAO-HNS 1995). Of the various symptoms of Ménière’s disease, vertigo is usually the most troublesome, at least in the acute phase, due to its unpredictable nature (Cohen et al 1995; Kentala 1996). Vertigo spells can last from several minutes to several days and are frequently debilitating. Anxiety, depression, and space and motion disorder (an inability to orientate oneself and the difficulties encountered in large spaces or by moving surroundings) often develop (Anderson and Harris 2001). During vertigo attacks, patients are quite unable to undertake normal work or social activities. Nausea is common and patients may vomit during an attack. Sleepiness may follow for several hours, and an off-balance sensation may last for a number of days. Quality of life is frequently seriously diminished (Cohen et al 1995). During later stages of the disease, hearing loss, sound distortion, recruitment, and tinnitus further affect quality of life (Kinney et al 1997).
Epidemiology
Together with back pain and headaches, dizziness and vertigo are among the most common symptoms causing patients to visit their physician. For example, a community survey among 50- to 60-year-olds revealed that a quarter currently suffered from giddiness or dizziness (Stephens et al 1990, 1991). Such problems tend to become more common and more severe with age, so that by age 80 years, up to two-thirds of women and one third of men will have experienced episodes of vertigo. (Luxon 1984). Ménière’s disease is a common cause of dizziness and vertigo spells. In most cases only one ear is involved, but both ears may be affected in about one third of patients. If both ears are affected, the disease generally starts in the second ear within the five first years of onset of the first ear. Ménière’s disease typically starts between the ages of 20 and 50 years, a diagnosis rate that increases with age up to 60 years (Wladislavosky et al 1980). Men and women are equally affected.
There is considerable disagreement in the world literature about the incidence and prevalence of Ménière’s disease with estimates varying by as much as a factor of 10 from country to country. Ranges of prevalence vary from over 200 cases/100,000 in the USA (with an incidence of over 15 cases/100,000 persons/year) (Cawthorne and Hewlett 1954) to prevalences as low as fewer than 20/100,000 in Japan (Wanatabe et al 1995; Shojaku and Watanabe 1997), with a prevalence level of about 46/100,000 in Scandinavia (Stahle et al 1978; Kotimaki et al 1999), making Ménière’s disease four times more common than otosclerosis. Although vertigo symptoms may decline with time leading to a “burn-out” of the inner ear, Havia and Kentala (2004) have shown a progression of the intensity of the vertigo symptoms during the longitudinal course of Ménière’s disease. In a prospective study of 243 consecutive patients admitted to their hospital, they found that the proportion of patients reporting severe or very severe attacks increased with the duration of symptoms. Nausea was also more common later in the disease than at the beginning. Sudden falls due to otolythic crisis (Tumarkin 1936) may arise many years after the last vertigo spell. Up to 25% of patients with Ménière’s disease may eventually require a surgical procedure to control the vertigo attacks (Filipo and Barbara 1977). Overall this suggests that untreated Ménière’s disease worsens over time.
Pathophysiology
Although the underlying cause of Ménière’s disease remains obscure and ELH is one of the common final physiopathological responses of the inner ear to many factors (Kiang 1990), Ménière’s disease nevertheless results from an abnormality in the fluid homeostasis of the inner ear (Andrews 2004). The proposed two phase model in which there is an imbalance between endolymph production and resorption (Dunnebier et al 1997, 2002) leading to volume expansion without pressure increase (Warmerdam et al 2003), is complemented with round membrane characteristics (Feijen et al 2004), perilymphatic regulating mechanisms (Laurens-Thalen et al 2004), and aquaporin involvement.
With the increase of ELH, there is a risk of a rupture in the membranous labyrinth, with the consequent mixing of endolymph and perilymph. The changing levels of potassium are presumed to give rise to the vestibular symptoms. The rupture of the membrane with hydrops is thought to explain the amelioration of the cochlear symptoms when vertigo appears. The repetition of hydrops crises and repeated rupture of the membrane are thought, in turn, to be responsible for the progressive destruction of the labyrinth and give rise to the evolution of profound deafness and disappearance of vertigo. Although the evidence for ELH as the pathophysiological basis of Ménière’s disease is well supported by scientific evidence, there is also evidence to suggest that it is not the only mechanism (Merchant et al 2005). For instance ganglionitis has been reported to contribute to Ménière’s disease (Selamani et al 2005).
Therapeutic management
Acute treatment
The first goal in the acute phase is to symptomatically control the vertigo spells by stopping the rotational sensation and accompanying nausea and neurovegetative symptoms. Drug treatment with anti-emetics (eg, domperidone), vestibulosedative anti-histamines (eg, meclizine), and central sedative drugs with vestibulo-suppressive or anti-emetic effect (eg, diazepam, sulpiride, dihydrobenzperidol, and phenothiazine) are useful during this phase of treatment. Nausea and vomiting frequently require that such drugs are given by intra-rectal or parenteral routes. Although widely used, there is little evidence to guide the physician in choosing one of these medications over another for a particular patient.
Long-term treatment
Long-term treatment has as its goal to prevent or diminish further spells of vertigo, to assist the patient with compensation for the vestibular deficit, to ameliorate or assist with the hearing loss and associated symptoms. Treatment modalities will include counselling, life style adaptation, drug therapy, rehabilitation, and surgery according to the stage and progression of the illness.
Because of the uncertainties of the illness, counseling is required for all patients. General life style adaptations consist of salt restriction and avoidance of caffeine, alcohol, tobacco, and coping with stress. Patients can frequently identify an event that provokes attacks and can be counseled to avoid such situations.
Chronic drug therapy
Drug therapy can play an important role in the treatment of most Ménière’s disease patients (for overview: Claes and Van de Heyning 1997, 2000). Betahistine is of benefit and, unlike sedative alternatives, does not interfere with the development of vestibular compensation. Diuretics can also be of benefit (Klockhoff and Lindblom 1967; Van Deelen and Huizing 1986), although the use of acetazolamide in Ménière’s disease is still controversial. When the transition from acute to chronic treatment fails to alleviate symptoms, mild vestibular sedatives such as cinnarizine may be of help.
Where an inflammatory component is suspected in bilateral Ménière’s disease, short courses of systemic glucocorticoids may be appropriate. It recently has been shown that glucocorticoids not only influence inflammatory process in Ménière’s disease, but also alter fluid dynamics via an interaction with the sodium pumps in the semicircular canals (Ponduglula et al 2004). Intra-tympanic application of corticosteroids appears to have only temporary effects (Dodson 2004) and is probably not recommended (Doyle et al 2004).
Anti-depressive treatment (eg, selective serotonin reuptake inhibitors) may improve the psychological handicap aspect of vertigo in pre-existing depression, but is of no benefit to vertigo itself nor in patients without pre-existing depression (Horii et al 2004). Where anxiety and stress susceptibility exacerbate the condition, short courses of benzodiazepines (eg, alprazolam or serenase) can be administered, but dependence is a concern during the longer term.
Vestibular rehabilitation
Vestibular rehabilitative therapy is useful for central vestibular compensation of peripheral vestibular asymmetry and consequent motion intolerance. Evidence is accumulating that Ménière’s disease patients can benefit from this therapy with a general or customized training program (Clendaniel and Tucci 1997; Dowdol-Ostorn 2002). When choosing drug therapy it is important to consider its effect on vestibular compensation; sedative drugs can seriously impair the process and are to be avoided if at all possible.
Betahistine
Mechanism of action
Histamine (HA) is a neuromodulatory transmitter that regulates various cerebral functions (wakefulness: Lin 1994; cardio-vascular regulation: Laurikainen et al 1993, 1998). In mammals, the histaminergic neurons are exclusively located in the tuberomammillary (TM) nuclei of the posterior hypothalamus (Pollard and Schwartz 1987) but the histaminergic nerve endings show a wide distribution throughout the whole brain. Three types of HA receptors have been identified: the postsynaptic histamine H1 and H2 receptors (Schwartz 1979) and the presynaptic histamine H3 receptors (Arrang et al 1983; Schwartz et al 1990). HA and histamine H3 receptor agonists inhibit HA turnover while HA receptor antagonists increase HA turnover and release in the central nervous system (CNS). The HA-containing perikarya of the TM nuclei send axonal projections to the whole vestibular nuclei (VN) complex in many species (Airaksinen and Panula 1988: guinea pig; Panula et al 1989: rat; Tighilet and Lacour 1996: cat). In addition, all three categories of HA receptors are present in the VN and reciprocal connections between the VN and the TM nuclei are supported by electrophysiological (Horii et al 1993), immunohistochemical (Tighilet et al 2006), molecular (Gustave dit Duflo et al 1999, 2000), and behavioral (Takeda et al 1987) investigations. Taken together, the data strongly suggest that HA regulates also the vestibular functions (see Lacour and Sterkers 2001 for a review). Indeed, HA modulation of second-order vestibular neurons was found both in vitro (Jaju and Wang 1971) and in vivo (Phelan et al 1990; Inverarity et al 1993; Serafin et al 1993) preparations. Moreover, chronic infusion of HA receptor ligands in the VN on one side induces behavioral modifications (Yabe et al 1993). Postural and oculomotor deficits similar to those seen after a unilateral vestibular loss were found following H2 receptor antagonists or H3 receptor agonists perfusion whereas the vestibular syndrome was reversed after local administration of H2 receptor agonists or H3 receptor antagonists.
Betahistine in vestibular disorders
Betahistine dihydrochloride is one of the drugs currently prescribed in patients with vestibular loss for their symptomatic treatment of vertigo, and especially in Ménière’s patients (Rascol et al 1995). It is a structural analog of HA with weak histamine H1 receptor agonist and more potent histamine H3 receptor antagonist properties (Arrang et al 1985; Timmerman 1991). Some sites of action of betahistine are known since a long time while new basic mechanisms at the CNS level were elucidated in a recent past only (see Lacour and Sterkers 2001).
Peripheral mechanisms
Until recently, it was generally believed that the efficacy of betahistine was due to vascular mechanisms in the inner ear. Increase in cochlear blood flow was reported after systemic administration of betahistine (Meyer et al 1974; Laurikainen et al 1993, 1998). The authors found that betahistine antagonizes the H3 heteroreceptors of the cochleo-vestibular vascular tree through the activation of autonomic alpha-2 receptors. Vasodilatation of the anterior inferior cerebellar artery could be involved, suggesting that the betahistine-induced increase in cochlear blood flow was associated with an increase in vascular conductivity and a decrease in systemic blood pressure. This effect appears to involve histamine H1 receptors, histamine presynaptic H3 heteroreceptors, and autonomic alpha-2 receptors, at least in the guinea pig inner ear.
Another peripheral mechanism was recently put forward by Botta et al (1998) in the frog. The authors examined the effects of betahistine on isolated preparations of frog posterior semicircular canals and found a decrease of the resting discharge of the ampullar receptors when the drug was administrated via the perilymphatic fluid. The effect could involve HA receptors in the peripheral vestibular system, a mechanism that remains, however, to be confirmed. More recently, H3 antagonists such as thioperamide and betahistine were found to decrease the electrical discharge of afferents neurons in the axolotl by interfering with the postsynaptic response to excitatory amino acid agonists (Chavez et al 2005). The results support the idea that antivertigo action of HA-released drugs may be caused, at least in part, by a decrease in the sensory input from the vestibular endorgans.
Whatever the real nature of the mechanism elicitated at the peripheral level after administration of betahistine, it seems that it contributes, at least partly, to the antivertigo property of the drug.
Central mechanisms
There is today a growing body of data indicating that HA acts also on central neuronal networks involved in the recovery process after vestibular loss (Lacour and Sterkers 2001), and that betahistine-induced improvements in vestibular disorders and vertigo result from such central effects at various CNS levels.
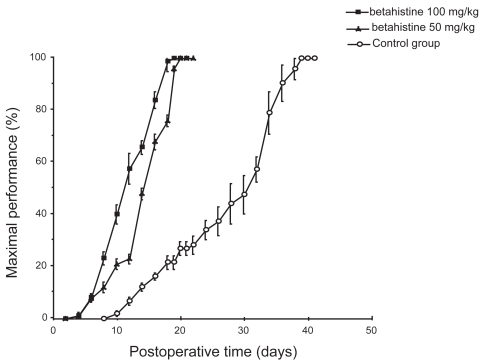
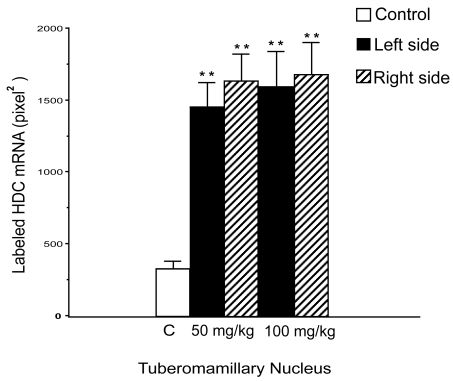
It has clearly been shown that the behavioral recovery process after unilateral vestibular neurectomy (UVN) in the cat is strongly accelerated under betahistine treatment (Tighilet et al 1995). Betahistine treatment reduces the time to recovery by 2 weeks for both static (posture) and kinetic (locomotor equilibrium) functions compared with controls (untreated and double blind, placebo-controlled animals (Figure 1). Immunohistochemical investigations performed in the same cat model suggest that improvement in vestibular compensation is related to changes in HA synthesis and release (Tighilet and Lacour 1997). Further studies using an in situ hybridization method for quantifying the messenger RNA for histidine decarboxylase (HDC), the enzyme synthesizing HA, pointed to an up-regulation of HDC mRNA in the TM nuclei of cats treated with HA-like drugs (Tighilet et al 2002) (Figure 2). In addition, receptor binding studies showed a downregulation of histamine H3 receptors in both the VN and TM nuclei. It seems very likely, therefore, that betahistine upregulates HA turnover by blocking the presynaptic histamine H3 receptors. This hypothesis is corroborated by the well known role of these receptors in mediating autoinhibition of brain HA release (Arrang et al 1983) and autoregulation of HA synthesis (Arrang et al 1987, 1992). In rat brain slices, agonists and antagonists of the histamine H3 receptors reduce and enhance HA release, respectively (Arrang et al 1983, 1987; Garbag et al 1989).
Figure 1.
Mean recovery curves illustrating maximal performance of the cat on the rotating beam. Results are expressed in percent of the preoperative maximal performance (on the ordinates) as a function of the postoperative time in days (on the abscissae). The betahistine groups (50 mg/kg and 100 mg/kg) are shown as solid squares and triangles, respectively, and the control group as open circles. Standard errors of the mean are shown as vertical lines. Note the acceleration of the recovery time under betahistine treatment compared with the controls, and the shortened time required to achieve a full compensation (3 weeks instead of 6 weeks).
Figure 2.
Quantification of the histidine decarboxylase (HDC) mRNA labeled surface in the right (hatched columns) and left (solid columns) tuberomammillary (TM) nuclei for the two groups of betahistine-treated cats compared with the control, untreated cats (open columns). Note that HDC mRNA labeled surface is significantly increased in the TM nuclei of betahistine-treated cats. ** p < 0.0001.
The VN are known to play a crucial role in the recovery process after vestibular lesion (Lacour et al 1989; Smith and Curthoys 1989) and are involved in an histaminergic vestibulo-hypothalamo-vestibular loop. It has been demonstrated recently that this histaminergic loop was implicated in the adaptive response to vestibular lesion (Tighilet et al 2006). HDC mRNA expression was: up-regulated in the ipsilateral TM nucleus in UVN cats; up-regulated in the contralateral TM nucleus in compensated UVN cats submitted to a second vestibular nerve section on their intact side (two steps bilateral vestibular neurectomy); and unchanged in one step bilateral vestibular neurectomized cats. The effectiveness of betahistine in the treatment of vertigo and vestibular disease can be seen therefore by the effects of HA release on second-order vestibular neurons. HA depolarizes the medial VN cells in vitro in rats (Phelan et al 1990; Inverarity et al 1993) and guinea pigs (Sérafin et al 1993; Wang and Dutia 1995). The VN control the vestibulo-ocular and vestibulo-spinal reflexes (Wilson and Melvill Jones 1979; Lacour and Borel 1993) and plays a major role in gaze stabilization, a function that is strongly impaired after vestibular loss and alteration of the peripheral vestibular system.
Antihistamines have been found effective in vertigo treatment probably due to central depressive effects. HA (Fischer 1991) and HA-like drugs such as betahistine dihydrochloride have been used also as antivertigo agents for over 20 years (see Rascol et al 1995 for a review). A major advantage of betahistine is that no sedative effects were observed following its administration in animal models (Lin 1994) or in healthy subjects (Betts et al 1991). On the contrary, HA-induced excitatory effects on the neuronal activity of cortical and subcortical structures in the cat by activating histamine H1 receptors (Lin 1994). These other central targets of HA very likely participate to the restoration of vestibular functions, indirectly. Indeed, brain arousal facilitates sensorimotor activity, a factor that was found crucial for the recovery process after vestibular loss in the cat (Xerri and Lacour 1980; Lacour et al 1989).
Finally, a dose-dependent and duration-dependent effect of betahistine treatment on HA turnover in the cat has been reported (Tighilet et al 2005). With a low dose (2 mg/kg) given over a long time period (3 months), similar to the dosing used clinically and reported to have anti-vertigo effects (Oosterveld et al 1987; Constantinescu et al 1996; Kingma et al 1997), HA synthesis and release were significantly increased in our animal model. The data are in favor of symptomatic treatment of vertigo in humans by means of HA-like drugs.
Clinical efficacy
Over the years, betahistine has acquired a dominant position in the pharmacotherapeutic armamentarium for Ménière’s disease and other disorders of the inner ear. It is associated with the beneficial effects of histamine, but, unlike histamine, is does not cause headaches, flushing, blurred vision, vomiting, or sedation. Thus patients whose Ménière’s disease is controlled by betahistine can maintain their lifestyles without fear of fall and fractures, and, most importantly betahistine does not interfere with vestibular rehabilitation.
Betahistine has been widely used for management of a range of disorders including Ménière’s disease vertigo of various origin and tinnitus, with good results and widespread acceptance by physicians for many years. However, until relatively recently this widespread use was not based fully supported by modern standards of evidence based medicine. However, in recent years a number of high-quality studies have been completed which better confirm the efficacy of betahistine in Ménière’s disease. The time is therefore opportune to review the evidence base for betahistine.
Clinical studies on betahistine up to the year 2000 have been reviewed by Mira (2001). In this period there were more than 20 controlled clinical studies of betahistine and, while none could be considered methodologically flawless, taken together they strongly suggest that betahistine is effective in reducing the frequency, severity and duration of vertigo and possibly neurovegitative symptoms in vertigo of various aetiologies, but particularly in Ménière’s disease. It is also apparent that tinnitus and hearing loss are far less amenable to treatment with betahistine. In addition to these clinical efficacy studies, laboratory studies on patients with vertigo have also yielded interesting results; a dose-dependent effect of betahistine on the vestibulo-ocular reflex was demonstrated (Kingma et al 1997) in paroxysmal vertigo patients who had previously responded to betahistine.
Comparative studies with betahstine during the early years of its investigation suggested that it was less effective than trimetazidine (Kluyskens et al 1990; Martini and De Domenico 1990), and flunarizine (Elbaz 1988) (though this has been challenged in later studies [Albera et al 2003]) and at least as effective as cinnarizine (Deering et al 1986) and prochlorperazine (Aantaa and Skinhoj 1976). However, few of these studies are altogether convincing and fewer still have been replicated.
Betahistine continued to be used in many territories on this less than ideal evidence base for many years, although the Food and Drug Administration were unconvinced by the data and withdrew the drug’s licence. It remains unlicensed in the US, although widely available elsewhere and anecdotal evidence suggests that patients in the US frequently obtain the drugs overseas.
More robust clinical evidence is available from recent clinical trials and the remainder of this review will focus on these studies, some very recent and others as yet unpublished. These studies are evaluated below and summarized in Table 1.
Table 1.
Summary of recent placebo and active control trials of betahistine
| Reference | N | Methodology | Diagnosis | Treatment | Duration | |
|---|---|---|---|---|---|---|
| Albera et al 2003 | (52) | Double-blind, randomized, muliticenter | Recurrent vetibular vertigo | Betahistine Flunarazne | 8 weeks | Betahistine significantly more effective then flunarizine on DHI and physical, functional and functional subscores. |
| Mira et al 2003 | 144 | Double-blind, multi-center, randomized, parallel group | Ménière’s ‘s disease or paroxysmal position vertigo of possible vascular origin | Placebo Betahistine 16 mg tid | 3 months | Frequency, intensity and duration of attacks significantly reduced compared with placebo. Physician’s judgment and patient’s opinion of efficacy and acceptability favored betahistine |
| Novotny and Kostrica 2002 | 82 | randomized, double-blind | Ménière’s disease | Betahistine 12 mg tid cinnarizine, 20 mg + dimenhydrinate, 40 mg tid | 12 weeks | Highly significant reduction in vertigo symptoms, no difference between treatments. Significant improvement in hearing function noted for cinnarizine + dimenhydrinate |
| Cirek et al 2005 | 61 | double-blind, comparative, single center | peripheral vestibular vertigo | Betahistine 12 mg tid cinnarizine 20 mg + dimenhydrinate 40 mg tid | 4 weeks | Significantly greater reduction in vertigo score with cinnarizine+ dimenhydrinate than with betahistine |
Placebo-controlled trials
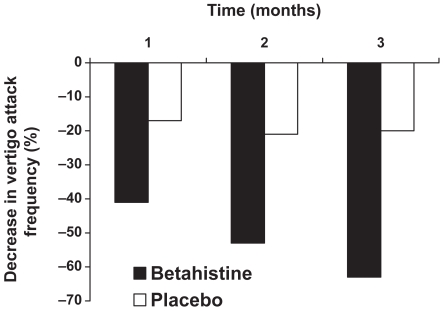
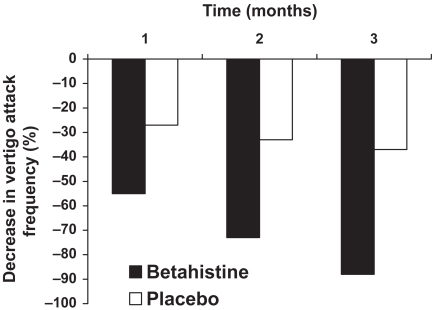
In a double-blind, parallel group study undertaken at 11 centers in Italy, a total of 144 patients with peripheral vestibular vertigo (including Meniere’s disease and paroxyxsmal peripheral vertigo of suspected vascular origin) were randomized to receive either placebo or betahistine 8 mg twice daily for up to 3 months (Mira et al 2003). Efficacy was determined by the frequency, severity and duration of vertigo attacks, the GISFaV self-rating scale and Dizziness Handicap Inventory (DHI). After 3 months of treatment the mean frequency of vertigo attacks was reduced from 6.7 per month at baseline to 2.06 per month in Meniere’s disease patients and from 6.9 per month to 1.91 per month in PPV patients. The difference between betahistine and placebo was significant from the end of the first month’s treatment onwards (Figure 3 and Figure 4). The vertigo intensity score was more frequently improved on betahistine than on placebo, a difference that was significant from day 15 onwards and associated symptoms (tinnitus, aural fullness, nausea, and vomiting was also more frequently improved on betahistine than on placebo. GISFaV scores improved in 70% of betahisitine patients, but in only 30% of the placebo group, moreover, the proportion of patients GISFaV of zero at 3 months was 57% in the betahistine-treated patients compared to 3% in those given placebo. Similarly the six of the seven items in the Dizziness Assessment Rating Scale were improved more by betahistine than by placebo. Betahistine significantly reduced DHI scores significantly more freqently than placebo. The physican’s rating of treatment was good or very good in 74% of betahistine-treated patients compared with 27% of placebo-treated patients.
Figure 3.
Percentage improvement in frequency of vertigo attacks – Menière’s disease. Reproduced with permission from Mira E, Guidetti G, Ghilardi L, et al 2003. Betahistine dihydrochloride in the treatment of peripheral vestibular vertigo. Eur Arch Otorhinolaryngol, 260:73–7. Copyright © 2003 Springer.
Figure 4.
Percentage improvement in frequency of vertigo attacks – peripheral paroxysmal vertigo. Reproduced with permission from FMira E, Guidetti G, Ghilardi L, et al 2003. Betahistine dihydrochloride in the treatment of peripheral vestibular vertigo. Eur Arch Otorhinolaryngol, 260:73–7. Copyright © 2003 Springer.
Comparative studies
Dizziness Handicap Score was used as the primary efficacy variable in a study by Albera and colleagues (Albera et al 2003). Seventy-eight patients who were severely handicapped vertigo of peripheral vestibular origin were randomized to receive 8 weeks treatment with either betahistine 16 mg tid or flunarizine 10 mg od. Although DHI score decreased significantly in both groups during treatment, the decrease on betahistine treatment was significantly greater than that on flunarizine (decrease from baseline of 12.4 for betahistine vs 8.8 for flunarizine, p < 0.01). Vegetative symptoms (of which nausea was the most common) decreased significantly in both groups, without significant difference between the treatments. Tinnitus was the most refractory symptom with no significant reduction by either treatment. Betahistine also appeared to be somewhat quicker in reducing the handicap associated with vertigo. Although there was no significant difference between the treatments in their reduction of vegetative symptoms, it was the physical (rather than functional or emotional) subscores of the DHI that showed the greatest difference between betahistine and flunarizine (5.7 vs 6.0 for betahistine and flunarizine respectivly, p < 0.01). Moreover, there was a clear relationship between the improvement of DHI scores and the improvement in vegetative symptoms.
An earlier study by Fraysse and colleagues (Fraysse et al 1991) yielded similar results. In this multicenter, double-blind, randomized trial, in which 55 patients received either betahistine 48 mg/day or flunarizine 10 mg/day, betahistine was significantly superior to flunarizine for frequency and duration of vertigo attacks and the presence of vegetative symptoms. The results of these two studies are in contrast to an earlier 2-month double-blind comparison of betahistine 8 mg tid and flunarizine 10 mg od in 117 patients with vestibular vertigo, which found flunarizine to be significantly superior to betahistine. It is not clear to what extent the lower dose of betahistine used in this study might have contributed to the lack of efficacy that was observed.
Two studies have compared betahistine with the fixed combination of cinnarizine and dimenhydrinate. In the first study by Novotny and Kostrica (2002), patients with Méniere’s disease were entered into a randomized, double-blind, parallel group study lasting 12 weeks. Eighty-two patients were randomized to treatment with betahistine 12 mg tid or cinnarizine plus dimendydrinate. The intensity of vertigo, as measured by the patient on a visual analogue scale was markedly reduced by both treatments from a baseline score of 2.4–0.31 and 0.4 for betahistine and cinnarizine plus dimendydrinate, respectively, there was no statistically significant difference between the two treatments. Tinnitus and vegetative symptoms were also significantly, and approximately equally, reduced by the two treatments.
Betahistine was used as a reference therapy in the study of a new fixed combination of cinnarizine 20 mg plus dimenhydrinate 40 mg in patients with vertigo of peripheral vestibular origin (Cirek et al 2005). Sixty-one patients were entered into the single center study and were randomized to 4 weeks of treatment with betahistine 12 mg or cinnarizine plus dimenhydrinate. Both betahistine and cinnarizine plus dimenhydrinate reduced mean vertigo scores, although cinnarizine plus dimenhydrinate was significantly more effective than betahistine. Vertigo-associated symptoms were also reduced more effectively by cinnarizine plus dimenhydrinate than by betahistine.
Open studies
The efficacy of betahistine has also been confirmed in open naturalistic studies. An as yet unpublished study by Novotny and Kostrica (2002) found symptoms disappeared or improved in 93% (15% and 78% respectively) of 293 patients with Ménière’s disease of at least 3 months duration who were treated with betahistine 16 mg tid.
Safety and tolerability
Adverse events appear to be rare during betahistine therapy, mild skin reactions are the most common and epigastric upset is reported occasionally as is headache and nausea (the latter also a symptom of the illness being treated). Although formal safety and tolerability studies have not been undertaken to modern standards the drug has been used for many decades and tens of millions of patients have been exposed without significant safety or tolerability concerns having arisen.
Discussion
Ménière’s is a relatively common and debilitating otological condition. Whilst many drug therapies are employed, the great majority hamper vestibular adaptation and, whilst reducing the distressing symptoms of vertigo, may hinder long-term recovery. Betahistine has been a staple of the treatment of Ménière’s disease and other disorders that include vertigo as a cardinal symptom. Until recently however, evidence for its efficacy has not been available to modern standards of proof. The past 10 years have seen significant advances, not only in clarifying the efficacy of betahistine, but also in our understanding of its mechanism of action – a remarkable situation for a drug that has been in routine clinical use for more than 40 years.
Besides vascular action in the inner ear, modulation of the peripheral vestibular sensory cells, and excitatory effect on the neuronalactivity of corticaland subcortical structures, betahistine interacts strongly with the histaminergic system increasing histamine synthesis and release in the tuberomammillary nuclei of the posterior hypothalamus. The action of histamine on the vestibular cells on the affected side may contribute to a rebalancing of neuronalactivity between the two sides; a key mechanism in the vestibular recovery process. Vestibular deficits require a considerable period for compensation to occur and these long-term adaptive mechanisms can be facilitated pharmacologically using histaminergic-like drugs such as betahistine. Animal studies show clearly that betahistine does not interfere with vestibular adaptation in the way that drugs with sedative effects do.
The situation concerning the clinical efficacy of betahistine is now somewhat regularized in that randomized placebo and active controlled clinical trials have confirmed that betahistine is effective in the treatment of Ménière’s disease and related conditions. However, compared with modern standards of evidence-based medicine, further trials, randomized, placebo-controlled studies in particular, would be welcome. Nevertheless, the available data from recent well-conducted trials shows that betahistine is more effective than placebo or flunarizine. Comparative studies of betahistine with the fixed combination of cinnarizine and dimenhydrinate are equivocal, whilst there are clear and significant reductions in symptoms with both treatments, their relative efficacy is not clear. More studies are needed, but it is now clear that betahistine is effective in Ménière’s disease – after 40 years of clinical use it would have been surprising if this were not the case – its freedom from sedative properties that interfere with vestibular adaptation is a major clinical advantage compared with many drugs employed in this field.
Although it remains true that there is still much to learn about betahistine, important advances have been in made in understanding its place in the therapy of Ménière’s disease and related illnesses and in elucidating its mechanism of action.
References
- Aantaa E, Skinhoj A. Controlled clinical trial comparing the effect of betahistine hydrochloride and prochlorperazine maleate on patients with Ménière’s disease. Ann Clin Res. 1976;8:284–7. [PubMed] [Google Scholar]
- AAO-HNS. Committee on hearing and equilibrium. Guidelines for the diagnosis and evaluation of therapy in Ménière’s disease. Otolaryngol Head Neck Surg. 1995;113:181–5. doi: 10.1016/S0194-5998(95)70102-8. [DOI] [PubMed] [Google Scholar]
- Airaksinen MS, Panula P. The histaminergic system in the guinea pig central nervous system: an immunocytochemical mapping study using an antiserum against histamine. J Comp Neurol. 1988;273:163–6. doi: 10.1002/cne.902730204. [DOI] [PubMed] [Google Scholar]
- Albera R, Ciuffolotti R, Di Cicco M, et al. Double-blind, randomized, multicenter study comparing the effect of betahistine and flunarizine on the dizziness handicap in patients with recurrent vestibular vertigo. Acta Otolaryngol. 2003;123:588–93. doi: 10.1080/00016480310001475. [DOI] [PubMed] [Google Scholar]
- Allum JHJ, Keshner EA, Honegger F, et al. Indicators of the influence a peripheral vestibular deficit has on vestibulo-spinal reflex responses controlling postural stability. Acta Otolaryngol. 1988;106:252–63. doi: 10.3109/00016488809106433. [DOI] [PubMed] [Google Scholar]
- Anderson JP, Harris JP. Impact of Ménière’s disease on quality of life. Otol Neurotol. 2001;22:888–94. doi: 10.1097/00129492-200111000-00030. [DOI] [PubMed] [Google Scholar]
- Andrews JC. Intralabyrinthine fluid dynamics: Meniere disease. Curr Opin Otolaryngol Head Neck Surg. 2004;12:408–12. doi: 10.1097/01.moo.0000136872.18760.3b. [DOI] [PubMed] [Google Scholar]
- Arrang JM, Garbag M, Quatch TT, et al. Actions of betahistine at histamine receptors in the brain. Eur J Pharmacol. 1985;111:73–84. doi: 10.1016/0014-2999(85)90115-3. [DOI] [PubMed] [Google Scholar]
- Arrang JM, Garbag M, Schwartz JC. Autoregulation of histamine synthesis in brain mediated by presynaptic H3 receptors. Neuroscience. 1987;23:149–57. doi: 10.1016/0306-4522(87)90279-x. [DOI] [PubMed] [Google Scholar]
- Arrang JM, Garbarg M, Schwartz JC. Auto-inhibition of brain histamine release mediated by a novel class H3. of histamine receptor. Nature. 1983;302:832–7. doi: 10.1038/302832a0. [DOI] [PubMed] [Google Scholar]
- Arrang JM, Garbag M, Schwartz JC. H3-receptors and control of histamine release. In: Schwartz JC, Haas HL, editors. The histamine receptors. New York: Wiley-Liss; 1992. pp. 145–60. [Google Scholar]
- Betts T, Harris D, Gadd E. The effects of two antivertigo drugs betahistine and prochlorperazine. on driving skills. Br J Clin Pharmacol. 1991;32:455–8. doi: 10.1111/j.1365-2125.1991.tb03930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel L, Harlay F, Lopez C, et al. Walking performance of vestibular-defective patients before and after unilateral vestibular neurotomy. Behav Brain Res. 2004;150:191–200. doi: 10.1016/S0166-4328(03)00257-2. [DOI] [PubMed] [Google Scholar]
- Borel L, Harlay F, Magnan J, et al. Deficits and recovery of head and trunk orientations and stabilization after unilateral vestibular loss. Brain. 2002;125:880–94. doi: 10.1093/brain/awf085. [DOI] [PubMed] [Google Scholar]
- Botta L, Mira E, Valli S, et al. Effects of betahistine on vestibular receptors of the frog. Acta Otolaryngol. 1998;118:519–23. doi: 10.1080/00016489850154658. [DOI] [PubMed] [Google Scholar]
- Brantberg K, Fransson PA, Bergenius J, et al. Tilt suppression, OKAN, and head-shaking nystagmus at long-term follow-up after unilateral vestibular neurectomy. J Vest Res. 1996;6:235–41. [PubMed] [Google Scholar]
- Chávez H, Vega R, Soto E. Histamine (H3) receptors modulate the excitatory amino acid receptor response of the vestibular afferents. Brain Res. 2005;7:1–9. doi: 10.1016/j.brainres.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Cirek Z, Schwarz M, Baumann W, et al. Efficacy and tolerability of a fixed combination of cinnarizine and dimenhydrinate versus betahistine in the treatment of otogenic vertigo: a double-blind, randomised clinical study. Clin Drug Invest. 2005;26:377–89. doi: 10.2165/00044011-200525060-00003. [DOI] [PubMed] [Google Scholar]
- Claes J, Van de Heyning PH. Medical therapy of Ménière’s disease : a review of literature. Acta Otolaryngol Suppl. 1997;526:37–42. doi: 10.3109/00016489709124019. [DOI] [PubMed] [Google Scholar]
- Claes J, Van de Heyning PH. A review of medical treatment for Meniere’s disease. Acta Otolaryngol Suppl. 2000;544:34–9. doi: 10.1080/000164800750044461. [DOI] [PubMed] [Google Scholar]
- Clendaniel RA, Tucci DL. Vestibular rehabilitation strategies in Meniere’s disease. Otolaryngol Clin North Am. 1997;30:1145–58. [PubMed] [Google Scholar]
- Cohen H, Ewell LR, Jenkins HA. Disability in Ménière’s disease. Arch Otolaryngol Head Neck Surg. 1995;121:29–33. doi: 10.1001/archotol.1995.01890010017004. [DOI] [PubMed] [Google Scholar]
- Constantinescu L, Schneider D, Claussen CF. 3rd EUFOS. Bologna: Monduzzi Editore; 1996. The influence of beta-histine on the vestibular evoked potentials in patients with peripheral vestibular disorders; pp. 95–98. [Google Scholar]
- Cawthorne T, Hewlett AB. Ménière’s disease. Proc R Soc Med. 1954;47:663–70. [PMC free article] [PubMed] [Google Scholar]
- Curthoys IS, Dai MJ, Halmagyi GM. Human ocular torsional position before and after unilateral vestibular neurectomy. Exp Brain Res. 1991;85:218–25. doi: 10.1007/BF00230003. [DOI] [PubMed] [Google Scholar]
- Dai MJ, Curthoys IS, Hamlagyi GM. Linear acceleration perception in the roll plane before and after unilateral vestibular neurectomy. Exp Brain Res. 1989;77:315–28. doi: 10.1007/BF00274989. [DOI] [PubMed] [Google Scholar]
- Deering RB, Prescott P, Simmons RL, et al. A double-blind crossover study comparing betahistine and cinnarizine in the treatment of recurrent vertigo in patients in general practice. Curr Med Res Opin. 1986;10:209–14. doi: 10.1185/03007998609110440. [DOI] [PubMed] [Google Scholar]
- Dodson KM, Sismanis A. Intratympanic perfusion for the treatment of tinnitus. Otolaryngol Clin North Am. 2004;37:991–1000. doi: 10.1016/j.otc.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Dowdal-Osborn M. Early vestibular rehabilitation in patients with Meniere’s disease. Otolaryngol Clin North Am. 2002;35:683–90. doi: 10.1016/s0030-6665(02)00026-9. [DOI] [PubMed] [Google Scholar]
- Doyle KJ, Bauch C, Battista R, et al. Intratympanic steroid treatment: a review. Otol Neurotol. 2004;25:1034–9. doi: 10.1097/00129492-200411000-00031. [DOI] [PubMed] [Google Scholar]
- Dunnebier EA, Segenhout JM, Wit HP, et al. Two-phase endolymphatic hydrops: a new dynamic guinea pig model. Acta Otolaryngol. 1997;117:13–19. doi: 10.3109/00016489709117984. [DOI] [PubMed] [Google Scholar]
- Dunnebier EA, Segenhout JM, Dijk F, et al. Cochlear ultrastructure in two-phase endolymphatic hydrops in the guinea-pig. Eur Arch Otorhinolaryngol. 2002;259:17–23. doi: 10.1007/pl00007522. [DOI] [PubMed] [Google Scholar]
- Elbaz P. Flunarizine and betahistine. Two different therapeutic approaches in vertigo compared in a double-blind study. Acta Otolaryngol Suppl. 1988;460:143–8. [PubMed] [Google Scholar]
- Feijen RA, Segenhout JM, Albers FW, et al. Cochlear aqueduct flow resistance depends on round window membrane position in guinea pigs. J Assoc Res Otolaryngol. 2004;5:404–10. doi: 10.1007/s10162-004-5001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipo R, Barbara M. Natural history of Méniere’s disease: staging the patients or their symptoms? Acta Otolaryngol Suppl. 1997;526:10–13. doi: 10.3109/00016489709124013. [DOI] [PubMed] [Google Scholar]
- Fischer AJ. Histamine in the treatment of vertigo. Acta Otolaryngol Suppl. 1991;479:24–8. doi: 10.3109/00016489109121145. [DOI] [PubMed] [Google Scholar]
- Fraysse B, Bebear JP, Dubreuil C, et al. Betahistine dihydrochloride versus flunarizine. A double-blind study on recurrent vertigo with or without cochlear syndrome typical of Ménière’s disease. Acta Otolaryngol Suppl. 1991;490:1–10. [PubMed] [Google Scholar]
- Garbag M, Trung Tuong MD, Gros C, et al. Effects of histamine H3 receptor ligands on various biochemical indices of histaminergic neuron activity in rat brain. Eur J Pharmacol. 1989;164:1–11. doi: 10.1016/0014-2999(89)90225-2. [DOI] [PubMed] [Google Scholar]
- Gustave dit Duflo S, Gestreau C, Lacour M. Fos expression in the rat brain after exposure to gravito-inertial force changes. Brain Res. 2000;861:333–44. doi: 10.1016/s0006-8993(00)02044-8. [DOI] [PubMed] [Google Scholar]
- Gustave dit Duflo S, Gestreau C, Tighilet B, et al. Fos expression in the cat brainstem after unilateral vestibular neurectomy. Brain Res. 1999;824:1–17. doi: 10.1016/s0006-8993(99)01172-5. [DOI] [PubMed] [Google Scholar]
- Halmagyi GM, Curthoys IS, Cremer PD, et al. The human horizontal vestibulo-ocular reflex in response to high-acceleration stimulation before and after unilateral vestibular neurectomy. Exp Brain Res. 1990;81:479–90. doi: 10.1007/BF02423496. [DOI] [PubMed] [Google Scholar]
- Havia M, Kentala E. Progression of symptoms of dizziness in Ménière’s disease. Arch Otolaryngol Head Neck Surg. 2004;130:431–5. doi: 10.1001/archotol.130.4.431. [DOI] [PubMed] [Google Scholar]
- Horii A, Takeda N, Matsunaga T, et al. Effect of unilateral vestibular stimulation on histamine release from the hypothalamus of rats in vivo. J Neurophysiol. 1993;70:1822–6. doi: 10.1152/jn.1993.70.5.1822. [DOI] [PubMed] [Google Scholar]
- Horii A, Mitani K, Kitahara T, et al. Paroxetine, a selective serotonin reuptake inhibitor, reduces depressive symptoms and subjective handicaps in patients with dizziness. Otol Neurotol. 2004;25:536–43. doi: 10.1097/00129492-200407000-00022. [DOI] [PubMed] [Google Scholar]
- Inverarity DJ, Johnston AR, McQueen DS, et al. Effects of histamine on rat medial vestibular nucleus neurones in vitro. J Physiol. 1993;459:466. [Google Scholar]
- Jaju B, Wang SC. Effects of diphenhydramine and dimenhydrinate on vestibular neuronalactivity of cat: a search for the locus of their antimotion sickness action. J Pharmacol Exp Therap. 1971;176:718–24. [PubMed] [Google Scholar]
- Kentala E. Characteristics of six otologic diseases involving vertigo. Am J Otol. 1996;17:883–92. [PubMed] [Google Scholar]
- Kiang NY. Curious oddments of auditory-nerve studies. Hear Res. 1990;49:1–16. doi: 10.1016/0378-5955(90)90091-3. [DOI] [PubMed] [Google Scholar]
- Kingma H, Bonink M, Meulenbroeks, et al. The dose-dependent effect of betahistine on vestibulo-ocular reflex: a double-blind, placebo controlled study in patients with paroxysmal vertigo. Acta Otolaryngol. 1997;117:1–6. doi: 10.3109/00016489709113454. [DOI] [PubMed] [Google Scholar]
- Kinney SE, Sandridge SA, Newman CW. Long-term effects of Ménière’s disease on hearing and quality of life. Am J Otol. 1997;18:67–73. [PubMed] [Google Scholar]
- Klockhoff I, Lindblom U. Ménière’s disease and hydrochlorothiazide (Dichlotride)—a critical analysis of symptoms and therapeutic effects. Acta Otolaryngol. 1967;63:347–65. doi: 10.3109/00016486709128769. [DOI] [PubMed] [Google Scholar]
- Kluyskens P, Lambert P, D’Hooge D. Trimetazidine versus betahistine in vestibular vertigo. A double blind study. Ann Otolaryngol Chir Cervicofac. 1990;107(Suppl 1):11–9. [PubMed] [Google Scholar]
- Kotimaki J, Sorri M, Aantaa E, et al. Prevalence of Ménière disease in Finland. Laryngoscope. 1999;109:748–53. doi: 10.1097/00005537-199905000-00013. [DOI] [PubMed] [Google Scholar]
- Lacour M, Borel L. Vestibular controlof posture and gait. Arch Ital Biol. 1993;131:81–104. [PubMed] [Google Scholar]
- Lacour M, Sterkers O. Histamine and betahistine in the treatment of vertigo: elucidation of mechanisms of action. CNS Drugs. 2001;15:853–70. doi: 10.2165/00023210-200115110-00004. [DOI] [PubMed] [Google Scholar]
- Lacour M, Toupet M, Denise P, et al. Vestibular compensation Facts, theories and clinical perspectives . Paris: Elsevier; 1989. [Google Scholar]
- Laurens-Thalen EO, Wit HP, Segenhout JM, et al. Direct measurement of the flow resistance of the aqueduct in the guinea pig. Acta Otolaryngol. 2004;124:1–5. doi: 10.1080/00016480410017530. [DOI] [PubMed] [Google Scholar]
- Laurikainen EA, Miller JM, Nuttall AL, et al. The vascular mechanisms of action of betahistine in the inner ear of the guinea pig. Eur Arch Otorhinolaryngol. 1998;255:119–23. doi: 10.1007/s004050050025. [DOI] [PubMed] [Google Scholar]
- Laurikainen EA, Miller JM, Quirk WS, et al. Betahistine-induced vascular effects in the rat cochlea. Am J Otol. 1993;14:24–30. [PubMed] [Google Scholar]
- Lin JS. Système histaminergique centralet états de vigilance chez le chat. Thèse Doct Sciences, Université Claude Bernard; Lyon: 1994. [Google Scholar]
- Lopez C, Borel L, Magnan J, et al. Torsional optokinetic nystagmus after unilateral vestibular loss: asymmetry and compensation. Brain. 2005 doi: 10.1093/brain/awh504. [DOI] [PubMed] [Google Scholar]
- Luxon LM. The anatomy and physiology of vestibular system. In: Dix MR, Hood J, editors. Vertigo. New York: John Wiley and Sons; 1984. pp. 1–36. [Google Scholar]
- Martini A, De Domenico F. Trimetazidine versus betahistine in Ménière’s disease. A double blind method. Ann Otolaryngol Chir Cervicofac. 1990;107(Suppl 1):20–7. [PubMed] [Google Scholar]
- Mira E. Betahistine in the treatment of vertigo. History and clinical implications of recent pharmacological researches. Acta Otorhinolaryngol Ital. 2001;21(Suppl 66):1–7. [PubMed] [Google Scholar]
- Mira E, Guidetti G, Ghilardi L, et al. Betahistine dihydrochloride in the treatment of peripheral vestibular vertigo. Eur Arch Otorhinolaryngol. 2003;260:73–7. doi: 10.1007/s00405-002-0524-4. [DOI] [PubMed] [Google Scholar]
- Merchant SN, Adams JC, Nadol JB., Jr Physiopathology of ménière’s syndrome: are symptoms caused by endolymphatic hydrops? Otol Neurotol. 2005;26:74–81. doi: 10.1097/00129492-200501000-00013. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Mathew NT, Hartmann A, et al. Orally administrated betahistine and regional cerebral blood flow in cerebrovascular disease. J Clin Pharmacol. 1974;14:280–5. doi: 10.1002/j.1552-4604.1974.tb02314.x. [DOI] [PubMed] [Google Scholar]
- Novotny M, Kostrica R. Fixed combination of cinnarizine and dimen-hydrinate versus betahistine dimesylate in the treatment of Ménière’s disease: a randomized, double-blind, parallel group clinical study. Int Tinnitus J. 2002;8:115–23. [PubMed] [Google Scholar]
- Oosterveld WJ. Betahistine dihydrochloride in the treatment of vertigo of peripheral vestibular origin. A double-blind placebo-controlled study. J Laryngol Otol. 1984;98:37–41. doi: 10.1017/s0022215100146158. [DOI] [PubMed] [Google Scholar]
- Oosterveld WJ. Effect of betahistine dihydrochloride on induced vestibular nystagmus: a double blind study. Clin Otolaryngol Allied Sci. 1987;12:131–5. doi: 10.1111/j.1365-2273.1987.tb00175.x. [DOI] [PubMed] [Google Scholar]
- Panula P, Pirvola U, Auvinen S, et al. Histamine-immunoreactive nerve fibers in the rat brain. Neuroscience. 1989;28:585–610. doi: 10.1016/0306-4522(89)90007-9. [DOI] [PubMed] [Google Scholar]
- Phelan KD, Nakamura J, Gallagher JP. Histamine depolarizes rat medial vestibular nucleus neurons recorded intracellularly in vitro. Neurosci Lett. 1990;109:287–92. doi: 10.1016/0304-3940(90)90009-x. [DOI] [PubMed] [Google Scholar]
- Pollard H, Schwartz JC. Histamine neuronal pathways and their function. Trends Neurosci. 1987;10:86–9. [Google Scholar]
- Pondugula SR, Sanneman JD, Wangemann P, et al. Glucocorticoids stimulate cation absorption by semicircular canal duct epithelium via epithelial sodium channel. Am J Physiol Renal Physiol. 2004;286:F1127–35. doi: 10.1152/ajprenal.00387.2003. [DOI] [PubMed] [Google Scholar]
- Rascol O, Hain TC, Brefel C, et al. Antivertigo medications and drug-induced vertigo. Drugs. 1995;50:777–91. doi: 10.2165/00003495-199550050-00002. [DOI] [PubMed] [Google Scholar]
- Scholtz AW, Schwarz M, Baumann W, et al. Treatment of vertigo due to acute unilateral vestibular loss with a fixed combination of cinnarizine and dimenhydrinate: a double-blind, randomized, parallel-group clinical study. Clin Ther. 2004;26:866–77. doi: 10.1016/s0149-2918(04)90130-0. [DOI] [PubMed] [Google Scholar]
- Schwartz JC. Histamine receptors in brain. Life Sci. 1979;25:895–912. doi: 10.1016/0024-3205(79)90495-8. [DOI] [PubMed] [Google Scholar]
- Schwartz JC, Arrang JM, Garbarg M, et al. A third histaminergic receptor subtype: characterisation, location and functions of the H3-receptor. Agents Actions. 1990;30:13–23. doi: 10.1007/BF01968988. [DOI] [PubMed] [Google Scholar]
- Selmani Z, Marttila T, Pyykkö I. Incidence of virus infection as a cause of Ménière’s disease or endolymphatic hydrops assessed by electrocochleography. Eur Arch Otorhinolaryngol. 2005;262:331–4. doi: 10.1007/s00405-004-0816-y. [DOI] [PubMed] [Google Scholar]
- Serafin M, Khateb A, Vibert N, et al. Medial vestibular nucleus in the guinea-pig: histaminergic receptors. I. An in vitro study. Exp Brain Res. 1993;93:242–8. doi: 10.1007/BF00228391. [DOI] [PubMed] [Google Scholar]
- Shojaku H, Watanabe Y. The prevalence of definite cases of Méniere’s disease. Acta Otolaryngol. 1997;528:94–6. [PubMed] [Google Scholar]
- Smith PF, Curthoys IS. Mechanisms of recovery following unilateral labyrinthectomy: a review. Brain Res Brain Res Rev. 1989;14:155–80. doi: 10.1016/0165-0173(89)90013-1. [DOI] [PubMed] [Google Scholar]
- Smith WK, Sankar V, Pfleiderer AG. A national survey amongst UK otolaryngologists regarding the treatment of Ménière’s disease. J Laryngol Otol. 2005;119:102–5. doi: 10.1258/0022215053419871. [DOI] [PubMed] [Google Scholar]
- Stahle J, Stahle C, Arenberg IK. Incidence of Ménière’s disease. Arch Otolaryngol. 1978;104:99–102. [PubMed] [Google Scholar]
- Stephens SD, Lewis PA, Charny MC, et al. Characteristics of self-reported hearing problems in a community survey. Audiology. 1990;29:93–100. doi: 10.3109/00206099009081650. [DOI] [PubMed] [Google Scholar]
- Stephens SD, Hogan S, Meredith R. The desynchrony between complaints and signs of vestibular disorders. Acta Otolaryngol. 1991;111:188–92. doi: 10.3109/00016489109137373. [DOI] [PubMed] [Google Scholar]
- Takeda N, Morita M, Kubo T, et al. Histaminergic projection from the posterior hypothalamus to the medial vestibular nucleus of rats and its relation to motion sickness. In: Graham MD, Kemink JL, editors. The vestibular system: Neurophysiologic and clinical research. New York: Raven Press; 1987. pp. 601–17. [Google Scholar]
- Tighilet B, Lacour M. Distribution of histaminergic axonal fibres in the vestibular nuclei of the cat. Neuroreport. 1996;7:873–8. doi: 10.1097/00001756-199603220-00008. [DOI] [PubMed] [Google Scholar]
- Tighilet B, Lacour M. Histamine immunoreactivity changes in vestibular-lesioned and histaminergic-treated cats. Eur J Pharmacol. 1997;330:65–77. doi: 10.1016/s0014-2999(97)10124-8. [DOI] [PubMed] [Google Scholar]
- Tighilet B, Leonard J, Lacour M. Betahistine dihydrochloride treatment facilitates vestibular compensation in the cat. J Vest Res. 1995;5:53–66. [PubMed] [Google Scholar]
- Tighilet B, Trottier S, Lacour M. Dose and duration-dependent effects of betahistine dihydrochloride treatment on histamine turnover in the cat. Eur J Pharmacol. 2005;523:54–63. doi: 10.1016/j.ejphar.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Tighilet B, Trottier S, Mourre C, et al. Betahistine dihydrochloride interaction with the histaminergic system in the cat: neurochemical and molecular mechanisms. Eur J Pharmacol. 2002;446:63–73. doi: 10.1016/s0014-2999(02)01795-8. [DOI] [PubMed] [Google Scholar]
- Tighilet B, Trottier S, Mourre C, et al. Changes in histaminergic system during vestibular compensation in the cat. J Physiol Lond. 2006 doi: 10.1113/jphysiol.2006.107805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman H. Histamine agonists and antagonists. Acta Otolaryngol Suppl. 1991;479:5–11. doi: 10.3109/00016489109121143. [DOI] [PubMed] [Google Scholar]
- Tumarkin A. The otolythic catastrophe: a new syndrome. BMJ. 1936;2:175–7. doi: 10.1136/bmj.2.3942.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Heyning PH, Boudewyns A, Wuyts F. Surgical treatment of Ménière’s disease. Curr Opn Neurol. 2005;18:23–28. doi: 10.1097/00019052-200502000-00006. [DOI] [PubMed] [Google Scholar]
- van Deelen GW, Huizing EH. Use of a diuretic (Dyazide) in the treatment of Ménière’s disease. A double-blind cross-over placebo-controlled study. ORL J Otorhinolaryngol Relat Spec. 1986;48:287–92. doi: 10.1159/000275884. [DOI] [PubMed] [Google Scholar]
- Vibert D, Häusler R. Long-term evolution of subjective visual vertical after vestibular neurectomy and labyrinthectomy. Acta Otolaryngol. 2000;120:620–2. doi: 10.1080/000164800750000432. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Mizokoshi K, Shojaku H, et al. Epidemiological and clinical characteristics of Ménière’s disease in Japan. Acta Otolaryngol Suppl. 1995;519:206–10. doi: 10.3109/00016489509121906. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Dutia MB. Effects of histamine and betahistine on rat medial vestibular nucleus neurones: possible mechanism of action of anti-histaminergic drugs in vertigo and motion sickness. Exp Brain Res. 1995;105:18–24. doi: 10.1007/BF00242178. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Melvill Jones G. Mammalian vestibular physiology. New York: Plenum Press; 1979. [Google Scholar]
- Warmerdam T J, Schroder FH, Wit HP, et al. Perilymphatic and endolymphatic pressure during endolymphatic hydrops. Eur Arch Otorhinolaryngol. 2003;260:9–11. doi: 10.1007/s00405-002-0518-2. [DOI] [PubMed] [Google Scholar]
- Wladislavosky-Waserman P, Facer G, et al. Ménière’s disease: a 30-year epidemiologic and clinical study in Rochester, MN, 1951–1980. Laryngoscope. 1984;94:1098–02. doi: 10.1288/00005537-198408000-00020. [DOI] [PubMed] [Google Scholar]
- Xerri C, Lacour M. Compensation des déficits posturaux et cinétiques après neurectomie vestibulaire unilatérale chez le chat. Rôle de l’activité sensorimotrice. Acta Otolaryngol. 1980;90:414–20. [PubMed] [Google Scholar]
- Yabe T, de Waele C, Serafin M, et al. Medial vestibular nucleus in the guinea-pig: histaminergic receptors. II. An in vivo study. Exp Brain Res. 1993;93:249–58. doi: 10.1007/BF00228392. [DOI] [PubMed] [Google Scholar]