Abstract
Flavonoids can protect against inflammatory diseases such as atherosclerosis by decreasing vascular endothelial cell activation. Plasma microdomains called caveolae may be critical in regulating endothelial activation. Caveolae are particularly abundant in endothelial cells and play a major role in endothelial trafficking and the regulation of signaling pathways associated with the pathology of vascular diseases. We hypothesize that flavonoids can down-regulate endothelial inflammatory parameters by modulating caveolae-regulated cell signaling. We focused on the role of caveolae and its major protein, caveolin-1, in mechanisms of linoleic acid-induced endothelial cell activation and protection by the catechin EGCG. Exposure to linoleic acid for 6 h induced both caveolin-1 and COX-2 expression. Pretreatment with EGCG blocked fatty acid-induced caveolin-1 and COX-2 expression in a time and concentration-dependent manner. Similar results were observed with NF-κB DNA binding activity, which was also reduced by caveolin-1 silencing. Exposure to linoleic acid rapidly increased phosphorylation of several kinases, including p38 MAPK, ERK1/2, and Akt, with maximal induction at about 10 min. Inhibitors of ERK1/2and Akt down-regulated the linoleic acid-induced increase in COX-2 protein, which also occurred after pretreatment with EGCG. Caveolin-1 silencing blocked linoleic acid-induced phosphorylation of ERK1/2 and protein expression of COX-2, suggesting that specific MAPK signaling is caveolae-dependent. Our data provide evidence that caveolae may play a critical role in regulating vascular endothelial cell activation and protection by flavonoids such as EGCG.
Keywords: Flavonoids, EGCG, protection, linoleic acid, inflammation, endothelial cell, caveolae, cell signaling
Introduction
The lining of blood vessels is protected by the endothelium, and dysfunction of endothelial cells is a critical underlying cause of the initiation of cardiovascular diseases [1]. In addition to endothelial barrier dysfunction, another functional change leading to atherosclerosis is the activation of the endothelium by pro-inflammatory mediators that regulate the vascular entry of leukocytes. Cyclooxygenase inhibitors can be applied to reduce inflammation, to relieve from pain, or to prevent atherothrombotic complications in cardiovascular diseases [2]. The inducible isoform of cyclooxygenase-2 (COX-2) plays a role in inflammation and is constitutively expressed in tissues such as the kidney or vascular endothelium [3].
There is clear evidence that hypertriglyceridemia is an independent risk factor of cardiovascular diseases such as atherosclerosis [4, 5]. Even though diets high in omega-6 fatty acids may lead to a decrease in serum cholesterol [6], replacing saturated with unsaturated omega-6 rich lipids may not be desirable because of their ability to easily oxidize. High intake of linoleic acid-rich oils or fats will lead to an increase in cellular oxidative stress, which has been implicated in most chronic diseases. Omega-6 fatty acids, and especially linoleic acid can cause endothelial cell dysfunction as well as potentiate tumor necrosis factor-α (TNF-α)-mediated endothelial injury [7]. We have recently demonstrated that both the extracellular signal regulated kinase (ERK1/2) and phosphoinositide-3 kinase/amino kinase terminal (PI3K/Akt) signaling pathways can contribute to the effect of linoleic acid on nuclear factor-kappa B (NF-κB)-dependent transcription and endothelial cell activation [8].
There is much evidence suggesting diets high in various nutrients and phytochemicals (e.g., flavonoids) are associated with a reduced risk of chronic diseases, such as cardiovascular diseases and cancer, by affecting molecular mechanisms involved in the initiation and progression of these diseases [9, 10]. Flavonoids constitute a subclass of bioactive compounds rich in fruits and vegetables, soy food, legumes, tea and cocoa [11]. Many flavonoids are composed of a polyphenol structure (i.e., several hydroxyl groups on aromatic rings), and these polyphenols are often classified according to structural similarities [12]. Examples of flavonoids include flavonols (e.g., quercetin), isoflavones (e.g., genistein), flavonones (e.g., hesperetin), and flanan-3-ols (e.g., catechins). Many of these bioactive food components are lipophilic, suggesting they may have a possible interaction with membrane domains or cellular lipid components such as caveolae.
There is increasing evidence that caveolae play a critical role in the pathology of atherosclerosis [13] and that the lack of the caveolin-1 gene may provide protection against the development of atherosclerosis [14]. Caveolae are particularly abundant in endothelial cells, where they are believed to play a major role in the regulation of endothelial vesicular trafficking as well as the uptake of lipids and related lipophilic compounds [15], possibly including bioactive food components such as flavonoids. There is evidence that fatty acids can alter localization and function of caveolae-associated signaling proteins in mouse colonic mucosa [16]. Caveolins also have been reported to co-localize with cyclooxyenase, suggesting that caveolins play a role in regulating the function of this enzyme [17, 18]. Besides their role in cellular uptake of lipophilic substances, caveolae house an array of cell signaling molecules involved in endothelial cell dysfunction and inflammation [13].
Little is known about the involvement of caveolae in gene regulation by bioactive compounds. In hypertensive rats, quercetin prevented up-regulation of endothelial nitric oxide synthase (eNOS) expression [19], an enzyme associated with caveolae and involved in vascular tone regulation. Genistein has been shown to decrease the expression of caveolin-1 in ovariectomized rat hearts [20]. Furthermore, the internalization of caveolae can be suppressed by tyrosine kinase inhibitors such as staurosporine [21]. Flavonoids have been described to inhibit these kinases [22, 23], which suggests that these bioactive compounds can modulate the function of caveolae.
A major objective of the current study was to explore specific mechanisms involved in anti-inflammatory properties of bioactive compounds like flavonoids within the vascular endothelium. We hypothesized that the regulation of signaling pathways induced by bioactive compounds is associated within caveolae and linked to caveolae function and associated gene inductions. Our data provide evidence that linoleic acid-induced inflammatory parameters (e.g., COX-2 expression) can be down-regulated by bioactive food components such as epigallocatechin-3-gallate (EGCG) and that these metabolic events are linked to caveolae signaling.
Materials and Methods
Materials
Linoleic acid (>99% pure) was obtained from Nu-Chek Prep (Elysian, MN) and epigallocatechin gallate (EGCG, >98% pure) was purchased from Cayman Chemical (Ann Arbor, MI). Inhibitors LY294002, PD98059, and SB 203580 as well as antibodies used for immunoblotting including anti-Akt, anti-phospho Akt, anti-ERK1/2, anti-phospho ERK1/2, anti-p38, anti-phospho p38, and anti-rabbit Ig horseradish peroxidase linked were obtained from Cell Signaling Technology (Danvers, MA). Anti-caveolin-1 antibody was obtained from Affinity BioReagents (Golden, MO). Anti-COX-2 and anti-p65 NF-κB antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-β-actin antibody was purchased from Sigma (Saint Louis, MO). Supplies and reagents for SDS-PAGE were purchased from Bio-Rad (Hercules, CA).
Cell culture
EAhy926 cells were a gift from Dr. C. S. Edgell (University of North Carolina). The EAhy926 line was derived by fusing human umbilical vein endothelial cells with the permanent human cell line A549 [24]. The culture medium consisted of Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad, CA), 10% fetal bovine serum (FBS; Hyclone, Logan, UT), and antibiotics. Cell cultures were grown until confluent, and then synchronized by maintaining in 1% serum for 16 h before treatment for various times periods. Experimental media contained 5% FBS and was supplemented with EGCG for 12 h, and followed by linoleic acid at the final concentration of 90 μM, for 6 h. Optimal experimental conditions, such as concentration of linoleic acid (90 μM) and time exposure (6 h), to activate endothelial cells were established previously [25]. Experimental media were enriched with linoleic acid as described earlier [25].
Caveolin-1 siRNA and transfection
The caveolin-1 gene silencer was designed according to previously described methods [26]. Cells were transfected with control siRNA or caveolin-1 siRNA at a final concentration of 80 nM using GeneSilencer (Genlantis, San Diego, CA) with Optimem I medium (Invitrogen, Carlsbad, CA). Cells were incubated with transfection mixtures for 4 h and then replaced with 10% serum medium. Cells were synchronized overnight after 48 h transfection, pre-treated with EGCG for 12 h, and then treated with linoleic acid or vehicle.
Immunoblotting
Cells were treated with either vehicle (0.1% DMSO) or EGCG (0–40 μM) followed by linoleic acid (90 μM) for immunoblot analysis of caveolin-1 and COX-2 protein activation. Treatment with DMSO (vehicle) alone for up to 48 h did not affect the expression of both caveolin-1 and COX-2 (data not shown). To analyze the expression of linoleic acid-induced phospho-Akt, -ERK1/2, and -p38, cells were treated with linoleic acid (90 μM, 0–20 min), or vehicle (0.1% DMSO). To analyze the effects of MAPK inhibitors on linoleic acid induced COX-2 expression, cells were treated with Akt, ERK1/2, and p38 inhibitors (LY294002, PD98059, and SB 203580, respectively) for 1 h, followed by the treatment of linoleic acid (90 μM, 6 h), or vehicle (0.1% DMSO). Cell protein was extracted as described before [8]. Equal amounts of protein (20 μg) were fractionated by SDS-PAGE (12% acrylamide) and transferred to nitrocellulose membranes. The membrane was blocked 1 h at room temperature with 5% nonfat milk in Tris-buffered saline (TBS, pH 7.6) containing 0.05% Tween-20, and then washed with TBS-Tween. Membranes were incubated overnight with the primary antibody (1,000-fold diluted in TBST containing 5% bovine serum albumin) at 4 °C and for 1 h with HRP-conjugated secondary antibody (~5,000-fold diluted) at room temperature. Bands were visualized using the appropriate horseradish peroxidase-conjugated secondary antibodies followed by ECL immunoblotting detection reagents (Amersham Biosciences, Buckinghamshire, England).
Electrophoretic mobility shift assays of NF-κB DNA binding
Nuclear extracts from endothelial cells were prepared as previously described [27]. Synthetic 5′-biotinylated complementary oligonucleotides were purchased from Integrated DNA Technologies (Coralville, IA). Nuclear extracts were incubated at room temperature for 20 min with biotin-labeled oligonucleotide probes containing the enhancer DNA element for NF-κB (5′-AGTTGAGGGGACTTTCCCAGGC -3′). Gel mobility shift assay was performed to demonstrate the shifted DNA-protein complexes for NF-κB using a LightShiftTM chemiluminescent EMSA kit (Pierce, Rockford, IL) [28].
Real-time reverse-transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA), according to the manufacturer’s protocol. Reverse transcription was performed using the AMV reverse transcription system (Promega, Madison, WI). The levels of mRNAs and the PCR-products were then assessed by real-time PCR using 7300 Real time PCR system (Applied Biosystems, CA). Samples were mixed with SYBR Green Master Mix (Applied Biosystems CA), and caveolin-1 or 18S specific primers. The sequences for human caveolin-1 gene were designed by Primer Express Software 3.0 for real-time PCR (Applied Biosystems CA), and the sequences were as followed: sense, 5′ TCAACCGCGACCCTAAACAC3′; antisense, 5′ CCTTCCAAATGCCGTCAAAA 3′. The house keeping gene was 18S (sense, 5′ TCGGAACTGAGGCCATGATT 3′, antisense, 5′ TTTCGCTCTGGTCCGTCTTG 3′).
Statistical analysis
Values are reported as mean ± standard error of the mean (SEM) of at least three independent groups. Data were analyzed using Sigma Stat software (Jandel Corp., Wan Rafael, CA). One way ANOVA followed by post hoc least significant difference (LSD)’s pairwise multiple comparison procedure were used for statistical analysis of the original data. A statistical probability of p< 0.05 was considered significant.
Results
EGCG decreases caveolin-1 levels in endothelial cells
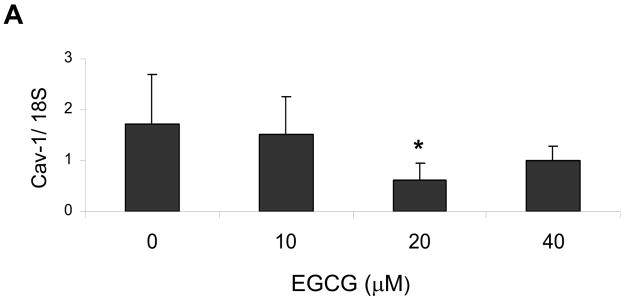
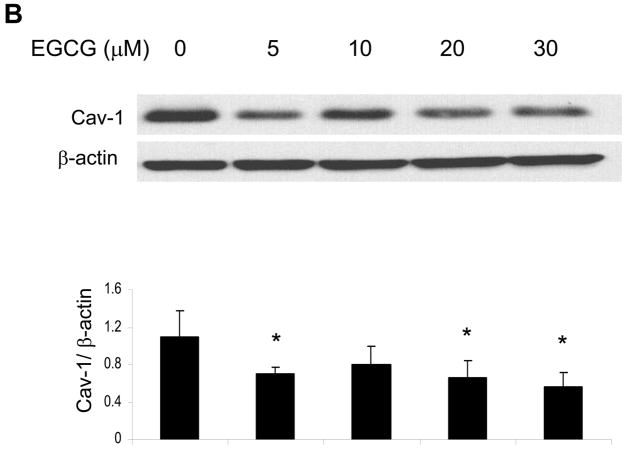
There is evidence that the lack of the caveolin-1 gene may provide protection against the development of atherosclerosis [14]. Thus, we tested the possibility that EGCG can down-regulated base-line levels of caveolin-1. Indeed, pretreatment of endothelial cells with increasing concentrations of EGCG down-regulated base-line levels of caveolin-1. A maximum decrease in caveolin-1 mRNA occurred in cells treated with 20 μM EGCG (Figure 1A). Protein expression of caveolin-1, however, reached the lowest values in cultures exposed to 5 and 20–30 μM EGCG (Figure 1B).
Figure 1.
EGCG decreases caveolin-1 levels in endothelial cells. Cells were treated with either vehicle (0.1% DMSO) or EGCG (0–40 μM) for 12 h before determining caveolin-1 (Cav-1) expression by real-time PCR (Figure 1A) and western blot analysis (Figure 1B). The western blot shown represents one of three experiments. Densitometry results shown in parallel represent the mean ± SEM of three independent experiments. *Significantly different compared to control cultures.
EGCG protects against linoleic acid-induced caveolin-1 expression
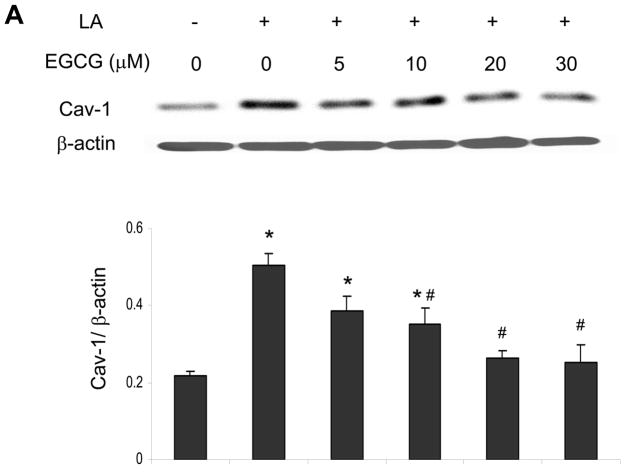
Exposing endothelial cells for 6 h to linoleic acid (90 μM) markedly induced caveolin-1 (Figure 2A) protein expression. To test whether EGCG can down-regulate the fatty acid-mediated induction of caveolin-1, endothelial cells were pretreated with EGCG for 12 h, followed by exposure to linoleic acid. A concentration-dependent protective effect of EGCG against linoleic acid-induced caveolin-1 was observed, with a 20 μM EGCG pretreatment completely blocking the fatty acid affect.
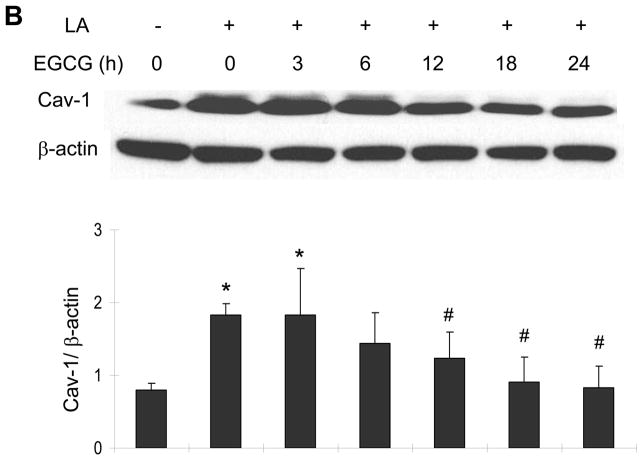
Figure 2.
EGCG protects against linoleic acid-induced caveolin-1 expression. Cells were pretreated with either vehicle (0.1% DMSO) or EGCG (0–30 μM) for 12 h, followed by exposure to linoleic acid (LA, 90 μM) for an additional 6 h (Figure 2A). To assess the time-dependent protective effect of EGCG (Figure 2B), some cultures were pretreated for up to 24 h with EGCG (20 μM) before exposure to LA for an additional 6 h. Caveolin-1 (Cav-1) protein was determined by western blot analysis. Each western blot shown represents one of three experiments. Densitometry results shown in parallel represent the mean ± SEM of three independent experiments. *Significantly different compared to vehicle control. #Significantly different compared to cultures treated only with LA.
To assess the time-dependent protective effects of EGCG, endothelial cultures were pretreated for up to 24 h with EGCG (20 μM), before exposure to linoleic acid for an additional 6 h. The fatty acid-mediated induction of caveolin-1 was completely blocked when cultures were pretreated with EGCG for 12 h (Figure 2B).
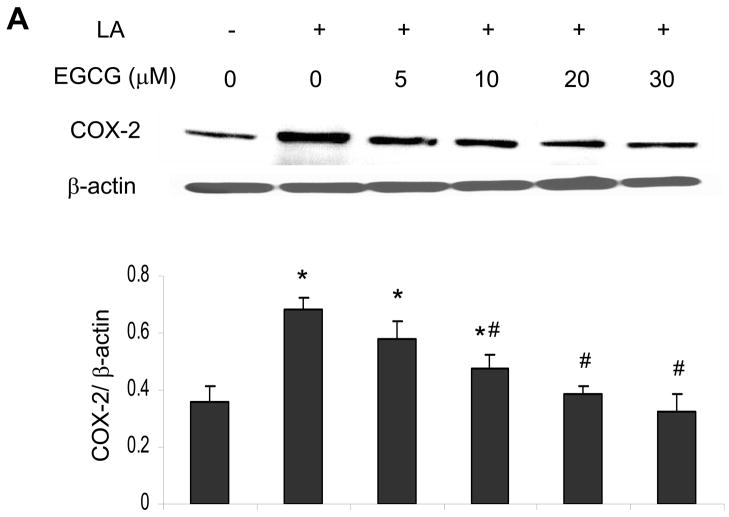
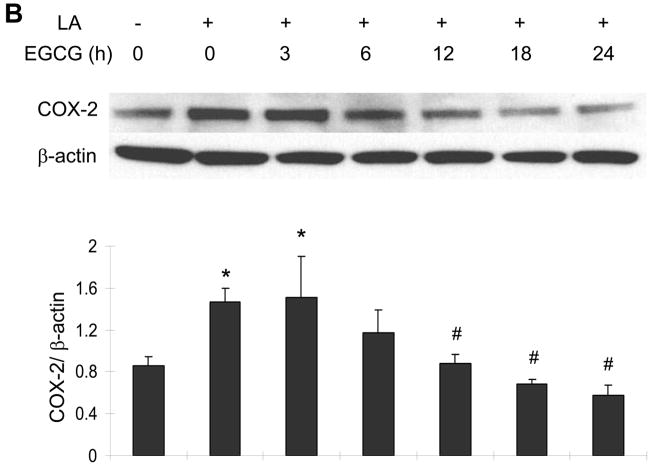
EGCG attenuates linoleic acid-mediated upregulation of COX-2
Similar to the caveolin-1 data, exposing endothelial cells to linoleic acid markedly induced COX-2 protein expression (Figure 3A). Pretreatment with 10 to 30 μM EGCG for 12 h markedly attenuated the fatty acid-induced COX-2 protein expression (Figure 3A). Similar to the caveolin-1 data, the linoleic acid-induced induction of COX-2 was time-dependent and completely blocked after a minimum of 12 h pre-exposure to EGCG (Figure 3B).
Figure 3.
EGCG attenuates linoleic acid-mediated upregulation of COX-2. Similar to Figure 2, cells were pretreated with either vehicle (0.1% DMSO) or EGCG (0–30 μM) for 12 h, followed by exposure to linoleic acid (LA, 90 μM) for an additional 6 h (Figure 3A). Some cultures were pretreated for up to 24 h with EGCG (20 μM) before exposure to LA for an additional 6 h (Figure 3B. COX-2 protein expression was determined by western blot analysis. Each western blot shown represents one of three experiments. Densitometry results shown in parallel represent the mean ± SEM of three independent experiments. *Significantly different compared to vehicle control. #Significantly different compared to cultures treated only with LA.
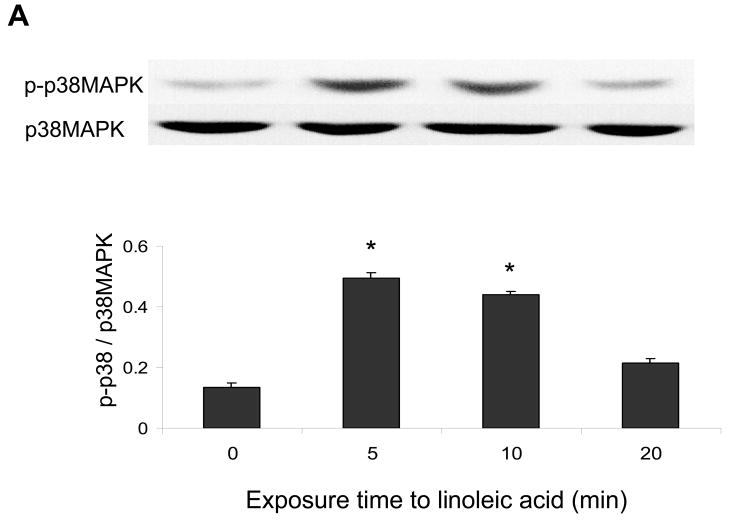
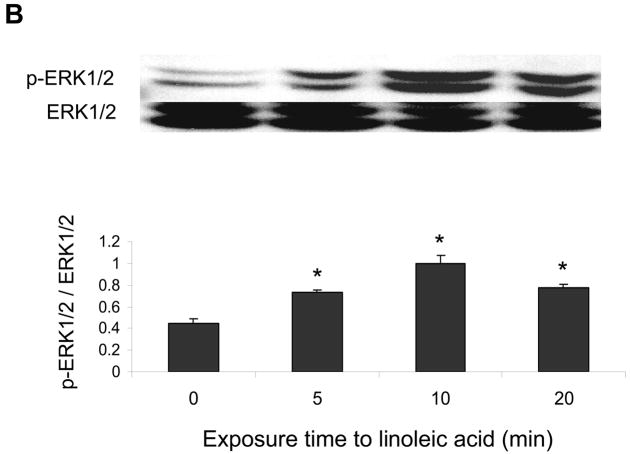
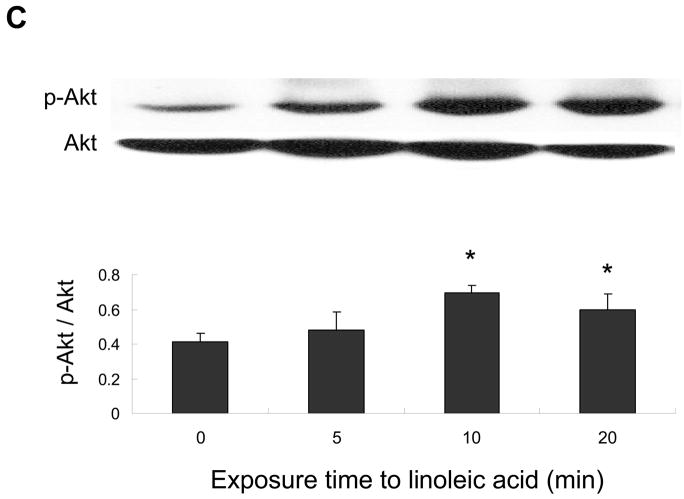
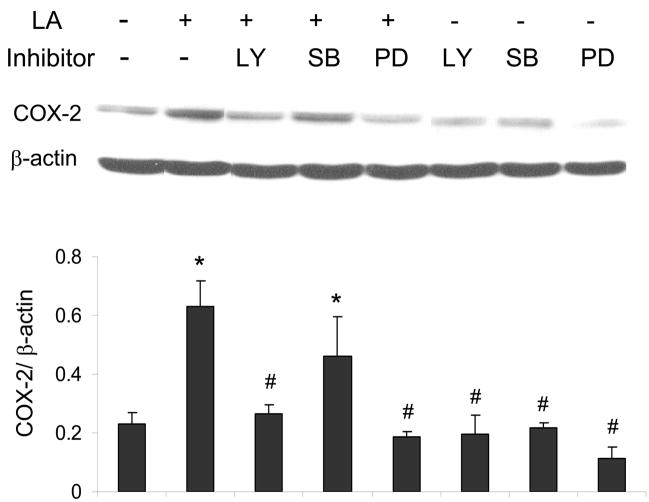
ERK1/2 and Akt but not p38 MAPK are involved in linoleic acid-induced upregulation of COX-2
The p38 MAPK, Akt and/or ERK1/2 pathways may regulate COX-2 expression. Therefore, endothelial cells were exposed to linoleic acid, and the levels of phosphorylated p38 MAPK (p-p38), Akt (p-Akt) and ERK1/2 (p-ERK1/2) were assessed by western blotting. As indicated in Figures 4, linoleic acid rapidly increased activation of all kinases in a time-dependent manner, with maximum phosphorylation at 5 min for p-p38 MAPK (Figure 4A), 5 min for p-ERK1/2 (Figure 4B), and 10 min for p-Akt (Figure 4C). In order to determine which of these kinases is involved in linoleic acid-mediated COX-2 expression, endothelial cells were pretreated for 1 h with specific pharmacological inhibitors of individual kinases, such as LY294002 for Akt, SB203580 for p38 MAPK, and PD98059 for ERK1/2. Subsequently, cells were incubated with or without 90 μM linoleic acid for 6 h. As indicated in Figure 5, LY294002 and PD98059, but not SB203580, effectively blocked linoleic acid-induced activation of COX-2. Treatment with inhibitors alone had no effect on activation of COX-2 or expression of caveolin-1 (data not shown).
Figure 4.
Linoleic acid activates p38 MAPK, Akt, and ERK1/2 signaling in vascular endothelial cells. Cells were exposed to linoleic acid (90 μM) for 5, 10, or 20 min. Total p38 MAPK, Akt, or ERK1/2 and phosphorylated p38 MAPK, Akt, or ERK1/2 were detected by western blot using specific antibodies. The western blots shown for each phosphorylated kinase represent one of three experiments. Densitometry results shown in parallel represent the mean ± SEM of three independent experiments. *Significantly different compared to control cultures.
Figure 5.
ERK1/2 and Akt but not p38 MAPK are involved in linoleic acid-induced upregulation of COX-2. Endothelial cells were pretreated with or without the Akt inhibitor LY294002 (LY, 10 μM for 1 h), the p38 MAPK inhibitor SB 203580 (SB, 10 μM for 1 h) or the ERK1/2 inhibitor PD98059 (PD, 20μM for 1 h), followed by exposure to linoleic acid (LA, 90 μM) for 6 h. Activation of COX-2 was determined by western blot analysis (Figure 5). The western blot shown represents one of three experiments. Densitometry results shown in parallel represent mean ± SEM of three independent experiments. *Significantly different compared to control cultures. #Significantly different compared to cultures treated only with linoleic acid.
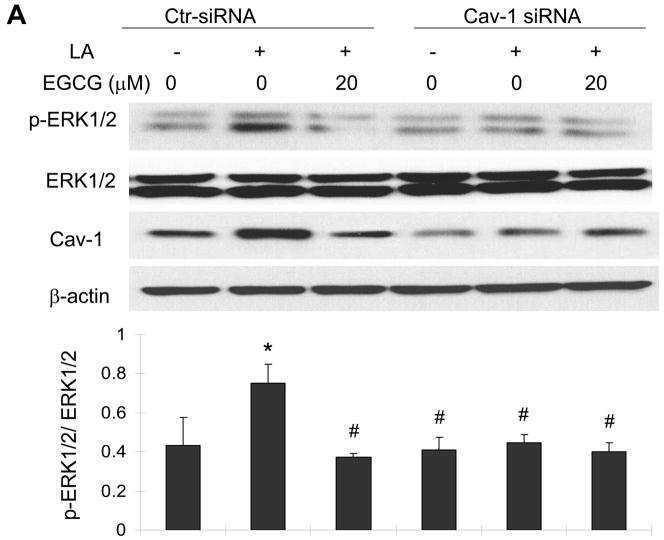
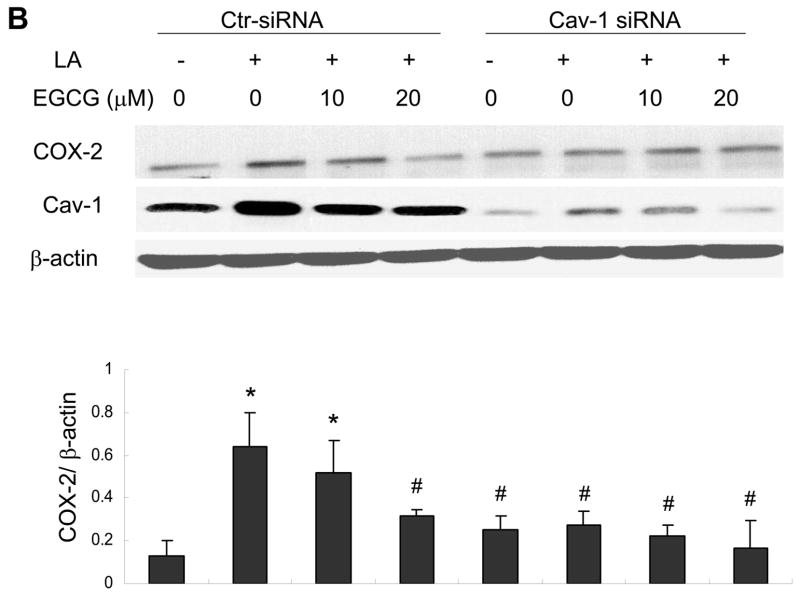
Caveolin-1 silencing mimics the protective effects of EGCG on linoleic acid-induced ERK1/2 phosphorylation and COX-2 expression
Knowing that the ERK1/2 and Akt pathways are involved in linoleic acid-mediated induction of COX-2, we next determined the role of caveolin-1 and EGCG in activation of these signaling cascades. In these experiments, we utilized small interfering RNA to specifically silence caveolin-1. This procedure reduced caveolin-1 expression by ~80 % as compared with control cells without changing β-actin and total ERK1/2 levels (Figure 6A). Most importantly, caveolin-1 silencing significantly protected against linoleic acid-induced phosphorylation of ERK1/2 (Figure 6A); however, it did not affect linoleic acid-mediated phosphorylation of Akt (data not shown). Pretreatment with EGCG blocked linoleic acid-mediated phosphorylation of both ERK1/2 (Figure 6A) and Akt (data not shown). Similar to the ERK1/2 phosphorylation data, both pretreatment with EGCG or caveolin-1 silencing effectively blocked linoleic acid-mediated induction of COX-2 (Figure 6B).
Figure 6.
Caveolin-1 silencing mimics the protective effects of EGCG on linoleic acid-induced ERK1/2 phosphorylation (Figure 6A) and activation of COX-2 (Figure 6B). Endothelial cells were transfected with siRNA for caveolin-1 (Cav-1 siRNA) or with control siRNA (Ctr- siRNA) and treated with EGCG (20 μM) for 12 h, followed by exposure to linoleic acid (LA, 90 μM) for 10 min (Figure 6A) or 6 h (Figure 6B). Cell lysates were probed with caveolin-1 (Cav-1), COX-2 and β-actin antibodies or with anti-p-ERK1/2 and anti-ERK1/2. Protein expression was determined by western blot analysis. The western blot shown in each figure represents one of three experiments. Densitometry results shown in parallel represent the mean ± SEM of three independent experiments. *Significantly different compared to control cultures. #Significantly different compared to cultures treated only with LA (Ctr-siRNA).
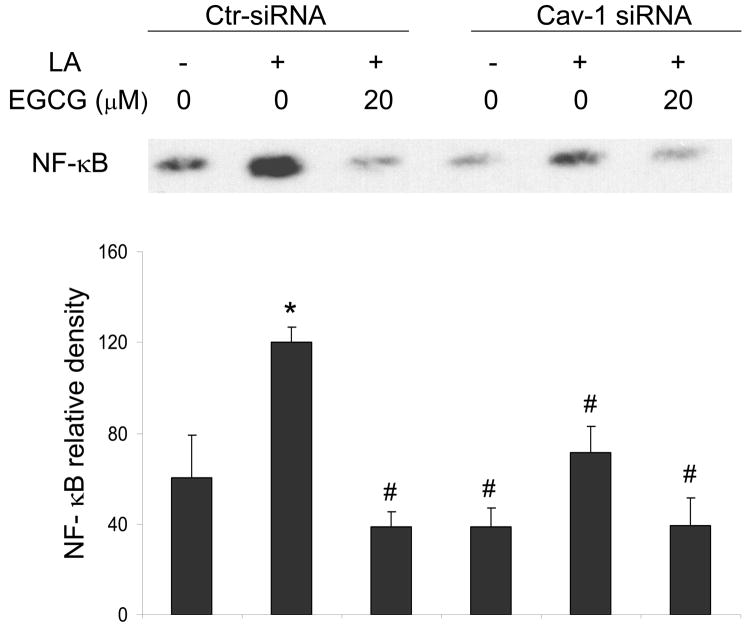
Caveolin-1 silencing reduces linoleic acid-induced NF-κB DNA binding
NF-κB is a transcriptional regulator of COX-2 induction. Therefore, our studies were completed by determination of the role of EGCG and caveolin-1 in linoleic acid-induced activation of NF-κB. Linoleic acid significantly increased NF-κB DNA binding activity, which was blocked when cells were pre-treated with EGCG (Figure 7). In addition, caveolin-1 silencing mimicked the effects of EGCG and significantly decreased the linoleic acid-induced activation of NF-κB (Figure 7).
Figure 7.
EGCG and caveolin-1 silencing both reduce linoleic acid induced NF-κB DNA binding. Endothelial cells were transfected with siRNA for caveolin-1 (Cav-1 siRNA) or with control siRNA (Ctr-siRNA) and treated with EGCG (20 μM) for 12 h, followed by exposure to linoleic acid (LA, 90 μM) for 3 h. Electrophoretic mobility shift assay for NF-κB was performed with nuclear proteins extracted from endothelial cells. The western blot shown represents one of three experiments. Densitometry results shown in parallel represent the mean ± SEM of three independent experiments. *Significantly different compared to vehicle control. #Significantly different compared to cultures treated only with LA (Ctr-siRNA).
Discussion
Bioactive compounds such as flavonoids are known to have anti-inflammatory properties and to provide protection against inflammatory diseases such as atherosclerosis [11]. Endothelial cells line the inner layer of blood vessels and play a critical role in the overall dynamics of vascular physiology. Activation and subsequent dysfunction of the endothelium is considered an early event in the etiology of cardiovascular diseases such as the pathology of atherosclerosis [1]. Endothelial cells are constantly exposed to blood components, including food-derived lipids, toxicants, etc., and are thus highly susceptible to insult and activation, leading to inflammatory interactions with cytokines and increased uptake of activated leukocytes into the vasculature.
In the current study, endothelial cells were activated with linoleic acid to mimic a postprandial hyperlipidemic state. High-fat or high-energy diets can lead to hypertriglyceridemia and increased exposure of the endothelium to free fatty acid anions, metabolic events known to activate endothelial cells [29, 30]. We have previously demonstrated that endothelial cell exposure to fatty acids, and especially linoleic acid, markedly induced an endothelial inflammatory response [31]. We also have demonstrated that the ERK1/2 signaling pathway can contribute to the effect of linoleic acid on NF-κB-dependent transcription [8]. In the current study, inhibitors of ERK1/2 and Akt down-regulated the linoleic acid-induced increase in COX-2 protein, demonstrating the involvement of MAPK signaling in our model of endothelial cell activation.
Most interestingly, linoleic acid induced both caveolin-1 and COX-2, which is significant because of the link between caveolins (caveolae) and the pathology of atherosclerosis [13]. Caveolin-1 has been reported to co-localize with interleukin-1beta-induced COX-2 [30], suggesting the dependence of COX-2 induction on functional caveolae. Recent evidence suggests that high-fat diets can up-regulate caveolin-1 expression in aorta of diet-induced obese rats [32], suggesting that our fatty acid data may mimic an in vivo response by activating COX-2. High-fat diets contribute to hypertriglyceridemia, and the vascular endothelium can be exposed to significant levels of free fatty acids derived from lipoprotein lipase-mediated hydrolysis of triglyceride-rich lipoproteins [7].
Protective mechanisms of plant-derived bioactive compounds (e.g., flavonoids) against endothelial activation and inflammation are not well understood. EGCG, the most abundant and effective polyphenol of green tea, has been shown to inhibit the expression of COX-2 and the production of prostaglandin E2 [30, 33]. In the current study, pretreatment with EGCG blocked both fatty acid-induced COX-2 and caveolin-1 protein expression in a time and concentration-dependent manner. We also found that EGCG can down-regulate baseline levels of the caveolin-1 gene at both the mRNA and protein level. These data suggest that the anti-inflammatory properties of EGCG may reside at or be initiated at the cellular level of caveolae and associated signaling molecules. In fact, down-regulation of COX-2 by EGCG was mimicked by selective inhibitors of kinases such as ERK1/2 and Akt. Others also have reported EGCG-mediated down-regulation of MAPK pathways such as ERK1/2 and decreased COX-2 activity in cancer cell lines [33, 34] and in human vascular smooth muscle cells [35]. MAPKs such as ERK1/2 are known to regulate NF-κB [36], and there is evidence that a decrease in COX-2 by EGCG may be through inhibition of NF-κB [37]. Indeed, our data show that EGCG can decrease linoleic acid-induced activation of NF-κB. Most importantly, our data suggest that caveolae may provide a critical signaling platform for both induction and protection of inflammatory genes. EGCG concentrations utilized in our cell culture model system were relatively high compared to plasma levels attainable through diets [38]. Thus, further studies are needed in animal models to examine the effects of EGCG on inflammatory genes.
We provide evidence in the current study that EGCG-mediated down-regulation of both NF-κB and COX-2 is dependent on functional caveolin-1, the main structural protein of caveolae. This is significant, because caveolae are highly expressed in endothelial cells [15]. We provide novel data, demonstrating that silencing of the caveolin-1 gene can markedly down-regulate linoleic acid-induced phosphorylation of ERK1/2, NF-κB and COX-2, suggesting that specific MAPK signaling is caveolae-dependent. The specificity for ERK signaling may be due to the co-localization of Ras, a small GTPase upstream of ERK, with caveolae [39–41].
In summary, data from the current study strongly support the hypothesis that caveolae are involved in regulation of inflammatory signaling pathways in vascular endothelial cells. In addition, these signaling mechanisms can be modulated by the interaction of bioactive compounds, such as EGCG, with the cellular lipid milieu.
Acknowledgments
This research was supported in part by grants from NIEHS, NIH (P42ES07380) and the University of Kentucky AES.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Sohn HY, Krotz F. Cyclooxygenase inhibition and atherothrombosis. Curr Drug Targets. 2006;7:1275–1284. doi: 10.2174/138945006778559102. [DOI] [PubMed] [Google Scholar]
- 3.Cipollone F, Fazia ML. Cyclooxygenase-2 inhibition: vascular inflammation and cardiovascular risk. Curr Atheroscler Rep. 2006;8:245–251. doi: 10.1007/s11883-006-0080-2. [DOI] [PubMed] [Google Scholar]
- 4.Austin MA, McKnight B, Edwards KL, Bradley CM, McNeely MJ, Psaty BM, Brunzell JD, Motulsky AG. Cardiovascular disease mortality in familial forms of hypertriglyceridemia: A 20-year prospective study. Circulation. 2000;101:2777–2782. doi: 10.1161/01.cir.101.24.2777. [DOI] [PubMed] [Google Scholar]
- 5.Malloy MJ, Kane JP. A risk factor for atherosclerosis: triglyceride-rich lipoproteins. Adv Intern Med. 2001;47:111–136. [PubMed] [Google Scholar]
- 6.Psota TL, Gebauer SK, Kris-Etherton P. Dietary omega-3 fatty acid intake and cardiovascular risk. Am J Cardiol. 2006;98:3i–18i. doi: 10.1016/j.amjcard.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Hennig B, Toborek M, McClain CJ. High-energy diets, fatty acids and endothelial cell function: implications for atherosclerosis. J Am Coll Nutr. 2001;20:97–105. doi: 10.1080/07315724.2001.10719021. [DOI] [PubMed] [Google Scholar]
- 8.Hennig B, Lei W, Arzuaga X, Ghosh DD, Saraswathi V, Toborek M. Linoleic acid induces proinflammatory events in vascular endothelial cells via activation of PI3K/Akt and ERK1/2 signaling. J Nutr Biochem. 2006;17:766–772. doi: 10.1016/j.jnutbio.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Horia E, Watkins BA. Comparison of stearidonic acid and alpha-linolenic acid on PGE2 production and COX-2 protein levels in MDA-MB-231 breast cancer cell cultures. J Nutr Biochem. 2005;16:184–192. doi: 10.1016/j.jnutbio.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Munoz-Espada AC, Watkins BA. Cyanidin attenuates PGE2 production and cyclooxygenase-2 expression in LNCaP human prostate cancer cells. J Nutr Biochem. 2006;17:589–596. doi: 10.1016/j.jnutbio.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Kris-Etherton PM, Lefevre M, Beecher GR, Gross MD, Keen CL, Etherton TD. Bioactive compounds in nutrition and health-research methodologies for establishing biological function: the antioxidant and anti-inflammatory effects of flavonoids on atherosclerosis. Annu Rev Nutr. 2004;24:511–538. doi: 10.1146/annurev.nutr.23.011702.073237. [DOI] [PubMed] [Google Scholar]
- 12.Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 13.Frank PG, Woodman SE, Park DS, Lisanti MP. Caveolin, caveolae, and endothelial cell function. Arterioscler Thromb Vasc Biol. 2003;23:1161–1168. doi: 10.1161/01.ATV.0000070546.16946.3A. [DOI] [PubMed] [Google Scholar]
- 14.Frank PG, Lee H, Park DS, Tandon NN, Scherer PE, Lisanti MP. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:98–105. doi: 10.1161/01.ATV.0000101182.89118.E5. [DOI] [PubMed] [Google Scholar]
- 15.Matveev S, Li X, Everson W, Smart EJ. The role of caveolae and caveolin in vesicle-dependent and vesicle-independent trafficking. Adv Drug Deliv Rev. 2001;49:237–250. doi: 10.1016/s0169-409x(01)00138-7. [DOI] [PubMed] [Google Scholar]
- 16.Ma DW, Seo J, Davidson LA, Callaway ES, Fan YY, Lupton JR, Chapkin RS. n-3 PUFA alter caveolae lipid composition and resident protein localization in mouse colon. Faseb J. 2004;18:1040–1042. doi: 10.1096/fj.03-1430fje. [DOI] [PubMed] [Google Scholar]
- 17.Cha SH, Jung NH, Kim BR, Kim HW, Kwak JO. Evidence for cyclooxygenase-1 association with caveolin-1 and -2 in cultured human embryonic kidney (HEK 293) cells. IUBMB Life. 2004;56:221–227. doi: 10.1080/15216540410001699312. [DOI] [PubMed] [Google Scholar]
- 18.Kwak JO, Lee WK, Kim HW, Jung SM, Oh KJ, Jung SY, Huh YH, Cha SH. Evidence for cyclooxygenase-2 association with caveolin-3 in primary cultured rat chondrocytes. J Korean Med Sci. 2006;21:100–106. doi: 10.3346/jkms.2006.21.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez M, Galisteo M, Vera R, Villar IC, Zarzuelo A, Tamargo J, Perez-Vizcaino F, Duarte J. Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. J Hypertens. 2006;24:75–84. doi: 10.1097/01.hjh.0000198029.22472.d9. [DOI] [PubMed] [Google Scholar]
- 20.Tang YB, Wang QL, Zhu BY, Huang HL, Liao DF. Phytoestrogen genistein supplementation increases eNOS and decreases caveolin-1 expression in ovariectomized rat hearts. Sheng Li Xue Bao. 2005;57:373–378. [PubMed] [Google Scholar]
- 21.Nabi IR, Le PU. Caveolae/raft-dependent endocytosis. J Cell Biol. 2003;161:673–677. doi: 10.1083/jcb.200302028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pignatelli P, Di Santo S, Buchetti B, Sanguigni V, Brunelli A, Violi F. Polyphenols enhance platelet nitric oxide by inhibiting protein kinase C-dependent NADPH oxidase activation: effect on platelet recruitment. Faseb J. 2006;20:1082–1089. doi: 10.1096/fj.05-5269com. [DOI] [PubMed] [Google Scholar]
- 23.Choi YJ, Jeong YJ, Lee YJ, Kwon HM, Kang YH. (−)Epigallocatechin gallate and quercetin enhance survival signaling in response to oxidant-induced human endothelial apoptosis. J Nutr. 2005;135:707–713. doi: 10.1093/jn/135.4.707. [DOI] [PubMed] [Google Scholar]
- 24.Edgell CJ, McDonald CC, Graham JB. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci U S A. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toborek M, Lee YW, Kaiser S, Hennig B. Measurement of inflammatory properties of fatty acids in human endothelial cells. Methods Enzymol. 2002;352:198–219. doi: 10.1016/s0076-6879(02)52020-6. [DOI] [PubMed] [Google Scholar]
- 26.Repetto S, Salani B, Maggi D, Cordera R. Insulin and IGF-I phosphorylate eNOS in HUVECs by a caveolin-1 dependent mechanism. Biochem Biophys Res Commun. 2005;337:849–852. doi: 10.1016/j.bbrc.2005.09.125. [DOI] [PubMed] [Google Scholar]
- 27.Lim EJ, Kim CW. Functional characterization of the promoter region of the chicken elongation factor-2 gene. Gene. 2007;386:183–190. doi: 10.1016/j.gene.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Sauzeau V, Rolli-Derkinderen M, Marionneau C, Loirand G, Pacaud P. RhoA expression is controlled by nitric oxide through cGMP-dependent protein kinase activation. J Biol Chem. 2003;278:9472–9480. doi: 10.1074/jbc.M212776200. [DOI] [PubMed] [Google Scholar]
- 29.Hennig B, Chung BH, Watkins BA, Alvarado A. Disruption of endothelial barrier function by lipolytic remnants of triglyceride-rich lipoproteins. Atherosclerosis. 1992;95:235–247. doi: 10.1016/0021-9150(92)90027-e. [DOI] [PubMed] [Google Scholar]
- 30.Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med. 2005;56:45–62. doi: 10.1146/annurev.med.56.082103.104751. [DOI] [PubMed] [Google Scholar]
- 31.Toborek M, Lee YW, Garrido R, Kaiser S, Hennig B. Unsaturated fatty acids selectively induce an inflammatory environment in human endothelial cells. Am J Clin Nutr. 2002;75:119–125. doi: 10.1093/ajcn/75.1.119. [DOI] [PubMed] [Google Scholar]
- 32.Yang N, Ying C, Xu M, Zuo X, Ye X, Liu L, Nara Y, Sun X. High-fat diet up-regulates caveolin-1 expression in aorta of diet-induced obese but not in diet-resistant rats. Cardiovasc Res. 2007 doi: 10.1016/j.cardiores.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 33.Jeong YI, Jung ID, Lee JS, Lee CM, Lee JD, Park YM. (−)-Epigallocatechin gallate suppresses indoleamine 2,3-dioxygenase expression in murine dendritic cells: evidences for the COX-2 and STAT1 as potential targets. Biochem Biophys Res Commun. 2007;354:1004–1009. doi: 10.1016/j.bbrc.2007.01.076. [DOI] [PubMed] [Google Scholar]
- 34.Kundu JK, Na HK, Chun KS, Kim YK, Lee SJ, Lee SS, Lee OS, Sim YC, Surh YJ. Inhibition of phorbol ester-induced COX-2 expression by epigallocatechin gallate in mouse skin and cultured human mammary epithelial cells. J Nutr. 2003;133:3805S–3810S. doi: 10.1093/jn/133.11.3805S. [DOI] [PubMed] [Google Scholar]
- 35.Park JS, Kim MH, Chang HJ, Kim KM, Kim SM, Shin BA, Ahn BW, Jung YD. Epigallocatechin-3-gallate inhibits the PDGF-induced VEGF expression in human vascular smooth muscle cells via blocking PDGF receptor and Erk-1/2. Int J Oncol. 2006;29:1247–1252. [PubMed] [Google Scholar]
- 36.Adhikari N, Charles N, Lehmann U, Hall JL. Transcription factor and kinase-mediated signaling in atherosclerosis and vascular injury. Curr Atheroscler Rep. 2006;8:252–260. doi: 10.1007/s11883-006-0081-1. [DOI] [PubMed] [Google Scholar]
- 37.Peng G, Dixon DA, Muga SJ, Smith TJ, Wargovich MJ. Green tea polyphenol (−)-epigallocatechin-3-gallate inhibits cyclooxygenase-2 expression in colon carcinogenesis. Mol Carcinog. 2006;45:309–319. doi: 10.1002/mc.20166. [DOI] [PubMed] [Google Scholar]
- 38.Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, Lambert G, Mohr S, Yang CS. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomarkers Prev. 2002;11:1025–1032. [PubMed] [Google Scholar]
- 39.Prior IA, Harding A, Yan J, Sluimer J, Parton RG, Hancock JF. GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nat Cell Biol. 2001;3:368–375. doi: 10.1038/35070050. [DOI] [PubMed] [Google Scholar]
- 40.Garrean S, Gao XP, Brovkovych V, Shimizu J, Zhao YY, Vogel SM, Malik AB. Caveolin-1 regulates NF-kappaB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J Immunol. 2006;177:4853–4860. doi: 10.4049/jimmunol.177.7.4853. [DOI] [PubMed] [Google Scholar]
- 41.Chen HQ, Tannous M, Veluthakal R, Amin R, Kowluru A. Novel roles for palmitoylation of Ras in IL-1 beta-induced nitric oxide release and caspase 3 activation in insulin-secreting beta cells. Biochem Pharmacol. 2003;66:1681–1694. doi: 10.1016/s0006-2952(03)00549-5. [DOI] [PubMed] [Google Scholar]