Abstract
Little is known about the association between brain white matter (WM) structure and motor function in humans. This study investigated complexity of brain WM interior shape as determined by magnetic resonance imaging (MRI) and its relationship with upper-extremity (UE) motor function in patients post stroke. We hypothesized that (1) the WM complexity would decrease following stroke, and (2) higher WM complexity in non-affected cortical areas would be related to greater UE motor function. Thirty-eight stroke patients (16 with left-hemisphere lesions) underwent MRI anatomical brain scans. Fractal dimension (FD), a quantitative shape metric, was applied onto skeletonized brain WM images to evaluate WM internal structural complexity. Wolf Motor Function Test (WMFT) and Fugl-Meyer Motor Assessment (FM) scores were measured to assess motor function of the affected limb. The WM complexity was lower in the stroke-affected hemisphere. The FD was associated with better motor function in two subgroups: with left-subcortical lesions, FD values of the lesion-free areas of the left hemisphere were associated with better FM scores; with right-cortical lesions, FD values of lesion-free regions were robustly associated with better WMFT scores. These findings suggest that greater residual WM complexity is associated with less impaired UE motor function, which is more robust in patients with right-hemisphere lesions. No correlations were found between lesion volume and WMFT or FM scores. This study addressed WM complexity in stroke patients and its relationship with UE motor function. Measurement of brain WM reorganization may be a sensitive correlate of UE function in people recovering from stroke.
Keywords: Fractal dimension, Magnetic resonance imaging, Recovery, Human
1. Introduction
Brain white matter (WM) alteration detected by magnetic resonance imaging (MRI) is commonly seen in normal aging and frequently found among elderly patients with risk factors for stroke or a history of stroke, motor and cognitive disabilities, and dementia (Meyer et al., 1992). Understanding WM adaptations post stroke is important for several reasons. First, WM structural changes may share common pathophysiological mechanisms with stroke (Inzitari, 2003). Second, the appearance of WM reorganization on imaging is associated with a history of stroke and can be used to predict stroke recurrence (Leys et al., 1998; Yamauchi et al., 2002; Inzitari, 2003). Third, WM degeneration may be correlated with functional disability, such as post-stroke dementia (Leys et al., 1998), balance impairment, and gait disturbance (Longstreth et al., 1996).
Despite the fact that WM measurements can be used to predict recovery from stroke, quantitative metrics of brain WM reorganization are limited in clinical settings. Currently, the clinical diagnosis of WM degeneration depends primarily on visual rating scales, employing features such as patterns or locations of lesions as auxiliary parameters (Mantyla et al., 1997) that are subjective and labor intensive. Various quantitative approaches based on conventional MRI and diffusion tensor imaging (DTI) have been developed recently to evaluate WM structure. These include voxel-based analysis of WM signal abnormalities such as WM hyperintensities on T2-weighted images (Wen and Sachdev, 2004) and use of a fractional anisotropy (FA) map on DTI images (Medina et al., 2006). Other approaches include region of interest (ROI)-based methods, such as mean FA measures in an ROI (Lie et al., 2004; Wang et al., 2006; Liang et al., 2007), volume measurement of such WM abnormalities as T2-weighted WM hyperintensities (Baloh et al., 2003; Wen and Sachdev, 2004; Sachdev et al., 2005), T1- weighted hypointensities (Viswanthan et al., 2007), and WM shape complexity (Zhang et al., 2006; Esteban et al., 2007). Among these quantitative approaches, many compute the intensity of the WM image signal and the size of WM lesions. However, studies using quantitative analysis for evaluation of the WM structural shape complexity are limited due to methodological challenges (Zhang et al., 2006).
“Fractal” is a term coined by Mandelbrot (1982) to describe the irregular but self-similar shapes of natural objects. A fractal is defined as any rough and irregular object composed of smaller versions of itself. Fractal geometry was developed to help study irregular shapes of natural objects (e.g., brain) by accommodating the complexity information of these shapes. Fractal geometry is unlike Euclidean geometry, which provides information regarding regular shapes (e.g., circles).
Compared to the topological dimensions in Euclidean geometry (e.g., one-dimensional line, two-dimensional [2D] plane, three-dimensional [3D] cube), fractal dimension (FD) in fractal geometry is fractional, implying that a shape is neither one-, two- or three-dimensional, but somewhere in between the dimensions. FD is a quantitative measurement used to characterize the morphometric complexity and variability of WM structure.
There are several reasons why fractal analysis may be a useful paradigm for analyzing brain WM shape: (1) FD can capture very complicated structures in a simple description (a fractional number). (2) FD can characterize the way in which the WM fills up the space. (3) FD has the ability to analyze and summarize the information over different scales, the largest scale being the entire WM structure of the whole brain, and the smallest scale being determined by resolution of the method based on which the structure is established (e.g., MRI).
The quantification of brain morphology (shape and size) has recently emerged as a promising field in clinical neuroscience and may provide a window to better understand the structural plasticity of the brain (May and Gaser, 2006). Furthermore, shape analysis in morphology studies may provide new information on brain WM structural adaptation versus conventional volumetric measurements (Gerig et al, 2001). FD is a sensitive metric for detecting WM changes in normal aging (Zhang et al., 2007a) and in pathological states such as multiple sclerosis (Esteban et al., 2007), epilepsy (Cook et al., 1995), and psychiatric disorders (Bullmore et al., 1994). To date, no investigators have used FD to characterize WM complexity post stroke. FD characterizes internal shape complexity (Zhang et al., 2006) and therefore complements other quantification metrics and visual scales; thus providing a sensitive framework for detecting stroke and/or recovery-related brain WM structural reorganization.
To be clinically useful and functionally relevant, FD should be correlated with commonly used clinical examinations. In this study, the relationship between FD and upper-extremity (UE) motor function of stroke patients was investigated. Among multiple functional impairments, motor function deficit is a common consequence of stroke, and the presence of motor deficit and WM abnormalities have been shown to predict physical dependence (Samuelsson et al., 1996). Despite the potential link between WM structure and function, few studies have evaluated the relationship between WM reorganization and functional disability following stroke. Among these, several investigators have reported that WM degeneration is related to abnormalities of lower-extremity function (Longstreth et al., 1996; Guo et al., 2000; Pantoni et al., 2005). However, information regarding the relationship between WM structure and UE function is controversial. In two studies using conventional MRI, a significant positive association between severity of WM degeneration (i.e., volume of WM hyperintensities) and reduced UE function (including fine motor dexterity [Sachdev et al., 2005] and speed of motor performance [e.g., time required to button a shirt or open a lock; Longstreth et al., 1996]) were reported. DTI studies have revealed positive correlations between the integrity of the corticospinal tract and increased motor function recovery following stroke (Kunimatsu et al., 2003, 2007; Lie et al., 2004; Cho et al., 2007; Liang et al., 2007). Other studies did not find a correlation between UE motor function and WM structure in able-bodied older adults (Guo et al., 2000; Baloh et al., 2003).
The controversial relationship between WM structure and UE motor function could partially be due to the use of subjective methods, such as visual scales (Mantyla et al., 1997), insensitive measures, such as WM abnormality volume (Baloh et al., 2003; Wen and Sachdev, 2004; Sachdev et al., 2005), or evaluation of a restricted portion of the WM, such as the corticospinal tract (Kunimatsu et al., 2003, 2007; Lie et al., 2004; Cho et al., 2007; Liang et al., 2007). New methods by which to examine the entire brain WM structure have provided greater precision and objectivity and may lead to a more accurate characterization of the WM-motor function relationship in clinical populations (Zhang et al., 2006).
The purpose of this study was to (1) investigate brain WM internal structural shape complexity in chronic stroke patients, (2) explore the relationship between the WM structure complexity with UE motor function of the affected limb assessed by the commonly used Wolf Motor Function Test (WMFT) and Fugl-Meyer Motor Assessment (FM) examination, and (3) examine the usefulness of the FD metric in people recovering from stroke. The correlation of lesion volume to UE motor function was also examined as a secondary measurement. It was hypothesized that (1) WM complexity would decrease following stroke, (2) higher WM complexity identified in non-affected cortex would correlate with greater UE motor function, and (3) FD is able to detect WM structural changes post stroke.
2. Results
2.1 WM FD changes in patients following stroke
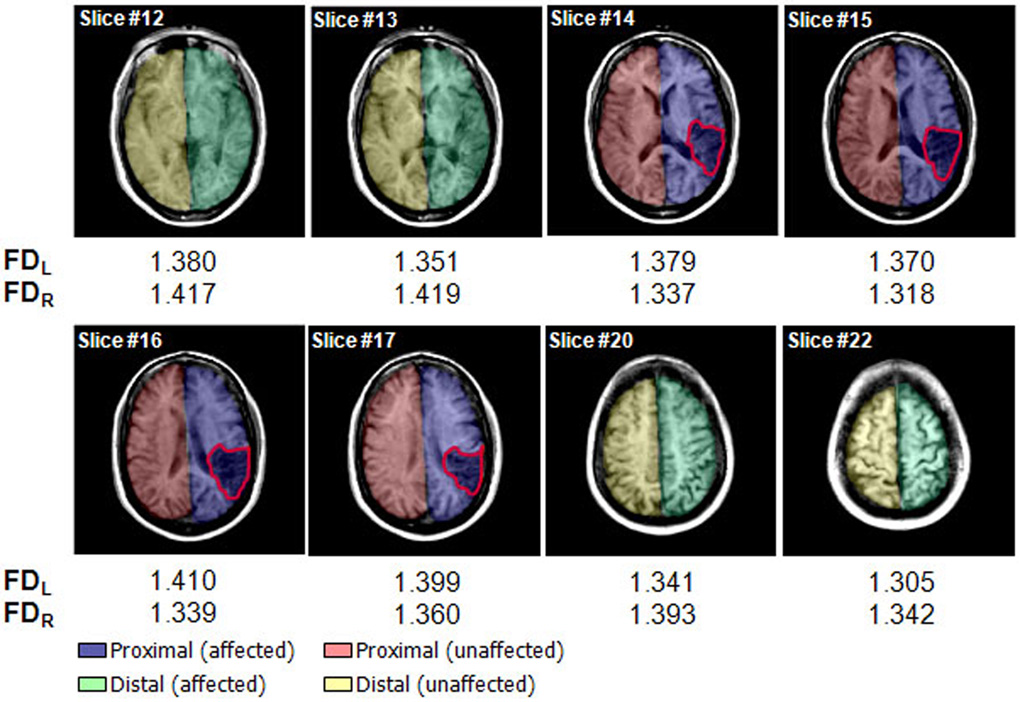
The WM FD changes were used to assess WM complexity degeneration quantitatively in patients following stroke. Fig. 1 demonstrates FD values in eight sample slices from one participant with a lesion in the right parietal region (outlined in red). Brain images where there is no apparent lesion (slice #12, #13, #20 and #22) had smaller FD values in the left than right hemisphere, compared to the four slices with a clearly visible lesion (#14–#17), which had greater FD values in the left than right hemisphere.
Fig. 1.
Illustration of WM FD values of the left and right hemispheres in eight horizontal slices from one subject with a right-hemisphere lesion (outlined in red). FD values of the left hemisphere were smaller than those of the right hemisphere in slices without apparent lesions (slices #12 and #13, #20 and #22). FD values of the left hemisphere were greater than those of the right hemisphere in slices with a lesion (slices #14–#17). Images are displayed in neurological convention, with the left side corresponding to the left hemisphere. FDL, FD of the left WM skeleton; FDR, FD of the right WM skeleton. The colors were designated to illustrate regions of interest (ROIs). Blue – proximal region on the affected side, red - proximal region on the unaffected side, green - distal region on the affected side, and yellow - distal region on the unaffected side. Note proximal region indicates slices with lesion and distal region specifies slices without lesion.
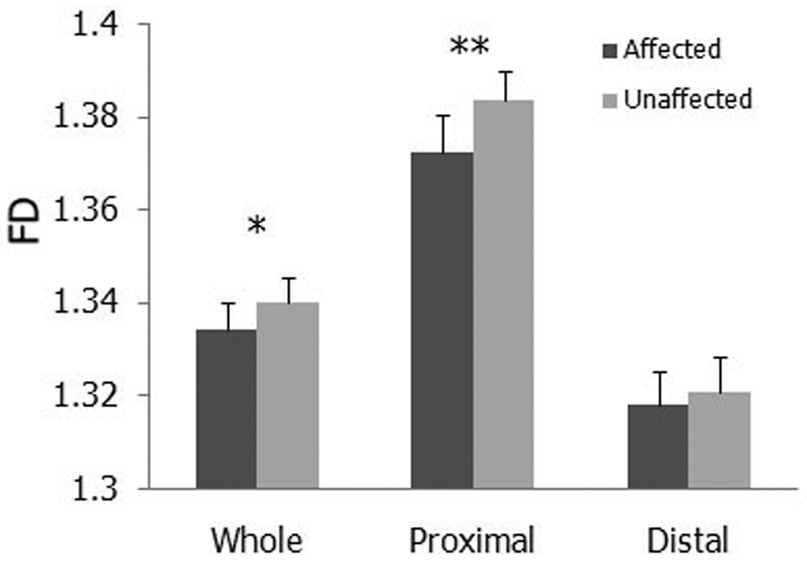
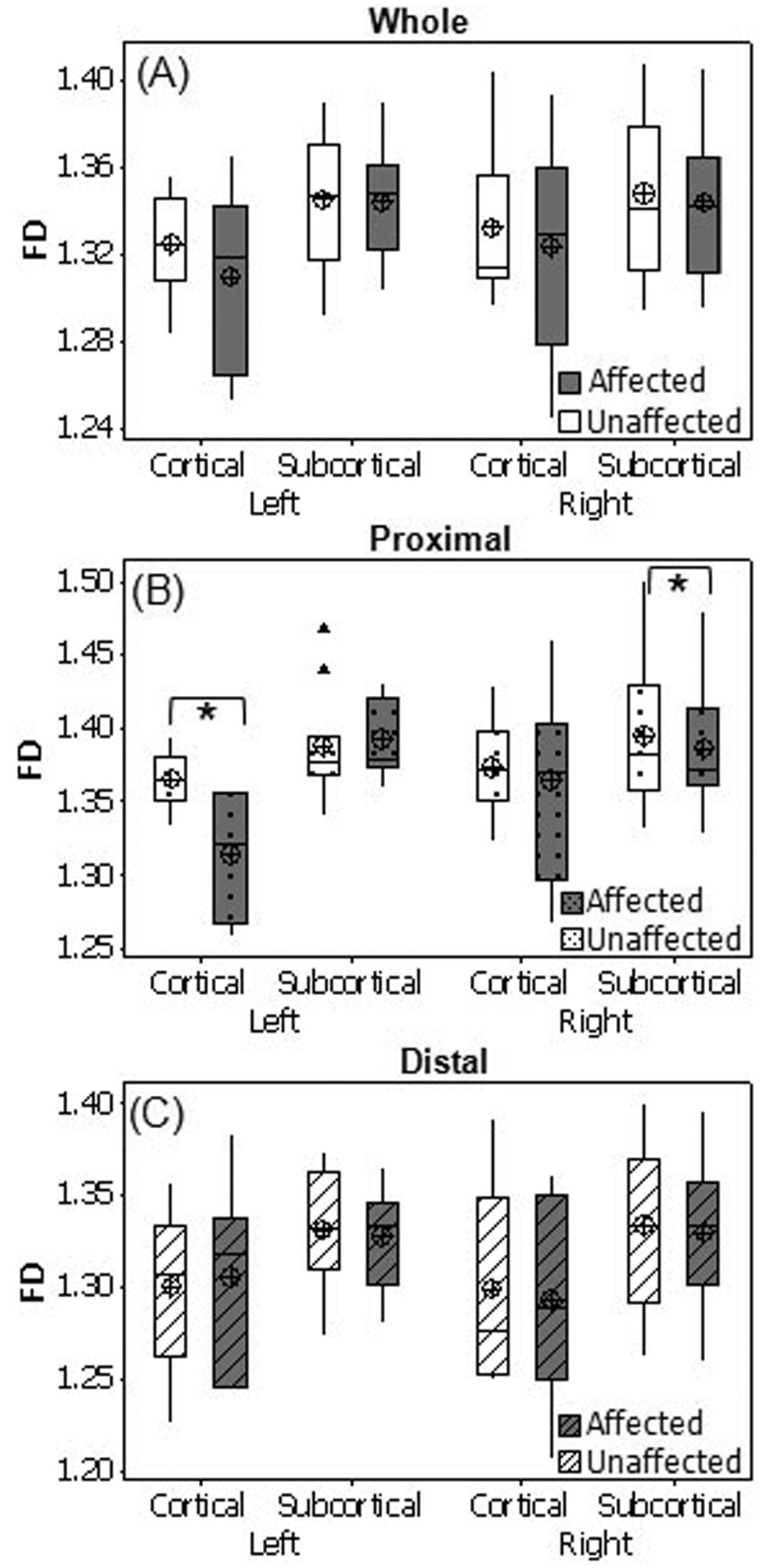
In the entire brain, a significant main effect was found for side (p = 0.0358) with a higher FD in the unaffected (1.340) than the affected hemisphere (1.334) (Fig. 2), suggesting that FD decreases in the affected hemisphere after stroke. No FD differences were found between unaffected and affected hemispheres of the entire brain after further categorizing the patients according to location of lesions (cortical or subcortical, left or right; Fig. 3A). In the area proximal to lesions, MANOVA revealed significant main effects for side (p = 0.0064; Fig. 2) and location_of_lesion (p=0.0092), and interactions for side × location_of_lesion (p = 0.0148) and side × location_of_lesion × affected_hemiphere (p=0.0166). Post-hoc paired t-test found significantly higher FD values in the unaffected than the affected side of the regions proximal to lesions for patients with left-cortical lesions (p = 0.0368) and with right-subcortical lesions (p = 0.0495; Fig. 3B). A significant main effect for location of lesion (p = 0.0351) was observed in the regions distal to lesions, indicating that cortical lesions have a greater effect on WM FD values than subcortical lesions. No significant differences between affected and unaffected sides were observed in the regions distal to lesions (Fig. 2, Fig. 3C). These results, together with those in Figure 1, indicate that WM structural complexity decreases in the affected hemisphere following stroke.
Fig. 2.
WM is more complex in the unaffected than the affected hemisphere. Bar charts of WM FD values of the affected and unaffected sides of the whole hemisphere (Whole), regions proximal to lesions (Proximal), and regions distal to lesions (Distal) are shown. *p < 0.05, **p < 0.01.
Fig. 3.
WM is more complex in the unaffected than the affected hemisphere. Box plots of WM FD of cortical and subcortical lesioned groups for the affected and unaffected sides of the whole hemisphere (A), regions proximal to lesions (B) and regions distal to lesions (C) of patients with left and right hemisphere lesions are shown. (⊕) Group mean; ▲, outliers, *p < 0.05.
2.2 Association between WM FD and UE motor function
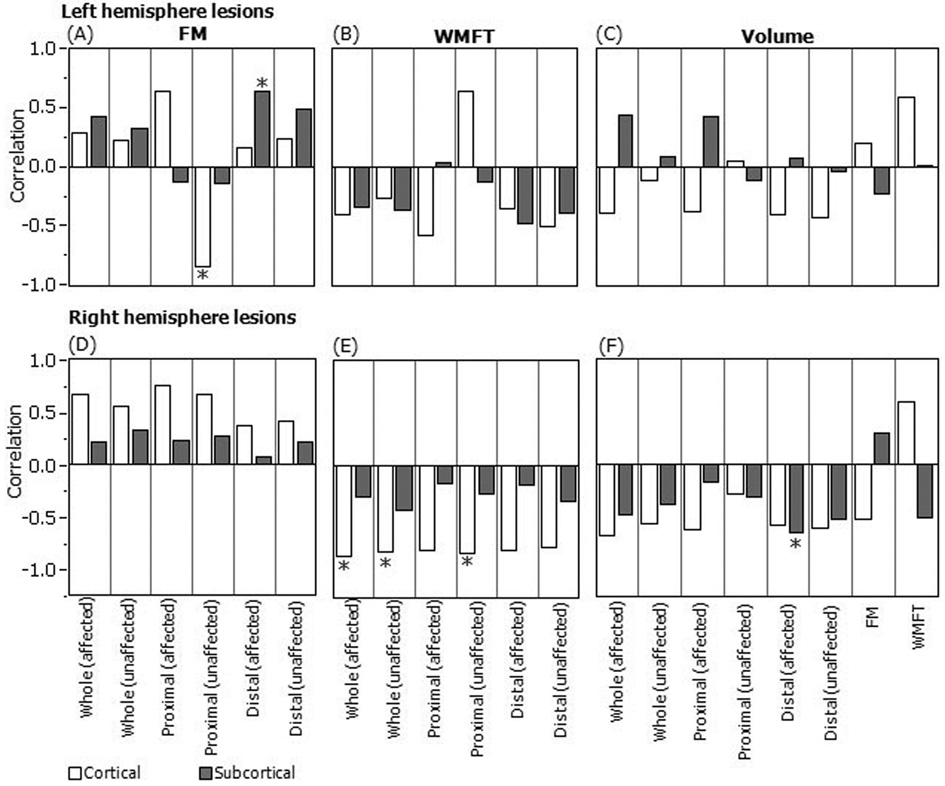
The association between WM complexity (FD) and UE motor function is illustrated in Figure 4, which shows the correlation of WM FD and lesion volume with WMFT and FM scores. Patients were classified as those with cortical and/or subcortical lesions in the left (Fig. 4, top row) and right (Fig. 4, bottom row) hemispheres. For patients with left-subcortical lesions, FD values were positively associated with FM scores (Fig. 4A) in the affected side of the regions distal to lesions, suggesting that higher residual WM complexity in the affected side of the brain regions distal to lesions is associated with less impaired motor function. For patients with cortical lesions, the FD values were negatively associated with the FM scores in the unaffected side of the regions proximal to the lesions (Fig. 4A), indicating that greater complexity of the residual WM in the regions adjacent to lesions is related to greater motor-function impairment.
Fig. 4.
Correlations between WM FD values of six ROIs and FM (A, D), WMFT (B, E), lesion volume (C, F) for patients with cortical and subcortical lesions in the left (top row) and right (bottom row) hemispheres. *p < 0.05. The six ROIs are affected and unaffected sides of whole hemisphere (Whole), regions proximal to lesions (Proximal), and regions distal to lesions (Distal).
For patients with cortical lesions in the right hemisphere, significant negative correlations were found between WMFT times and FD values of the entire left and right hemispheres, and the unaffected side of the regions proximal to lesions (Fig. 4E). These results suggest that a more complex WM structure in the non-affected areas correlates with less impaired motor function for patients with right-cortical lesions. Positive correlations were found between FM and FD measures without reaching statistical significance (Fig. 4D).
2.3 Association between lesion volume and FD, lesion volume and UE motor function
FD value of the affected side of the regions distal to lesions was negatively correlated with lesion volume for patients with right-subcortical lesions (Fig. 4F), suggesting that greater lesion volume is associated with lower WM complexity of healthy area in the lesioned hemisphere. No significant correlations were found between lesion volume and UE motor function scores (Fig. 4C, F).
3. Discussion
The aim of this study was to investigate WM shape complexity using FD and describe its relationship with UE motor function in patients following stroke. The outcomes suggest that (1) WM complexity is lower in the stroke-affected than unaffected hemisphere, indicating lesion side-dependent WM degeneration post stroke; (2) greater residual WM complexity is associated with less impaired UE motor function in patients with left-subcortical lesions and those with right-cortical lesions; and (3) FD might be a useful clinical metric for quantifying WM structural reorganization after stroke or other diseases and medical intervention.
3.1 FD-characterized complexity provides new brain WM morphological information
FD of skeletonized WM (white lines in Fig. 5C) measures the internal shape complexity of residual WM induced by pathology. The WM fiber network is a complicated multilayered twisting arborization of axons within the brain, composed of fiber-bundle crossings (two-, three-, or multilayer crossings) and bundle bifurcations. FD values represent different complexity levels throughout the brain. One factor that may lead to reduced WM FD values is the decreased number of crossings and/or reduced number of fiber bundles in each crossing. Decreased FD of the WM skeletons was observed in the current study. This could be an indication of reduced WM connectivity representing reduced fiber-bundle crossings/mixtures resulting from stroke. A second factor leading to reduced FD may be loss of higher-order bifurcations. Assuming a WM “tree limb” (skeletons, Fig. 5C) originates from the center of the corpus callosum, fiber bundles close to the corpus callosum are considered lower-order branches than those near the WM-gray matter (GM) interface. A postmortem study on aging brains has reported losses of higher-order fiber bundles with preservations of thicker lower-order branches (Marner et al., 2003), indicating a loss of higher-order WM fiber-bundle bifurcations. The loss of higher-order fiber bundles may lead to reductions in both the number of fiber bundles and bundle crossings in the WM “trunk.”
Fig. 5.
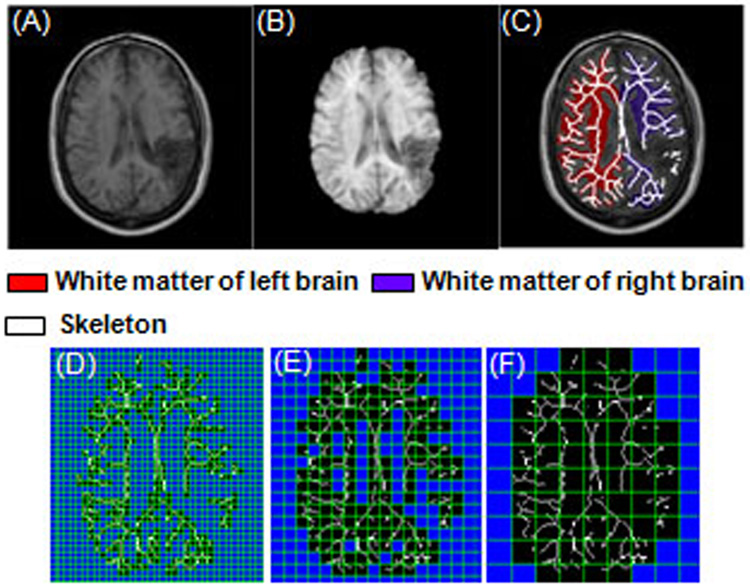
Upper panel: image processing procedure based on one sample slice from a stroke patient. (A) A T1-weighted transverse head image. (B) Brain segmentation result. (C) Brain WM segmentation and skeleton results overlaid on the original head image. Lower panel: illustration of 2D box-counting method. Mesh with different sizes (r) was put in the 2D sample WM skeleton slice (binary image). Number of boxes (N) which cover the WM skeleton (specified with black boxes) was counted. (D) r = 5 pixels, N = 362.0. (E) r = 10 pixels, N = 160.0. (F) r = 20 pixels, N = 58.2. The images were generated using the box-counting package from HarFA (Harmonic and Fractal Image Analyzer, Brno University of Technology, Brno, Czech Republic; http://www.fch.vutbr.cz/lectures/imagesci/).
Compared with other MR WM structural measures, FD of WM skeletons provides unique information about WM morphology. In the current study, we used FD to measure the internal shape complexity of residual WM after stroke-induced brain damage. In contrast, many other widely reported metrics are photometric parameters that quantify image intensity (or contrast) contributed by pathological vs. normal WM tissues (Lie et al., 2004; Wen and Sachdev, 2004; Medina et al., 2006; Wang et al., 2006; Liang 2007). Of these metrics, voxel-based intensity analysis is most commonly used to quantify regional WM pathology, such as distribution and extent of WM hyperintensities associated with lesions on T2-weighted images (Wen and Sachdev, 2004), and location and degree of FA reductions, which indicate loss of axonal fibers or myelin on DTI images (Medina et al., 2006).
Reports of WM morphological studies are limited because of the inherent technical difficulties associated with measuring shape complexity. Most studies based on conventional MRI have focused on measuring volumes (size) of WM lesions, such as T2-weighted WM hyperintensities (Baloh et al., 2003; Wen and Sachdev, 2004; Sachdev et al., 2005) and T1- hypointense lesions (Viswanthan et al., 2007). Only two reports have provided FD-measured WM-shape complexity data based on conventional T1-weighted MR brain images; these showed reduced WM complexity in multiple sclerosis (Esteban et al., 2007) and aging (Zhang et al., 2007a). In the aging study, we measured FD values of three 3D-WM structures: WM skeleton, gray-white matter boundary (WM surface), and WM general shape (Zhang et al., 2007a). In the current study, however, the FD value of 2D WM skeletons on each slice was calculated first and the values across the slices were subsequently averaged. Although the aging study provided the normal FD values (FD > 2) in healthy young and old individuals (Zhang et al., 2007a), FD values calculated in the current study (FD < 2) are not comparable to the normal aging values because these measurements were made on different structures: 3D-WM structures in the healthy aging study and 2D-WM structures in the current study. According to the fractal concept (Mandelbrot, 1982), FD values of 2D structures would be in a range of 1 to 2 whereas FD values of 3D structures would be in a range of 2 to 3.
ROI-based methods on DTI images examine the WM integrity locally by measuring mean values of directional water molecule distributions in WM bundles in terms of FA or mean diffusivity generated from manually selected ROIs (Lie et al., 2004; Wang et al., 2006; Liang et al., 2007). Although DTI provides unique information about the morphology of intra-WM structures, no DTI shape quantification studies have been found, owing to methodological challenges (Mori, 2007). The only studies focused on WM shape analysis based on DTI data in stroke examined the relationship between corticospinal tract integrity (FA values) and infarction volume by visualizing the course of the tract and extent of the infarction (Kunimatsu et al., 2003, 2007; Lie et al., 2004; Cho et al., 2007; Liang et al., 2007). Compared with the fiber orientation map of DTI, FD-based WM measurement provides information on fiber-branching patterns that is not measurable using current quantitative DTI measures. DTI also has difficulties in characterizing fiber crossing (Mori, 2007), whereas FD is able to uniquely characterize fiber crossing. Compared with the DTI studies that examined WM integrity of one or a few tracts by visual inspection, our study used a quantitative metric of WM (i.e., FD) that evaluated the complexity level of the entire brain’s WM branching structure and selected substructures. FD has even greater potential to be used in DTI fiber orientation maps to quantify the complexity of fiber networks in different orientations, a technique currently being explored in our laboratory.
3.2 WM structural complexity changes post stroke
In one patient with a right-hemisphere lesion, significantly smaller WM FD values were seen in the right hemisphere in slices with lesions (Fig. 1, p = 0.006), whereas FD values were greater in the right hemisphere in slices without lesions (Fig. 1, p = 0.007). This finding may suggest that complexity of the WM in the right hemisphere is reduced in the lesioned regions and their proximal surrounding areas. The finding that WM complexity is greater in the right than left hemisphere in slices without lesions is consistent with our previous finding of right-greater-than-left WM structural complexity in able-bodied people (Zhang et al., 2007a). In the group analysis, we observed significantly higher FD for the entire nonlesioned than the lesioned hemisphere – an observation seen in all patients (Fig. 2). Note the case with apparent lesions shown in Figure 1 and Figure 5 were used for demonstration; a majority of the patients did not have large lesions and apparent WM degeneration as shown in Figure 1 and Figure 5. Our previous study reported that the FD technique is able to detect WM changes even in healthy aging and WM structure asymmetry across hemispheres in young and elderly subjects (Zhang et al., 2007a). The fact that we could not observe differences by visual inspection but the FD measurement detected a significant difference in the WM structure between the left and right hemispheres suggests that the FD has the ability to detect subtle WM structural changes.
The FD is greater in regions proximal to the lesion in the unaffected than affected side for patients with left-cortical lesions and right-subcortical lesions (Fig. 2, 3B). These results suggest that WM complexity in regions adjacent to the lesion decrease following both left-cortical stroke and right-subcortical stroke. No significant FD differences were found between affected and unaffected sides in regions distal to the lesion (Fig. 2, 3C). These results support the idea that tissue damage caused by a stroke results in degeneration of the WM, which in turn lowers the brain’s structural complexity in areas proximal to the lesion. However, the effect of a lesion on WM complexity appears to be minimal in cortical regions relatively distal to the lesion.
The above-reported results depend on the location of the lesion, with cortical lesions having greater effects on WM structure complexity than subcortical lesions; significant WM structure degeneration is found after left-cortical or right-subcortical stroke. These findings might be explained by specificity of the structures and their unique functions. The specific reasons why the impact of the lesion on WM structure is location dependent need further investigation.
3.3 Association between WM complexity and UE motor function
A significant positive correlation between the FM score and FD value of the brain regions distal to the lesion of the affected side (Fig. 4A, gray bar with asterisk) suggests that greater residual WM complexity in these regions is associated with less impaired motor function for patients with left-subcortical lesions. Conversely, we found that the FD value in regions proximal to the lesion on the unaffected side were negatively associated with the FM score for patients with cortical lesions (Fig. 4A, white bar with asterisk). This finding indicates that greater WM complexity is correlated with more severely impaired UE function in regions proximal to the lesion in the unaffected hemisphere (regions in the unaffected hemisphere corresponding to lesioned regions in the affected hemisphere). These results seem to suggest that for patients with left-hemisphere lesions, the amount of residual WM is important for recovery of impaired motor function, whereas similar measures in the corresponding hemisphere do not predict functional recovery of the affected UE. A possible explanation for the latter finding is that patients with hemiparesis rely heavily on the use of their less-impaired UE. Consequently, WM in the cortical area controlling the less-impaired UE would be more developed. The prediction of reduced motor function of the affected UE following stroke is in agreement with the theory of learned nonuse (Taub, 1976). When applied to the model of human stroke, learned non-use may develop during the early stages following a stroke as the patient begins to compensate for difficulty using the impaired limb by increased reliance on the intact limb. This compensation has been shown to hinder recovery of function in the impaired limb. The idea that WM complexity is affected by or is a result of learned non-use needs further exploration.
For patients with right-hemisphere lesions, we observed negative correlations between WMFT scores and all FD values (Fig. 4E) and positive correlations between FM scores and FD for all cases (Fig. 4D). This strong finding demonstrates that for patients with stroke involving the right hemisphere, more complex WM structures in the brain are consistently associated with less-impaired motor function. The stronger association between the WM FD and WMFT (Fig. 4E) suggests that brain WM integrity might be particularly important for rapid motor performance of the upper limb; as the WMFT primarily measures the time required to accomplish various reaching tasks, “as fast as possible.” The homogeneous positive relationship between WM structure complexity in all ROIs and motor function of the affected UE has potential application in movement rehabilitation strategies. For stroke patients with lesions involving the right hemisphere, therapies that take advantage of existing WM or possibly restore WM structure in any brain region may facilitate motor function recovery. Preliminary results from our recent analysis suggest that WM complexity increases in stroke patients after constraint-induced therapy (Zhang et al., 2007b).
Although no studies have explored the relationship between WM complexity and UE motor function in hemiparetic patients recovering from stroke, four investigative groups have reported an inconsistent relationship between brain WM signal abnormalities and UE motor function based on conventional MRI. A significant correlation between WM signal abnormalities and UE motor functions was reported by two groups (Longstreth et al., 1996; Sachdev et al., 2005), but their finding were not confirmed by others (Guo et al., 2000; Baloh et al., 2003). A few DTI studies have revealed positive associations between the degree of corticospinal tract integrity and increased UE motor function recovery, including tolerance levels of muscles to resistance (Kunimatsu et al., 2003, 2007), ability of the hand to move and grasp objects (Cho et al., 2007), movement, coordination and reflex actions (Liang et al., 2007), and muscle strength (Lie et al., 2004).
It should be noted that our study has advantages over the previously discussed conventional MRI-based reports, although the findings may not be entirely comparable. First, different patient populations were studied. All participants in our study were patients with a history of stroke occurring 3–24 months prior to enrollment. In contrast, earlier studies used only a sample of stroke and non-stroke survivors (Longstreth et al., 1996; Guo et al., 2000; Baloh et al., 2003; Sachdev et al., 2005). In the study by Sachdev et al. (2005), only a small portion of the volunteers with a history of stroke participated in the study (19 of 476 participants); a majority of the participants were patients with hypertension or a history of heart disease (Sachdev et al., 2005). In the population-based longitudinal Cardiovascular Health Study (Longstreth et al., 1996), individuals with silent stroke (one third of the 3301 participants) were included. Guo and coworkers (2000) examined 248 women, of whom only 4% had been diagnosed with stroke. Only normal elderly individuals were included in the study by Baloh et al. (2003). Our investigation is unique because we enrolled only patients diagnosed with stroke excluding confounding effects from other diagnoses and healthy populations.
Second, different metrics and imaging techniques were used to evaluate the WM. We used FD to quantify WM morphology (shape complexity) on MR brain images, whereas the other groups evaluated MRI- or computed tomography (CT)-based WM signal abnormalities. Sachdev et al., (2005) and Baloh et al., (2003) performed a volumetric analysis of WM hyperintensities on MR images, whereas Guo et al., (2000) and Longstreth and coworkers (1996) evaluated WM hyperintensities using visual scales. WM changes were defined as low-density areas in the periventricular or subcortical WM on CT images (Guo et al., 2000) and evaluated as the total volume of WM signal abnormalities using 10-grade visual scales (Longstreth et al., 1996). We believe our study, which employed automated WM-complexity assessment, is more objective than studies using visual scales (Longstreth et al., 1996; Guo et al., 2000) and provides new shape information about WM structure compared with studies measuring volume of WM hyperintensities (Baloh et al., 2003; Sachdev et al., 2005).
Third, the variables used to assess UE motor function in the current study were very specific to upper-limb impairment. We used validated tests of UE motor function. The Purdue Pegboard test was administered to assess manual dexterity in two studies (Baloh et al., 2003; Sachdev et al., 2005), and the postural-location-manual test was employed in the study by Guo et al. (2000). No details were given regarding tests of motor function in the Cardiovascular Health Study (Longstreth et al., 1996).
3.4 Association between lesion volume and FD, lesion volume and UE motor function
In general, the lesion volume was not significantly correlated with the FD (Fig. 4C, F), suggesting that FD is an independent structural measure and can provide additional structural information compared with volume measurement. Negative correlations were observed between lesion volume and FD values for patients with right-hemisphere lesions (Fig. 4F). This finding demonstrates that a larger lesion volume in the right hemisphere is homogeneously associated with less complicated WM structures in all analyzed brain regions. These results seem logical, as a larger lesion is expected to more negatively influence WM structure. The WM FD correlation with an anatomical variable (lesion volume; Fig. 4F) can be readily linked to its correlation with a functional variable (WMFT; Fig. 4E), suggesting better motor function with greater WM complexity in patients with right-hemisphere stroke.
No significant correlation was found between lesion volume and UE motor function (Fig. 4C, F). This finding is not in agreement with the observation that lesion volume was moderately correlated with motor impairment (Schiemanck et al., 2005). This inconsistency may be related to variations in patient conditions in the two studies and to subjective manual determination of lesion volume. The findings of significant association between FD and UE motor function (Fig. 4A, E) but no such correlation between lesion volume and UE motor function (Fig. 4C, F) suggest that, at least in our study, FD measure is a more sensitive predictor of UE motor function post stroke than the lesion volume measurement.
3.5 Limitations and future work
This study used available low-resolution images (5 mm thick), which only allowed 2D fractal analysis of WM structure. Although 2D analysis is important in detecting local changes, structural information for the z-axis is missing. In future studies, we will use high-resolution images and 3D fractal analysis (Zhang et al., 2006) to improve the level of accuracy and sensitivity.
Although all patients in the study were defined clinically as having experienced a stroke, they were not classified according to stroke type. In other words, diverse groups of stroke patients were investigated (i.e., ischemic and hemorrhagic cases were not separated), and this may confound the relationship examined. As brain WM changes might share common pathophysiological mechanisms with stroke, particularly in the case of lacunar infarcts, more accurate and consistent results might be achieved if future studies were designed to compare separate cohorts of ischemic and hemorrhagic strokes. Finally, a longitudinal study is needed to monitor treatment- or time-related WM structural adaptations and their relationship with motor or other function.
3.6 Summary
We evaluated WM structural complexity using fractal analysis and upper limb motor function in a group of patients 3–24 months post stroke. In general, greater WM complexity was associated with better motor function of the affected UE in patients with left-subcortical lesions and those with right-cortical lesions. This association is more robust in patients with lesions in the right hemisphere, suggesting a functional asymmetry. No significant correlations were observed between lesion volume and UE motor function. Our study adds new information regarding the relationship between WM structure and UE motor function post stroke. FD can potentially serve as a useful clinical metric to monitor the level of residual WM structure following stroke or any accompanying neural plastic changes to guide future therapeutic interventions.
4. Experimental procedures
4.1 Data acquisition
Approval for this study was obtained from the Institutional Review Boards of each of the three participating universities. T1-weighted MR head images of 38 right-handed stroke survivors were collected at three academic medical centers in the United States using standard 1.5 T scanners (Philips Intera, Siemans Magnetom Sonata, GE Signa). Images were acquired at the time of the initial clinical data assessment using the MPRAGE imaging sequence (TR [repetition time] / TE [echo time] = 30 / 4 ms, slice thickness = 5 mm, in plane resolution = 1 × 1 mm2). Patient demographic and clinical data are listed in Table 1 and Table 2. Inclusion criteria required that participants were at least 18 years old, had survived a first-time stroke in the past 24 months, could actively lift hand from a drooping position and elevate the thumb and at least two fingers, could independently transfer to toilet and stand, could maintain their balance for at least 2 minutes with arm support, and were not participating in formal physical rehabilitation programs.
Table 1.
Individual patient demographic and clinical data
| Patient No. | Age (years) | Sex | Days after stroke | Affected brain | FM | WMFT | Site of lesion |
|---|---|---|---|---|---|---|---|
| 1 | 62 | M | 86 | Left | 32 | 2.80 | internal capsule |
| 2 | 65 | M | 229 | Left | 58 | 1.65 | thalamus, internal capsule |
| 3 | 51 | F | 121 | Left | 53 | 1.60 | internal capsule, thalamus |
| 4 | 79 | M | 282 | Left | 30 | 2.73 | basal ganglia |
| 5 | 49 | M | 100 | Left | 45 | 2.33 | internal capsule, thalamus |
| 6 | 81 | F | 106 | Left | - | 2.33 | internal capsule, basal ganglia |
| 7 | 62 | F | 144 | Left | 51 | 1.90 | corona radiata, internal capsule |
| 8 | 57 | F | 137 | Left | 51 | 2.28 | internal capsule |
| 9 | 50 | F | 224 | Left | 29 | 2.73 | internal capsule |
| 10 | 74 | M | 179 | Left | 50 | 2.65 | corona radiata |
| 11 | 48 | M | 581 | Left | 56 | 1.52 | internal capsule, basal ganglia, middle frontal gyrus |
| 12 | 49 | M | 158 | Left | 32 | 2.41 | frontal and parietal cortex, corona radiata |
| 13 | 52 | F | 539 | Left | 52 | 2.42 | frontal and temporal cortex |
| 14 | 66 | M | 495 | Left | 48 | 1.93 | parietal white matter |
| 15 | 51 | M | 229 | Left | 48 | 2.51 | frontal corona radiata |
| 16 | 54 | M | 183 | Left | 49 | 1.72 | precentral gyrus, middle frontal gyrus hemorrhage |
| 17 | 57 | M | 176 | Right | 37 | 2.63 | thalamus, internal capsule |
| 18 | 36 | F | 132 | Right | 53 | 1.83 | internal capsule, basal ganglia |
| 19 | 57 | M | 463 | Right | - | - | internal capsule, thalamus, basal ganglia |
| 20 | 65 | M | 616 | Right | 25 | 2.89 | basal ganglia |
| 21 | 60 | F | 250 | Right | 39 | - | internal capsule, corona radiata, basal ganglia |
| 22 | 32 | F | 126 | Right | 26 | 2.85 | internal capsule |
| 23 | 65 | M | 631 | Right | 50 | 2.21 | internal capsule |
| 24 | 55 | M | 451 | Right | 35 | 2.03 | thalamus, internal capsule |
| 25 | 64 | M | 465 | Right | 34 | 2.64 | internal capsule, thalamus |
| 26 | 43 | M | 295 | Right | - | 2.89 | internal capsule, basal ganglia |
| 27 | 61 | M | 521 | Right | 14 | 3.20 | internal capsule, basal ganglia |
| 28 | 70 | M | 125 | Right | 36 | 2.74 | putamen |
| 29 | 33 | F | 132 | Right | 57 | 2.06 | thalamus |
| 30 | 34 | F | 105 | Right | 57 | 1.65 | corona radiata, internal capsule |
| 31 | 75 | M | 150 | Right | 36 | 2.76 | precentral gyrus, temporal lobe, basal ganglia, internal capsule |
| 32 | 56 | F | 550 | Right | 40 | 2.30 | temporal cortex, parietal white matter |
| 33 | 45 | F | 99 | Right | 43 | 2.33 | precentral gyrus, corona radiata |
| 34 | 71 | F | 720 | Right | - | - | frontal and parietal corona radiate |
| 35 | 61 | M | 590 | Right | 54 | 1.86 | postcentral gyrus, parietal white matter |
| 36 | 34 | F | 647 | Right | - | - | postcentral gyrus, parietal white matter |
| 37 | 79 | F | 351 | Right | 23 | 3.00 | parietal and temporal cortex and white matter |
| 38 | 67 | M | 138 | Right | 54 | 2.51 | parietal and temporal hemorrhage |
Table 2.
Summary of demographic data and patient characteristics
| Characteristic | Left-hemisphere lesions (n = 16) | Right-hemisphere lesions (n = 22) | ||
|---|---|---|---|---|
| Lesion location | Subcortical | Cortical | Subcortical | Cortical |
| (n = 10) | (n = 6) | (n = 14) | (n = 8) | |
| Age | 63.00 (11.79) | 53.33 (6.56) | 52.29 (13.67) | 61.00 (15.43) |
| Days after stroke | 160.80 (63.31) | 364.17 (194.06) | 326.00 (203.48) | 413.00 (263.50) |
| WMFT* | 2.30 (0.45) | 2.08 (0.42) | 2.47 (0.49) | 2.45 (0.40) |
| FM | 44.33 (11.05) | 47.5 (8.19) | 38.58 (13.51) | 41.67 (11.74) |
Mean (Standard Deviation).
Log transformations of WMFT scores.
4.2 Data analysis
4.2.1 Image processing
Fig. 5A–C demonstrates the image-processing procedure based on one sample slice from a stroke patient with a lesion in the right parietal cortex (Fig. 5A). Brain images were segmented from head images using the brain extraction tool (BET; Smith, 2002) part of the FMRIB Software Library (FSL, www.fmrib.ox.ac.uk/fsl). This approach uses a triangular tessellated surface model that evolves itself to fit the brain’s surface by application of a set of locally adaptive model forces. The extraction result is shown in Fig. 5B. The left and right hemispheres of the brain were segmented manually using MEDx 3.4.1 tracing tool (Sensor Systems, Inc., Sterling, VA), and mask images corresponding to each hemisphere were generated. The segmentation is illustrated in Fig. 5C with WM of left (highlighted in red) and right hemispheres (highlighted in purple) shown.
WM areas were subsequently segmented from the brain images using FSL FAST (Zhang et al., 2001) and recorded as binary images. The FAST method labels pixels according to image intensity histograms and spatial neighborhood information coded by a hidden Markov random field.
A 2D thinning method in MATLAB 7.2 (MathWorks, Natick, MA) image-processing toolbox was applied to the binary images, and skeletons of the WM in each slice were obtained (white lines in Fig. 5C). The skeleton image is a descriptor that represents the interior shape of the object. The thinning method iteratively deleted successive layers of pixels on the boundary of WM images until only a 1-pixel-wide skeleton remained (Lam and Suen, 1992). The mask images were finally applied to the skeleton images to obtain WM skeletons of the left and right hemispheres.
4.2.2 Complexity measurement (FD calculation)
WM complexity was evaluated using FD measurement. FD analysis used in this study was a 2D version modified from our 3D fractal analysis method (Zhang et al., 2006) and implemented by custom-designed software written in MATLAB. This technique reportedly can detect WM changes in aging (Zhang et al., 2007a) and multiple sclerosis (Esteban et al., 2007). FD was determined by performing linear regression analysis of the logarithmic function of the box sizes (r) and number of boxes (N), which was the slope of the function
| (Eq. 1) |
K is a constant in this equation. The entire procedure included three steps: (1) overlaying different-sized 2D meshes (r) onto the WM skeleton images and counting the number of boxes (N) needed to cover these skeletons (Fig. 5D–F); (2) performing single slope analysis to find the linear portion of the function (Eq. 1) and obtain the range of box sizes; and (3) performing linear regression analysis based on the chosen range of box sizes to obtain FD. Complete details about the fractal analysis can be found elsewhere (Zhang et al., 2006).
4.2.3 Lesion volume measurement
Lesions were outlined manually on all slices of the individual MRI using tools within MRIcro (Rorden and Brett, 2000) by two investigators (KP, SS; see Acknowledgment). The Analysis of Brain Lesions (ABLe) toolbox (Makale et al., 2002) was then used to determine lesion volume and location. The image of the brain was normalized to Talairach space (Talairach and Tournoux, 1988), and lesions were assigned to cytoarchitectonic areas. The templates of Damasio and Damasio (1989) were chosen for use within ABLe to quantify any Brodmann area involvement of the lesion. Lesion locations assigned with ABLe were then verified by both investigators using an atlas of human brain structure (Duvernoy, 1991). The location of each lesion was categorized as cortical (or subcortical) if greater than 50% of the lesion region was enveloped in the cortical (or subcortical) site.
4.2.4 Region of interest (ROI) analysis
WM FD values were calculated on six ROIs. For each ROI, FD values of each slice in the region were calculated and averaged across slices. Six FD means corresponding to the 6 ROIs in each patient were used for statistical analysis.
Classification of the ROIs is illustrated in Figure 1. The MR brain slices were divided into two parts, slices with identified lesions and slices without lesions. Slices with lesions were defined as the ROI proximal to lesions (e.g., slices #14, #15, #16, #17 in Fig. 1), including two ROIs: affected side (highlighted in blue) and unaffected side (highlighted in red) of the region. Slices without lesions were defined as the ROI distal to lesions (e.g., slices #12, #13, #20, #22 in Fig. 1), including two ROIs, affected side (highlighted in green) and unaffected side (highlighted in yellow) of the region. The last two ROIs were the entire affected hemisphere and the entire unaffected hemisphere.
4.2.5 Upper-extremity motor function assessment
The WMFT and FM test scores were used to assess UE motor function of the affected limb. WMFT evaluates the amount of time used by a patient to complete single- and multiple-joint motor tasks (Wolf et al., 2001). Faster WMFT times indicate less impairment. Log transformations of the WMFT means were used because of skewness of the timed data. The FM contains items measuring movement, coordination, and reflex actions at various joints of the upper and lower extremities (Gladstone et al., 2002). It consists of two subscales, one to evaluate motor function of the upper extremity and the other, of the lower extremity (Fugl-Meyer et al., 1975). Only the upper-extremity FM scores were analyzed in this study. The scoring range for the arm section is 0 to 66 points; a higher score indicates better performance. Adequate psychometric properties for the FM have been presented (Platz et al., 2005).
4.2.6 Statistical analysis
Multivariate analysis of variance (MANOVA) with three factors (within factor: side [affected, unaffected]; between factors: affected_hemisphere [left, right], location_of_lesion [cortical, subcortical]) was performed separately on three pairs of regions (entire hemisphere [affected vs. unaffected], regions proximal to lesions [affected vs. unaffected], and regions distal to lesions [affected vs. unaffected]) to investigate FD differences between affected and unaffected regions. A subcortical lesion was operationally defined as a lesion completely in the WM or deep nuclei on one side of the brain or brainstem, whereas a cortical lesion was defined as a lesion affecting gray matter (GM) of the cerebral cortex, including GM-WM overlap lesions. Paired t tests were used to test the FD differences between affected and unaffected regions for patients with left (or right) cortical (or subcortical) lesions. Stroke chronicity and age were considered as potential confounds on WM FD. The MANOVA showed no significant effects of these factors on the FD and were therefore ignored in the subsequent statistical analysis. Pearson correlation was performed to investigate the relationship between WM structure measures (FD and lesion volume) and UE motor function (WMFT and FM scores).
Acknowledgements
We thank the FICIT study investigators David Good, M.D., Krish Sathian, M.D., Ph.D., Steve Wolf, Ph.D., P.T., and Deborah Nichols Larson, Ph.D., P.T. We are grateful to our site coordinators, evaluators and members of the data management center, without whose tireless and diligent efforts this study could not have been initiated or completed. Special thanks to Hui Mao, Ph.D., Allison Fowlkes, Joe Maldjian, M.D., Lumy Sawaki, M.D., Ph.D., Jason Greenberg, M.D., Peter Wassenaar, and Petra Schmalbrock PhD for their assistance with data collection and Sarah Sydnor, and Katie Pepper for data analysis This work was supported in part by National Institutes of Health (NIH) grants 1T32 AR050959, R01 HD40984 and R01 HD36725.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baloh RW, Ying SH, Jacobson KM. A longitudinal study of gait and balance dysfunction in normal older people. Arch. Neurol. 2003;60:835–839. doi: 10.1001/archneur.60.6.835. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Brammer M, Harvey I, Persaud R, Murray R, Ron M. Fractal analysis of the boundary between white matter and cerebral cortex in magnetic resonance images: A controlled study of schizophrenic and manic-depressive patients. Psychol. Med. 1994;24:771–781. doi: 10.1017/s0033291700027926. [DOI] [PubMed] [Google Scholar]
- Cho SH, Kim DG, Kim DS, Kim YH, Lee CH, Jang SH. Motor outcome according to the integrity of the corticospinal tract determined by diffusion tensor tractography in the early stage of corona radiata infarct. Neurosci. Lett. 2007;426:123–127. doi: 10.1016/j.neulet.2007.08.049. [DOI] [PubMed] [Google Scholar]
- Cook MJ, Free SL, Manford MRA, Fish DR, Shorvon SD, Stevens JM. Fractal description of cerebral cortical patterns in frontal lobe epilepsy. Eur. Neurol. 1995;35:327–335. doi: 10.1159/000117155. [DOI] [PubMed] [Google Scholar]
- Damasio H, Damasio AR. Lesion analysis in neuropsychology. New York: Oxford University Press; 1989. [Google Scholar]
- Duvernoy H. The Human Brain: Surface, Three-dimensional Sectional Anatomy and MRI. New York: Springer-Verlag; 1991. [Google Scholar]
- Esteban FJ, Sepulcre J, de Mendizábal NV, Goñi J, Navas J, de Miras JR, Bejarano B, Masdeu JC, Villoslada P. Fractal dimension and white matter changes in multiple sclerosis. Neuroimage. 2007;36:543–549. doi: 10.1016/j.neuroimage.2007.03.057. [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand. J.Rehabil. Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Gerig G, Styner M, Shenton ME, Lieberman JA. Shape versus size: Improved understanding of the morphology of brain structures. Proc MICCAI. LNCS 2208. 2001:24–32. [Google Scholar]
- Gladstone DJ, Danells CJ, Black SE. The fugl-meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil. Neural Repair. 2002;16:232–240. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- Guo X, Skoog I, Matousek M, Larsson L, Palsson S, Sundh V, Steen B. A population-based study on motor performance and white matter lesions in older women. J. Am. Geriatr. Soc. 2000;48:967–970. doi: 10.1111/j.1532-5415.2000.tb06896.x. [DOI] [PubMed] [Google Scholar]
- Inzitariz D. Leukoaraiosis: An independent risk factor for stroke? Stroke. 2003;34:2067–2071. doi: 10.1161/01.STR.0000080934.68280.82. [DOI] [PubMed] [Google Scholar]
- Kunimatsu A, Aoki S, Masutani Y, Abe O, Mori H, Ohtomo K. Three-dimensional white matter tractography by diffusion tensor imaging in ischaemic stroke involving the corticospinal tract. Neuroradiology. 2003;45:532–535. doi: 10.1007/s00234-003-0974-4. [DOI] [PubMed] [Google Scholar]
- Kunimatsu A, Itoh D, Nakata Y, Kunimatsu N, Aoki S, Masutani Y, Abe O, Yoshida M, Minami M, Ohtomo K. Utilization of diffusion tensor tractography in combination with spatial normalization to assess involvement of the corticospinal tract in capsular/pericapsular stroke: Feasibility and clinical implications. J. Magn. Reson. Imaging. 2007;26:1399–1404. doi: 10.1002/jmri.20945. [DOI] [PubMed] [Google Scholar]
- Lam L, Lee SW, Suen CY. Thinning methodologies - a comprehensive survey. IEEE Trans. Patt. Anal. Machine Intell. 1992;14:869–885. [Google Scholar]
- Leys D, Henon H, Pasquier F. White matter changes and poststroke dementia. Dement. Geriatr. Cogn. Disord. 1998;9 Suppl 1:25–29. doi: 10.1159/000051186. [DOI] [PubMed] [Google Scholar]
- Liang Z, Zeng J, Liu S, Ling X, Xu A, Yu J, Ling L. J. Neurol. Neurosurg. Psychiatry. 2007;78:581–586. doi: 10.1136/jnnp.2006.099077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie C, Hirsch JG, Rossmanith C, Hennerici MG, Gass A. Clinicotopographical correlation of corticospinal tract stroke: a color-coded diffusion tensor imaging study. Stroke. 2004;35:86–92. doi: 10.1161/01.STR.0000106912.09663.EB. [DOI] [PubMed] [Google Scholar]
- Longstreth WT, Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O'Leary D, Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The cardiovascular health study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- Makale M, Solomon J, Patronas NJ, Danek A, Butman JA, Grafman J. Quantification of brain lesions using interactive automated software. Behav. Res. Methods Instrum. Comput. 2002;34:6–18. doi: 10.3758/bf03195419. [DOI] [PubMed] [Google Scholar]
- Mandelbrot BB. The Fractal Geometry of Nature. New York: Freeman; 1982. [Google Scholar]
- Mantyla R, Erkinjuntti T, Salonen O, Aronen HJ, Peltonen T, Pohjasvaara T, Standertskjold-Nordenstam CG. Variable agreement between visual rating scales for white matter hyperintensities on MRI. Comparison of 13 rating scales in a poststroke cohort. Stroke. 1997;28:1614–1623. doi: 10.1161/01.str.28.8.1614. [DOI] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J. Comp. Neurol. 2003;462:144–152. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- May A, Gaser C. Magnetic resonance-based morphometry: a window into structural plasticity of the brain. Curr. Opin. Neurol. 2006;19:407–411. doi: 10.1097/01.wco.0000236622.91495.21. [DOI] [PubMed] [Google Scholar]
- Medina D, Detoledo-Morrell L, Urresta F, Gabrieli JD, Moseley M, Fleischman D, Bennett DA, Leurgans S, Turner DA, Stebbins GT. White matter changes in mild cognitive impairment and AD: A diffusion tensor imaging study. Neurobiol. Aging. 2006;27:663–672. doi: 10.1016/j.neurobiolaging.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Kawamura J, Terayama Y. White matter lesions in the elderly. J. Neurol. Sci. 1992;110:1–7. doi: 10.1016/0022-510x(92)90002-3. [DOI] [PubMed] [Google Scholar]
- Mori S. Introduction to diffusion tensor imaging. 1st ed. UK: Elsevier; 2007. [Google Scholar]
- Pantoni L, Basile AM, Pracucci G, Asplund K, Bogousslavsky J, Chabriat H, Erkinjuntti T, Fazekas F, Ferro JM, Hennerici M, O'Brien J, Scheltens P, Visser MC, Wahlund LO, Waldemar G, Wallin A, Inzitari D. Impact of age-related cerebral white matter changes on the transition to disability -- the LADIS study: Rationale, design and methodology. Neuroepidemiology. 2005;24:51–62. doi: 10.1159/000081050. [DOI] [PubMed] [Google Scholar]
- Platz T, Pinkowskiq C, van Wijck F, Kim IH, di Bella P, Johnson G. Reliability and validity of arm function assessment with standardized guidelines for the fugl-meyer test, action research arm test and box and block test: A multicentre study. Clin. Rehabil. 2005;19:404–411. doi: 10.1191/0269215505cr832oa. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav. Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Sachdev PS, Wen W, Christensen H, Jorm AF. White matter hyperintensities are related to physical disability and poor motor function. J. Neurol. Neurosurg. Psychiatry. 2005;76:362–367. doi: 10.1136/jnnp.2004.042945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson M, Soderfeldt B, Olsson GB. Functional outcome in patients with lacunar infarction. Stroke. 1996;27:842–846. doi: 10.1161/01.str.27.5.842. [DOI] [PubMed] [Google Scholar]
- Schiemanck SK, Post MW, Kwakkel G, Witkamp TD, Kappelle LJ, Prevo AJ. Ischemic lesion volume correlates with long-term functional outcome and quality of life of middle cerebral artery stroke survivors. Restor. Neurol. Neurosci. 2005;23:257–263. [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum. Brain Mapp. 2003;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotactic atlas of the human brain. New York: Thieme-Verlag; 1988. [Google Scholar]
- Taub E. Movement in nonhuman primates deprived of somatosensory feedback. Exerc. Sport Sci. Rev. 1976;4:335–374. [PubMed] [Google Scholar]
- Viswanathan A, Gschwendtner A, Guichard JP, Buffon F, Cumurciuc R, O'Sullivan M, Holtmannspötter M, Pachai C, Bousser MG, Dichgans M, Chabriat H. Lacunar lesions are independently associated with disability and cognitive impairment in CADASIL. Neurology. 2007;69:172–179. doi: 10.1212/01.wnl.0000265221.05610.70. [DOI] [PubMed] [Google Scholar]
- Wang C, Stebbins GT, Nyenhuis DL, deToledo-Morrell L, Freels S, Gencheva E, Pedelty L, Sripathirathan K, Moseley ME, Turner DA, GAbrieli JD, Gorelick PB. Longitudinal changes in white matter following ischemic stroke: a three-year follow-up study. Neurobiol. Aging. 2006;27:1827–1833. doi: 10.1016/j.neurobiolaging.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Wen W, Sachdev PS. Extent and distribution of white matter hyperintensities in stroke patients: the Sydney Stroke Study. Stroke. 2004;35:2813–2819. doi: 10.1161/01.STR.0000147034.25760.3d. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke. 2001;32:1635–1639. doi: 10.1161/01.str.32.7.1635. [DOI] [PubMed] [Google Scholar]
- Yamauchi H, Fukuda H, Oyanagi C. Significance of white matter high intensity lesions as a predictor of stroke from arteriolosclerosis. J. Neurol. Neurosurg. Psychiatry. 2002;72:576–582. doi: 10.1136/jnnp.72.5.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Liu JZ, Dean D, Sahgal V, Yue GH. A three-dimensional fractal analysis method for quantifying white matter structure in human brain. J. Neurosci. Methods. 2006;150:242–253. doi: 10.1016/j.jneumeth.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Zhang L, Dean D, Liu JZ, Sahgal V, Wang X, Yue GH. Quantifying degeneration of white matter in normal aging using fractal dimension. Neurobiol. Aging. 2007a;28:1543–1555. doi: 10.1016/j.neurobiolaging.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Zhang L, Butler AJ, Wang X, Sahgal V, Yue GH. White matter complexity and motor function changes after constraint induced therapy in stroke. Soc. Neurosci. Abstr. 2007b;900:19. [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]