Abstract
Alzheimer's disease (AD) is a progressive neurodegenerative disease marked by a constellation of cognitive disturbances, the earliest and most prominent being impaired episodic memory. Episodic memory refers to the memory system that allows an individual to consciously retrieve a previously experienced item or episode of life. Many recent studies have focused on characterizing how AD pathology impacts particular aspects of episodic memory and underlying mental and neural processes. This review summarizes the findings of those studies and discusses the effects of current and promising treatments for AD on episodic memory. The goal of this review is to raise awareness of the strides that cognitive neuroscientists have made in understanding intact and dysfunctional memory. Knowledge of the specific memorial processes that are impaired in AD may be of great value to basic scientists developing novel therapies and to clinical researchers assessing the efficacy of those therapies.
Keywords: Alzheimer's disease, memory, memory disorder
Alzheimer's disease (AD) is a progressive neurodegenerative disease that accounts for more than two-thirds of all cases of dementia [1]. The most important risk factor for AD is age, followed by an APOE4 genotype. A 2007 report released by the Alzheimer's Association estimated that more than 5 million Americans are currently diagnosed with AD, while a Delphi consensus study projected that the global prevalence of AD will quadruple by the year 2040 to over 80 million cases in total [2,3]. Just as this disease is often devastating at the individual and family levels, the high prevalence of AD means that it is also economically and societally burdensome. Indeed, AD represented the third most costly health condition in the USA in 2000, and is of growing financial relevance for health policy planning in other industrialized and developing nations [4-6]. Perhaps due to mounting evidence regarding the gravity of the situation, there has been a crescendo of research interest in AD over the past decade, with 50% more papers published on the topic in the year 2007 than 1997 (Pubmed keyword search, MeSH term: Alzheimer disease). Throughout this period, one major area of research in AD has focused on the cognitive impairments exhibited by patients.
Clinicians and researchers have identified six cognitive domains that are commonly disturbed in patients suffering from AD: memory, executive functioning, language, visuospatial functioning, attention and affect. Of these disturbances, memory impairment is the central problem. Memory problems are among the most frequent reasons for admission to residential nursing facilities [7]. A recent prospective, longitudinal study reported that delaying the onset of nursing home care for elderly adults with dementing illnesses by 1 month would result in annual savings of US$4 billion for the USA [8]. Moreover, it has been suggested that community-dwelling individuals diagnosed with AD score higher on scales of quality of life than institutionalized patients at every stage of the disease [9]. It has also been suggested that caregivers are more likely to avoid depression and to receive support from family and friends when patients exhibit fewer functional limitations [10]. While preventive, disease-modifying and curative therapies for AD must be aggressively pursued as the over-riding goals of pharmaceutical research, in the interim drugs that effectively treat the memory impairments associated with AD may benefit patients, their families and society at large.
Treatments for AD
Reversible acetylcholinesterase inhibitors represent the first and most widely prescribed class of pharmaceuticals approved by the US FDA for treatment of the cognitive disturbances caused by AD. Drugs in this class include donepezil, galantamine, rivastigmine and tacrine. Acetylcholinesterase inhibitors are thought to improve global cognitive functioning by increasing the neurotransmitter concentration at cholinergic synapses at many sites throughout the brain [11]. The only other FDA-approved treatment for the cognitive manifestations of AD is memantine, a noncompetitive, low-affinity NMDA receptor antagonist. It has been widely reported that memantine works by preventing excitotoxicity (the death of neurons resulting from over stimulation by glutamate), although this putative mechanism is unproven in humans. Memantine may also improve cognitive function by modulating NMDA receptors to sharpen the neural signal and decrease background noise. However, studies have suggested that the clinical effects of memantine may be due, at least in part, to its role as a dopaminergic agonist [12]. Acetylcholinesterase inhibitors and memantine are frequently prescribed in tandem, although memantine is only approved for the treatment of moderate and severe AD (Table 1) [13].
Table 1.
Current therapies for Alzheimer's disease.
| Drug(s) | Proposed mechanism(s) | Status |
|---|---|---|
| Donepezil; galantamine; rivastigmine; tacrine |
Increasing the neurotransmitter concentration at cholinergic synapses at many sites throughout the brain |
US FDA-approved for the symptomatic treatment of mild and moderate AD; donepezil is also approved for use in severe AD |
| Memantine | Preventing glutamate excitotoxicity and/or stimulating dopamine receptors |
FDA approved for the symptomatic treatment of moderate and severe AD |
AD: Alzheimer's disease.
In addition to these two established therapies, dozens of possible drugs for AD are in various stages of development [14]. Many of the possible disease-modifying therapies are based on the amyloid hypothesis, which posits that the cognitive disturbances of AD result directly or indirectly from β-amyloid (Aβ), either soluble as dimers and oligomers, or deposited in amyloid plaques in particular regions of the CNS [15]. It should be noted that synaptic loss and cognitive dysfunction have been most closely associated with tau pathology in AD, and much less well correlated with amyloid pathology. Tau pathology and amyloid pathology are both thought to disrupt neuroplasticity, the process of forming and removing synapses that is believed to underlie memory. Recently, both tramiprosate, designed to bind to and maintain Aβ peptides in their nontoxic soluble form, and tarenflurbil, designed to selectively lower the concentration of Aβ42 by modulating γ-secretase activity, have failed high-profile Phase III clinical trials [16,17]. With the failure of these drugs, one of the most prominent possible therapies for AD is administration of monoclonal antibodies or antibody fragments to Aβ peptides [18].
Memory systems
Understanding the specific memory deficits that AD patients experience is essential to designing and assessing the efficacy of novel pharmaceuticals for the treatment of memory impairments. Although once thought to be a simple concept, memory is now considered to be a collection of mental abilities that use different systems and components within the brain. Memory research that began with neuropsychological studies of patients with focal brain lesions and today includes techniques such as PET, functional MRI (fMRI) and event-related potentials (ERPs) has provided the rationale for a more refined and improved classification scheme [19]. Six major memory systems have been characterized (Figure 1):
Episodic memory is the memory system employed when consciously remembering a particular episode of one's life, such as sharing a meal with a friend;
Semantic memory represents the store of conceptual and factual knowledge that is not related to any specific memory, such as the color of broccoli or for what purpose a fork is used;
Simple classical conditioning involves the pairing of two stimuli, an unconditioned stimulus and a conditioned stimulus. When paired together repeatedly, the response associated with the conditioned stimulus can be elicited by the unconditioned stimulus alone;
Procedural memory is the ability to learn cognitive and behavioral skills that operate at an automatic and unconscious level, such as learning to ride a bicycle or to play the piano;
Bringing together the traditional fields of attention, concentration and short-term memory, working memory refers to the ability to temporarily maintain and manipulate information that one needs to keep in mind;
Priming occurs when a prior encounter with a particular item changes the response to the current item.
Figure 1.


Major memory systems in everyday life.
In studies of AD patients, cognitive neuroscientists have found some of the six major memory systems to be severely impaired and others to be relatively preserved [20]. Studies have consistently shown semantic memory to be disrupted in AD, with patients exhibiting particular deficits in naming categorized items [21]. The disruption of semantic memory in AD patients has often been attributed to pathology in the anterior and inferolateral temporal lobes, and the frontal lobes, which presumably causes a loss of neuronal dendritic trees in these cortical regions [22,23]. Studies indicate that several forms of classical conditioning may be impaired in AD patients, including amygdala-dependent fear conditioning and eyeblink conditioning that may be supported by inputs from the entorhinal cortex to the hippocampus [24,25]. Experiments testing procedural memory suggest that while this memory system is diminished compared with healthy age-matched controls, it may be relatively intact compared with other forms of memory in AD patients [26,27]. Findings from working memory paradigms indicate that the ability to keep information in mind is very vulnerable to manipulations that divide or interrupt attention in AD patients [28]. Deficits in working memory may reflect damage to the frontal lobes in AD patients [29,30].
Studies of priming have reported mixed findings in AD patients. In repetition priming experiments the participant studies a word or picture and is later presented with the identical stimulus. If priming is intact, then the stimulus should be processed more quickly when viewed for the second time than the first time. Several researchers have reported preserved repetition priming in AD patients [31-33]. Repetition priming is thought to be supported by the frontal lobes [34]. In its simplest form, semantic priming involves presentation of an item, such as the word ‘dog’, and subsequent presentation of a semantically related item, such as the word ‘cat’. The experience of processing the original stimulus (dog) is expected to lead to faster processing of the semantically related stimulus (cat). In AD, some studies have reported semantic priming to be normal [35], while others have found diminished semantic priming [36], and still others have found greater-than-normal semantic priming [37].
Clinical importance of episodic memory in AD
Of the six major memory systems, episodic memory is the most clinically relevant for AD patients. Disruptions to the episodic memory system are among the earliest signs and symptoms of AD [38]. Early in the disease, such disruptions may result in misplaced keys, missed appointments and late bills. Individuals and their families may attribute these occasional and seemingly innocuous incidents of forgetfulness to fatigue, distraction or ‘senior moments’. However, the episodic memory system is also essential for remembering more critical events, such as whether or not the stove has been turned off and if medications have been taken. Clinical observations suggest that potentially dangerous incidents of forgetfulness often precipitate the initial visit to a behavioral neurologist, geriatric- or neuropsychiatrist, geriatrician, neuropsychologist or other health professional with expertise in memory disorders. Making a confident clinical diagnosis of probable AD is a complex process that is based on patient history, a report from a family member or friend of the patient, physical examination, and laboratory and neuroimaging studies [39]. Standardized neuropsychological tests may be used to confirm the diagnosis of dementia and to assess the severity of the patient's cognitive impairments [40]. Brief neuropsychological tests used to directly assess episodic memory in the clinical setting include the Montreal cognitive assessment (MoCA [201]), the blessed information–memory–concentration test [41], the drilled word span test [42], the Mini-Cog [43], the mini-mental state examination [44], the 7-min screen [45], the three words-three shapes memory test [42] and the word list memory test of the Consortium to Establish a Registry for Alzheimer's Disease [46].
From a clinical perspective, episodic memory deficits continue to represent one of the most significant functional problems as a patient progresses through the mild and moderate stages of AD. Disruptions to the episodic memory system usually follow Ribot's law, which states that events and items experienced just prior to an ictus are more vulnerable to decay than remote memories [47]. Thus, as episodic memory abilities decline in AD patients, events from the distant past are relatively better remembered than events that occurred after or shortly before the onset of the disease [48]. Of benefit, this means that watching movies or discussing life events from youth or early adulthood may orient and soothe a patient with AD. However, vivid remote memories may sometimes be confused with psychotic delusions or hallucinations. For example, caregivers may become concerned when a patient claims to have recently seen and interacted with a long-dead friend or family member. Inevitably, the inability to remember recent events or learn new information leads to functional deficits that are devastating for the patient and the caregiver.
Episodic memory from a cognitive neuroscience perspective
Owing to its clinical importance, the episodic memory system is among the most thoroughly researched topics in cognitive neuroscience. From early lesion studies and more recent work in neuroimaging, it is apparent that the episodic memory system is supported by the medial temporal lobes, especially the hippocampus (Figure 2) [49,50]. Other structures that appear to be involved in the episodic memory system in humans include the anterior and dorsomedial nuclei of the thalamus [51], the fornix [52], the mammillary bodies [53], the mammillothalamic tract [54] and the retrosplenial cortex [55]. Findings from animal studies suggest that several more structures may play roles in episodic memory, including the diagonal band of Broca's area [56] and the presubiculum [57].
Figure 2.
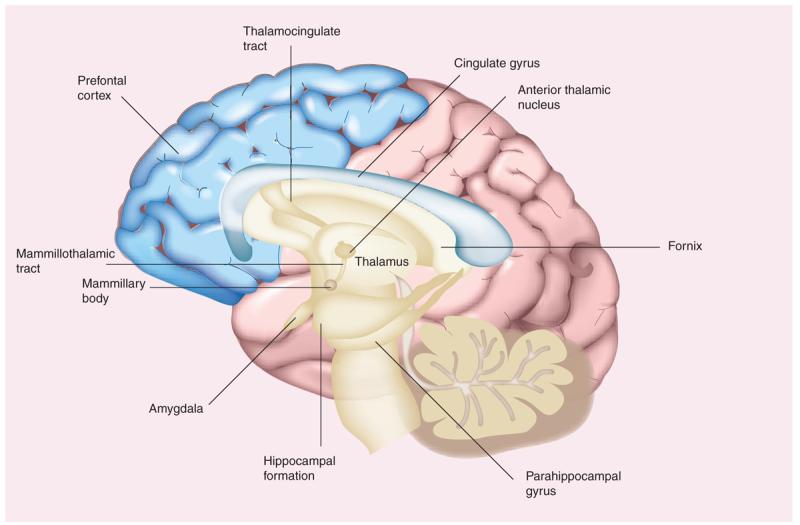
Selected brain regions involved in episodic memory.
Researchers have found evidence of changes in many of these anatomical areas in AD patients. The hippocampus has long been viewed as one of the sites most severely damaged in AD patients. Studies have demonstrated hippocampal atrophy, and alterations in hippocampal shape and surface structure in AD patients compared with nondemented older adults [58-60]. Neurons in the vertical limb of the diagonal band of Broca's area, a major source of innervation to the hippocampus, are paradoxically more metabolically active in AD patients than in age-matched controls [61].
MRI findings have suggested that the fornix and the mammillary bodies are atrophied in AD patients compared with healthy controls and with patients with mild cognitive impairment (MCI).[62], a condition thought to represent a transition state between healthy aging and AD [63]. Neuropathological studies have shown that regions including the entorhinal transition area and the presubiculum contain exceptionally high levels of amyloid plaques in AD patients [64].
Cognitive neuroscientists have not yet completely elucidated the mechanisms by which the medial temporal lobes store and retrieve memories of items and events. However, certain aspects of the neural processes underlying episodic memory are generally accepted [50]. Sensory information, emotions and thoughts present at the time of an event are processed in various regions of the cerebral cortex. The entorhinal cortex, located in the anterior portion of the parahippocampal gyrus, is in reciprocal communication with these cortical areas and serves to transmit information about organization relevance to other regions. From the entorhinal cortex, the signals are sent to the dentate gyrus and then to the CA3 region of the hippocampus via the mossy fiber pathway. Neurobiological and computational modeling studies support the theory that the CA3 region is essential for creating the so-called hippocampal index, the distinct record of an item or event that facilitates subsequent retrieval [65-67]. Studies have suggested that the entorhinal cortex [68], the dentate gyrus [69] and the CA3 region of the hippocampus [70] are all altered to some degree in AD patients.
Memory retrieval is often initiated by experiencing an environmental or internal cue that shares specific features with a stored memory. The cue, whether sensory, emotional or based in thought, travels from various cortical locations to the entorhinal cortex and then to the CA3 region of the hippocampus. By some mechanism, the cue activates the hippocampal index associated with the index of the original stored memory – not the memory itself – in the CA3 region. This activation leads to the reinstatement of much of the neural pattern of activity associated with the original event in the CA1 region of the hippocampus, the subiculum and various cortical regions [66,67]. This reinstatement of cortical activity leads to the experience of ‘remembering’ the sights, sounds, smells, tastes, emotions and thoughts that were present at the time of the original episode. It must be noted that reactivation of the hippocampal index may only be necessary for neural reinstatement of episodic memories that have not yet been consolidated [71]. Consolidation refers to the process by which the distributed pattern of cortical neural activity associated with a particular memory is directly linked together. It is thought that once consolidation has occurred, encountering an environmental or internal cue may lead to retrieval of the memory directly from cortical–cortical connections without the need for the hippocampus [72]. Studies indicate that sleep may be critical for consolidation [73]. Sleep spindles, a defining feature of stage two sleep, are thought to be particularly important [74] and may be decreased in number in patients with AD compared with controls [75]. That AD patients retain the ability to recall remote memories, but are unable to learn new information or recall relatively recent events is consistent with impairments in medial temporal lobe function, consolidation or both.
Cognitive neuroscientists have explored several types of episodic memory supported by the medial temporal lobes. Recall and recognition represent the two quintessential tests of episodic memory. In a standard recall experiment, an individual studies a series of items (e.g., the words fence, kangaroo and truck) and is subsequently asked to recall the items without their re-presentation, and often without cues of any kind. In a typical recognition memory experiment, an individual is presented with a series of items (e.g., fence, kangaroo and truck) during the ‘study phase’. In the ‘test phase’, the individual is presented with a randomly ordered series of items (e.g., truck, basketball, tree, fence, kangaroo and pencil), some studied and some unstudied. The individual is asked to declare each item to be ‘old’ (i.e., previously studied) or ‘new’ (i.e., novel). Both recall and recognition memory tests are used for assessing episodic memory in AD patients, and each has its strengths and weaknesses. Recall tests are quite sensitive for detecting memory deficits, but are not specific as recall is affected by many factors, including, for example, frontal lobe dysfunction. Although not very sensitive, performing poorly on a recognition memory test more specifically suggests a failure of the hippocampus or other medial temporal lobe structures that are affected in AD, provided that information has been successfully learned or encoded.
Box 1. Six reasons to consider cognitive neuroscience in the development of therapeutics for Alzheimer's disease.
Knowledge of the anatomical correlates of episodic memory may be critical to delivering anatomically targeted immunotherapies based on the amyloid hypothesis.
Scientists developing new drugs for symptomatic relief of the cognitive disturbances associated with Alzheimer's disease (AD) would benefit from a detailed understanding of the neural correlates and neurochemistry of episodic memory in AD patients.
Tests that measure particular aspects of episodic memory may be more useful tools in assessing the efficacy of new therapies for AD than current cognitive tests.
Outcome measures that directly measure brain physiology while individuals are performing a memory task might reduce the costs and human risks associated with clinical trials.
Measuring an AD patient's brain physiology during a memory task might also be a valuable tool for assessing the usefulness of approved therapies in that individual.
Tests of episodic memory taking advantage of the discoveries of cognitive neuroscience may prove useful for the early diagnosis of mild cognitive impairment and mild AD.
Researchers have suggested that two distinct neural processes, recollection and familiarity, support recognition memory [76]. Recollection refers to the retrieval of specific context-bound information about an item or event, while familiarity is defined as a more general, acontextual sense that an item or event has been previously encountered. These two constructs are sometimes consciously experienced in daily life. For example, the unexpected sight of a particular woman on a crowded city street may elicit an immediate feeling of knowing her without being able to produce any specific details about who she is or how she is known. After a moment of thought, these details may come into mind and the woman's identity – for example, the nice clerk at the record store you visited last Tuesday – becomes apparent. Familiarity describes the initial feeling of knowing the woman without being able to place her, while recollection captures the subsequent remembering of the specific details of her identity. Findings from a variety of recognition memory paradigms indicate that both processes are impaired in AD patients, with a greater decrement in recollection than familiarity reported by many researchers [77,78]. In theory, relying on one's sense of familiarity, and not on a firm recollection, would depress the hits (i.e., ‘old’ responses to studied items) and increase false alarms (i.e., ‘old’ responses to novel items). Indeed, this is exactly the pattern typically reported in recognition memory studies of AD patients [79]. Studies suggest that recollection requires an intact hippocampus, while familiarity appears to be supported by the perirhinal and lateral entorhinal cortex [80,81]. By carefully dissociating the processes underlying recognition memory, cognitive neuroscientists have gained insight into the patterns of medial temporal lobe dysfunction in AD patients.
In addition to the medial temporal lobes, the frontal lobes are also important for episodic memory. The frontal lobes play key roles in the acquisition and encoding of information, the retrieval of information in the absence of contextual cues, the recollection of the source of information, and the assessment of the temporal sequence and recency of events. Dysfunction of the frontal lobes can lead to distortions of episodic memory [82,83]. In mild cases, new information may become associated with the wrong context or incorrect specific details, a phenomenon that has been called both a provoked confabulation [84] and a false memory [79]. Studies have suggested that mild AD patients are more likely than healthy controls to exhibit false memories when tested on personal episodic memory [85-87]. More severe damage to the frontal lobes can lead to spontaneous confabulation [88]. Spontaneous confabulation refers to the formation of a ‘memory’ for an event that did not occur, but is merely consistent with current information. For example, an individual who spontaneously confabulates may not remember that she has rearranged the furniture in the living room. Upon seeing the furniture in locations that do not match her memory, she may create a new ‘memory’ that involves someone breaking into the house and rearranging the items. Such confabulations frequently occur in mild AD, even in the absence of frontal lobe pathology, simply because these patients cannot remember that their memory is impaired. They show inappropriately high confidence in their memory, and therefore assume that any discrepancy between their memory and the external world must be related to a problem in the world and not with their memory. Researchers have reported that some AD patients spontaneously confabulate as a result of their illness [89]. Spontaneous confabulation in AD may be associated with delusions and aggression [90], behaviors caused by frontal lobe dysfunction in AD patients [83,91,92].
Expert commentary
Deterioration of episodic memory is the central clinical feature of AD, a disease associated with tremendous personal suffering and financial costs. As the number of cases increases, research interest in therapies for AD is booming [14]. Many involved in the development of therapies for AD have focused their efforts on treating the neuropathology thought to be responsible for the progressive cognitive changes associated with the disease. It follows logically that diminishing the concentration of the Aβ plaques and neurofibrillary tangles commonly observed in the brains of AD patients at autopsy will lessen episodic memory impairments and other cognitive disturbances. Ultimately, the goal of treatment will be to prevent the formation of this pathology entirely. We offer six compelling, specific arguments for why it also may be useful for pharmaceutical scientists, directors of clinical trials involving AD patients and clinicians to be aware of the strides that cognitive neuroscientists have made in understanding episodic memory in healthy and memory-impaired individuals (Box 1).
First, knowledge of the anatomical correlates of episodic memory may be critical to delivering anatomically targeted immunotherapies based on the amyloid hypothesis. Therapies based on the amyloid hypothesis have been designed to cross the BBB and then be distributed throughout the CNS, both to sites where plaques are present and to regions where they are absent. There is evidence that some anti-amyloid immunotherapies have caused deleterious effects when distributed throughout the brain and spinal cord. For example, the Phase IIa trial of an Aβ vaccine known as AN-1792 was terminated when several of the 360 patients who were given the vaccine developed meningoencephalitis [93]. Although the exact cause of the meningoencephalitis was never determined, some have suggested that it was due to an immune response not just to Aβ, but also to vascular amyloid [94]. Remarkably, autopsy of one of the AN-1792 clinical trial patients who developed meningoencephalitis revealed clearing of Aβ throughout the brain [95]. As mentioned earlier, administration of monoclonal antibodies or antibody fragments against Aβ is considered to be among the most promising potential therapies for AD [96]. However, it has been suggested that in high concentration, passive immunotherapy may also lead to brain inflammation and hemorrhage [18]. If passive immunotherapy proves to be an effective method of clearing Aβ, but is also dangerous when distributed throughout the CNS, then it may be beneficial to develop techniques for delivering antibodies or antibody fragments directly to the neuroanatomical sites where plaque deposition is contributing to cognitive impairment. Awareness of the neural correlates of episodic memory would be essential to any such effort.
Second, scientists developing new drugs for symptomatic relief of the cognitive disturbances associated with AD would benefit from a detailed understanding of the neural correlates and neurochemistry of episodic memory in AD patients. With increasing numbers of highly touted potential therapies failing clinical trials, it is possible that drugs based on the amyloid hypothesis (and other current theories of the basis of AD) will not have a major effect on the clinical course of AD. If this proves to be the case, then there may be renewed interest in developing treatments for episodic memory deficits and other cognitive impairments. Such drugs might augment the cholinergic system in new ways or more specifically target the glutamatergic NMDA and dopaminergic receptors that may be responsible for the cognitive benefits of memantine. Novel therapies based on additional neurotransmitter systems may be developed as neuroscientists learn more about the molecular underpinnings of various aspects of intact and impaired episodic memory.
Third, tests that measure particular aspects of episodic memory may be more useful tools in assessing the efficacy of new therapies for AD than current cognitive tests. Tramiprosate, the anti-amyloid therapy designed to maintain Aβ peptides in a nontoxic form, failed in a large multicenter Phase III trial in 2007. A press release issued by the manufacturer of tramiprosate suggested that very high statistical variance among centers on the primary behavioral end points, the Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-cog) [97] and the Clinical Dementia Rating-Sum of Boxes (CDR-SB) [98], may have been partly responsible for the failure of the trial [202]. The ADAS-cog and CDR-SB were designed to assess a broad assortment of cognitive abilities in AD patients, including episodic memory, through questions about activities of daily living and some cognitive testing. These scales are widely used in AD clinical trials. However, they are not designed to provide information about recollection and familiarity, false memories or other issues in episodic memory. Indeed, some cognitive neuroscientists have already proposed new versions of the recognition memory section of ADAS-cog that would take false memory into account [99]. It is possible that particular tests of episodic memory drawn from the cognitive neuroscience literature might have illuminated differences in the treatment group versus the placebo group in the tramiprosate trial, as well as other recently failed trials. The feasibility of using an episodic memory paradigm as an outcome measure in a clinical trial has already been demonstrated [100]. In that clinical trial, researchers testing the efficacy of a substance known as AIT-082 assessed the performance of AD patients on a false-memory paradigm before and after treatment [100]. As more is learned about the details of episodic memory (e.g., the neural correlates of recollection vs familiarity), more specific tests and elements of larger scales such as the ADAS-cog can be devised, validated and implemented in clinical trials.
Fourth, outcome measures that directly measure brain physiology while individuals are performing a memory task might reduce the costs and human risks associated with clinical trials. Owing to the tremendous statistical variance associated with current outcome measures such as the ADAS-cog, AD clinical trials typically require enrollment of several hundred to more than 1000 patients to achieve statistical significance. Implementing a more specific test of episodic memory as an outcome measure, as discussed previously, might lead to somewhat lower variance. However, variance would likely be decreased to a much greater extent by using a specific test of episodic memory that is time-locked with a physiologic measure sensitive to improvements in memory, such as ERPs or fMRI, as an outcome measure. Researchers could design smaller, less costly clinical trials that would expose fewer AD patients to experimental medications if they had reasonable expectations of low statistical variance.
Fifth, measuring an AD patient's brain physiology during a memory task might also be a valuable tool for assessing the usefulness of approved therapies in that individual. It is has been suggested that the cognitive response of individual AD patients to acetylcholinesterase inhibitor therapy varies from dramatic to minimal [101]. However, response to therapy cannot always be determined clinically. Studies have reported that AD patients who were thought not to have responded to acetylcholinesterase inhibitor therapy displayed a decline in function when the medication was discontinued [102]. Since the financial costs of acetylcholinesterase inhibitor therapy are not insignificant, a cost effective physiologic method for determining the response to therapy in individual patients would be helpful.
Event-related potentials may be able to serve as such a method to measure the brain physiology during the performance of a memory task for either an approved therapy as part of standard clinical care, or an experimental therapy as part of a clinical trial. Many ERP studies indicate that on a recognition memory test, studied words elicit a larger late positive component over parietal scalp areas than do unstudied words [103]. This parietal ‘old/new effect’ is of greater amplitude when test items are consciously remembered [104], when there are numerous study–test repetitions [105] and when correctly recognized items are subsequently recalled [106]. These findings have led researchers to associate the parietal old/new effect with the process of recollection. Previous research from our laboratory has suggested that the parietal effect amplitude is decreased in mild AD patients compared with controls, consistent with behavioral findings suggesting that recollection is markedly impaired in AD patients [79,107]. Thus, measuring parietal effect amplitude prior to initiating treatment and then again after some period of treatment might provide a physiologic measurement of episodic memory improvement. Future studies might assess the physiological effects of donezepil and other acetylcholinesterase inhibitors, memantine and combined acetylcholinesterase inhibitor, and memantine therapy in AD patients. Eventually, such testing might become routine in the clinical setting for devising individualized therapeutic protocols.
Sixth, tests of episodic memory taking advantage of the discoveries of cognitive neuroscience may prove useful for the early diagnosis of MCI and mild AD. At present, widespread screening of elderly adults for memory impairment is not typically recommended, as the social and financial costs are thought to outweigh the benefits of early diagnosis [108-110]. If more effective therapies for AD become available, then it may be advisable for most or all community-dwelling elderly adults to be screened. Potential approaches to episodic memory-based screening include very brief neuropsychological tests administered by telephone [111] or in the primary care setting [43] and lengthier web-based assessments [112]. Testing by phone or in the primary care setting has the advantage of brevity (e.g., <3 min are required to administer the Mini-cog developed by Borson and colleagues) and easy access, but perhaps at the cost of specificity. Web-based cognitive tests require internet access and out-of-pocket payment. Moreover, such testing requires the elderly individual or a family member to actively pursue screening. Despite these drawbacks, web-based testing methods may eventually represent the most efficacious screening strategy, as they allow for longer tests drawn directly from the cognitive neuroscience literature.
Five-year view
Cognitive neuroscientists will continue to make refinements in their understanding of episodic memory in healthy individuals and in AD patients. We foresee particular advancements in delineating the neural correlates of episodic memory using electro physiological and neuroimaging techniques. Technological advances may allow multiple techniques (e.g., ERPs and fMRI) to be used simultaneously during memory tests, fostering insight into the temporal and spatial relationships of the neural regions involved. Future clinical trials may assess the efficacy of AD therapies using paradigms drawn from the cognitive neuroscience literature. Such trials may implement episodic memory tests as standalone outcome measures or time-locked with techniques that measure brain physiology. Finally, technologies that might aid memory-impaired patients with activities of daily living, such as paper organizers, personal digital assistants [113] and even neural prostheses [114], may be developed and offered to AD patients.
Key issues.
Alzheimer's disease (AD) is a progressive neurodegenerative illness that accounts for more than two-thirds of all cases of dementia and represents an increasingly important public health problem.
Memory impairment is the most common and debilitating cognitive impairment associated with mild and moderate AD.
Of the six major memory systems, the episodic memory system is the most clinically relevant for AD patients, as impairments in this system lead to poor memory for recent events, which results in functional deficits.
The pattern of episodic memory impairments displayed by AD patients is consistent with damage to areas of the medial temporal and frontal lobes.
Awareness of the neuroanatomical and physiological correlates of episodic memory may prove essential to pharmaceutical scientists developing targeted immunotherapies and other novel therapies for AD.
Episodic memory tests drawn from the cognitive neuroscience literature may be implemented as outcome measures in future AD clinical trials.
Physiologic techniques such as event-related potentials and functional MRI may allow for assessment of treatment-related memory improvements in individual AD patients.
Footnotes
Financial & competing interests disclosure
AE Budson receives honoraria for speaking on behalf of Eisai, Pfizer, Novartis and Forest Laboratories. This material is in part the result of work supported with resources and the use of facilities at the Edith Nourse Rogers Memorial Veterans Hospital in Bedford, MA, USA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Nussbaum RL, Ellis CE. Alzheimer's disease and Parkinson's disease. N. Engl. J. Med. 2003;348(14):1356–1364. doi: 10.1056/NEJM2003ra020003. [DOI] [PubMed] [Google Scholar]
- 2.Alzheimer's Association Alzheimer's disease facts and figures 2007. 2007 [Google Scholar]
- 3.Ferri C, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fillit H, Hill J. Economics of dementia and pharmacoeconomics of dementia therapy. Am. J. Geriatr. Pharmacother. 2005;3(1):39–49. doi: 10.1016/j.amjopharm.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Allegri RF, Butman J, Arizaga RL, et al. Economic impact of dementia in developing countries: an evaluation of costs of Alzheimer-type dementia in Argentina. Int. Psychogeriatr. 2007;19(4):705–718. doi: 10.1017/S1041610206003784. [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Bastida J, Serrano-Aguilar P, Perestelo-Perez L, Oliva-Moreno J. Social–economic costs and quality of life of Alzheimer disease in the Canary Islands, Spain. Neurology. 2006;67:2186–2191. doi: 10.1212/01.wnl.0000249311.80411.93. [DOI] [PubMed] [Google Scholar]
- 7.Andel R, Hyer K, Slack A. Risk factors for nursing home placement in older adults with and without dementia. J. Aging Health. 2007;19:213–228. doi: 10.1177/0898264307299359. [DOI] [PubMed] [Google Scholar]
- 8.Clipp E. US Department of Veterans Affairs HSR&D Study NRI 95-218, Informal Caregivers of Veterans with Dementia: Cost, QOL, and Service Use. 2005 [Google Scholar]
- 9.Leon J, Neumann PJ, Hermann RC, et al. Health-related quality-of-life and service utilization in Alzheimer's disease: a cross-sectional study. Am. J. Alzheimers Dis. Other Demen. 2000;15(2):94–108. [Google Scholar]
- 10.Clyburn LD, Stones MJ, Hadjistavropoulos T, Tuokko H. Predicting caregiver burden and depression in Alzheimer's disease. J. Gerontol. 2000;55(1):S2–S13. doi: 10.1093/geronb/55.1.s2. [DOI] [PubMed] [Google Scholar]
- 11.Stahl SM. The new cholinesterase inhibitors for Alzheimer's disease, part 2: illustrating their mechanisms of action. J. Clin. Psychiatry. 2000;61(11):813–814. doi: 10.4088/jcp.v61n1101. [DOI] [PubMed] [Google Scholar]
- 12.Seeman P, Caruso C, Lasaga M. Memantine agonist action at dopamine D2High receptors. Synapse. 2008;62(2):149–153. doi: 10.1002/syn.20472. [DOI] [PubMed] [Google Scholar]
- 13.Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann. Intern. Med. 2008;148(5):379–397. doi: 10.7326/0003-4819-148-5-200803040-00009. [DOI] [PubMed] [Google Scholar]
- 14.Van Marum RJ. Current and future therapy in Alzheimer's disease. Fundamen. Clin. Pharmacol. 2008;22(3):265–274. doi: 10.1111/j.1472-8206.2008.00578.x. [DOI] [PubMed] [Google Scholar]
- 15.Masters CL, Beyreuther K. Alzheimer's centennial legacy: prospects for rational therapeutic intervention targeting the Aβ amyloid pathway. Brain. 2006;129(11):1763–1769. doi: 10.1093/brain/awl251. [DOI] [PubMed] [Google Scholar]
- 16.Aisen PS, Gauthier S, Vellas B, et al. Alzhemed: a potential treatment for Alzheimer's disease. Curr. Alzheimer Res. 2007;4(4):473–478. doi: 10.2174/156720507781788882. [DOI] [PubMed] [Google Scholar]
- 17.Wilcock G, Black S, Hendrix S, Zavitz K, Swabb E, Laughlin M. Efficacy and safety of tarenflurbil in mild to moderate Alzheimer's disease: a randomised Phase II trial. Lancet Neurol. 2008;7(6):483–493. doi: 10.1016/S1474-4422(08)70090-5. [DOI] [PubMed] [Google Scholar]
- 18.Lichtlen P, Mohajeri MH. Antibody-based approaches in Alzheimer's research: safety, pharmacokinetics, metabolism and analytical tools. J. Neurochem. 2008;104(4):859–874. doi: 10.1111/j.1471-4159.2007.05064.x. [DOI] [PubMed] [Google Scholar]
- 19.Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiol. Learn. Mem. 2004;82:171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 20•.Budson AE, Price BH. Memory dysfunction. N. Engl. J. Med. 2005;352:692–699. doi: 10.1056/NEJMra041071. Discusses how different memory systems become dysfunctional in different disease states. [DOI] [PubMed] [Google Scholar]
- 21.Tippett L, Meier S, Blackwood K, Diaz-Asper C. Category specific deficits in Alzheimer's disease: fact or artefact? Cortex. 2007;43(7):907–920. doi: 10.1016/s0010-9452(08)70690-7. [DOI] [PubMed] [Google Scholar]
- 22.Davies RR, Graham KS, Xuereb JH, Williams GB, Hodges JR. The human perirhinal cortex and semantic memory. Eur. J. Neurosci. 2004;20(9):2441–2446. doi: 10.1111/j.1460-9568.2004.03710.x. [DOI] [PubMed] [Google Scholar]
- 23.Starr JM, Loeffler B, Abousleiman Y, et al. Episodic and semantic memory tasks activate different brain regions in Alzheimer disease. Neurology. 2005;65:266–269. doi: 10.1212/01.wnl.0000168907.44632.55. [DOI] [PubMed] [Google Scholar]
- 24.Hamann S, Monarch ES, Goldstein FC. Impaired fear conditioning in Alzheimer's disease. Neuropsychologia. 2002;40(8):1187–1195. doi: 10.1016/s0028-3932(01)00223-8. [DOI] [PubMed] [Google Scholar]
- 25.Woodruff-Pak DS. Eyeblink classical conditioning differentiates normal aging from Alzheimer's disease. Integr. Physiol. Behav. Sci. 2001;36:87–108. doi: 10.1007/BF02734044. [DOI] [PubMed] [Google Scholar]
- 26.Libon DJ, Bogdanoff B, Cloud BS, et al. Declarative and procedural learning, quantitative measures of the hippocampus, and subcortical white alterations in Alzheimer's disease and ischaemic vascular dementia. J. Clin. Exp. Neuropsychol. 1998;20(1):30–41. doi: 10.1076/jcen.20.1.30.1490. [DOI] [PubMed] [Google Scholar]
- 27.Poe MK, Seifort LS. Implicit and explicit tests: evidence for dissociable motor skills in probable Alzheimer's dementia. Percept. Mot. Skills. 1997;85(2):631–634. doi: 10.1177/003151259708500201. [DOI] [PubMed] [Google Scholar]
- 28.Belleville S, Chertkow H, Gauthier S. Working memory and control of attention in persons with Alzheimer's disease and mild cognitive impairment. Neuropsychology. 2007;21(4):458–469. doi: 10.1037/0894-4105.21.4.458. [DOI] [PubMed] [Google Scholar]
- 29.Kalpouzos G, Eustache F, de la Sayette V, Viader F, Chetalat G, Desgranges B. Working memory and FDG-PET dissociate early and late onset Alzheimer disease patients. J. Neurol. 2005;252(5):548–558. doi: 10.1007/s00415-005-0685-3. [DOI] [PubMed] [Google Scholar]
- 30.Yetkin FZ, Rosenberg RN, Weiner MF, Purdy PD, Cullum CM. fMRI of working memory in patients with mild cognitive impairment and probable Alzheimer's disease. Eur. Radio. 2006;16(1):193–206. doi: 10.1007/s00330-005-2794-x. [DOI] [PubMed] [Google Scholar]
- 31.Fleischman DA, Wilson RS, Gabrieli JDE, Schneider JA, Bienias JL, Bennett DA. Implicit memory and Alzheimer's disease neuropathology. Brain. 2005;128:2006–2015. doi: 10.1093/brain/awh559. [DOI] [PubMed] [Google Scholar]
- 32.Golby A, Silverberg G, Race E, et al. Memory encoding in Alzheimer's disease: an fMRI study of explicit and implicit memory. Brain. 2005;128(4):773–787. doi: 10.1093/brain/awh400. [DOI] [PubMed] [Google Scholar]
- 33.Lustig C, Buckner R. Preserved neural correlates of priming in old age and dementia. Neuron. 2004;42(5):865–875. doi: 10.1016/j.neuron.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Wig GS, Grafton ST, Demos KE, Kelley WM. Reductions in neural activity underlie behavioral components of repetition priming. Nat. Neurosci. 2005;8:1228–1233. doi: 10.1038/nn1515. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura H, Nakanishi M, Hamanaka T, Nakaaki S, Yoshida S. Semantic priming in patients with Alzheimer and semantic dementia. Cortex. 2000;36(2):151–162. doi: 10.1016/s0010-9452(08)70521-5. [DOI] [PubMed] [Google Scholar]
- 36.Hernandez M, Costa A, Juncadella M, Sebastian-Galles N, Rene R. Category-specific semantic deficits in Alzheimer's disease: a semantic priming study. Neuropsychologia. 2008;46(4):935–946. doi: 10.1016/j.neuropsychologia.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 37.Chertkow H, Bub D, Bergman H, Bruemmer A, Merling A, Rothfleisch J. Increased semantic priming in patients with dementia of the Alzheimer's type. J. Clin. Exp. Neuropsychol. 1994;16(4):608–622. doi: 10.1080/01688639408402672. [DOI] [PubMed] [Google Scholar]
- 38.Hodges JR. Memory in the dementias. In: Tulving E, Craik FIM, editors. The Oxford Handbook of Memory. Oxford University Press; NY, USA: 2000. [Google Scholar]
- 39.Geldmacher DS, Whitehouse PJ. Differential diagnosis of Alzheimer's disease. Neurology. 1997;48:2S–9S. doi: 10.1212/wnl.48.5_suppl_6.2s. [DOI] [PubMed] [Google Scholar]
- 40.McKhann G, Drachman D, Folstein M, Katzman R, Price D. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 41.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br. J. Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 42.Mesulam MM. Principles of Behavioral and Cognitive Neurology. 2nd Edition Oxford University Press; NY, USA: 2000. [Google Scholar]
- 43.Borson S, Scanlan JM, Watanabe J, Tu SP, Lessig M. Simplifying detection of cognitive impairment: comparison of the Mini-Cog and the Mini-Mental State Examination in a multiethnic sample. J. Am. Geriatr. Soc. 2005;53(5):871–874. doi: 10.1111/j.1532-5415.2005.53269.x. [DOI] [PubMed] [Google Scholar]
- 44.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 45.Solomon PR, Hirschoff A, Kelly B, et al. A 7 minute neurocognitive screening battery highly sensitive to Alzheimer's disease. Arch. Neurol. 1998;55:349–355. doi: 10.1001/archneur.55.3.349. [DOI] [PubMed] [Google Scholar]
- 46.Welsh KA, Butters N, Hughes JP, et al. Detection and staging of dementia in Alzheimer's disease. Use of the neuropsychological measures developed for the Consortium to Establish a Registry for Alzheimer's Disease. Arch. Neurol. 1992;49:448–452. doi: 10.1001/archneur.1992.00530290030008. [DOI] [PubMed] [Google Scholar]
- 47.Ribot T. Les Maladies de la Mémoire. Librairie Germer Baillière; Paris, France: 1882. [Google Scholar]
- 48.Sagar HJ, Cohen NJ, Sullivan EV, Corkin S, Growdon JH. Remote memory function in Alzheimer's disease and Parkinson's disease. Brain. 1988;111:185–206. doi: 10.1093/brain/111.1.185. [DOI] [PubMed] [Google Scholar]
- 49.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampus lesions. J. Neurol. Neurosurg. Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Squire LR, Clark RE, Bayley PJ. Medial temporal lobe function and memory. In: Gazzaniga M, editor. The Cognitive Neurosciences. 3rd Edition MIT Press; Cambridge, MA, USA: 2004. [Google Scholar]
- 51.Stenset V, Grambaite R, Reinvang I, et al. Diaschisis after thalamic stroke: a comparison of metabolic and structural changes in a patient with amnesic syndrome. Acta Neurologica Scandinavica. 2007;187:68–71. doi: 10.1111/j.1600-0404.2007.00851.x. [DOI] [PubMed] [Google Scholar]
- 52.Hamani C, McAndrews MP, Cohn M, et al. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann. Neurol. 2008;63(1):119–123. doi: 10.1002/ana.21295. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka Y, Miyazawa Y, Akaoka F, Yamada T. Amnesia following damage to the mammillary bodies. Neurology. 1997;48(1):160–165. doi: 10.1212/wnl.48.1.160. [DOI] [PubMed] [Google Scholar]
- 54.Yoneoka Y, Takeda N, Inoue A, et al. Acute Korsakoff syndrome following mammillothalamic tract infarction. Am. J. Neuroradiol. 2004;25(6):964–968. [PMC free article] [PubMed] [Google Scholar]
- 55.Epstein RA, Higgins JS. Differential parahippocampal and retrosplenial involvement in three types of visual scene recognition. Cereb. Cortex. 2007;17(7):1680–1693. doi: 10.1093/cercor/bhl079. [DOI] [PubMed] [Google Scholar]
- 56.Pang KC, Nocera R, Secor AJ, Yoder RM. GABAergic septohippocampal neurons are not necessary for spatial memory. Hippocampus. 2001;11(6):814–827. doi: 10.1002/hipo.1097. [DOI] [PubMed] [Google Scholar]
- 57.Malkova L, Mishkin M. One-trial memory for object–place associations after separate lesions of hippocampus and posterior parahippocampal region in the monkey. J. Neurosci. 2003;23(5):1956–1965. doi: 10.1523/JNEUROSCI.23-05-01956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barnes J, Godbolt AK, Frost C, et al. Atrophy rates of the cingulate gyrus and hippocampus in AD and FTLD. Neurobiol. Aging. 2007;28(1):20–28. doi: 10.1016/j.neurobiolaging.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 59.Scher AI, Xu Y, Korf ES, et al. Hippocampal shape analysis in Alzheimer's disease: a population-based study. Neuroimage. 2007;36(1):8–18. doi: 10.1016/j.neuroimage.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 60.Wang L, Miller JP, Gado MH, et al. Abnormalities of hippocampal surface structure in very mild dementia of the Alzheimer type. Neuroimage. 2006;30(1):52–60. doi: 10.1016/j.neuroimage.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ishunina TA, Swaab DF. Increased neuronal metabolic activity and estrogen receptors in the vertical limb of the diagonal band of Broca in Alzheimer's disease: relation to sex and aging. Exp. Neurol. 2003;183(1):159–172. doi: 10.1016/s0014-4886(03)00138-9. [DOI] [PubMed] [Google Scholar]
- 62.Petersen RC. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 63.Copenhaver BR, Rabin LA, Saykin AJ, et al. The fornix and mammillary bodies in older adults with Alzheimer's disease, mild cognitive impairment, and cognitive complaints: a volumetric MRI study. Psychiatry Res. 2006;147(2):93–103. doi: 10.1016/j.pscychresns.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 64.Wisniewski HM, Sadowski M, Jakubowska-Sadowska K, Tarnawski M, Wegiel J. Diffuse, lake-like amyloid-β deposits in the parvopyramidal layer of the presubiculum in Alzheimer disease. J. Neuropathol. Exp. Neurol. 1998;57(7):674–683. doi: 10.1097/00005072-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 65.Nakazawa K, Quirk MC, Chitwood RA, et al. Requirement for hippocampal CA3 NMDA repceptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shastri L. Episodic memory and cortico–hippocampal interactions. Trends Cogn. Sci. 2002;6(4):162–168. doi: 10.1016/s1364-6613(02)01868-5. [DOI] [PubMed] [Google Scholar]
- 67.Teylor TJ, Rudy JW. The hippocampal indexing theory and episodic memory: updating the index. Hippocampus. 2007;17(12):1158–1169. doi: 10.1002/hipo.20350. [DOI] [PubMed] [Google Scholar]
- 68.Di Paola M, Macaluso E, Carlesimo GA, et al. Episodic memory impairment in patients with Alzheimer's disease is correlated with entorhinal cortex atrophy. J. Neurol. 2007;254(6):774–781. doi: 10.1007/s00415-006-0435-1. [DOI] [PubMed] [Google Scholar]
- 69.Ohm TG. The dentate gyrus in Alzheimer's disease. Prog. Brain Res. 2007;163:723–740. doi: 10.1016/S0079-6123(07)63039-8. [DOI] [PubMed] [Google Scholar]
- 70.Adachi M, Kawakatsu S, Hosoya T, et al. Morphology of the inner structure of the hippocampal formation in Alzheimer disease. Am. J. Neuroradiol. 2003;24(8):1575–1581. [PMC free article] [PubMed] [Google Scholar]
- 71.McGaugh JL. Memory: a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 72.Wiltgen B, Brown R, Talton L, Silva A. New circuits for old memories: the roles of the neocortex in consolidation. Neuron. 2004;44(1):101–108. doi: 10.1016/j.neuron.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 73.Axmacher N, Haupt S, Fernandez G, Elger CE, Fell J. The role of sleep in declarative memory consolidation – direct evidence by intracranial EEG. Cereb. Cortex. 2008;18(3):500–507. doi: 10.1093/cercor/bhm084. [DOI] [PubMed] [Google Scholar]
- 74.Schabus M, Gruber G, Parapatics S, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27(8):1443–1445. doi: 10.1093/sleep/27.7.1479. [DOI] [PubMed] [Google Scholar]
- 75.Petit D, Gagnon J, Fantini M, FeriniStrambi L, Montplaisir J. Sleep and quantitative EEG in neurodegenerative disorders. J. Psychosom. Res. 2004;56(5):487–496. doi: 10.1016/j.jpsychores.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 76•.Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. J. Mem. Lang. 2002;46:441–517. Lengthy paper that represents the definitive review of recognition memory. [Google Scholar]
- 77.Smith JA, Knight RG. Memory processing in Alzheimer's disease. Neuropsychologia. 2002;46:666–682. doi: 10.1016/s0028-3932(01)00137-3. [DOI] [PubMed] [Google Scholar]
- 78.Gallo DA, Sullivan AL, Daffner KR, Schacter DL, Budson AE. Associative recognition in Alzheimer's disease: evidence for impaired recall-to-reject. Neuropsychology. 2004;18:556–563. doi: 10.1037/0894-4105.18.3.556. [DOI] [PubMed] [Google Scholar]
- 79.Budson AE, Daffner KR, Desikan R, Schacter DL. When false recognition is unopposed by true recognition: gist based memory distortion in Alzheimer's disease. Neuropsychology. 2000;14:277–287. doi: 10.1037//0894-4105.14.2.277. [DOI] [PubMed] [Google Scholar]
- 80.Brown CM, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat. Rev. Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- 81.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu. Rev. Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buckner RL, Schacter DL. Neural correlates of memory's successes and sins. In: Gazzaniga M, editor. The Cognitive Neurosciences. 3rd Edition MIT Press; Cambridge, MA, USA: 2004. [Google Scholar]
- 83.Budson AE, Sullivan AL, Mayer E, Daffner KR, Black PM, Schacter DL. pression of false recognition in Alzheimer's disease and in patients with frontal lobe lesions. Brain. 2002;125:2750–2765. doi: 10.1093/brain/awf277. [DOI] [PubMed] [Google Scholar]
- 84.Schnider A. Spontaneous confabulation, reality monitoring, and the limbic system. Brain Res. Rev. 2001;36:150–160. doi: 10.1016/s0165-0173(01)00090-x. [DOI] [PubMed] [Google Scholar]
- 85.Budson AE, Simons JS, Sullivan AL, et al. Memory and emotions for the September 11, 2001, terrorist attacks in patients with Alzheimer's disease, patients with mild cognitive impairment, and healthy older adults. Neuropsychology. 2004;18:315–327. doi: 10.1037/0894-4105.18.2.315. [DOI] [PubMed] [Google Scholar]
- 86.Budson AE, Simons JS, Waring JD, Sullivan AL, Hussion T, Schacter DL. Memory for the September 11, 2001, terrorist attacks one year later in patients with Alzheimer's disease, patients with mild cognitive impairment, and healthy older adults. Cortex. 2007;43:875–888. doi: 10.1016/s0010-9452(08)70687-7. [DOI] [PubMed] [Google Scholar]
- 87.Cooper JM, Shanks MF, Venneri A. Provoked confabulations in Alzheimer's disease. Neuropsychologia. 2006;44(10):1697–1707. doi: 10.1016/j.neuropsychologia.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 88.Gilboa A, Alain C, Stuss DT, Melo B, Miller S, Moscovitch M. Mechanisms of spontaneous confabulations: a strategic retrieval account. Brain. 2006;129(6):1399–1414. doi: 10.1093/brain/awl093. [DOI] [PubMed] [Google Scholar]
- 89.Lee E, Meguro K, Hashimoto R, et al. Confabulations in episodic memory are associated with delusions in Alzheimer's disease. J. Geriatr. Psychiatry Neurol. 2007;20(1):34–40. doi: 10.1177/0891988706292760. [DOI] [PubMed] [Google Scholar]
- 90.Lee E, Akanuma K, Meguro M, Ishii H, Yamaguchi S, Meguro K. Confabulations in remembering past and planning future are associated with psychiatric symptoms in Alzheimer's disease. Arch. Clin. Neuropsychol. 2007;22(8):949–956. doi: 10.1016/j.acn.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 91.Geroldi C, Bresciani L, Zanetti O, Frisoni GB. Regional brain atrophy in patients with mild Alzheimer's disease and delusions. Int. Psychogeriatr. 2002;14(4):365–378. doi: 10.1017/s1041610202008566. [DOI] [PubMed] [Google Scholar]
- 92.Senanarong V, Cummings JL, Fairbanks L, et al. Agitation in Alzheimer's disease is a manifestation of frontal lobe dysfunction. Dement. Geriatr. Cogn. Disord. 2004;17(1):14–20. doi: 10.1159/000074080. [DOI] [PubMed] [Google Scholar]
- 93.Check E. Nerve inflammation halts trial for Alzeimer's drug. Nature. 2002;415:462. doi: 10.1038/415462a. [DOI] [PubMed] [Google Scholar]
- 94.Greenberg SM, Bacskai BJ, Hyman BT. Alzheimer disease's double-edged vaccine. Nat. Med. 2003;9:389–390. doi: 10.1038/nm847. [DOI] [PubMed] [Google Scholar]
- 95.Nicoll JAR, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-β peptide: a case report. Nat. Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 96.Mohajeri MH. The underestimated potential of the immune system in prevention of Alzheimer's disease pathology. Bioessays. 2007;29(9):927–932. doi: 10.1002/bies.20630. [DOI] [PubMed] [Google Scholar]
- 97.Mohs RC, Rosen WG, Davis KL. The Alzheimer's disease assessment scale: an instrument for assessing treatment efficacy. Psychopharmacol. Bull. 1983;19(3):448–450. [PubMed] [Google Scholar]
- 98.Lynch CA, Walsh C, Blanco A, et al. The clinical dementia rating sum of box score in mild dementia. Dement. Geriatr. Cogn. Disord. 2006;21(1):40–43. doi: 10.1159/000089218. [DOI] [PubMed] [Google Scholar]
- 99.Kinjo H. Improving sensitivity of the recognition task in the Alzheimer's disease assessment scale. Psychol. Rep. 2007;100(2):420–426. doi: 10.2466/pr0.100.2.420-426. [DOI] [PubMed] [Google Scholar]
- 100•.Budson AE, Michalska KJ, Rentz DM, et al. Use of a false recognition paradigm in an Alzheimer's disease clinical trial: a pilot study. Am. J. Alzheimers Dis. Other Demen. 2002;17:93–100. doi: 10.1177/153331750201700204. Demonstrates the feasibility of using a false-recognition experiment as an outcome measure in an Alzheimer's disease clinical trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cummings JL. Alzheimer's disease. N. Engl. J. Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 102.Holmes C, Wilkinson D, Dean C, et al. The efficacy of donepezil in the treatment of neuropsychiatric symptoms in Alzheimer's disease. Neurology. 2004;63(2):214–219. doi: 10.1212/01.wnl.0000129990.32253.7b. [DOI] [PubMed] [Google Scholar]
- 103•.Friedman D, Johnson R. Event-related potential studies of memory encoding and retrieval: a selective review. Microsc. Res. Tech. 2000;51:6–28. doi: 10.1002/1097-0029(20001001)51:1<6::AID-JEMT2>3.0.CO;2-R. A review of the neurophysiological correlates of episodic memory, including the late positive component, a possible marker of treatment-related improvements in recollection. [DOI] [PubMed] [Google Scholar]
- 104.Ally BA, Simons JS, McKeever JD, Peers PV, Budson AE. Parietal contributions to recollection: electrophysiological evidence from aging and patients with parietal lesions. Neuropsychologia. 2008;46:1800–1812. doi: 10.1016/j.neuropsychologia.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Johnson R, Kreiter K, Russo B, Zhu J. A spatio–temporal analysis of recognition-related event-related brain potentials. Int. J. Psychophysiol. 1998;29:83–104. doi: 10.1016/s0167-8760(98)00006-3. [DOI] [PubMed] [Google Scholar]
- 106.Rugg MD, Schloerscheidt AM, Mark RE. An electrophysiological comparison of two indices of recollection. J. Mem. Lang. 1998;39:47–69. [Google Scholar]
- 107.Wolk DA, Signoff ED, DeKosky ST. Recollection and familiarity in amnestic mild cognitive impairment: a global decline in recognition memory. Neuropsychologia. 2008;46(7):1965–1978. doi: 10.1016/j.neuropsychologia.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ashford J, Borson S, O'Hara B, et al. Should older adults be screened for dementia? Alzheimers Dement. 2006;2(2):76–85. doi: 10.1016/j.jalz.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 109.Ashford JW. Screening for memory disorder, dementia, and Alzheimer's disease. Aging Health. 2008;4(4):399–432. [Google Scholar]
- 110.Ashford JW, Borson S, O'Hara R, et al. Should older adults be screened for dementia? It is important to screen for evidence of dementia. Alzheimers Dement. 2007;3:75–80. doi: 10.1016/j.jalz.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lipton RB, Katz MJ, Kuslansky G. Screening for dementia by telephone using the memory impairment screen. J. Am. Geriatr. Soc. 2008;51(10):1382–1390. doi: 10.1046/j.1532-5415.2003.51455.x. [DOI] [PubMed] [Google Scholar]
- 112.Kaushik T, Schafer A, Webbe F, Freeman J, Erlanger D. Validation of the HeadMinder dementia screening battery (DSB): a 10-minute computerized screening for primary care. Arch. Clin. Neuropsychol. 2004;19:972. [Google Scholar]
- 113.Becker SA, Webbe FM. Designing for older adult users of handheld technology. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2006:3297–3300. doi: 10.1109/IEMBS.2006.260370. [DOI] [PubMed] [Google Scholar]
- 114.Berger TW, Glanzman DL. Toward Replacement Parts for the Brain: Implantable Biomimetic Electronics as Neural Prostheses. MIT Press; Cambridge, MA, USA: 2005. [Google Scholar]
Websites
- 201.Montreal Cognitive Assessment www.mocatest.org.
- 202.Neurochem announces results from tramiprosate (ALZHEMED™) North American Phase III clinical trial. www.neurochem.com/PR214.htm.


