Abstract
Background
Obstetric complications, particularly fetal hypoxia, are associated with increased risk for schizophrenia later in life. Such factors are also related to increased severity of certain neuropathological features of schizophrenia, including hippocampal and cortical gray matter reduction, among individuals with a genetic susceptibility to the disorder. However, the molecular mechanisms underlying these associations are unknown. Here we sought to determine whether neurotrophic factors, which are stimulated as part of a neuroprotective response to fetal distress, are differentially expressed in cord blood samples at the time of birth following fetal hypoxia, maternal hypertension/small for gestational age status, and/or prematurity among individuals who developed schizophrenia as adults, as compared with controls.
Methods
One hundred and eleven cases with psychotic disorders (70 with schizophrenia) and 333 controls matched for gender, race, and date of birth, were drawn from the Philadelphia cohort of the National Collaborative Perinatal Project, in a nested case-control study. Brain derived neurotrophic factor (BDNF) was assayed from cord and maternal blood samples taken at delivery and stored at −20 C for 45–50 years.
Results
Among controls, birth hypoxia was associated with a significant (10%) increase in BDNF in cord samples, while among cases, hypoxia was associated with a significant (20%) decrease in BDNF. This differential response to fetal hypoxia was specific to schizophrenia and was not explained by other obstetric complications or by the BDNF Val66Met polymorphism.
Conclusions
These findings provide serologically based prospective evidence of disrupted neurotrophic signaling in response to birth hypoxia in the molecular pathogenesis of schizophrenia.
Keywords: Schizophrenia, Hypoxia, Brain Derived Neurotrophic Factor, Neuroprotection
Introduction
Numerous well-designed population-based studies have demonstrated increased risk for schizophrenia among individuals exposed to disruptive influences in utero, particularly complications related to fetal hypoxia (1–3). Such complications are associated with a 1.5 to 3 times increased risk for schizophrenia in the offspring (4). The molecular mechanisms by which these and other obstetric risk factors contribute to schizophrenia are unknown, but are likely to be at least in part gene-dependent (2; 5; 6). For example, hypoxia-related obstetric complications are more strongly related to hippocampal and cortical pathology among individuals with schizophrenia and their relatives than among the general population (7–11).
Neurotrophins represent prominent candidates for mediating the relationship between obstetric complications and schizophrenia, in part because these factors play critical roles in protecting against neuronal damage following disruptive intrauterine events in general (12–15),and in part because a number of these proteins are known to be dysregulated in patients with schizophrenia (16; 17). Neurotrophins are secreted proteins that signal neurons to survive, differentiate, or grow. In particular, brain-derived neurotrophic factor (BDNF) supports growth and differentiation of new neurons by stimulating axonal and dendritic sprouting, and is critical for survival of existing neurons under stressful conditions such as fetal hypoxia, effects that depend on participation of the tyrosine kinase B receptor (TrkB) (18; 19). The neural sequelae of fetal hypoxia vary from alterations in neurite outgrowth to neuronal cell death, depending on the severity and timing of insult (20). In many cases, immature neurons survive the hypoxic insult but still have a compromised elaboration of synaptic interconnections (20), resulting in reduced cortical thickness and increased cortical neuronal density, without observable neuronal loss (21).These effects parallel the cellular pathology characteristic of schizophrenia, which is associated with preserved cell number in cortex but reduced dendritic complexity and decreased spine and synapse density (22–24). Altered BDNF function would be expected to play a role in this pathology, given that postmortem studies have observed reduced mRNA expression of BDNF receptors TrkB and TrkC in the prefrontal cortex of schizophrenia patients (17; 25), and in animal models, a reduction in TrkB expression is associated with dendritic atrophy and decreased spine density in pyramidal neurons within kainic acid induced lesion sites (26).
Based on the foregoing, we hypothesized that the BDNF response to hypoxia would be inhibited in cord serum samples from individuals who developed schizophrenia later in life as compared with demographically matched controls.
Methods and Materials
Description of Birth Cohort and Perinatal Risk Indicators
The study sample was drawn from the Philadelphia cohort of the National Collaborative Perinatal Project (NCPP). The NCPP was a large-scale, prospective, multi-site study of pregnant women and their offspring born from 1959–1965, selected to be representative of patients receiving prenatal care at each study site (27). The Philadelphia cohort includes 9,236 surviving offspring of a sample of 6,753 pregnant women (2). As part of the original NCPP study, data from examinations and interviews were recorded by trained staff beginning at the time of maternal registration for prenatal care, using standardized protocols, through to the delivery, with follow-up evaluations of offspring health at 8 months, 4 years and 7 years of age (27). Maternal blood samples were collected throughout pregnancy and infant cord samples at delivery and stored at −20°C in a repository. Three obstetric risk factors were included in this analysis: birth hypoxia, maternal toxemia/small for gestational age (SGA) status, and prematurity. Birth hypoxia is one of the most replicated obstetric risk factors for schizophrenia in both cohort and case-control studies (4; 28); it was coded as positive if the infant was blue during the first five minutes of life (i.e., rating of 0 on Apgar scale for skin color from 1 to 5 minutes) and/or required resuscitation at delivery. We used only the color component of the APGAR score so as focus the measure on fetal blood oxygenation. This index is a very general measure of hypoxia, reflecting a relatively broad range of hypoxia severity; thus it has a base rate that is much higher than that of true asphyxia. Sensitivity to a broader range of hypoxia severity and a higher base rate were considered advantageous in this context given the sample size available. Maternal toxemia/SGA status has been linked to psychotic disorder outcomes in the Boston-Providence cohort of the NCPP and may imply a risk for chronic fetal hypoxia (1); following the definition used in our prior study, this factor was coded as positive if the mother had toxemia or hypertension during pregnancy or the infant was born small-for-gestational age (i.e., birth weight below the 10th percentile for a given gestational age). Premature birth, defined as a gestational age less then 37 weeks, has been associated with risk for schizophrenia in some studies (4) and may moderate the effects of hypoxia and other obstetric insults (9; 20).
Diagnostic Follow-Up and Case-Control Selection
As described in detail elsewhere (2), a two-stage diagnostic assessment procedure was used to ascertain offspring who had developed schizophrenia and affective psychoses by adulthood. Briefly, stage one screening utilized a citywide psychiatric database, identifying 339 study offspring who had been treated and diagnosed with some form of psychotic disorder. Psychiatric records were located and reviewed for 144 of these individuals. Six experienced diagnosticians performed the chart reviews, assigning diagnoses using DSM-IV criteria. Of the 144 charts reviewed, 113 received confirmed diagnoses of major psychotic disorders, including 62 individuals with schizophrenia (n=62), 10 with schizoaffective disorder, depressed type, and 41 received a confirmed diagnosis of psychotic bipolar disorder (n=10) or major depression with psychotic features (n=31). The inter-rater reliability for primary chart-review diagnoses (based on independent reviews by two experienced clinicians for a random sample of 94 cases) was 93% (κ=.85). The agreement between primary chart-review diagnoses and diagnoses based on direct interview for 15 of the cases was 92.8%.
The pool of potential controls was composed of all the remaining members of the cohort. These subjects were grouped into four strata according to gender (male/female) and race/ethnicity (Caucasian/African American), and within strata sorted by month and day of birth. The three controls closest for month and day of birth within each gender X race stratum were selected for each case.
Human subjects approval was granted by review groups at the University of Pennsylvania, the City of Philadelphia, the University of California, Los Angeles, NICHD, the Johns Hopkins School of Medicine, and local psychiatric facilities. Written consent was obtained from all interviewed study participants.
A maternal blood sample was obtained from the NIH study repository for 111 of the cases (70 with schizophrenia or schizoaffective disorder-depressed type; 41 with psychotic bipolar disorder or major depression with psychotic features) and their 333 controls. A cord blood sample was obtained for 64 cases (40 with schizophrenia or schizoaffective disorder, 24 with affective psychosis) and 188 controls. Demographic characteristics of the cases and controls are given in Table 1.
Table 1.
Demographic and obstetric characteristics of the case and control groups by primary diagnosis.
| Characteristic | Schizophrenia | Affective Psychosis | ||
|---|---|---|---|---|
| Cases (N=70) | Controls (N=210) | Cases (N=41) | Controls (N=123) | |
| Female, N (%) | 23 (32.8%) | 69 (32.8%) | 24 (58.5%) | 72 (58.5%) |
| African American | 68 (97.1%) | 204 (97.1%) | 35 (85.4%) | 105 (85.4%) |
| Birth Hypoxia | 22 (31.4%) | 60 (28.6%) | 8 (19.5%) | 31 (25.2%) |
| Maternal Toxemia/SGA | 12 (17.1%) | 52 (24.8%) | 8 (19.5%) | 18 (14.6%) |
| Prematurity | 14 (20.0%) | 37 (17.6%) | 8 (19.5%) | 22 (17.9%) |
| T/C or T/T Val66Met | 3 (7.9%) | 9 (7.8%) | 0 (0.0%) | 5 (8.1%) |
| Birth year, mean (SD) | 1962 (2.1) | 1962 (2.1) | 1962 (2.0) | 1962 (2.0) |
| Mother’s age, yrs | 24.4 (6.6) | 24.5 (6.3) | 24.2 (7.4) | 23.8 (5.9) |
| SES | 2.9 (1.9) | 3.4 (1.9) | 3.3 (1.9) | 3.5 (1.9) |
| Maternal education, yrs completed | 10.4 (2.4) | 10.8 (2.6) | 9.7 (1.9) | 10.7 (3.5) |
| BDNF, infant, pg/ml | 434.2 (128.6) | 432.3 (141.9) | 465.9 (165) | 457.6 (127.3) |
| BDNF, mother, pg/ml | 406.3 (65.5) | 399.8 (65.4) | 392.1 (69.2) | 386.4 (65.6) |
SGA=small for gestational age.
Antibody Measurements and Genotyping
BDNF was measured in the serum samples using a solid phase immunoassay as previously described (29). Reagents were obtained from Promega Corporation, Madison Wisconsin, and the assay was performed following manufacturers instructions (30). This assay employs a monoclonal antibody with <3% cross-reactivity with other trophins and has a coefficient of variation ranging from 2.2–8.8%. The assay is engineered to require reactivity to 2 distinct epitopes of intact protein. The Val66Met polymorphism of the BDNF gene (rs6265) was genotyped using the Taqman system and PCR as previously described (31) on DNA extracted from cord blood. The minor allele (T) at this marker (resulting in a valine to methionine amino acid substitution) is associated with lower depolarization-induced secretion of BDNF and a failure of BDNF localization to secretory granules and synapses in transfected cell culture (32).
Statistical Analysis
To test our hypothesis, we conducted mixed model analysis of variance that took into account the matching of three controls per case to test for differences in cord and maternal BNDF levels between the case and control groups as a function of birth hypoxia, maternal toxemia/SGA status, and prematurity. We fit random effects models that assessed the main effects and interactions of case-control status and each of the obstetric risk factors, treating each “quartet” of one case and three matched controls as a random effect (33).
These obstetric factors have previously associated with increased risk for schizophrenia in the Philadelphia and New England cohorts of the NCPP or in other studies (1; 2; 4; 28), so analyses of their association with diagnostic outcome will not be repeated here. Rather, we seek to identify molecular mechanisms underlying these associations by determining whether each risk factor was associated with a differential profile of neurotrophin response in cases versus controls.
Results
Obstetric risk factors and BDNF
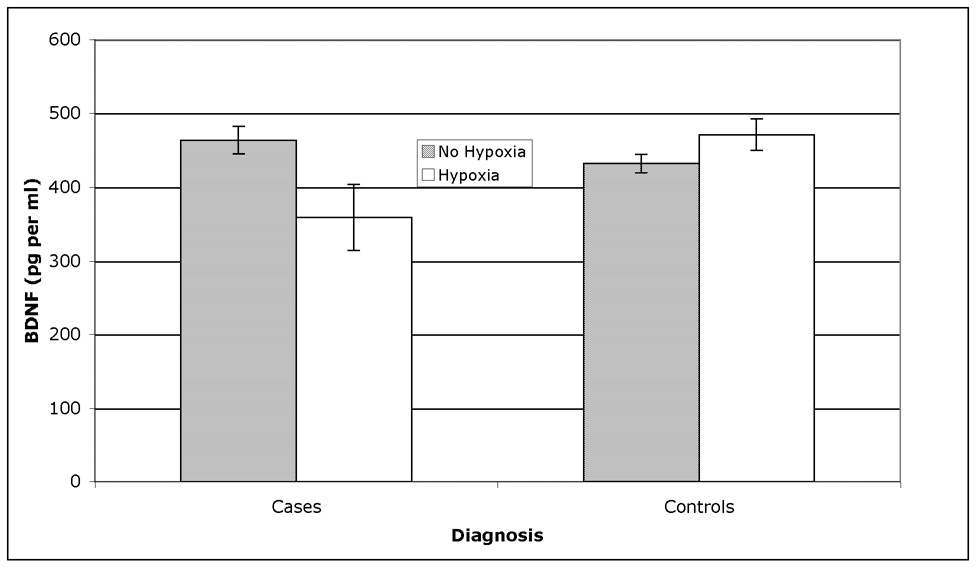
The results of the mixed model analyses (see Table 2) showed that BDNF in cord blood samples was differentially expressed in cases versus controls as a function of birth hypoxia, but not as a function of maternal toxemia/SGA status or prematurity. As shown in Figure 1, BDNF was significantly increased (by 10%) in cord samples among controls exposed to hypoxia, but significantly decreased (by 20%) among future cases (all psychoses) exposed to hypoxia. This effect was specific to the cord samples – BDNF was not differentially expressed among cases and controls according to hypoxia in maternal serum samples at the time of birth (Table 2). Maternal and cord BDNF levels were not significantly correlated in this sample overall or among cases and controls separately. There was also a significant effect of prematurity on cord BDNF levels (Table 2), with infants born prematurely showing a significantly lower level of BDNF (367.3±24.3) than those born at term (435.8±15.9).
Table 2.
Mixed model analysis of variance1 results for diagnosis and obstetric predictors on cord and maternal blood levels of BDNF.
| Predictor4 | Cord BDNF2 | Maternal BDNF3 | ||
|---|---|---|---|---|
| F | p | F | p | |
| Diagnosis | 0.65 | 0.42 | 1.23 | 0.27 |
| Birth Hypoxia | 3.37 | 0.07 | 0.05 | 0.82 |
| Maternal Toxemia/SGA | 0.81 | 0.37 | 0.33 | 0.56 |
| Prematurity | 8.18 | 0.005 | 0.79 | 0.37 |
| Diagnosis × Birth Hypoxia | 5.04 | 0.02 | 1.27 | 0.26 |
| Diagnosis × Maternal Toxemia/SGA | 0.52 | 0.47 | 0.05 | 0.83 |
| Diagnosis × Prematurity | 0.21 | 0.65 | 0.00 | 0.99 |
Each case was matched with 3 control subjects on age, gender, and race, and sociodemographic stratum was modeled as a random variable.
Each effect tested with 1 and 173 degrees of freedom.
Each effect tested with 1 and 317 degrees of freedom.
All predictors were modeled simultaneously, thereby controlling for overlap among predictors.
SGA=Small for gestational age.
Figure 1.
Least-square mean (± SEM) BDNF levels in cord blood samples by fetal hypoxia in cases versus controls (as derived from the full mixed model analysis of variance shown in Table 2). While controls showed an increase in BDNF as a function of exposure to hypoxia, cases exposed to hypoxia showed a significant decrease in BDNF.
In secondary analyses, the differential downregulation in BDNF as a function of birth hypoxia in cases compared with controls was found to be specific to the cases with a diagnosis of schizophrenia or schizoaffective disorder-depressed type (F[1,111]=4.45, p=0.03), and was not observed in the cases with psychotic bipolar disorder or major depression with psychotic features (F[1,55]=0.06, p=0.80). This analysis was done because prior work has in general shown greater relevance of obstetric risk factors to outcomes of schizophrenia than to outcomes of affective psychosis (34).
BDNF genotype as a potential moderator
The frequency of the minor allele (T) of the BDNF Val66Met polymorphism was 4% in both the patient and control groups (χ2=0.59, df=1, p=.43) and did not differ significantly between cases with schizophrenia versus affective psychosis (i.e., 4% vs 0%, χ2=1.91, df=1, p=.17). Further, the interaction between schizophrenia and birth hypoxia on cord BDNF levels remained after excluding subjects with the T allele (F[1,90]=5.13, p=.026).
Tests of Bias
We conducted a series of analyses to determine whether the cases included in the analysis (whose charts were available for evaluation and diagnostic classification) differed in some way from the larger group of potential cases identified through the psychiatric register. The 72 cases with chart-review-based DSM-IV diagnoses of schizophrenia or schizoaffective disorder did not differ from the remaining 121 probands with register diagnoses of schizophrenia or schizoaffective disorder (whose charts were not reviewed and who were thus not included as cases in the serological study) in terms of gender (65% vs. 59% male, respectively; χ2=0.9, df=1, p=0.33), race (3% vs. 6% Caucasian; χ2=0.9, df=1, p=0.34), season of birth (24% vs. 27% winter-born; χ2=0.3, df=1, p=0.57), year of birth (1962 ± 2.2 vs. 1962 ± 1.9; t=−0.2, df=191 p=0.81), birth order (3.6 ± 2.7 vs. 3.1 ± 1.9; t=1.4, df=191, p=0.16), mother’s age at birth (24.2 ± 6.6 vs. 23.8 ± 6.3; t=0.4, df=191, p=0.67), or years of maternal education (10.4 ± 2.4 vs. 10.7 ± 2.5; t=−0.6, df=191, p=0.49). The groups also did not differ significantly from each other in terms of birth hypoxia (χ2=0.48, df=1, p=0.49), maternal toxemia/SGA status (χ2=0.11, df=1, p=0.75), or prematurity (χ2=0.06 df=1, p=0.81).
Discussion
These results provide evidence that a critical cell signaling mechanism is disrupted at the time of birth in individuals destined to develop schizophrenia as adults, such that neurotrophic factors fail to be recruited during biological stress invoked by birth hypoxia, potentially leading to dendritic atrophy and disruption of synaptogenesis in the fetus.
The downregulation of BDNF was specific to the fetus and was not observed in maternal samples obtained at the time of delivery. The specificity of this effect to the cord samples held regardless of whether the analysis of maternal samples was limited to those subjects who also had cord samples available. This pattern suggests that a fetus-specific vulnerability factor results in dysregulation of neurotrophic signaling in response to birth hypoxia. Notably, the downregulation of BDNF was specific to birth hypoxia – there were no differences in expression of BDNF in either cord or maternal samples in relation to maternal toxemia/SGA status or prematurity. Some degree of specificity of acute perinatal hypoxia is to be expected given that BDNF levels in cord samples from the time of delivery should index perinatal complications more directly than complications arising earlier in the course of pregnancy. It remains to be determined whether the latter may also be associated with failure to mount a fetal neuroprotective response via BDNF. Notably, however, a similar profile of specificity has been observed in the association of birth hypoxia, and not SGA status or prematurity, with gray matter reduction in schizophrenia patients and their siblings, and differentially so compared with normal controls (9).
The factors responsible for the differential pattern of neurotrophin expression in cases versus controls as a function of hypoxia remain to be identified. We examined and ruled out the Val66Met polymorphism in the BDNF gene as moderating this effect, although it should be noted that the Met allele frequency observed in this predominantly African-American sample is much lower than has been seen in predominantly Caucasian samples from the US population (35) but is comparable to other populations of African origin (see http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?rs=6265). Given that circulating BDNF may emanate from platelets, brain neurons, vascular endothelial cells, activated T cells, B cells and monocytes (36; 37), it is not possible to isolate the tissue source of the reduced BDNF levels observed in this study to brain neurons. Nevertheless, it is worth noting that postmortem studies have observed reduced mRNA expression of BDNF receptors TrkB and TrkC in the prefrontal cortex of schizophrenia patients (17; 25), and in animal models, a reduction in TrkB expression is associated with dendritic atrophy and decreased spine density in pyramidal neurons within kainic acid induced lesion sites (26), while there are no reports examining its expression in platelets or vascular endothelial cells. Further, in patients with chronic schizophrenia, circulating BDNF levels are lower in serum but not whole blood, suggesting a deficit in neurotrophic factor release (38). BDNF neuroprotection is regulated by the Ras-MAPK signaling pathway and depends on sustained activation of the cAMP response element-binding protein (CREB) and ERK 1/2 phosphorylation (18; 19). It is of interest in this regard that several putative susceptibility genes for schizophrenia are involved in Ras-MAPK and ERK signaling (39–44) and along with BDNF and its receptors may thus be considered candidate genes for mediating the differential BDNF response to obstetric insults in individuals who develop schizophrenia as adults. Given that cord blood BNDF may reflect immune system function (45), and considering that immune cell BDNF secretion is associated with white matter volume in multiple sclerosis (46), it is also possible that an immunological mechanism is involved in the lower BDNF levels among cases exposed to hypoxia.
Overall, the rate of birth hypoxia was equivalent in the cases and controls in this study. However, in the overall cohort from which the cases and controls were drawn, exposure to birth hypoxia was associated with an increased risk for an early onset form of schizophrenia (2). In the subset of cases and controls used in this study, the same degree of association between hypoxia and early-onset schizophrenia was present, but with the smaller sample size, at only a trend level of significance (p=0.08). A specific association between hypoxia-associated obstetric complications and an early onset of form of schizophrenia has also been replicated in an independent cohort from Finland (3). In view of this pattern, and considering the differential BDNF response among cases versus controls, birth hypoxia may not increase the risk for developing schizophrenia in the absence of a particular biological vulnerability, such as failure to mount a neurotrophin protective response. However, the presence of both this biological vulnerability and birth hypoxia may contribute to an increased risk for a form of schizophrenia characterized by an early age at onset. While discussion of the potential mechanisms involved is necessarily speculative at this point, such a pattern could reflect a reduced degree of dendritic complexity and synaptic density arising at birth, which then interacts with subsequent regressive brain developmental processes during adolescence (e.g., synaptic pruning) in pushing individuals below some critical threshold of interneuronal connectivity associated with expression of psychosis (5; 47). If this interpretation is correct, it is possible that administration of neurotrophins (perhaps modified chemically to promote increased CNS penetration) during critical stages of brain development may have some protective benefits in individuals who are genetically susceptible to schizophrenia.
It is also possible that, rather than moderating the effect of birth hypoxia on cord BDNF levels, genetic factors associated with vulnerability to future schizophrenia cause both a higher rate of birth hypoxia and lower BDNF levels in fetal serum. This interpretation is somewhat less likely, however, given that presence of a family history of schizophrenia is not correlated with birth hypoxia independently of maternal health risk behaviors (48).
One limitation of this study is that the majority of cases were diagnosed by chart review rather than by direct interview. The chart-review diagnoses were found to be reliable; independent evaluations of a random sample of medical records by different reviewers produced a high rate of diagnostic agreement (κ=.85, 93% simple agreement), and there was high agreement between the chart-review-based diagnoses and those based on the Structured Clinical Interview for DSM-IV diagnoses in the 15 cases who were interviewed directly (i.e., 92.8%).
Another limitation is that ascertainment of psychiatric morbidity relied on a recorded history of local psychiatric service utilization, which missed cases who were deceased or had not yet come to treatment at the time of follow-up, who had been treated at facilities that were no longer operating or did not cooperate with the chart reviews, or who, because of emigration or changes in social class, utilized psychiatric facilities other than those whose patient rolls were screened. Our use of a case-control (rather than a cohort analytic) design mitigates the potential biasing effects of incomplete ascertainment to the extent that the cases used in the study are representative of the overall pool of affected individuals in the birth cohort. One indication that the cases who were included in this study are likely to be representative is that they were found to be comparable in terms of demographics and obstetric history to the 121 probands with a treated history of schizophrenia or schizoaffective disorder whose charts were not available for review and were thus not included in the serological study. Of note, the control sample for this study included all remaining members of the cohort, not simply unaffected controls. This helps mitigate the concern that the observed differences between cases and controls could be attributable to atypical patterns of BDNF expression in the controls. While some cases of untreated or otherwise unascertained schizophrenia or affective psychosis could have been included as controls, the rate of ascertained morbidity for these diagnoses in this cohort was quite high and comparable to that in similar populations (2). Further, the existence of any such misclassified controls would result in an under-estimation (rather than over-estimation) of the BDNF effect in the cases.
Although the samples of schizophrenia cases and controls were large and sufficient for detecting statistical differences between groups, the numbers of affective psychosis cases with and without birth hypoxia were relatively small, and small sample sizes could have mitigated against detecting effects in this group.
The samples were stored for 40+ years at −20° C. Protease inhibitors were not added to the samples, but serum contains a number of protease inhibitors such as alpha-1 antitrypsin so the amount of proteolytic cleavage while frozen is limited. Steroid protein measurements from NCPP samples are consistent with values from published studies of fresh samples collected at similar points in gestation (49), and the measured values of BDNF from this study are comparable to those from other studies using fresh samples conducted by the Yolken lab. The fact that the samples for cases and controls were collected and stored in an identical fashion (blindly with respect to adult psychiatric outcome) makes it very unlikely that the results of this study could be due to storage artifacts.
Given the higher rate of schizophrenia among males in this cohort (2), it could be argued that there is an under-ascertainment of female schizophrenia patients, such that the present results may generalize only to male schizophrenia patients. Arguing against this interpretation, however, is the fact that the hypoxia effect on risk for schizophrenia in this cohort was not differential according to gender (2), and the differential BDNF response to hypoxia in cases versus controls detected in this study was independent of gender.
Rates of schizophrenia are known to vary by factors such as urban residence, social class, and minority status, factors whose variability is greatly truncated in this cohort compared with the general population. Unfortunately, the truncated racial diversity did not permit us to examine race as a modifier of the differential BDNF response to birth hypoxia in cases versus controls. A race-specific effect seems unlikely, however, since hypoxia-related OCs have been found to be associated with schizophrenia in several Scandinavian countries whose populations are nearly entirely Caucasian (3; 4).
In conclusion, we found that individuals exposed to fetal hypoxia and who developed schizophrenia as adults showed a decreased level of BDNF in cord blood samples from the time of birth, findings that suggest dysregulation of neurotrophic signaling in the pathogenesis of schizophrenia and suggest novel molecular targets for preventive intervention.
Acknowledgements
This work was supported by grants from the Stanley Medical Research Institute, March of Dimes, and National Institute of Mental Health, as well as a gift from the Staglin Music Festival for Mental Health. In addition to the authors, the following persons are investigators in the Collaborative Study Group on the Perinatal Origins of Severe Psychiatric Disorders and participated in this study: J. Goldstein, L. Seidman, and M. Tsuang, Harvard Medical School; T. Hadley, C. Bearden (currently at UCLA), and I. Rosso (currently at Harvard), University of Pennsylvania; and D. Dhavale, Stanley Medical Research Institute.
Financial Disclosures
Dr Cannon reports that he currently or in the past 5 years has received investigator-initiated research funding support from multiple not-for-profit entities including the National Institute for Mental Health, the Stanley Medical Research Foundation, the National Alliance for Research on Schizophrenia and Depression, the March of Dimes, and the Staglin Music Festival for Mental Health. Dr Cannon reports that he has served as a consultant for Janssen Pharmaceuticals and Eli Lilly. Dr. Buka reports that he currently or in the past 5 years has received investigator-initiated research funding support from the National Institutes of Health, the Stanley Medical Research Institute, and the Flight Attendants Medical Research Institute. Dr Torrey reports that he currently or in the past 5 years has received investigator-initiated funding from the Stanley Medical Research Institute. Dr. Yolken he currently or in the past 5 years has received investigator-initiated funding from the Stanley Medical Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buka SL, Tsuang MT, Lipsitt LP. Pregnancy/delivery complications and psychiatric diagnosis. A prospective study. Arch Gen Psychiatry. 1993;50:151–156. doi: 10.1001/archpsyc.1993.01820140077009. [DOI] [PubMed] [Google Scholar]
- 2.Cannon TD, Rosso IM, Hollister JM, Bearden CE, Sanchez LE, Hadley T. A prospective cohort study of genetic and perinatal influences in the etiology of schizophrenia. Schizophr Bull. 2000;26:351–366. doi: 10.1093/oxfordjournals.schbul.a033458. [DOI] [PubMed] [Google Scholar]
- 3.Rosso IM, Cannon TD, Huttunen T, Huttunen MO, Lonnqvist J, Gasperoni TL. Obstetric risk factors for early-onset schizophrenia in a Finnish birth cohort. Am J Psychiatry. 2000;157:801–807. doi: 10.1176/appi.ajp.157.5.801. [DOI] [PubMed] [Google Scholar]
- 4.Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002;159:1080–1092. doi: 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- 5.Cannon TD, van Erp TG, Bearden CE, Loewy R, Thompson P, Toga AW, et al. Early and late neurodevelopmental influences in the prodrome to schizophrenia: contributions of genes, environment, and their interactions. Schizophr Bull. 2003;29:653–669. doi: 10.1093/oxfordjournals.schbul.a007037. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt-Kastner R, van Os J, WMS H, Schmitz C. Gene regulation by hypoxia and the neurodevelopmental origin of schizophrenia. Schizophr Res. 2006;84:253–271. doi: 10.1016/j.schres.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Cannon TD, Mednick SA, Parnas J. Genetic and perinatal determinants of structural brain deficits in schizophrenia. Arch Gen Psychiatry. 1989;46:883–889. doi: 10.1001/archpsyc.1989.01810100025005. [DOI] [PubMed] [Google Scholar]
- 8.Cannon TD, Mednick SA, Parnas J, Schulsinger F, Praestholm J, Vestergaard A. Developmental brain abnormalities in the offspring of schizophrenic mothers. I. Contributions of genetic and perinatal factors. Arch Gen Psychiatry. 1993;50:551–564. doi: 10.1001/archpsyc.1993.01820190053006. [DOI] [PubMed] [Google Scholar]
- 9.Cannon TD, van Erp TG, Rosso IM, Huttunen M, Lonnqvist J, Pirkola T, et al. Fetal hypoxia and structural brain abnormalities in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry. 2002;59:35–41. doi: 10.1001/archpsyc.59.1.35. [DOI] [PubMed] [Google Scholar]
- 10.van Erp TG, Saleh PA, Huttunen M, Lonnqvist J, Kaprio J, Salonen O, et al. Hippocampal volumes in schizophrenic twins. Arch Gen Psychiatry. 2004;61:346–353. doi: 10.1001/archpsyc.61.4.346. [DOI] [PubMed] [Google Scholar]
- 11.Van Erp TG, Saleh PA, Rosso IM, Huttunen M, Lonnqvist J, Pirkola T, et al. Contributions of genetic risk and fetal hypoxia to hippocampal volume in patients with schizophrenia or schizoaffective disorder, their unaffected siblings, and healthy unrelated volunteers. Am J Psychiatry. 2002;159:1514–1520. doi: 10.1176/appi.ajp.159.9.1514. [DOI] [PubMed] [Google Scholar]
- 12.Gilmore JH, Jarskog LF, Vadlamudi S. Maternal infection regulates BDNF and NGF expression in fetal and neonatal brain and maternal-fetal unit of the rat. J Neuroimmunol. 2003;138:49–55. doi: 10.1016/s0165-5728(03)00095-x. [DOI] [PubMed] [Google Scholar]
- 13.Gilmore JH, Jarskog LF, Vadlamudi S. Maternal poly I:C exposure during pregnancy regulates TNF alpha, BDNF, and NGF expression in neonatal brain and the maternal-fetal unit of the rat. J Neuroimmunol. 2005;159:106–112. doi: 10.1016/j.jneuroim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Marx CE, Vance BJ, Jarskog LF, Chescheir NC, Gilmore JH. Nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3 levels in human amniotic fluid. Am J Obstet Gynecol. 1999;181:1225–1230. doi: 10.1016/s0002-9378(99)70113-4. [DOI] [PubMed] [Google Scholar]
- 15.Rocamora N, Massieu L, Boddeke HW, Palacios JM, Mengod G. Differential regulation of the expression of nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3 mRNAs in adult rat brain after intrahippocampal injection of quinolinic acid. Brain Res Mol Brain Res. 1994;26:89–98. doi: 10.1016/0169-328x(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 16.Shoval G, Weizman A. The possible role of neurotrophins in the pathogenesis and therapy of schizophrenia. Eur Neuropsychopharmacol. 2005;15:319–329. doi: 10.1016/j.euroneuro.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2003;8:592–610. doi: 10.1038/sj.mp.4001308. [DOI] [PubMed] [Google Scholar]
- 18.Lee HT, Chang YC, Wang LY, Wang ST, Huang CC, Ho CJ. cAMP response element-binding protein activation in ligation preconditioning in neonatal brain. Ann Neurol. 2004;56:611–623. doi: 10.1002/ana.20259. [DOI] [PubMed] [Google Scholar]
- 19.Meng M, Zhiling W, Hui Z, Shengfu L, Dan Y, Jiping H. Cellular levels of TrkB and MAPK in the neuroprotective role of BDNF for embryonic rat cortical neurons against hypoxia in vitro. Int J Dev Neurosci. 2005;23:515–521. doi: 10.1016/j.ijdevneu.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Nyakas C, Buwalda B, Luiten PG. Hypoxia and brain development. Prog Neurobiol. 1996;49:1–51. doi: 10.1016/0301-0082(96)00007-x. [DOI] [PubMed] [Google Scholar]
- 21.Rees S, Mallard C, Breen S, Stringer M, Cock M, Harding R. Fetal brain injury following prolonged hypoxemia and placental insufficiency: a review. Comp Biochem Physiol A Mol Integr Physiol. 1998;119:653–660. doi: 10.1016/s1095-6433(98)01001-0. [DOI] [PubMed] [Google Scholar]
- 22.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 23.Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 24.Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. discussion 819–820. [DOI] [PubMed] [Google Scholar]
- 25.Weickert CS, Ligons DL, Romanczyk T, Ungaro G, Hyde TM, Herman MM, et al. Reductions in neurotrophin receptor mRNAs in the prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2005;10:637–650. doi: 10.1038/sj.mp.4001678. [DOI] [PubMed] [Google Scholar]
- 26.Ampuero E, Dagnino-Subiabre A, Sandoval R, Zepeda-Carreno R, Sandoval S, Viedma A, et al. Status epilepticus induces region-specific changes in dendritic spines, dendritic length and TrkB protein content of rat brain cortex. Brain Res. 2007 doi: 10.1016/j.brainres.2007.02.089. [DOI] [PubMed] [Google Scholar]
- 27.Niswader KR, Gordon M. The Collaborative Perinatal Study of the National Institute of Neurological Diseases and Stroke: The women and their pregnancies. Philadelphia, PA: W.B. Saunders; 1972. [Google Scholar]
- 28.Cannon TD. On the nature and mechanisms of obstetric influences in schizophrenia: A review and synthesis. International Review of Psychiatry. 1997;9:387–397. [Google Scholar]
- 29.Nawa H, Carnahan J, Gall C. BDNF protein measured by a novel enzyme immunoassay in normal brain and after seizure: partial disagreement with mRNA levels. Eur J Neurosci. 1995;7:1527–1535. doi: 10.1111/j.1460-9568.1995.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki K, Narita N, Sakuta R, Miyahara T, Naruse H, Okado N, Narita M. Serum neurotrophin concentrations in autism and mental retardation: a pilot study. Brain Dev. 2004;26:292–295. doi: 10.1016/S0387-7604(03)00168-2. [DOI] [PubMed] [Google Scholar]
- 31.Geller B, Badner JA, Tillman R, Christian SL, Bolhofner K, Cook EH., Jr Linkage disequilibrium of the brain-derived neurotrophic factor Val66Met polymorphism in children with a prepubertal and early adolescent bipolar disorder phenotype. Am J Psychiatry. 2004;161:1698–1700. doi: 10.1176/appi.ajp.161.9.1698. [DOI] [PubMed] [Google Scholar]
- 32.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 33.Brown H, Prescott R. Applied mixed models in medicine. New York: John Wiley & Sons; 1999. [Google Scholar]
- 34.Bain M, Juszczak E, McInneny K, Kendell RE. Obstetric complications and affective psychoses. Two case-control studies based on structured obstetric records. Br J Psychiatry. 2000;176:523–526. doi: 10.1192/bjp.176.6.523. [DOI] [PubMed] [Google Scholar]
- 35.Surtees PG, Wainwright NW, Willis-Owen SA, Sandhu MS, Luben R, Day NE, Flint J. The brain-derived neurotrophic factor Val66Met polymorphism is associated with sense of coherence in a non-clinical community sample of 7335 adults. J Psychiatr Res. 2007;41:707–710. doi: 10.1016/j.jpsychires.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 36.Nakahashi T, Fujimura H, Altar CA, Li J, Kambayashi J, Tandon NN, Sun B. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000;470:113–117. doi: 10.1016/s0014-5793(00)01302-8. [DOI] [PubMed] [Google Scholar]
- 37.Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toyooka K, Asama K, Watanabe Y, Muratake T, Takahashi M, Someya T, Nawa H. Decreased levels of brain-derived neurotrophic factor in serum of chronic schizophrenic patients. Psychiatry Res. 2002;110:249–257. doi: 10.1016/s0165-1781(02)00127-0. [DOI] [PubMed] [Google Scholar]
- 39.Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- 40.Cannon TD, Hennah W, van Erp TG, Thompson PM, Lonnqvist J, Huttunen M, et al. Association of DISC1/TRAX haplotypes with schizophrenia, reduced prefrontal gray matter, and impaired short-and long-term memory. Arch Gen Psychiatry. 2005;62:1205–1213. doi: 10.1001/archpsyc.62.11.1205. [DOI] [PubMed] [Google Scholar]
- 41.Shinoda T, Taya S, Tsuboi D, Hikita T, Matsuzawa R, Kuroda S, et al. DISC1 regulates neurotrophin-induced axon elongation via interaction with Grb2. J Neurosci. 2007;27:4–14. doi: 10.1523/JNEUROSCI.3825-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci. 2004;7:575–580. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- 43.Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu Z, Jiang Q, Fu AK, Ip NY, Yan Z. Regulation of NMDA receptors by neuregulin signaling in prefrontal cortex. J Neurosci. 2005;25:4974–4984. doi: 10.1523/JNEUROSCI.1086-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malamitsi-Puchner A, Economou E, Rigopoulou O, Boutsikou T. Perinatal changes of brain-derived neurotrophic factor in pre-and fullterm neonates. Early Hum Dev. 2004;76:17–22. doi: 10.1016/j.earlhumdev.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Weinstock-Guttman B, Zivadinov R, Tamano-Blanco M, Abdelrahman N, Badgett D, Durfee J, et al. Immune cell BDNF secretion is associated with white matter volume in multiple sclerosis. J Neuroimmunol. 2007;188:167–174. doi: 10.1016/j.jneuroim.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 47.McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Archives of General Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- 48.Ellman LM, Huttunen M, Lonnqvist J, Cannon TD. The effects of genetic liability for schizophrenia and maternal smoking during pregnancy on obstetric complications. Schizophr Res. 2007 doi: 10.1016/j.schres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Stroud LR, Solomon C, Shenassa E, Papandonatos G, Niaura R, Lipsitt LP, et al. Long-term stability of maternal prenatal steroid hormones from the National Collaborative Perinatal Project: still valid after all these years. Psychoneuroendocrinology. 2007;32:140–150. doi: 10.1016/j.psyneuen.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]