Abstract
Numerous studies have reported a relationship between folate status, the methylenetetrahydrofolate reductase (MTHFR) 677C→T variant and disease risk. Although folate and choline metabolism are inter-related, only limited data are available on the relationship between choline and folate status in humans. This study sought to examine the influences of folate intake and the MTHFR 677C→T variant on choline status. Mexican-American women (n =43; 14 CC, 12 CT and 17 TT) consumed 135 μg/day as dietary folate equivalents (DFE) for 7 weeks followed by randomization to 400 or 800 μg DFE/day for 7 weeks. Throughout the study, total choline intake remained unchanged at ∼350 mg/day. Plasma concentrations of betaine, choline, glycerophosphocholine, phosphatidylcholine and sphingomyelin were measured via LC-MS/MS for Weeks 0, 7 and 14. Phosphatidylcholine and sphingomyelin declined ( P=.001, P=.009, respectively) in response to folate restriction and increased ( P=.08, P=.029, respectively) in response to folate treatment. The increase in phosphatidylcholine occurred in response to 800 ( P=.03) not 400 ( P=.85) μg DFE/day (week×folate interaction, P=.017). The response of phosphatidylcholine to folate intake appeared to be influenced by MTHFR C677T genotype. The decline in phosphatidylcholine during folate restriction occurred primarily in women with the CC or CT genotype and not in the TT genotype (week×genotype interaction, P=.089). Moreover, when examined independent of folate status, phosphatidylcholine was higher ( P <.05) in the TT genotype relative to the CT genotype. These data suggest that folate intake and the MTHFR C677T genotype influence choline status in humans.
Keywords: Folate, Choline, Phosphatidylcholine, MTHFR, Betaine, Women, Human
1. Introduction
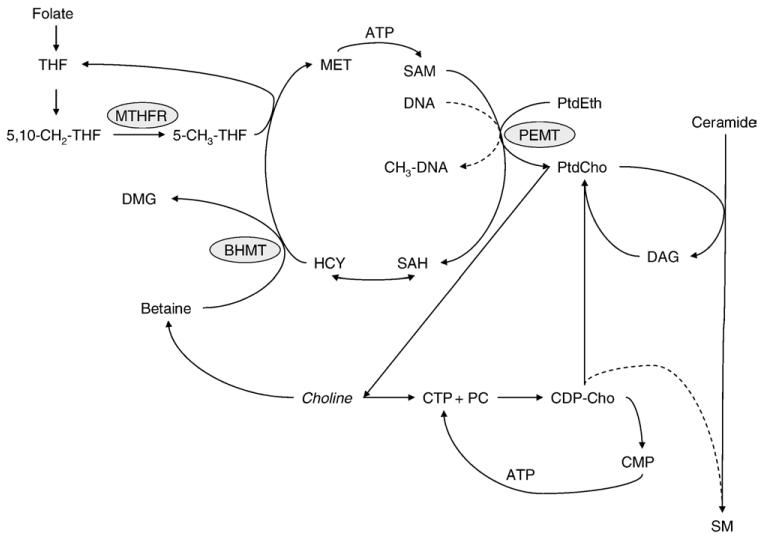
In 1998, choline was classified as an essential nutrient for humans and adequate intakes (AI) of 425 and 550 mg/day were established for adult women and men, respectively [1]. Choline is found in plant and animal products as free choline (small amounts) or as derivatives of choline including phosphatidylcholine (most abundant), phosphocholine, sphingomyelin and glycerophosphocholine [2,3]. Betaine is also a derivative of choline but in contrast to the choline compounds listed above it cannot be converted back to choline [2]. Choline, specifically, phosphatidylcholine can also be synthesized de novo in a reaction catalyzed by phosphatidylethanolamine N-methyltransferase (PEMT) utilizing phosphatidylethanolamine and three methyl groups derived from S-adenosylmethionine (SAM; Fig. 1).
Fig. 1.
Choline metabolism, phosphatidylcholine and sphingomyelin synthesis, and the role of betaine and folate in one-carbon metabolism. BHMT, betaine homocysteine S-methyltransferase; CDP-Cho, cytidinediphosphocholine; CMP, cytidinemonophosphate; CTP, cytidinetriphosphate, DAG, diacylglycerol; DMG, dimethylglycine; HCY, homocysteine; MET, methionine; MTHFR, methylenetetrahydrofolate reductase; PC, phosphocholine; PtdCho, phosphatidylcholine; PtdEth, phosphatidylethanolamine; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; SM, sphingomyelin; THF, tetrahydrofolate.
Choline serves as a precursor for compounds possessing diverse functions including the neurotransmitter important in brain function, acetylcholine; phospholipids needed for membrane integrity and cellular signaling; and betaine, a one-carbon donor for SAM and folate-dependent one-carbon transfer reactions [2,4]. In regard to one-carbon transfer reactions, choline may be oxidized primarily in liver and kidney to betaine [2]. In a subsequent reaction catalyzed by betaine homocysteine S-methyltransferase, one of the three methyl groups may be used for homocysteine remethylation to methionine. Alternatively, 5-methyl-tetrahydrofolate (THF) may serve as the methyl donor in a reaction catalyzed by methionine synthase which occurs in all tissues. In humans, choline intake/status is associated with the risk of neural tube defects [5]; plasma homocysteine [6], a metabolite that has been linked to numerous developmental and chronic diseases; and DNA damage [7]. In addition, choline availability has also been shown to alter site-specific and global DNA methylation in human neuroblastoma cells [8].
In animal models, choline-deficient diets promote folate deficiency and folate-deficient diets lower choline status [9,10]. However, only one study has assessed this relationship in humans [11] and more studies that include measurements of betaine, phosphatidylcholine (for women) and sphingomyelin are needed to more fully delineate the relationship between folate and choline nutriture.
Methylenetetrahydrofolate reductase (MTHFR) is an important enzyme in folate metabolism and catalyzes the reduction of 5,10-methylene-THF to 5-methyl-THF. In mice, MTHFR deficiency adversely impacts choline status [12]. In humans, a common genetic polymorphism involving a Cytosine (C) to thymine (T) transition at nucleotide 677 exists [13] and is associated with reduced enzyme activity [13], lower folate status [14] and altered risk for chronic and developmental diseases [13,15].
As an extension of previous work [14], the objective of the present study was to assess the influence of folate intake and the MTHFR C677T genotype on choline status (i.e., betaine, choline, phosphatidylcholine and sphingomyelin) in young Mexican-American women consuming controlled folate and choline intakes for 14 weeks. Women of Mexican descent were chosen as the study population because of the high prevalence of the MTHFR 677TT genotype in this ethnic group.
2. Subjects and methods
2.1. Human subjects
Women aged 18–45 years of self-reported Mexican descent defined as having two Mexican parents were selected for participation in this study and were recruited from staff and students at Cal Poly Pomona University and the surrounding community. At the initial screen, potential subjects completed a health history questionnaire and gave a fasting blood sample for MTHFR genotype determination. For those with the appropriate MTHFR genotype, another fasting blood sample was obtained for clinical chemistry evaluation. Additional inclusion criteria have been described elsewhere [14]. The screening and experimental procedures were reviewed and approved by the Institutional Review Board of Cal Poly Pomona University and informed consent was obtained from each participant.
2.2. Experimental design
This was a 14-week controlled feeding study in which subjects consumed a folate-restricted diet providing 135 μg dietary folate equivalents (DFE)/day for 7 weeks followed by consumption of 400 or 800 μg DFE/day as previously described in detail [14]. The diet provided 112 mg betaine and 174 mg total choline which remained constant throughout the duration of the study.
2.3. Diet and supplements
A low-folate five-day rotation menu consisting of non-folic acid fortified foods was used [14]. The mean folate content of the diet, as determined by tri-enzyme methodology [16,17] on three separate occasions, was ∼135 μg/day. Total choline and betaine content of the diet as determined by LC-MS/MS [3,18] were 174 and 112 mg/day (Table 1). Subjects were also given supplemental choline (350 mg; TwinLab, Twin Laboratories, Ronkonkoma, NY, USA) every other day to provide total choline intakes (food+supplement) of 349 mg/day. All other nutrients were provided in recommended amounts (IOM 1998) through the diet alone or via supplements as previously described [14]. Folic acid supplements were prepared from commercially available folic acid (Sigma Chemical Co., St. Louis, MO, USA) and were consumed at the morning and evening meals throughout repletion under the supervision of the investigators.
Table 1.
Choline, glycerophosphocholine (GPCho), phosphocholine (PCho), phosphatidylcholine (PtdCho), lysophosphatidylcholine (LPCho) and betaine content (mg/day) of the 5-day menua,b
| Metabolite | Menu |
|||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | Overall | |
| Choline | 52 (10.0) | 47.0 (6.3) | 28.5 (3.5) | 57.0 (11.8) | 24.0 (4.4) | 41.7 (7.2) |
| GPCho | 8.7 (0.7) | 25.8 (1.5) | 41.0 (4.6) | 43.0 (3.1) | 20.6 (2.2) | 27.8 (2.4) |
| PCho | 2.0 (0.9) | 3.6 (1.1) | 3.0 (1.3) | 4.0 (2.0) | 2.9 (0.1) | 3.1 (1.1) |
| PtdCho | 19.1 (0.7) | 59.8 (1.1) | 20.2 (1.3) | 32.0 (0.3) | 66.0 (19.8) | 39.4 (4.6) |
| LPCho | 93 (5.6) | 86.0 (6.5) | 72.5 (6.1) | 54.0 (4.7) | 3.2 (0.2) | 61.7 (4.6) |
| Total choline | 174.8 (17.9) | 222.2 (16.5) | 165.2 (16.8) | 190.0 (21.8) | 116.7 (26.7) | 173.7 (19.9) |
| Betaine | 104.0 (13.1) | 96.3 (13.8) | 118.0 (23.5) | 109.0 (9.4) | 132.0 (3.6) | 111.8 (12.7) |
Results are presented as mean (S.E.M.).
Sphingomyelin was not detected.
Betaine is not included in the total choline content of the menu.
2.4. Sample collection and blood processing
Fasting (10 h) venous blood samples were collected in serum separator gel and clot-activator tubes (SST, Vacutainer; Becton Dickinson, Rutherford, NY, USA) and EDTA tubes (Vacutainer). Serum was collected after centrifugation (650×g for 15 min at 21°C), dispensed into 1.5-ml microcentrifuge tubes containing ascorbic acid (10–15 mg) and stored at -80°C. Plasma and leukocytes were collected from EDTA blood that was immediately placed on ice and centrifuged within 1 h of the blood draw at 1800×g for 15 min at 4°C. Plasma for choline measurements was dispensed into 1.5-ml microcentrifuge tubes and stored at -80°C. For genotyping, the buffy layer representing peripheral leukocytes (∼500 μl) was removed, dispensed into 1.5-ml microcentrifuge tubes containing 50 μl dimethyl sulfoxide (Sigma), mixed by inversion and frozen at -80°C.
2.5. Analytical methods
2.5.1. Folate content of diet
The folate content of the diet was determined prior to starting the study and twice during the study using a tri-enzyme methodology [16] and double extraction [17].
2.5.2. MTHFR C677T Genotype
DNA for genotyping was extracted from leukocytes using a commercially available kit (QIAamp blood kit; Qiagen, Santa Clarita, CA, USA). Determination of the C677T MTHFR genotype involved polymerase chain reaction and Hinf1 restriction enzyme digestion as described by Frosst et al. [13].
2.5.3. Serum folate
Folate concentrations of serum were determined microbiologically by use of the microtiter plate adaptation with Lactobacillus casei [19]. The intra- and interassay CV were both 12%, based on the positive control.
2.5.4. Dietary and plasma choline measurements
Plasma and food betaine, choline, phosphocholine, glycerophosphocholine, phosphatidylcholine, sphingomyelin and food lysophosphatidylcholine were determined using the method developed by Koc et al. [3] and Choudhary et al. [18] with modifications based on our instrumentation. Choline compounds were spiked with deuterium-labeled internal standards of all the analytes and analyzed using liquid chromatography tandem mass spectrometry (LC-MS/MS). The LC-MS/MS system consisted of an LCQ Advantage (Thermo Finnigan) with electrospray ionization source, a Surveyor HPLC system (Thermo Finnigan) and a refrigerated Surveyor autosampler (Thermo Finnigan). The HPLC was equipped with an Alltech Adsorbosphere Silica guard column (4.6×25 mm, 5 μm) and an Alltech Solvent Miser Silica analytical column (2.1×150 mm, 5 μm). The mass spectrometer was operated in positive ion electrospray mode. The parent ion/daughter ion fragments monitored for the aqueous phase choline compounds were m/z 118/59 (betaine), m/z 104/60 (choline), m/z 258/104 (glycerophosphocholine) and m/z 184/125/86 (phosphocholine). The m/z’s monitored for deuterium-labeled internal standards were m/z 127/68 for betaine-d9, m/z 113/69 for choline-d9, m/z 267/113 for glycerophosphocholine-d9 and m/z 193/125/95 for phosphocholine-d9. For the organic phase, plasma phosphatidylcholine and sphingomyelin and food phosphatidylcholine, sphingomyelin and lysophosphatidylcholine were detected by monitoring m/z 184/125/86 after in-source fragmentation of phosphatidylcholine, sphingomyelin and lysophosphatidylcholine which produced phosphocholine. The m/z’s monitored for deuterium-labeled internal standards of phosphatidylcholine-d9 and sphingomyelin-d3 were m/z 193/125/95 and m/z 187/125/89, respectively (phosphatidylcholine-d9 was also used for analyses of lysophosphatidylcholine in food samples). Quantification of choline compounds in LC-MS/MS experiments was performed by comparing samples with the signal obtained from choline compound standards. Quality assurance was monitored through the use of duplicate sampling and in-house control materials. The intra- and inter-CV for each analyte measured ranged from 2% to 12%. In addition, for plasma, each run included all three MTHFR C677T genotypes and all three weeks (0, 7 and 14).
2.6. Statistical analysis
Choline and its derivatives (betaine, glycerophosphocholine, phosphatidylcholine, sphingomyelin) were tested for normality with the Shapiro—Wilks test (SAS PROC UNIVARIATE). Glycerophosphocholine and sphingomyelin were not normal, but were transformed to a normal distribution using an inverse power function identified by the Box-Cox method (SAS PROC TRANSREG). Choline was not normal and could not be transformed to normal. Transformed values were used in all ANOVA and ANCOVA procedures.
Baseline differences in plasma choline, its derivatives and serum folate between the MTHFR C677T genotypes were assessed by one-way ANOVA. The effect of folate treatment and the MTHFR C677T genotype through time on choline and its derivatives was analyzed using repeated-measures ANOVA (SAS PROC GLM). Separate analyses with each derivative as a response variable were performed. Derivative concentrations at Weeks 0, 7 and 14 were the levels of the within-subjects factor. Between-subjects factors were folate treatment (two levels: 400 or 800 μg DFE/day) and MTHFR C677T genotype (three levels: CC, CT, TT). All possible two-way interactions and the three-way interaction among the factors were included in the model. In each analysis, the assumptions of compound symmetry and homoscedasticity were assessed with a sphericity test of orthogonal components. When the assumptions were not met, the Huynh—Feldt correction was applied to all tests involving the within-subjects effect. When the week (within-subjects) effect was significant, profile contrasts between consecutive weeks (0 vs. 7; 7 vs. 14) were examined.
To examine the influence of the MTHFR C677T genotype during folate restriction on choline and its derivatives independent of folate status, an ANCOVA (SAS PROC MIXED) was performed using serum folate, measured previously [14], as the covariate. The relationship among the weeks (within the subjects) was modeled as residual covariance with a variance components structure. Parameter estimates were fit using restricted maximum likelihood methods. Significance of genotype using serum folate as the covariate was assessed by Type 3 tests.
The data were analyzed using SAS/STAT software, version 9.1.3 of the SAS System for Windows. Differences were considered to be significant at P <.05, whereas P values of .05 to .1 were considered to be indicative of a trend. Data are presented as mean±S.E.M.
3. Results
3.1. Subject characteristics
Forty-three Mexican-American women participated in this study pre-selected for the following MTHFR C677T genotypes: 14 wild type (CC), 12 heterozygous (CT) and 17 homozygous for the T variant (TT). The women were 25 years of age (range 18–44 years) with a body mass index (kg/m2)of 25.2 (range 19.5–32). No differences ( P>.05) in weight or age were detected among the MTHFR C677T genotypes. At baseline, no differences ( P<.05) in the measured variables existed between the MTHFR C677T genotypes although sphingomyelin tended ( P=.08) to be lower in women with the CT or TT genotype relative to the CC genotype.
3.2. Betaine, choline and glycerophosphocholine
Throughout the study, no effect of week, genotype or folate, or their interactions was detected on betaine, choline or glycerophosphocholine (Table 2).
Table 2.
Serum folate (nmol/L) and plasma concentrations (μmol/L) of betaine, choline, glycerophosphocholine (GPCho), phosphatidylcholine (PtdCho) and sphingomyelin (SM) in Mexican-American women differing in MTHFR C677T genotype (14 CC, 12 CT, 17 TT) at baseline (0), after 7 weeks of folate restriction with 135 μg dietary folate equivalents (DFE)/day (7–135), and after 7 weeks of folate treatment with 400 μg DFE/day (14–400; 7 CC, 6 CT, 9 TT) or 800 μg DFE/day (14–800; 7 CC, 6 CT, 8 TT) or 400 and 800 μg DFE/day combined (14—overall)
| Metabolite | Week-folate intake (μg DFE/day) |
||||
|---|---|---|---|---|---|
| 0 | 7–135 | 14–400 | 14–800 | 14—overall | |
| Betaine | |||||
| CC | 28.6 (2.5) | 27.4 (2.3) | 25.3 (2.6) | 26.3 (3.8) | 25.8 (2.2) |
| CT | 27.4 (2.5) | 28.7 (2.7) | 27.5 (2.4) | 24.0 (3.7) | 25.7 (2.2) |
| TT | 26.1 (2.4) | 23.6 (2.2) | 21.2 (2.7) | 25.8 (4.5) | 23.4 (2.5) |
| Overall | 27.3 (1.4) | 26.3 (1.4) | 24.2 (1.6) | 25.4 (2.3) | 24.8 (1.4) |
| Choline | |||||
| CC | 13.0 (1.4) | 11.7 (0.9) | 11.3 (1.2) | 10.5 (1.2) | 11.7 (0.9) |
| CT | 11.1 (1.3) | 11.6 (1.4) | 9.3 (2.0) | 12.9 (1.8) | 11.1 (1.1) |
| TT | 12.5 (0.9) | 11.9 (1.1) | 9.5 (1.3) | 14.2 (1.7) | 11.7 (1.1) |
| Overall | 12.3 (0.7) | 11.7 (0.6) | 10.5 (0.9) | 12.6 (0.9) | 11.5 (0.6) |
| GPCho | |||||
| CC | 28.5 (2.0) | 28.2 (2.3) | 26.5 (2.2) | 25.6 (3.3) | 26.0 (1.9) |
| CT | 29.0 (2.4) | 26.6 (2.3) | 27.5 (3.2) | 32.9 (5.3) | 30.2 (3.1) |
| TT | 27.9 (2.0) | 28.3 (1.9) | 27.6 (3.3) | 34.6 (7.3) | 30.9 (3.8) |
| Overall | 28.4 (1.2) | 27.8 (1.2) | 27.3 (1.7) | 31.1 (3.3) | 29.1 (1.8) |
| PtdCho | |||||
| CC | 1911 (93) | 1728 (78) | 1760 (187) | 1927 (118) | 1844 (109) |
| CT | 1727 (120) | 1533 (116) | 1476 (192) | 1744 (183) | 1610 (133) |
| TT | 1872 (82) | 1808 (66) | 1767 (101) | 1801 (84) | 1784 (63) |
| Overalla,b | 1844 (55) | 1705 (50)* | 1686 (92) | 1827 (71)+ | 1755 (57)† |
| SM | |||||
| CC | 718 (57) | 671 (57) | 679 (60) | 741 (108) | 710 (60) |
| CT | 603 (37) | 580 (29) | 592 (70) | 606 (50) | 599 (41) |
| TT | 593 (31) | 561 (28) | 586 (57) | 594 (47) | 590 (36) |
| Overalla | 637 (26) | 602 (24)* | 617 (36) | 646 (43) | 631 (27)* |
| Serum folate | |||||
| CC | 34.0 (3.6) | 16.5 (1.6) | 19.3 (1.4) | 42.1 (4.3) | 30.6 (3.9) |
| CT | 28.6 (2.9) | 11.6 (1.1) | 18.6 (1.6) | 34.0 (2.5) | 26.3 (2.7) |
| TT | 29.7 (2.3) | 10.9 (0.9) | 14.3 (2.0) | 38.5 (3.4) | 25.6 (3.6) |
| Overalla,b | 30.8 (1.7) | 12.9 (0.7)* | 17.0 (1.1) | 38.3 (2.1)+ | 27.4 (2.0)* |
Results are presented as mean (S.E.M.).
An overall week effect ( P <.05) was detected between the values in the same row (repeated measures ANOVA).
A week by folate interaction ( P <.05) was detected for this variable (repeated measures ANOVA).
Denote differences between Weeks 0 vs. 7 or 7 vs. 14 at P <.05.
Denote differences in the response variable to 800 vs. 400 μg DFE/day.
Denote differences at P≥.05 and P ≤.1.
3.3. Phosphatidylcholine
Phosphatidylcholine declined from Weeks 0 to 7 ( P=.001) and tended to increase from Weeks 7 to 14 ( P=.087; overall week effect, P=.001; Table 2). The observed increase during folate treatment (i.e., Weeks 7 to 14) occurred in response to 800 ( P=.03) rather than 400 ( P=.85) μg DFE/day (folate×week interaction, P=.017; Table 2). The MTHFR genotypes tended to respond differently to folate intake. The decrease in phosphatidylcholine during folate restriction and the increase in phosphatidylcholine during folate treatment occurred pre-dominately in the MTHFR CC and CT genotypes not in the TT genotype (week×genotype interaction, P=.089; genotype×folate interaction, P=.1; Table 2). Furthermore, when examined independent of folate status (i.e., serum folate), MTHFR C677T genotype was a predictor ( P=.027) of phosphatidylcholine concentration. Phosphatidylcholine was higher ( P=.032) or tended to be higher ( P=.067) in women with the TT or CC genotype respectively than in those with the CT genotype (Fig. 2).
Fig. 2.
The influence of the methylenetetrahydrofolate reductase C677T genotype on phosphatidylcholine (PtdCho) when assessed independent of serum folate (i.e., serum folate was used a covariate in the statistical analysis). Genotype was a predictor ( P=.027) of phosphatidylcholine concentration. Phosphatidylcholine was higher ( P=.032) or tended to be higher ( P=.067) in women with the TT or CC genotype respectively than in those with the CT genotype.
3.4. Sphingomyelin
Sphingomyelin declined from Weeks 0 to 7 ( P=.009) and increased from Weeks 7 to 14 ( P=.029; overall week effect P=.024; Table 2). No effect of genotype or the level of folate treatment (i.e., 400 or 800 μg DFE/day) or their interactions with each other or week was detected. However, when examined independent of folate status, the MTHFR C677T genotype was a predictor ( P=.01) of sphingomyelin. Women with the MTHFR 677 TT genotype had higher (P=0.009) sphingomyelin concentrations than women with the MTHFR 677 CC genotype, whereas women with the MTHFR 677 CT genotype had intermediate concentrations (data not shown).
3.5. Serum folate
These data were previously published in greater detail and with multiple measurements throughout folate restriction and treatment [14]. Here, we present data for Weeks 0, 7 and 14 in order to facilitate comparisons with choline status and demonstrate compliance to the study protocol. Serum folate decreased ( P<.001) after folate restriction and increased ( P <.001) after folate treatment particularly in the 800 vs. 400 μg DFE/day group (folate level interaction=P <.001; Table 2). Serum folate was lower ( P=.003) in women with the MTHFR 677 TT or CT genotype relative to the CC genotype after folate restriction and tended ( P=.08) to be lower in these women throughout the study duration. However, no significant interactions were detected between week and MTHFR C677T genotype or between MTHFR C677T genotype and folate treatment (i.e., 400 vs. 800 μg DFE/day).
4. Discussion
This controlled feeding study conducted in young Mexican-American women sought to assess the influence of the MTHFR 677C→T variant and folate intake on choline status. Compliance to the study protocol was demonstrated by the significant changes in serum folate in response to folate restriction and treatment. The study findings confirm the relationship between folate intake and phosphatidylcholine [11] and extend this relationship to sphingomyelin. Specifically, phosphatidylcholine and sphingomyelin decreased in response to folate restriction ( P=.001, and P=.009, respectively) and increased ( P=.087 and P=.028, respectively) in response to folate treatment. For phosphatidylcholine, the increase was in response to folate treatment with 800 ( P=.03) not 400 ( P=.85) μg DFE/day (week×folate interaction, P=.017). Importantly, the low folate diet utilized in the present study during folate restriction is commensurate with the folate intake of the vast majority of the world’s population residing in countries without mandated folic acid fortification programs. The adverse effects of low folate intake on choline status underscore the importance of including assessments of choline status in developmental anomalies and chronic diseases with ties to folate.
The mechanism by which folate restriction decreased plasma phosphatidylcholine and sphingomyelin concentrations cannot be determined by the present study. However, based upon what is known about the metabolic pathways of choline and folate, we propose that folate restriction decreased the availability of methyl groups for phosphatidylcholine synthesis through the PEMT pathway. Another possibility is that folate restriction increased the demand for betaine as the methyl donor in the methionine pathway. The latter possibility would favor the oxidation of choline to betaine at the expense of phosphatidylcholine synthesis through the Kennedy pathway. In contrast, folate treatment with 800 μg DFE/day may have increased the availability of methyl groups for phosphatidylcholine synthesis through the PEMT pathway and/or reduced the demand for betaine and favored the synthesis of phosphatidylcholine from choline. Since the majority of sphingomyelin is synthesized from phosphatidylcholine [20], it would be sensitive to changes in the phosphatidylcholine pool.
Data from the present study also suggest that the response of choline to changes in folate intake is modified by the MTHFR C677T genotype. An interaction ( P=.089) was detected between folate restriction and MTHFR C677T genotype on phosphatidylcholine concentration. The nature of the interaction was that the MTHFR 677TT genotype was less responsive to decreases in folate intake in women with the CT or CC genotypes. Furthermore, when examined independent of folate status (i.e., serum folate was used as a covariate in the analysis), women possessing the TT genotype had higher ( P=.03) phosphatidylcholine concentrations compared to the CT genotype and were similar to the CC genotype (Fig. 2). These data provide support for a possible compensatory mechanism that allows for maintenance of phosphatidylcholine under conditions of low folate intake in women with the MTHFR 677TT genotype at least over the short-term. Up-regulation of the PEMT pathway is one potential mechanism. In this regard, methyl groups derived from betaine and/or folate would be channeled toward phosphatidylcholine synthesis at the expense of other reactions such as DNA methylation. This possibility is supported by the following observations: global DNA methylation is lower in human leukocytes obtained from the MTHFR 677 TT genotype (relative to the CT or CC genotype) under conditions of low folate status [21-23]; flux through transmethylation reactions (i.e., SAM→S-adenosylhomocysteine) is higher in women with the MTHFR 677TT genotype relative to the CC [24]; global DNA is hypomethylated in tissues of MTHFR-deficient mice [25]; and liver phosphatidylcholine concentrations in MTHFR-deficient mice do not differ from those in wild-type mice despite lower betaine and choline concentrations [12].
An AI for choline of 425 and 550 mg/day for women and men, respectively, was recently established [1]. The AI was based on the amount of choline needed for the prevention of elevated liver enzymes in healthy young men consuming controlled choline and folate intakes. In the present study, consumption of ∼350 mg/day choline derived from dietary (174 mg/day) and supplemental (350 mg every second day) sources was sufficient in preventing liver dysfunction assessed via plasma concentrations of ALT and AST (data not shown). However, it was not enough to maintain or achieve baseline plasma phosphatidylcholine and sphingomyelin even when the folate RDA (i.e., 400 μg DFE/day) was consumed. Unfortunately, established norms for blood choline concentrations that would facilitate further assessment of our study findings are not available. Furthermore, while data on the concentrations of choline-containing compounds in common foods have been recently published [26,27], few studies have examined choline intake in the general population. Utilizing a food-frequency questionnaire, Cho et al. [6] reported an energy-adjusted mean choline intake of 312 and 314 mg/day for men and women, respectively, in the Framingham Offspring Cohort. These estimates are substantially lower than initial predictions of 730 to 1040 mg/day [1]. While the results of our controlled feeding study suggest that young women may require more than 350 mg/day choline, it is clear that additional studies are needed to more fully examine human requirements for choline.
The biological significance of the observed decline in plasma phosphatidylcholine in response to folate restriction is unknown. However, phosphatidylcholine in plasma is present primarily as low-density lipoproteins (LDL) and high-density lipoproteins (HDL) under fasting conditions. Thus, the decline in plasma phosphatidylcholine may suggest that less phosphatidylcholine is incorporated into these lipoproteins during their assembly within the liver and/or fewer lipoproteins are being exported from the liver [28]. Changes such as these may have implications on lipoprotein profiles as well as long-term liver function. Interestingly, in the present study, HDL-C tended ( P=.056) to decline from 1.52 to 1.39 mmol/L for women in the 400 μg DFE/day treatment group (data not shown). This modest decline in HDL-C was not observed among women in the 800 μg DFE/day treatment group nor was it observed for either LDL-C or total cholesterol. A decline in plasma phosphatidylcholine concentration and HDL was recently reported in a mouse model with targeted deletion of the hepatic CTP:phosphocholine cytidylytransferase gene, the product of which is critical to the Kennedy pathway and the synthesis of phosphatidylcholine from choline [29]. Thus, the possible link between folate, choline and HDL-C in young women warrants further investigation.
To more fully delineate the relationship between folate, the MTHFR C677T genotype and choline metabolism, studies assessing gene/protein expression in choline relevant pathways are needed as are investigations involving the use of stable isotopes. At present, it is clear that folate intake, and likely the MTHFR C677T genotype, modulates choline nutriture. Because of the interdependence between folate and choline, additional work is needed to examine the possible role of choline in diseases associated with folate nutriture.
Acknowledgments
The authors would like to thank Bradley Hart from Thermo Finnigan, Inc., for his assistance in establishing the methodology for quantifying choline and its derivatives via LC-MS/MS in our laboratory. We are also grateful to the women whose participation made this study possible.
Footnotes
Supported by the National Institute of Health S06GM53933 and funds from the California Agricultural Research Initiative.
References
- [1].Institute of Medicine . Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. National Academy Press; Washington, DC: 1998. [PubMed] [Google Scholar]
- [2].Zeisel SH. Dietary choline: biochemistry, physiology and pharmacology. Annu Rev Nutr. 1981;1:95–121. doi: 10.1146/annurev.nu.01.070181.000523. [DOI] [PubMed] [Google Scholar]
- [3].Koc H, Mar M-H, Ranasinghe A, Swenberg JA, Zeisel SH. Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal Chem. 2002;74:4734–40. doi: 10.1021/ac025624x. [DOI] [PubMed] [Google Scholar]
- [4].Zeisel SH, Blusztajn JK. Choline and human nutrition. Annu Rev Nutr. 1994;14:269–96. doi: 10.1146/annurev.nu.14.070194.001413. [DOI] [PubMed] [Google Scholar]
- [5].Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer DM. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am J Epidemiol. 2004;160:102–9. doi: 10.1093/aje/kwh187. [DOI] [PubMed] [Google Scholar]
- [6].Cho E, Zeisel SH, Jacques P, Selhub J, Dougherty L, Colditz GA, et al. Dietary choline and betaine assessed by food-frequency questionnaire in relation to plasma total homocysteine concentration in the Framingham Offspring Study. Am J Clin Nutr. 2006;83:905–11. doi: 10.1093/ajcn/83.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].da Costa KA, Niculescu MD, Craciunescu CN, Fisher LM, Zeisel SH. Choline deficiency increases lymphocyte apoptosis and DNA damage in humans. Am J Clin Nutr. 2006;84:88–94. doi: 10.1093/ajcn/84.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Niculescu MD, Yamamuro Y, Zeisel SH. Choline availability modulates human neuroblastoma cell proliferation and alters the methylation of the promoter region of the cyclin-dependent kinase inhibitor 3 gene. J Neurochem. 2004;89:1252–9. doi: 10.1111/j.1471-4159.2004.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Selhub J, Seyoum E, Pomforte EA, Zeisel SH. Effects of choline deficiency and methotrexate treatment upon liver folate content and distribution. Cancer Res. 1991;51:16–21. [PubMed] [Google Scholar]
- [10].Kim YI, Miller FW, daCosta KA, Nadeau M, Smith D, Selhub J, et al. Folate deficiency causes secondary depletion of choline and phosphocholine in liver. J Nutr. 1995;124:2197–203. doi: 10.1093/jn/124.11.2197. [DOI] [PubMed] [Google Scholar]
- [11].Jacob RA, Jenden DJ, Allman-Farinelli MA, Swendseid ME. Folate nutriture alters choline status of women and men fed low choline diets. J Nutr. 1999;129:712–7. doi: 10.1093/jn/129.3.712. [DOI] [PubMed] [Google Scholar]
- [12].Schwahn BC, Chen Z, Laryea MD, Wendel U, Lussier-Cacan S, Genest J, et al. Homocysteine-betaine interactions in a murine model of 5,10-methylenetetrahydrofolate deficiency. FASEB J. 2003;17(3):512–4. doi: 10.1096/fj.02-0456fje. [DOI] [PubMed] [Google Scholar]
- [13].Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthew RG, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nature Genet. 1995;10:111–3. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- [14].Guinotte CL, Burns MG, Axume JA, Hata H, Urrutia TF, Alamilla A, et al. Methylenetetrahydrofolate reductase 677C→T variant modulates folate status response to controlled folate intakes in young women. J Nutr. 2003;133:1272–80. doi: 10.1093/jn/133.5.1272. [DOI] [PubMed] [Google Scholar]
- [15].Rozen R. Folate and genetics. J Food Sci. 2004;69:SNQ65–7. [Google Scholar]
- [16].Tamura T, Mizuno Y, Johnston KE, Jacob RA. Food folate assay with protease, alpha amylase, and folate conjugase treatments. J Agric Food Chem. 1997;45:135–9. [Google Scholar]
- [17].Gregory JF, Engelhardt R, Bhandari SD, Bustafson SK. Adequacy of extraction techniques for determination of folates in foods and other biological materials. J Food Compos Anal. 1990;3:134–44. [Google Scholar]
- [18].Choudhary G, Hart B, Cho D, Caudill MA. App. Note 334: Determination of choline and its metabolites using a Finnigan LTQ linear ion trap mass spectrometer.
- [19].Tamura T. Microbiological assay of folates. In: Picciano MF, Stokstad JF, Gregory JF, editors. Folic acid metabolism in health and disease. John Wiley & Sons; New York: 1990. pp. 121–37. [Google Scholar]
- [20].Merrill AH, Jones DD. An update of the enzymology and regulation of sphingomyelin metabolism. Biochim Biophys Acta. 1990;1044:1–12. doi: 10.1016/0005-2760(90)90211-f. [DOI] [PubMed] [Google Scholar]
- [21].Friso S, Choi SW, Girelli D, Mason JB, Dolnikowski GG, Bagley PJ, et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci USA. 2002;99:5606–11. doi: 10.1073/pnas.062066299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Castro R, Rivera I, Ravasco P, Camilo ME, Jakobs C, Blom HJ, et al. 5,10-Methylenetetrahydrofolate reductase (MTHFR) 677 C→T and 1298 A→C mutations are associated with DNA hypomethylation. J Med Genet. 1991;41:454–8. doi: 10.1136/jmg.2003.017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Axume J, Smith SS, Pogribny IP, Moriarty DJ, Caudill MA. The methylenetetrahydrofolate reductase 677TT genotype and folate intake interact to lower global leukocyte DNA methylation in young Mexican American women. Nutr Res. 2007;27:13–7. doi: 10.1016/j.nutres.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Davis SR, Quinlivan EP, Shelnutt KP, Ghandour H, Capdevila A, Coats BS, et al. Homocysteine synthesis is elevated but total remethylation is unchanged by the methylenetetrahydrofolate reductase 677C→T polymorphism and by dietary folate restriction in young women. J Nutr. 2005;135:1045–50. doi: 10.1093/jn/135.5.1045. [DOI] [PubMed] [Google Scholar]
- [25].Chen Z, Karaplis AC, Ackerman SL, Pogribny IP, Melnyk S, Lussier-Cacan S, et al. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum Mol Genet. 2001;10:433–43. doi: 10.1093/hmg/10.5.433. [DOI] [PubMed] [Google Scholar]
- [26].Zeisel SH, Mar M-H, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr. 2003;133:1302–7. doi: 10.1093/jn/133.5.1302. [DOI] [PubMed] [Google Scholar]
- [27].Howe JC, Williams JR, Holden JM, Zeisel SH, Mar M-H. USDA database for the choline content of common foods. United States Department of Agricultural; http://www.nal.usda.gov/fnic/foodcomp/Data/Choline/Choline.html [Google Scholar]
- [28].Zeisel SH, Da Costa K-A, Franklin PD, Alexander EA, Lamont JT, Sheard NF, et al. Choline, an essential nutrient for humans. FASEB J. 1991;5:2093–8. [PubMed] [Google Scholar]
- [29].Jacobs RL, Devlin C, Tabas I, Vance DE. Targeted deletion of hepatic CTP: phosphocholine cytidylytransferase A in mice decreases plasma high density and very low density lipoproteins. J Biol Chem. 2004;279:47402–10. doi: 10.1074/jbc.M404027200. [DOI] [PubMed] [Google Scholar]