Abstract
BACKGROUND
Plasmodium falciparum malaria is a pressing global health problem. A previous study of the malaria vaccine RTS,S (which targets the circumsporozoite protein), given with an adjuvant system (AS02A), showed a 30% rate of protection against clinical malaria in children 1 to 4 years of age. We evaluated the efficacy of RTS,S given with a more immunogenic adjuvant system (AS01E) in children 5 to 17 months of age, a target population for vaccine licensure.
METHODS
We conducted a double-blind, randomized trial of RTS,S/AS01E vaccine as compared with rabies vaccine in children in Kilifi, Kenya, and Korogwe, Tanzania. The primary end point was fever with a falciparum parasitemia density of more than 2500 parasites per microliter, and the mean duration of follow-up was 7.9 months (range, 4.5 to 10.5).
RESULTS
A total of 894 children were randomly assigned to receive the RTS,S/AS01E vaccine or the control (rabies) vaccine. Among the 809 children who completed the study procedures according to the protocol, the cumulative number in whom clinical malaria developed was 32 of 402 assigned to receive RTS,S/AS01E and 66 of 407 assigned to receive the rabies vaccine; the adjusted efficacy rate for RTS,S/AS01E was 53% (95% confidence interval [CI], 28 to 69; P<0.001) on the basis of Cox regression. Overall, there were 38 episodes of clinical malaria among recipients of RTS,S/AS01E, as compared with 86 episodes among recipients of the rabies vaccine, with an adjusted rate of efficacy against all malarial episodes of 56% (95% CI, 31 to 72; P<0.001). All 894 children were included in the intention-to-treat analysis, which showed an unadjusted efficacy rate of 49% (95% CI, 26 to 65; P<0.001). There were fewer serious adverse events among recipients of RTS,S/AS01E, and this reduction was not only due to a difference in the number of admissions directly attributable to malaria.
CONCLUSIONS
RTS,S/AS01E shows promise as a candidate malaria vaccine. (ClinicalTrials.gov number, NCT00380393.)
Worldwide, the mortality and morbidity associated with Plasmodium falciparum malaria are high.1-3 Progress has been made in controlling malaria by introducing insecticide-treated nets4 and highly effective artemisinin-based combination treatments.5 There is evidence that the incidence of malaria is falling in some areas.6-10 These advances have renewed interest in the prospects for the control of malaria and even its elimination in areas in which P. falciparum was previously endemic.11 A safe and affordable vaccine providing protection against malaria would be an important addition to control strategies and should be assessed in the context of the use of insecticide-treated nets and the availability of artemisinin-based combination treatments.
The candidate pre-erythrocytic malaria vaccine RTS,S targets the circumsporozoite protein and has been evaluated in combination with two different adjuvant systems: AS01 and AS02. Clinical development of RTS,S in field trials began with the AS02 adjuvant system. Preliminary estimates of rates of efficacy against infection after curative antimalarial treatment were 34% (95% confidence interval [CI], 8 to 53) in adults12 and 66% (95% CI, 43 to 80) in infants.13 The rate of efficacy against the more clinically relevant end point of clinical malaria in children 1 to 4 years of age was 30% (95% CI, 11 to 45).14
Planning is now under way for a multicenter phase 3 trial. However, since preliminary data suggested better immunogenicity with the AS01 adjuvant,15-17 there was a need to evaluate RTS,S administered with the AS01 adjuvant system before selecting the vaccine formulation for phase 3. We evaluated the efficacy of RTS,S/AS01E against clinical malaria in children 5 to 17 months of age.
METHODS
STUDY DESIGN
The study was randomized, controlled, and double-blind and was prospectively registered at ClinicalTrials.gov. Approval was obtained from the Kenyan Medical Research Institute National Ethics Committee, the Tanzanian Medical Research Coordinating Committee, the Central Oxford Research Ethics Committee, the London School of Hygiene and Tropical Medicine Ethics Committee, and the Western Institutional Review Board in Seattle. An independent data and safety monitoring board and local safety monitors were appointed. The study was conducted in accordance with the Helsinki Declaration of 1964 (revised in 1996) and according to Good Clinical Practice guidelines.
GlaxoSmithKline Biologicals was the study sponsor. The database was managed by the sponsor and was opened to the principal investigators at the time of unblinding. Analysis was performed in parallel by an industry author who is an employee of the sponsor and an academic author. Two academic authors and the industry author vouch for the data and analysis. The first draft of the manuscript was written by an academic author, who subsequently implemented revisions from all the authors after their review.
GlaxoSmithKline and both study sites (Kilifi, Kenya, and Korogwe, Tanzania) received funding to undertake the work described in this report from the Program for Appropriate Technology in Health (PATH) Malaria Vaccine Initiative (MVI), which was involved in all aspects of the study design. Permission to submit the manuscript for publication was given by the directors of the Kenya Medical Research Institute and the National Institute for Medical Research of Tanzania. More details of the investigators' and sponsor's roles in the study are given in the Supplementary Appendix, available with the full text of this article at www.nejm.org.
STUDY PARTICIPANTS
We randomly assigned children to receive either the candidate malaria vaccine or a licensed rabies vaccine in a 1:1 ratio at the two study sites (for more details, see the Supplementary Appendix). Vaccinations took place over a 6-month period from March through August 2007. Active surveillance for malaria began 2.5 months after the first vaccination and continued throughout the follow-up period. Venous-blood samples were taken to screen for eligibility, 3 months after the first vaccination as well as in March 2008 (irrespective of the time of recruitment). The study was unblinded at the time of the final blood test, in March 2008. Hence, the duration of follow-up varied according to the time of recruitment, between 4.5 and 10.5 months (mean, 7.9). A locked database containing data collected through the time of the final blood test was unblinded and the data were analyzed in August 2008. The database of adverse events collected up to August 4, 2008, was locked, and the data were analyzed in August 2008.
The participating children were 5 to 17 months of age at the time of the first vaccination, were healthy, and were residents of the study area. Details of the screening are given in the Supplementary Appendix. Written informed consent in an appropriate language (Swahili, Chonyi, or Giriama) was obtained from each child's parent (or parents) or guardian before the study procedures were initiated. Nonliterate parents indicated consent using a thumbprint, and a signature was obtained from a literate witness.
VACCINES
Children were randomly assigned to receive three doses of RTS,S/AS01E or three doses of a human diploid-cell rabies vaccine (rabies vaccine BP, Sanofi-Pasteur). Both vaccines were administered intramuscularly in the deltoid, with one dose given each month for 3 months. Further details about the vaccines are given in the Supplementary Appendix.
RANDOMIZATION AND VACCINATION
The RTS,S/AS01E and rabies vaccines were packaged in identical boxes labeled with treatment numbers from a randomization list generated at GlaxoSmithKline Biologicals in Rixensart, Belgium, and then shipped to the trial sites. Block randomization was used, with stratification according to study site. Subjects were assigned treatment numbers on the basis of order of attendance at the clinic. Boxes containing the assigned vaccine were opened out of sight of the investigators who evaluated the study end points, the study subjects, and their parents or guardians. The syringe used to draw up the vaccine was masked. Personnel preparing the vaccines were aware of the treatment assignments but took no other part in study-related procedures and were instructed not to reveal the assignments to either the parents or guardians of the study subjects or the investigators.
MONITORING FOR EPISODES OF CLINICAL MALARIA
The primary end point was a clinical episode of malaria, defined as an axillary temperature of 37.5°C or higher with a P. falciparum density of more than 2500 parasites per microliter. The presence of any falciparum parasitemia density with a temperature of 37.5°C or higher was a secondary end point.
Active surveillance was performed through weekly visits by field-workers to the home to identify febrile children. Passive surveillance was performed by field-workers and by personnel in the local health facilities. Further details of these visits, as well as safety assessments and laboratory methods, are described in the Supplementary Appendix. The implementation of active surveillance was unintentionally delayed by 3 months in Korogwe, but passive surveillance was conducted during this period.
STATISTICAL ANALYSIS
The study was designed to have a statistical power of 90% to detect a 30% efficacy rate, at a significance level of 0.05, based on a projected malaria incidence of 36% among children receiving the rabies-vaccine doses during a 4-month surveillance period. This design required 800 subjects who could be evaluated, with a total enrollment of 890 subjects to allow for loss to follow-up. Before the beginning of the study, it was planned that the number of episodes of clinical malaria would be monitored in real time and that unblinding of the data would be delayed if fewer than 245 episodes had been recorded by the time this number was reached. In fact, the rates of malaria were considerably lower than expected. The unblinding was delayed, but by the time the transmission season was ending, just over 100 episodes had been recorded. We believed that the target of 245 episodes would not be reached in the near future, and the trial was therefore unblinded on August 4, 2008, after discussion among the investigators, sponsors, regulatory authorities, and data and safety monitoring board.
An analysis plan was agreed on by the data and safety monitoring board, sponsor, and investigators before the unblinding. The primary analysis was an estimate of the hazard ratio for the first or only episode of malaria involving fever and a parasite density above 2500 per microliter (the primary end point) for the group that received the RTS,S/AS01E vaccine as compared with the control group in the according-to-protocol cohort. Secondary analyses included episodes of malaria involving any parasitemia density with fever (a secondary end point) in the according-to-protocol cohort, as well as cases qualifying as primary and secondary end points in the intention-to-treat cohort (i.e., all children randomly assigned to receive vaccinations) and multiple episodes of malaria (analyzed by means of Poisson regression). The period for the according-to-protocol analysis was 2 weeks after the last vaccination until the final blood test. The period for the intention-to-treat analysis was the time from the first vaccination until the final blood test. More details are given in the Supplementary Appendix.
The effect of anticircumsporozoite antibodies on the risk of clinical malaria was examined with the use of titers measured 1 month after the third dose. Log-transformed titers (with undetectable levels scored as a value that was half the lower limit of detection) were used as a continuous variable in an adjusted Cox regression analysis for the children receiving the RTS,S/AS01E vaccine.
Safety data are presented for the intention-to-treat cohort, and efficacy data are presented for both the according-to-protocol cohort and the intention-to-treat cohort. Data were analyzed from the time of the first vaccination until the time of the final blood test for efficacy, but all the available safety data up to August 4, 2008 (the time of unblinding), were analyzed. Serious adverse events are described with the use of the preferred terms from the Medical Dictionary for Regulatory Activities (MedDRA).18
RESULTS
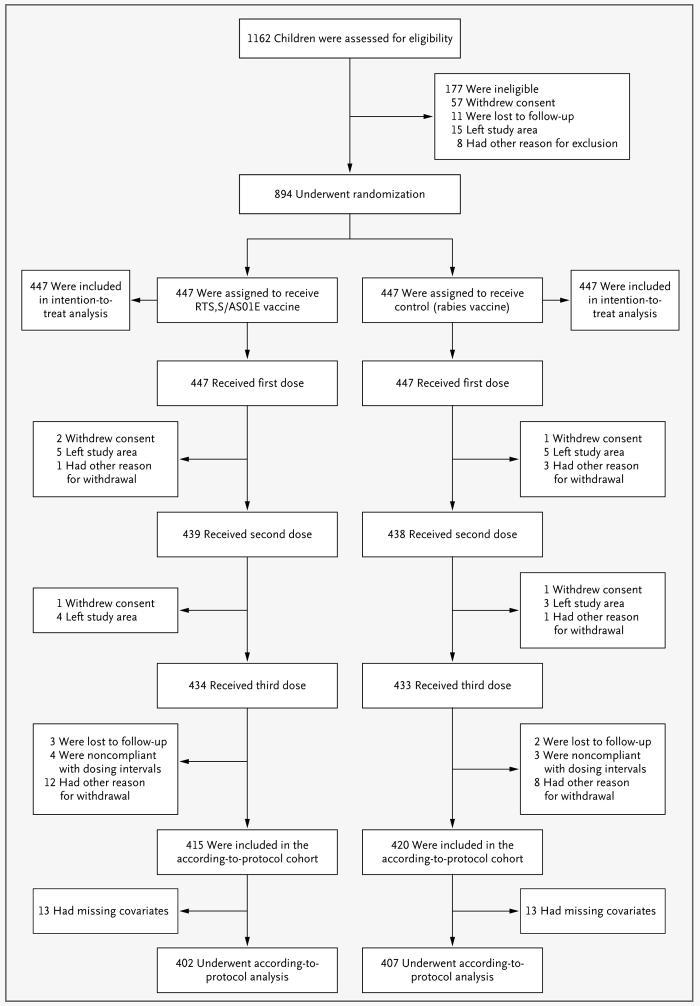
A total of 1162 children were screened; 894 were randomly assigned to a study group and received at least one vaccination, and data for 809 were included in the according-to-protocol analysis of efficacy (Fig. 1). The demographic characteristics of the participants were balanced between the two vaccine groups (Table 1).
Figure 1. Screening, Randomization, and Follow-up of Study Participants.
The 177 children who were deemed ineligible at screening had an age outside the acceptable range (67 children), an acute disease at enrollment (23), a serious illness on clinical screening (42), a laboratory result outside the acceptable limit (28), or another reason for ineligibility: a parent or guardian who was judged by the investigator to be unable to give consent, an inability to follow the protocol, a planned administration of another vaccination, or the use of blood products in the previous 3 months (17). The other reasons for ineligibility or withdrawal from the study were enrollment in other clinical trials (for the eight children excluded at screening only), missed vaccinations because of hospital admission, contraindications to further vaccination, medical conditions not permitted according to the protocol, and unavailability of documentation about concomitant vaccination.
Table 1. Baseline Characteristics of the Subjects, According to Vaccine Group.
| Characteristic | RTS,S/AS01E (N = 447) |
Rabies (N = 447) |
||
|---|---|---|---|---|
| Mean age — mo | 11.4 | 11.3 | ||
| Sex — no. (%) | ||||
| Female | 230 (51) | 222 (50) | ||
| Male | 217 (49) | 225 (50) | ||
| Site — no. (%) | ||||
| Kilifi, Kenya | 223 (50) | 224 (50) | ||
| Korogwe, Tanzania | 224 (50) | 223 (50) | ||
| Geographic cluster — no. (%) | ||||
| Kilifi, Kenya | ||||
| Junju West | 63 (14) | 48 (11) | ||
| Junju East | 52 (12) | 58 (13) | ||
| Pingilikani South | 54 (12) | 60 (13) | ||
| Pingilikani North | 54 (12) | 58 (13) | ||
| Korogwe, Tanzania | ||||
| Ngombezi | 41 (9) | 39 (9) | ||
| Makuyuni | 152 (34) | 150 (34) | ||
| Mbagai | 31 (7) | 34 (8) | ||
| Distance to dispensary — no. (%) | ||||
| <5 km | 299 (67) | 290 (65) | ||
| 5–10 km | 122 (27) | 131 (29) | ||
| >10 km | 26 (6) | 26 (6) | ||
| Bed net — no. (%) | ||||
| None | 80 (18) | 95 (21) | ||
| Treated, with no holes | 103 (23) | 99 (22) | ||
| Treated, with holes | 85 (19) | 69 (15) | ||
| Untreated, with no holes | 57 (13) | 54 (12) | ||
| Untreated, with holes | 95 (21) | 108 (24) | ||
| Missing data | 27 (6) | 22 (5) | ||
EFFICACY
In the according-to-protocol analysis (of data over a mean of 8 months of follow-up, beginning 2 weeks after the final vaccination), the cumulative incidence of the first or only malarial episode, as defined for the primary end point, was 8% (32 of 402 subjects) in the group that received the RTS,S/AS01E vaccine, as compared with 16% (66 of 407 subjects) in the group that received the rabies vaccine. Cox regression provided an adjusted efficacy of 53% (95% CI, 28 to 69; P<0.001) and an unadjusted efficacy of 55% (95% CI, 31 to 70; P<0.001) (Fig. 2).
Figure 2. Kaplan–Meier Estimates of the Time to the First or Only Episode of Clinical Malaria.
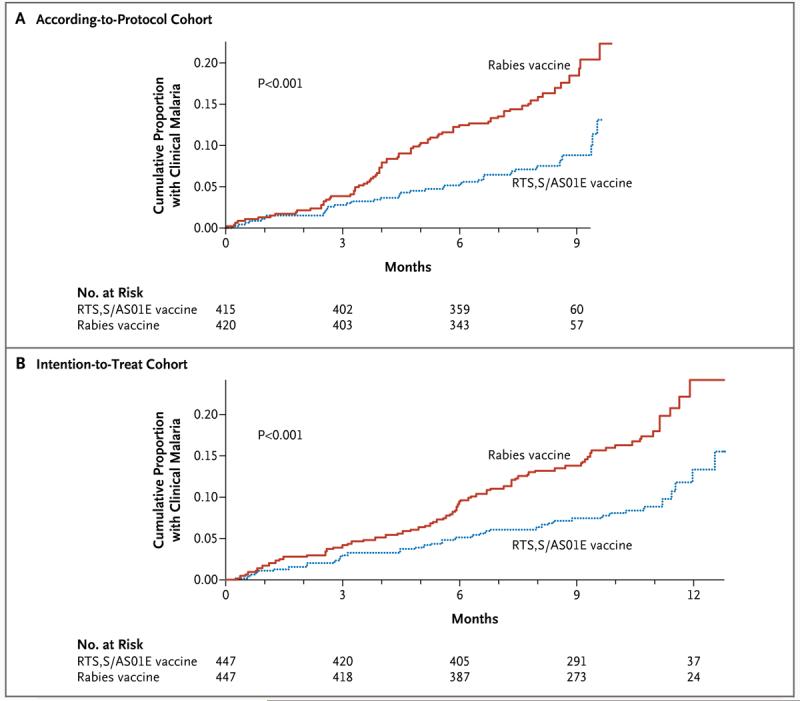
Clinical malaria was defined as fever with a parasite count of more than 2500 per microliter. Panel A shows data for the according-to-protocol analysis, beginning 2 weeks after the last vaccination; Panel B shows data for the intention-to-treat analysis, beginning at the time of the first vaccination. P values were calculated with the use of the log-rank test.
In the intention-to-treat analysis (of data collected over a mean of 10.5 months of follow-up, beginning with the first vaccination), the cumulative incidence of the first or only episode meeting the criteria for the primary end point was 9% (42 of 447 subjects) in the group receiving the RTS,S/AS01E vaccine and 17% (78 of 447) in the group receiving the rabies vaccine, with an unadjusted efficacy rate of 49% (95% CI, 26 to 65; P<0.001) on the basis of Cox regression.
In the according-to-protocol analysis, 6 of 402 subjects in the RTS,S/AS01E group (1%) and 14 of 407 subjects in the rabies-vaccine group (3%) had more than one episode of clinical malaria. In the intention-to-treat analysis, 6 of the 447 subjects in the RTS,S/AS01E group (1%) and 17 of 447 in the rabies-vaccine group (4%) had more than one clinical episode. The rate of efficacy against all clinical episodes meeting the criteria for the primary end point was 56% (95% CI, 31 to 72; P<0.001) for the RTS,S/AS01E vaccine on the basis of Poisson regression, which was similar to the rate of efficacy against first or only episodes. The results of the intention-to-treat analysis were similar (Table 2).
Table 2. Efficacy of the RTS,S/AS01E Vaccine against Episodes of Clinical Malaria.*.
| Episode | RTS,S/AS01E Vaccine | Rabies Vaccine | Adjusted Efficacy | Unadjusted Efficacy | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Subjects |
No. of Episodes |
Person-Yr at Risk |
Event Rate |
No. of Subjects |
No. of Episodes |
Person-Yr at Risk |
Event Rate |
% (95% CI) | P Value | % (95% CI) | P Value | ||
| According-to-protocol analysis | |||||||||||||
| First or only episode | |||||||||||||
| >2500 parasites/μl | 402 | 32 | 245 | 0.13 | 407 | 66 | 239 | 0.28 | 53 (28–69) | <0.001 | 55 (31–70) | <0.001 | |
| >0 parasites/μl | 402 | 33 | 245 | 0.13 | 407 | 70 | 237 | 0.30 | 55 (31–70) | <0.001 | 57 (34–71) | <0.001 | |
| All multiple episodes | |||||||||||||
| >2500 parasites/μl | 402 | 38 | 254 | 0.15 | 407 | 86 | 255 | 0.34 | 56 (31–72) | <0.001 | 58 (34–73) | <0.001 | |
| >0 parasites/μl | 402 | 40 | 254 | 0.16 | 407 | 94 | 255 | 0.37 | 58 (35–73) | <0.001 | 60 (37–74) | <0.001 | |
| Intention-to-treat analysis | |||||||||||||
| First or only episode | |||||||||||||
| >2500 parasites/μl | 447 | 42 | 351 | 0.12 | 447 | 78 | 339 | 0.23 | 49 (26–65) | <0.001 | |||
| >0 parasites/μl | 447 | 47 | 349 | 0.13 | 447 | 81 | 337 | 0.24 | 45 (21–62) | 0.001 | |||
| All multiple episodes | |||||||||||||
| >2500 parasites/μl | 447 | 49 | 364 | 0.13 | 447 | 107 | 365 | 0.29 | 54 (31–69) | <0.001 | |||
| >0 parasites/μl | 447 | 57 | 364 | 0.16 | 447 | 117 | 365 | 0.32 | 50 (26–67) | <0.001 | |||
Percentage efficacy was calculated by means of a Cox regression model for the risk of first episodes and a Poisson regression model for multiple episodes. The event rate was calculated by dividing the number of episodes by the person-years at risk. The according-to-protocol analysis was adjusted for the covariates use or nonuse of insecticide-treated net, age, distance from the dispensary, and village; data collection for this analysis began 2 weeks after the last vaccination and ended with the final blood test. The intention-to-treat analysis was unadjusted, and data collection began with the first vaccination and ended with the final blood test.
Estimates of efficacy according to the secondary end point of fever with any P. falciparum parasitemia density were similar to those according to the primary end point (Table 2). Two cases of parasitemia were identified in the rabies-vaccine group when clinicians, responding to the concerns of the parents, believed a malaria test was warranted despite the absence of fever. Including these two cases did not change the results appreciably. Estimates of efficacy were similar at each site (in Kilifi, 55%; 95% CI, 25 to 73; P=0.002; and in Korogwe, 56%; 95% CI, 4 to 80; P=0.04).
Asymptomatic parasitemia was detected infrequently at the time of the final blood test (in 2% of subjects in the RTS,S/AS01E group and 3% of those in the rabies-vaccine group), and the mean hemoglobin level was similar in the two vaccine groups (10.3 g per deciliter and 10.4 g per deciliter, respectively; P=0.22).
SAFETY
In all, 47 of the 447 children receiving the RTS,S/AS01E vaccine had one or more serious adverse events (11%; 95% CI, 8 to 14), as did 82 of the 447 children receiving the rabies vaccine (18%; 95% CI, 15 to 22). The lower rate of serious adverse events in the RTS,S/AS01E group was only partly accounted for by a reduction in admissions related to falciparum malaria. A total of 51 MedDRA-preferred terms were used to describe the serious adverse events. The numbers of children with the three most common diagnoses and those with serious adverse events not related to malaria are shown according to vaccine group in Table 3. Less-common serious adverse events (those occurring fewer than 10 times per group) are not shown, but there was no obvious imbalance between the two groups in the rates of these events. Laboratory safety tests did not reveal any significant difference in the frequency of out-of-range values between the two treatment groups. No out-of-range values were considered to be related to vaccination.
Table 3. Incidence of Adverse Events (AEs) and Serious Adverse Events (SAEs) in the Intention-to-Treat Cohort, According to the Vaccine Group.*.
| Events | RTS,S/AS01E | Rabies | |||
|---|---|---|---|---|---|
| No. of Subjects or Doses |
Percent (95% CI) | No. of Subjects or Doses |
Percent (95% CI) | ||
| SAEs, per-subject analysis | N = 447 | N = 447 | |||
| Any | 47 | 11 (8–14) | 82 | 18 (15–22) | |
| In absence of Plasmodium falciparum infection | 41 | 9 (8–14) | 61 | 14 (11–17) | |
| Pneumonia | 18 | 4 (2–6) | 26 | 6 (4–8) | |
| Gastroenteritis | 10 | 2 (1–4) | 21 | 5 (3–7) | |
| P. falciparum infection | 7 | 2 (1–3) | 21 | 5 (3–7) | |
| Death | 1 | <1 (0–1) | 1 | <1 (0–1) | |
| Related to vaccination | 1 | <1 (0–1) | 0 | 0 (0–1) | |
| Unsolicited AE, per-subject analysis | N = 447 | N = 447 | |||
| Any | 349 | 78 (74–82) | 332 | 74 (70–78) | |
| Gastroenteritis | 103 | 23 (19–27) | 83 | 19 (15–23) | |
| Pneumonia | 152 | 34 (30–39) | 143 | 32 (28–37) | |
| Upper respiratory tract infection | 81 | 18 (15–22) | 57 | 13 (10–16) | |
| Impetigo | 27 | 6 (4–9) | 20 | 5 (3–7) | |
| Lower respiratory tract infection | 27 | 6 (4–9) | 28 | 6 (4–9) | |
| Rhinitis | 27 | 6 (4–9) | 19 | 4 (3–7) | |
| Severity grade 3 | 36 | 8 (6–11) | 51 | 11 (9–15) | |
| Solicited local AEs, per-dose analysis | N = 1320 | N = 1320 | |||
| Pain | |||||
| Any | 172 | 13 (11–15) | 157 | 12 (10–14) | |
| Severity grade 3 | 0 | 0 (0–0) | 0 | 0 (0–0) | |
| Swelling | |||||
| Any | 34 | 3 (2–4) | 16 | 1 (1–2) | |
| Severity grade 3 | 3 | <1 (0–1) | 0 | 0 (0–0) | |
| Solicited systemic AEs, per-dose analysis | N = 1320 | N = 1318 | |||
| Drowsiness | |||||
| Any | 81 | 6 (5–8) | 64 | 5 (4–6) | |
| Severity grade 3 | 0 | 0 (0–0) | 1 | <1 (0–0) | |
| Related to vaccination | 69 | 5 (4–7) | 60 | 5 (4–6) | |
| Irritability | |||||
| Any | 59 | 5 (3–6) | 21 | 2 (1–2) | |
| Severity grade 3 | 0 | 0 (0–0) | 0 | 0 (0–0) | |
| Related to vaccination | 44 | 3 (2–4) | 16 | 1 (1–2) | |
| Loss of appetite | |||||
| Any | 64 | 5 (4–6) | 43 | 3 (2–4) | |
| Severity grade 3 | 0 | 0 (0–0) | 2 | <1 (0–1) | |
| Related to vaccination | 29 | 2 (2–3) | 16 | 1 (1–2) | |
| Fever† | |||||
| Any | 149 | 11 (10–13) | 409 | 31 (29–34) | |
| Severity grade 3 | 5 | <1 (0–1) | 12 | 1 (1–2) | |
| Related to vaccination | 132 | 10 (8–12) | 396 | 30 (28–33) | |
The three most common severe adverse events (SAEs) are listed individually, as are unsolicited adverse events (AEs) (those reported by parents spontaneously rather than in response to questions from field-workers) occurring in more than 5% of children in each group. SAEs are reported for the period from the first dose of vaccine until the unblinding of the database on August 4, 2008. Unsolicited AEs were recorded during the first 30 days (including the day of vaccination). Local AEs and solicited systemic AEs are reported for the first 7 days (including the day of vaccination) after each of the three vaccinations. Data on events reported per vaccine dose were available for the 447 subjects receiving the first dose in each group, for the 439 subjects in the RTS,S/AS01E group and the 438 in the rabies-vaccine group who received the second dose, and for the 434 subjects in the RTS,S/AS01E group and the 433 in the rabies-vaccine group who received the third dose — for a total of 1320 and 1318 doses in the respective groups.
Fever was defined as an axillary temperature of 37.5°C or greater.
Between the time of the first vaccination and the time of unblinding, one episode in the RTS,S/AS01E group met the criteria for severe malaria, as compared with eight episodes of severe malaria among seven children in the rabies-vaccine group. Of the children with severe malaria, two had cerebral malaria, one had severe anemia, and the remaining five had other types of severe malaria (defined in the Supplementary Appendix). All children treated for acute malarial infection recovered without sequelae.
One serious adverse event was judged to be related to vaccine: a simple febrile seizure, observed 1 day after vaccination with RTS,S/AS01E. The seizure terminated spontaneously, and there were no neurologic abnormalities detected 24 hours afterward. Despite the facilitated access to medical care during the study, two children died (one at home and one while being brought to the dispensary), with no clinical evaluation. One child, who had been vaccinated 9 months earlier with RTS,S/AS01E, had generalized convulsions and loss of consciousness. Traditional medicine was used, but the child died without being brought to the health center. The other child, who had been given the rabies vaccine and was subsequently withdrawn from the study because of the parents' anxiety about the blood sampling, had atypical prolonged seizures, lost consciousness, and died while being brought to the health center. Neither death was judged to be related to the study vaccine.
The frequency of local adverse events during the first 7 days after each of the three vaccinations is shown in Table 3. Unsolicited adverse events (those reported by parents spontaneously rather than in response to questions from field-workers) occurring in more than 5% of children per group are also shown. Unsolicited symptoms were recorded during the first 30 days. These were classified according to the MedDRA-preferred term, and 105 preferred terms were assigned. There was no obvious imbalance between the two vaccine groups with respect to these other, less-frequent adverse events, and all were expected events for the population. A total of 77% of children in the RTS,S/AS01E group and 76% of those in the rabies-vaccine group had unsolicited adverse events that were not related to malaria.
IMMUNOGENICITY
The geometric mean titer of anticircumsporozoite antibody 1 month after the third vaccination was 539.6 enzyme-linked immunosorbent assay units (EU) per milliliter (95% CI, 500.7 to 581.6) in the RTS,S/AS01E group, as compared with 0.3 EU per milliliter (95% CI, 0.3 to 0.3) in the rabies-vaccine group (with a limit of detection of 0.5 EU per milliliter). The geometric mean titer in the RTS,S/AS01E group had fallen by the time of the final cross-sectional survey at 4.5 to 10.5 months (71.9 EU per milliliter; 95% CI, 66 to 79) but remained higher than in the rabies-vaccine group (0.3 EU per milliliter; 95% CI, 0.2 to 0.3).
There was a wide range of antibody titers after vaccination (Fig. 3), but more than 99% of the children in the RTS,S/AS01E group had detectable anticircumsporozoite titers. There was no evidence that RTS,S/AS01E-vaccinated children with high titers had greater protection against clinical disease than those with lower titers. Each increase by a factor of 10 in the anticircumsporozoite antibody titer (assessed 1 month after the third dose) was associated with an increase in efficacy of 9% (95% CI, −25 to 34; P = 0.58).
Figure 3. Time Course of Anticircumsporozoite Antibody Titers for the According-to-Protocol Cohort, According to Vaccination Group.
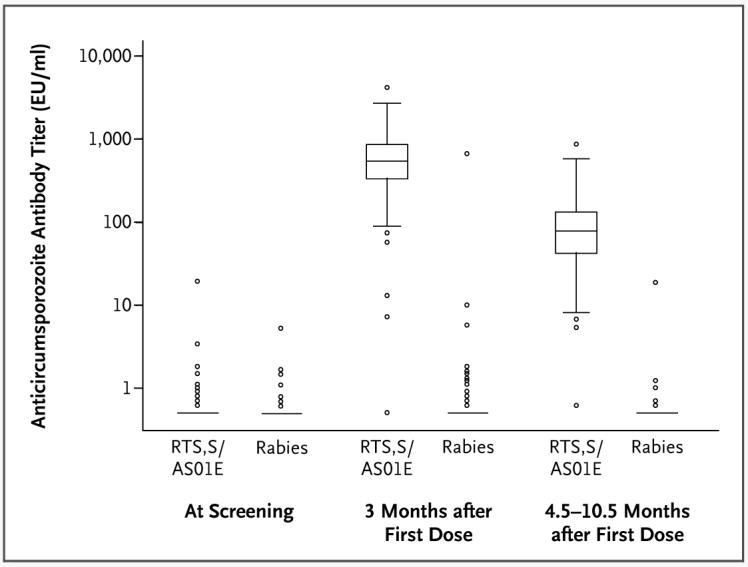
The box-and-whisker plots show the distribution of anticircumsporozoite antibodies according to time point and according to vaccination group. When only a horizontal line is shown, it represents the 25th, 50th, and 75th percentiles (all of which were identical). Otherwise, the horizontal line within each box represents the median, the top and bottom of each box represent the 25th and 75th percentiles, respectively, and the I bars represent the highest and lowest values within 1.5 times the interquartile range. The circles denote outliers. EU denotes enzyme-linked immunosorbent assay units.
All children had received the usual course of hepatitis B vaccine, with doses at 6, 10, and 14 weeks of age; the prevalence of hepatitis B virus surface antibody titers above 10 mIU per milliliter was 97% at screening and 100% one month after the third RTS,S/AS01E vaccination. There was no correlation between the hepatitis B virus surface antibody titer at screening and the circumsporozoite titer after vaccination (r = 0.06, P = 0.21), but there was a strong correlation between the hepatitis B virus surface antibody titer at screening and the titer after vaccination (r = 0.48, P<0.001).
DISCUSSION
The RTS,S/AS01E malaria vaccine has significant efficacy against clinical malaria in the field in a target group for licensure (i.e., children 5 to 17 months of age). Our estimate of efficacy against clinical malaria for RTS,S given with the AS01 adjuvant system is higher than previous estimates with the use of the AS02 adjuvant system.14 AS01 is a more immunogenic adjuvant than AS02 in comparative studies.15-17 The mean antibody titer in our study (539.6 EU per milliliter; 95% CI, 500.7 to 581.6) was higher than the antibody titers induced by RTS,S/AS02D in young infants (199.9 EU per milliliter; 95% CI, 150.9 to 264.7)13 and in children 1 to 4 years of age (158 EU per milliliter; 95% CI, 142 to 176).14 The antibody titer was 40 EU per milliliter (95% CI, 36 to 45) 6 months after vaccination with RTS,S/AS02D, but in our study, the titer was 71.9 EU per milliliter (95% CI, 65.7 to 78.6) 4.5 to 10.5 months (mean, 8) after vaccination with RTS,S/AS01E.
However, there are other differences between the studies. The intensity of malaria transmission is falling on the East African coast, probably as a result of increasing use of insecticide-treated nets.6,7 We used active and passive case detection rather than passive case detection alone and studied a younger group of children than those in Mozambique.14 In Mozambique, there was 47% efficacy against clinical malaria among children under 2 years of age, as compared with 30% overall efficacy among children 1 to 4 years of age, albeit with wide confidence intervals (15 to 67%) and without a significant interaction between age and vaccine efficacy. Whatever the explanation for the higher efficacy and immunogenicity, our data support the conduct of a phase 3 trial of RTS,S/AS01E.
We detected fewer serious adverse events in children receiving the RTS,S/AS01E vaccine than in children receiving the rabies vaccine. Although previous studies have shown a reduced rate of serious adverse events in children vaccinated with RTS,S/AS02A,19 our results suggest that not all of this reduction is directly attributable to malaria. This raises the possibility that preventing malaria through vaccination may have indirect as well as direct effects. Some trials have suggested indirect effects of the use of insecticide-treated nets,20 but this has not been confirmed in other studies.21 Indirect effects of malaria might be more likely to result from chronic parasitemia than from the acute, promptly treated episodes seen in our cohort,22 although a decreased rate of growth in children after an acute episode has also been observed.23 The possible indirect benefits of vaccination will be examined further in a phase 3 multicenter trial.
The immunologic correlate of protection remains unknown. Antibodies13 and cell-mediated immunity24,25 have been associated with protection against infection, but not against clinical disease,14 in previous studies. We found no correlation between the anticircumsporozoite antibody titer and protection against clinical disease in this study, despite the wide variation in anticircumsporozoite antibody responses among the vaccinated children. This result does not rule out a role for anticircumsporozoite antibody responses. For instance, in studies of immunity to blood-stage parasites, the functional properties of antibodies, but not the overall titer, determine the outcome.26 Studies of cell-mediated immunity in this cohort are still under way.
There was a 3-month delay in implementing active case detection at one site in our study, accounting for 87 person-years at risk out of a total of 491 person-years at risk for the two sites. During this period, passive case detection was implemented, and transmission appeared to be low (with identification of <25% of the total cases). Furthermore, during the rest of the study, 92% of malarial episodes were detected through passive rather than active case detection, and estimates of efficacy were similar at the two sites. Hence, it seems unlikely that a large number of cases were missed. Our findings indicate that the RTS,S/AS01E vaccine should be further tested in a phase 3, multicenter trial.
Supplementary Material
Acknowledgments
Supported by grants from the PATH Malaria Vaccine Initiative (MVI) and GlaxoSmithKline Biologicals.
Dr. Leach, Mr. Lievens, Dr. Vekemans, Ms. Dubois, Dr. Demoitié, Mr. Stallaert, and Dr. Cohen report being employees of GlaxoSmithKline Biologicals; Dr. Ballou reports having been an employee during the design and implementation of the study but reports becoming an employee of the Bill and Melinda Gates Foundation by the time the results were available. Dr. Leach, Dr. Gould, Ms. Dubois, Dr. Ballou, and Dr. Cohen report owning shares in GlaxoSmithKline. Drs. Ballou and Cohen report being listed as inventors of patented malaria vaccines. Ms. Vansadia, Mr. Carter, Ms. Savarese, and Dr. Villafana report being employees of MVI, which supports the development and testing of several malaria vaccines. Prof. Marsh reports receiving grant support from the Wellcome Trust. No other potential conflict of interest relevant to this article was reported.
We thank the participants and their parents and the village and district authorities for their cooperation; the data and safety monitoring board, chaired by Prof. Malcolm Molyneux, and the local safety monitors, Dr. Jay Berkley in Kilifi and Dr. Firimina Mberesero in Korogwe; Prof. Raimos Olomi for supervising pediatric care in Korogwe; Anna Randall, Denise Dekker, James Beard, Roly Gosling, and Hugh Reyburn for providing support in Korogwe; Thor Theander and Brian Greenwood for helping to set up the Joint Malaria Programme collaboration, and Brian Greenwood for advice regarding the design and execution of the trial; Dr. Norbert Peshu (unit director), Edna Ogada, Tabitha Mwangi, Jacinta Mutegi (site coordinator), and Dorothy Mwachiro (community liaison officer) for providing support in Kilifi; and the staff of the Malaria Project Team at GlaxoSmithKline — in particular, Nathalie Annez, Delphine Beauport, Sarah Benns (professional writer), Conor Cahill (professional writer), Philippe Dehottay, Issam Jaimai, Philippe Moris, Sarah Muthuri, Ezekiel Oenga, Opokua Ofori Anyinam, Srilakshmi Pranesh, Isabelle Ramboer, Marie-Sylvie Remacle (professional writer), Christine Swysen, Joelle Thonnard, Marie Chantal Uwamwezi, Wendy Valinski, and Laurence Vigneron.
REFERENCES
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–7. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hay SI, Guerra CA, Tatem AJ, Noor AM, Snow RW. The global distribution and population at risk of malaria: past, present, and future. Lancet Infect Dis. 2004;4:327–36. doi: 10.1016/S1473-3099(04)01043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snow RW, Craig M, Deichmann U, Marsh K. Estimating mortality, morbidity and disability due to malaria among Africa's non-pregnant population. Bull World Health Organ. 1999;77:624–40. [PMC free article] [PubMed] [Google Scholar]
- 4.Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004;2:CD000363. doi: 10.1002/14651858.CD000363.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Nosten F, White NJ. Artemisinin-based combination treatment of falciparum malaria. Am J Trop Med Hyg. 2007;77(Suppl 6):181–92. [PubMed] [Google Scholar]
- 6.Okiro EA, Hay SI, Gikandi PW, et al. The decline in paediatric malaria admissions on the coast of Kenya. Malar J. 2007;6:151. doi: 10.1186/1475-2875-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Meara WP, Mwangi TW, Williams TN, McKenzie FE, Snow RW, Marsh K. Relationship between exposure, clinical malaria, and age in an area of changing transmission intensity. Am J Trop Med Hyg. 2008;79:185–91. [PMC free article] [PubMed] [Google Scholar]
- 8.Barnes KI, Durrheim DN, Little F, et al. Effect of artemether-lumefantrine policy and improved vector control on malaria burden in KwaZulu-Natal, South Africa. PLoS Med. 2005;2(11):e330. doi: 10.1371/journal.pmed.0020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhattarai A, Ali AS, Kachur SP, et al. Impact of artemisinin-based combination therapy and insecticide-treated nets on malaria burden in Zanzibar. PLoS Med. 2007;4(11):e309. doi: 10.1371/journal.pmed.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nyarango PM, Gebremeskel T, Mebrahtu G, et al. A steep decline of malaria morbidity and mortality trends in Eritrea between 2000 and 2004: the effect of combination of control methods. Malar J. 2006;5:33. doi: 10.1186/1475-2875-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenwood BM, Fidock DA, Kyle DE, et al. Malaria: progress, perils, and prospects for eradication. J Clin Invest. 2008;118:1266–76. doi: 10.1172/JCI33996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bojang KA, Milligan PJ, Pinder M, et al. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet. 2001;358:1927–34. doi: 10.1016/S0140-6736(01)06957-4. [DOI] [PubMed] [Google Scholar]
- 13.Aponte JJ, Aide P, Renom M, et al. Safety of the RTS,S/AS02D candidate malaria vaccine in infants living in a highly endemic area of Mozambique: a double blind randomised controlled phase I/IIb trial. Lancet. 2007;370:1543–51. doi: 10.1016/S0140-6736(07)61542-6. [DOI] [PubMed] [Google Scholar]
- 14.Alonso PL, Sacarlal J, Aponte JJ, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364:1411–20. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- 15.Garçon N, Chomez P, Van Mechelen M. GlaxoSmithKline Adjuvant Systems in vaccines: concepts, achievements and perspectives. Expert Rev Vaccines. 2007;6:723–39. doi: 10.1586/14760584.6.5.723. [DOI] [PubMed] [Google Scholar]
- 16.Mettens P, Dubois PM, Demoitié MA, et al. Improved T cell responses to Plasmodium falciparum circumsporozoite protein in mice and monkeys induced by a novel formulation of RTS,S vaccine antigen. Vaccine. 2008;26:1072–82. doi: 10.1016/j.vaccine.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Stewart VA, McGrath SM, Dubois PM, et al. Priming with an adenovirus 35-circumsporozoite protein (CS) vaccine followed by RTS,S/AS01B boosting significantly improves immunogenicity to Plasmodium falciparum CS compared to that with either malaria vaccine alone. Infect Immun. 2007;75:2283–90. doi: 10.1128/IAI.01879-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MedDRA, version 11.1; International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH); Geneva. 2008; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alonso PL, Sacarlal J, Aponte JJ, et al. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet. 2005;366:2012–8. doi: 10.1016/S0140-6736(05)67669-6. [DOI] [PubMed] [Google Scholar]
- 20.Binka FN, Hodgson A, Adjuik M, Smith T. Mortality in a seven-and-a-half-year follow-up of a trial of insecticide-treated mosquito nets in Ghana. Trans R Soc Trop Med Hyg. 2002;96:597–9. doi: 10.1016/s0035-9203(02)90321-4. [DOI] [PubMed] [Google Scholar]
- 21.Nevill CG, Some ES, Mung'ala VO, et al. Insecticide-treated bednets reduce mortality and severe morbidity from malaria among children on the Kenyan coast. Trop Med Int Health. 1996;1:139–46. doi: 10.1111/j.1365-3156.1996.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 22.Snow RW, Korenromp EL, Gouws E. Pediatric mortality in Africa: Plasmodium falciparum malaria as a cause or risk? Am J Trop Med Hyg. 2004;71(Suppl 2):16–24. [PubMed] [Google Scholar]
- 23.Nyakeriga AM, Troye-Blomberg M, Chemtai AK, Marsh K, Williams TN. Malaria and nutritional status in children living on the coast of Kenya. Am J Clin Nutr. 2004;80:1604–10. doi: 10.1093/ajcn/80.6.1604. [DOI] [PubMed] [Google Scholar]
- 24.Sun P, Schwenk R, White K, et al. Protective immunity induced with malaria vaccine, RTS,S, is linked to Plasmodium falciparum circumsporozoite protein-specific CD4+ and CD8+ T cells producing IFN-gamma. J Immunol. 2003;171:6961–7. doi: 10.4049/jimmunol.171.12.6961. [DOI] [PubMed] [Google Scholar]
- 25.Reece WH, Pinder M, Gothard PK, et al. A CD4(+) T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat Med. 2004;10:406–10. doi: 10.1038/nm1009. [DOI] [PubMed] [Google Scholar]
- 26.Okech BA, Corran PH, Todd J, et al. Fine specificity of serum antibodies to Plasmodium falciparum merozoite surface protein, PfMSP-1(19), predicts protection from malaria infection and high-density parasitemia. Infect Immun. 2004;72:1557–67. doi: 10.1128/IAI.72.3.1557-1567.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.