Abstract
Trem2 is an orphan, DAP12 associated receptor constitutively expressed in vivo by subsets of microglia in the healthy adult murine CNS and in vitro by subsets of oligodendrocytes in neonatal mixed glial cultures. Loss of a functional Trem2 signaling pathway is the genetic cause of Nasu-Hakola disease. Whether the early onset cognitive dementia and myelin-pallor associated with this disorder are due to deficits in functional Trem2 signaling in microglia and/or oligodendrocytes is still being debated. Here, we find that Trem2/DAP12 expression is detected in embryonic day 14 CNS mRNA. Using dual immunohistochemistry/in situ hybridization, we find that both Trem2 and DAP12 expression always co-localized with markers of microglia/macrophages. However, Trem2/DAP12 positive microglia are found in very close apposition with CNP+ oligodendrocytes prior to myelination (post-natal day 1). In addition, CNS expression of TREM2 and DAP12 are not detected in PU.1KO which lack microglia and macrophages. Our data provide continuing support for Nasu-Hakola disease being identified as a cognitive disorder caused by a primary dysfunction of CNS microglia.
Keywords: TREM, PLOSL, Neuroinflammation, Myelin, Neurodegeneration
Introduction
Nasu-Hakola disease, also referred to as polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy (PLOSL) is a rare recessive human genetic disorder [1, 2]. Nasu-Hakola disease is characterized by early onset cognitive dementia and bone cysts (both evident by the third decade). Individuals with this disease die by their fourth or fifth decade. Seminal studies by Peltonen and colleagues identified loss of function mutations in two separate genes as the causes of this rare disorder: Triggering Receptor Expressed on Myeloid cells-2 (Trem2) and DNAX-activating protein of molecular mass 12 kDa (DAP12), also referred to as killer-cell activating receptor-associated protein (KARAP) and TYROBP [3–5].
Trem2 is an orphan receptor that has recently been implicated in limiting the pro-inflammatory activation state of macrophages and promoting expression of molecules associated with presenting antigen to T-lymphocytes [6–9]. Trem2 belongs to the class of receptors lacking an intracellular signaling tail. To date, only a single molecule has been identified that serves to mediate Trem2 triggered intracellular signaling: DAP12 [6, 10–12]. DAP12 is a transmembrane adaptor molecule that signals via immuno-receptor tyrosine-based activation motifs (ITAM) [11–14]. DAP12 is expressed by multiple immune cells in both the myeloid and lymphoid lineages and serves as a signaling partner for multiple receptors including Trem1, Trem2, NKG2D and KIR3DS1 [11–14]. Despite broad expression within the immune system, functional DAP12 deficiency in humans does not lead to overt widespread deficiencies and/or dysfunction in the immune system [4]. These data suggest that in Nasu-Hakola disease, the loss of functional Trem2 is the only non-redundant, non-compensated function associated with the human DAP12 mutations.
The cognitive dementia and psychosis observed in Nasu-Hakola disease suggested that the mutated genes causing the disease should normally be primarily expressed in neurons in healthy individuals. Surprisingly, we found that subsets of microglia were the only cells expressing Trem2 within the healthy adult murine CNS as assayed by in situ hybridization analysis in previously published studies [15]. Subsequent studies of adult rodent tissue have confirmed these initial findings [16–18]. In addition, Kiialainen et al. have observed by immunostaining that not all microglia in mixed glial cultures express detectable levels of Trem2 and DAP12 [19].
Two types of data suggest that Trem2 and DAP12 may play either a direct or indirect role in oligodendrocyte development and function. First, histological analysis of adult CNS tissue from DAP12 knock-out mice in studies by two separate groups revealed modest, diffuse hypomyelination in anterior brain regions that was associated with substantial reductions in CNS microglia [20–22]. Subsequent gene profiling studies of CNS mRNA isolated from DAP12KO mice by a third group revealed decreased expression of selected myelin transcripts [23]. These data were initially interpreted to suggest a fundamental role for microglia in supporting oligodendrocyte development and/or myelination. In support of this hypothesis, Trem2 and DAP12 expression near white matter tracts has been detected by in situ hybridization analysis in post-natal developing brain [19]. Unfortunately, the cell type(s) expressing Trem2 and DAP12 within the intact tissue was not determined in these studies.
A second series of in vitro studies have now raised an alternative possibility: that Trem2 and DAP12 might directly regulate oligodendrocyte development and myelination. Specifically, Trem2 and DAP12 expression was detected by RT-PCR and by immunostaining in oligoden-drocytes isolated from neonatal mixed glial cultures [19]. While highly suggestive, we and others have observed robust but aberrant gene expression in macroglia and microglia that differentiated in culture in the absence of normal in vivo cues provided by synaptically active neurons [24, 25]. The authors of these same studies raise an additional caution concerning the significance of their detected expression in cultured oligodendrocytes. These authors could not detect any differences in the numbers, rate or extent of oligodendrocyte development in mixed glial cultures established from neonatal wild-type and DAP12 mice [19].
In summary, loss of function mutations in the Trem2 pathway have been identified as the cause of a neurologic disorder. It is important to ascertain whether these clinical symptoms and neurologic dysfunction are due to primary deficits in microglial versus oligodendrocyte function during development. In the current study, we examined expression during early post-natal developmental ages corresponding to the developmental window modeled by in vitro mixed glial culture systems. Using dual immunohistochemical/in situ hybridization analysis, Trem2 or DAP12 mRNA expression was only found co-indcident with microglia/macrophages and with oligodendendrocytes.
Material and Methods
Microglia Isolation from Murine Neonatal Mixed Glial Cultures
Mixed glial cultures were prepared as previously described [15, 26]. Briefly, brains from newborn C57Bl/6J mice were stripped of meninges, mechanically dissociated, seeded into T-75 flasks and maintained in OM-5 media (D-MEM supplemented with 30 nM SeO2, 15 nM T3, 10 ng/ml biotin, 3.7 g/ml NaHCO3, 1.5 g/ml glucose, 10% FBS and 50 µg/ml gentimicin). After 2–4 weeks, mixed glial cultures were trypsinized and incubated as a single cell suspension in OM-5 media without phenol red for 30 min at 37°C to allow for the re-expression of trypsinized surface markers. Microglia were then purified to >98% purity by flow cytometry using PE-conjugated antibodies directed against FcR/CD16/CD32 (Pharmingen). Cytoplasmic mRNA was prepared from isolated cells immediately after isolation as previously described [15].
Peritoneal Macrophage Preparation
Peritoneal macrophages were prepared from C57Bl/6J mice as previously described [26]. Briefly, 3.0 ml of aged sterile thioglycolate broth were injected into the peritoneum (Difco, Detroit, MI). Mice were sacrificed 3 days post-injection and peritoneal macrophages were harvested after euthanasia by rinsing the peritoneal cavity with two 5 ml washes of OM5 media supplemented with 5.0 U/ml heparin (Sigma, St. Louis, MO). Exudate cells were plated, allowed to adhere. Nonadherent cells were removed by rinsing the cultures. Greater than 95% of the remaining adherent cells were macrophages (CD11b+ cells).
In situ Hybridization
In situ hybridization was performed on free-floating cryo-sections sections as previously described [15]. Briefly, coronal sections were hybridized at 55°C for 16 h with a 33P-labeled riboprobe (107 cpm/ml). Excess probe was removed by washing at room temperature for 30 min in 0.03 M NaCl, 0.003 M sodium citrate (2× SSC) containing 10 mM β-mercaptoethanol, followed by a 1 h incubation with 4 µg/ml ribonuclease, 0.5 M NaCl, 0.5 M EDTA, 0.05 M Tris–HCl (pH 7.5) at 37°C. Sections were than washed under high stringency conditions for 2 h at 55°C in 0.5× SSC, 50% formamide, 10 mM β-mercaptoethanol, followed by a 1 h at 68°C in 0.1× SSC, 5 mM β-mercaptoethanol, 5% sarkosyl. Myeloid cells (microglia and macrophages) and blood vessels were identified by their ability to bind biotinylated tomato lectin (Sigma). Bound biotinylated tomato lectin was visualized by standard strep-avidin, horseradish peroxidase (HRP) methodology [15]. Sections were mounted onto Fisherbrand superfrost/plus slides and dehydrated with ethanol and chloroform. Slides were exposed for 3 days to Kodak X-AR film and dipped in Ilford K-5 emulsion. After 3–4 weeks, slides were developed with Kodak D19 developer, fixed and counterstained with Mayer’s hematoxylin.
Northern Blot
For northern blots, 2 µg per lane of poly A+ RNA were resolved by electrophoresis in a 1.5% agarose/1.2 M formaldehyde gel, transferred to nylon membrane and hybridized with 32P-radiolabeled probes as previously described [15, 27]. Nylon membranes were exposed to Kodak X-AR film for 16 h (short exposure) and then for an additional 2 weeks (long exposure). Densitometry was quantified using ImageJ (NIH public domain software: http://rsb.info.nih.gov/ij/).
Results
Trem2 belongs to the class of receptors lacking an intra-cellular signaling tail and thus depends on the transmembrane adaptor molecule DAP12 to mediate Trem2 induced signaling [6, 8, 28]. We previously reported that Trem2 is readily detected in unstimulated microglia from murine neonatal mixed glial cultures isolated by flow cytometric cell sorting and in thioglycolate-elicited peritoneal macrophages [15]. By reprobing the same northern blot used to quantify Trem2 expression (Fig. 1 in Ref. [15]), we now report that DAP12 is expressed at very high levels in these same purified microglia and macrophage populations (Fig. 1). DAP12 expression is also downregulated in both cultured microglia and macrophages following 22 h of LPS/IFNg treatment (100 ng/10 Units per ml). The degree of Trem2 and DAP12 repression was not identical. While Trem2 expression was repressed by 60–70%, DAP12 expression was repressed by ~35–45% after 22 h of treatment in these same samples. In addition, the different exposure times required to visualize DAP12 and Trem2 expression on the same northern blot suggests that DAP12 is expressed at much higher levels than Trem2 in both microglia and macrophages. Trem2 expression could only be detected following exposure times of 2 weeks. Robust DAP12 expression was readily detected within 16 h exposure time following re-probing of the same northern blot.
Fig. 1.
DAP12 is abundantly expressed in microglia isolated from mixed glial culture. The same northern blots previously probed for Trem2 expression [15], were probed for DAP12 and exposed for only 16 h (a). This same blot was probed with cyclophillin (cycloph) as a mRNA loading control. DAP12 expression was quantified and normalized to the cyclophillin control (b). MG: microglia isolated from mixed glial cultures. MP: thioglycolate-elicited macrophages
Although the precise date of microglial appearance in the CNS and/or neuroectodermal tissue is not resolved, microglia are easily detected histologically in the CNS from embryonic day 14 onward [24]. We therefore used northern blot analysis to quantify DAP12 and Trem2 expression in mRNA isolated from whole brain tissue from embryonic day 14 through young adulthood (Fig. 2). DAP12 expression was readily detected in murine brain RNA at all ages (Fig. 2a). Trem2 expression was also detected at all ages, although the level of expression was substantially lower (Fig. 2a). DAP12 and Trem2 expression levels per sample were normalized to cyclophilin expression and quantified revealing only modest increases in expression as a function of increasing age.
Fig. 2.
DAP12 and Trem2 expression is detected within the CNS both pre- and post-natally but only modestly increases as a function of age. In panel a, the same developmental northern blot of whole brain mRNA is probed for DAP12 (24 h exposure), Trem2 (2 week exposure) and cylophilin (mRNA loading control). In panel b, DAP12 (dark bars) and Trem2 (grey bars) is quantified and normalized to cyclophillin expression (Note: For interpretation of the references to color in this figure legend, the reader is referred to the online version of this article)
We have previously demonstrated that only subsets of microglia express detectable levels of Trem2 in the adult CNS as analyzed by in situ hybridization analysis [15]. Subsequent studies have reported that oligodendrocytes differentiating in neonatal mixed glial cultures express DAP12 and Trem2 [19]. Here we directly examined the extent that Trem2 and DAP12 were expressed in vivo by oligodendrocytes and microglia and macrophages during the neonatal periods associated with oligodendrocyte development and early myelination: postnatal day 1 (Fig. 3) and postnatal day 10 (Fig. 4). In these experiment 2′3′ cyclic nucleotide phosphodiesterase (CNP) was used as a marker for oligodendrocytes (Fig. 3a, Fig. 4a), tomato lectin as a marker of blood vessels, microglia and macrophages (Fig. 3b, Fig. 4b). In tissue sections, we can readily distinguish between the tubular structure of blood vessels and the nucleated cellular structure of microglia and macrophages. Histologically, microglia and macrophages cannot be distinguished and will be considered a single class of CNS macrophages for the purposes of this study.
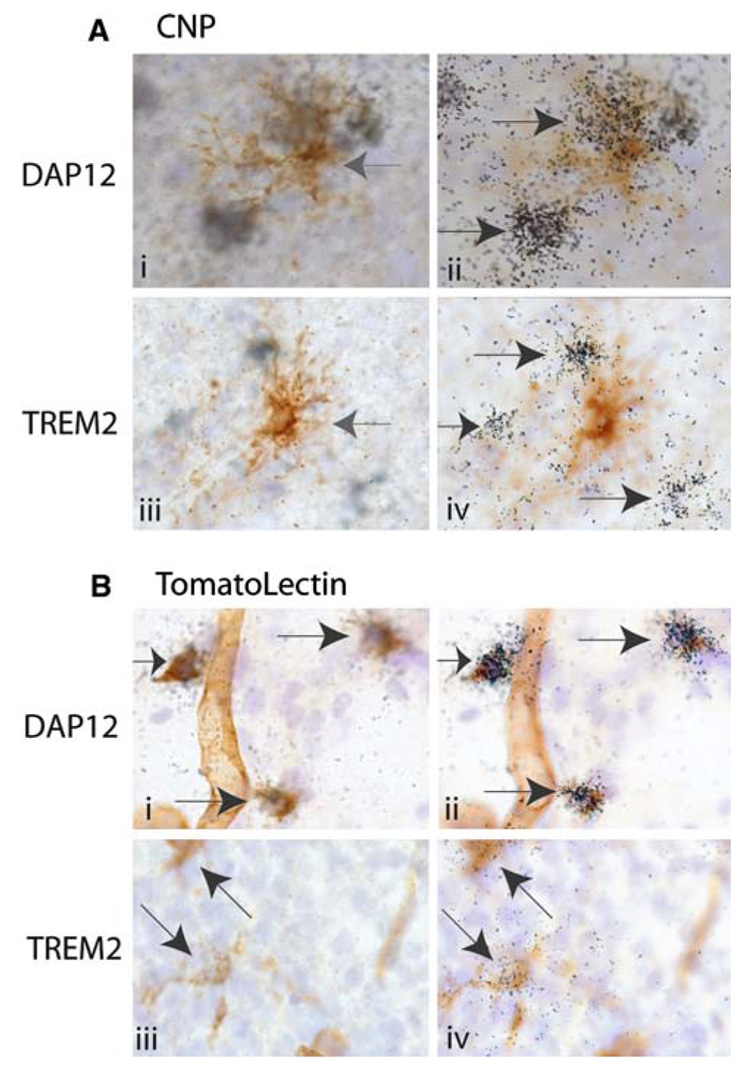
Fig. 3.
DAP12 and Trem2 expression is co-incident with microglia/macrophages at post-natal day 1. In all panels (a and b), nuclei are visualized in blue with hematoxylin. Panel a: Oligodendrocytes were visualized in brown with CNP antibodies (ai and aiii). Left pointing grey arrow indicates oligodendrocyte cell body. DAP12 (aii) and Trem2 (aiv) mRNA expression is visualized on a different focal but on the same tissue sections as depicted in ai and aiii respectively by the presence of photographic emulsion grains (black) with 33P labeled riboprobes. Black arrows indicate DAP12 (aii) and Trem2 (aiv) positive cells. Panel b: Microglia, macrophages and blood vessels are visualized in brown using tomato lectin. DAP12 and Trem2 expression is visualized as in (a). Arrows indicate DAP12+ (bi, bii) and Trem2+ (aiii, biv) cells (Note: For interpretation of the references to color in this figure legend, the reader is referred to the online version of this article)
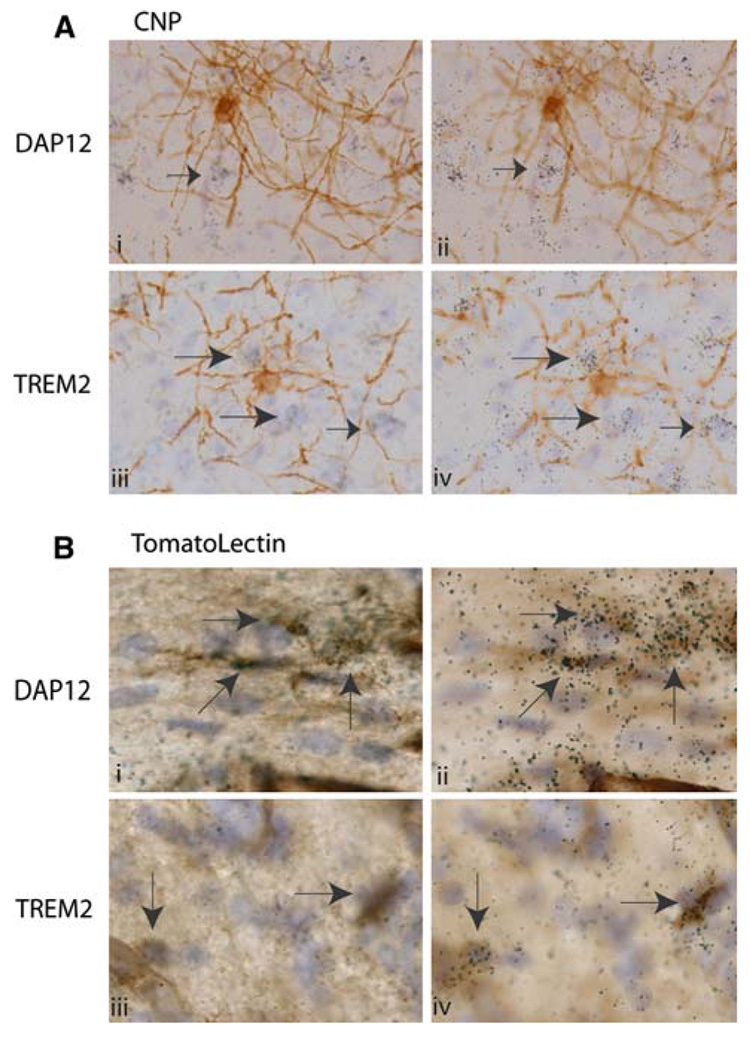
Fig. 4.
DAP12 and Trem2+ microglia/macrophages surround myelinating oligodendrocytes at post-natal day 10. In all panels (a and b), nuclei are visualized in blue with hematoxylin. Panel a: Oligoden-drocytes and myelinated axons were visualized in brown with CNP antibodies. DAP12 (aii) and Trem2 (aiv) mRNA expression is visualized on a different focal but on the same tissue sections as depicted in ai and aiii respectively by the presence of photographic emulsion grains (black) with 33P labeled riboprobes. Black arrows indicate DAP12 (3aii) and Trem2 (3aiv) positive cells. Panel b: Microglia, macrophages and blood vessels are visualized in brown using tomato lectin. DAP12 and Trem2 expression is visualized as in Fig. 3a. Arrows indicate DAP12+ (bi, bii) and Trem2+ (aiii, biv) cells (Note: For interpretation of the references to color in this figure legend, the reader is referred to the online version of this article)
Strikingly, at both post-natal days 1 and 10, DAP12 positive and Trem2 positive cells were frequently detected surrounding CNP positive cells (Fig. 3a and Fig. 4a), but DAP12/Trem2 expression were never completely coincident with CNP. At post-natal day 1, these DAP12 and Trem2 positive cells were often found in very close apposition to CNP+ cells. The apposition of DAP12 and CNP expression was occasionally so close that in some of our initial screens, rare CNP+ cells were classified as DAP12+ (Fig. 3a). However, conclusive co-expression of DAP12 and CNP was never detected when a careful comparison of the location of the CNP+ cell body (Fig. 3ai: grey left pointing arrow) and the DAP12 expression were made. For example, the two clusters of emulsion grains corresponding to DAP12 expression are located not over the CNP+ cell body but are located on hematoxylin+, CNP− cells very near the CNP+ cell (Fig. 3ai–ii). TREM-2 expression was similarly observed to cluster around CNP+ cells at post-natal day 1 (Fig. 3aiii–iv).
By contrast, DAP12 and Trem2 expression was completely coincident with tomato lectin positive cells (microglia and/or macrophages) (Fig. 3b). At this age, all tomato lectin positive cells expressed detectable levels of both DAP12 and Trem2. Conversely, in these same sections, DAP12/Trem2 expression were never detected on lectin negative cells (Fig. 3b). DAP12 and Trem2 expression was also not detected on tomato lectin positive blood vessels. The DAP12 and Trem2 studies depicted in Fig. 3b were performed together with the same reagents and allowed to develop during the same 4 week timeframe. Therefore, the lower level of emulsion grains detected in the P1 tissue sections treated with Trem2 riboprobes as compared to those treated with DAP12 riboprobes is consistent with lower level of Trem2 expression detected by the northern blot analysis (Fig. 2).
By post-natal day 10, CNP immunoreactivity visualizes not only the oligodendrocyte cell body but also the ongoing myelination of axons surrounding the oligodendrocyte (Fig. 4a). At this age, DAP12 and Trem2 expression is again observed in cells surrounding CNP+ cells, but is not observed co-incident with CNP expression (Fig. 4a). Conversely, DAP12 and Trem2 expression is again always observed co-incident with tomato lectin+ cells (Fig. 4b). Within the corpus collosum, these DAP12+, tomato lectin+ cells are located in very close apposition to tomato lectin negative cells (Fig. 4b). It is also important to note, that by post-natal day 10 the level of DAP12 and Trem2 expression per tomato lectin positive cell was no longer as uniform as that observed at post-natal day 1. DAP12 and Trem2 expression could still be detected on most tomato lectin positive cells at post-natal day 10. However, scattered tomato lectin postive, DAP12/TREM2 negative cells were now apparent throughout the CNS. The same general pattern of expression was observed in tissue from post-natal day 15 and 20 (data not shown).
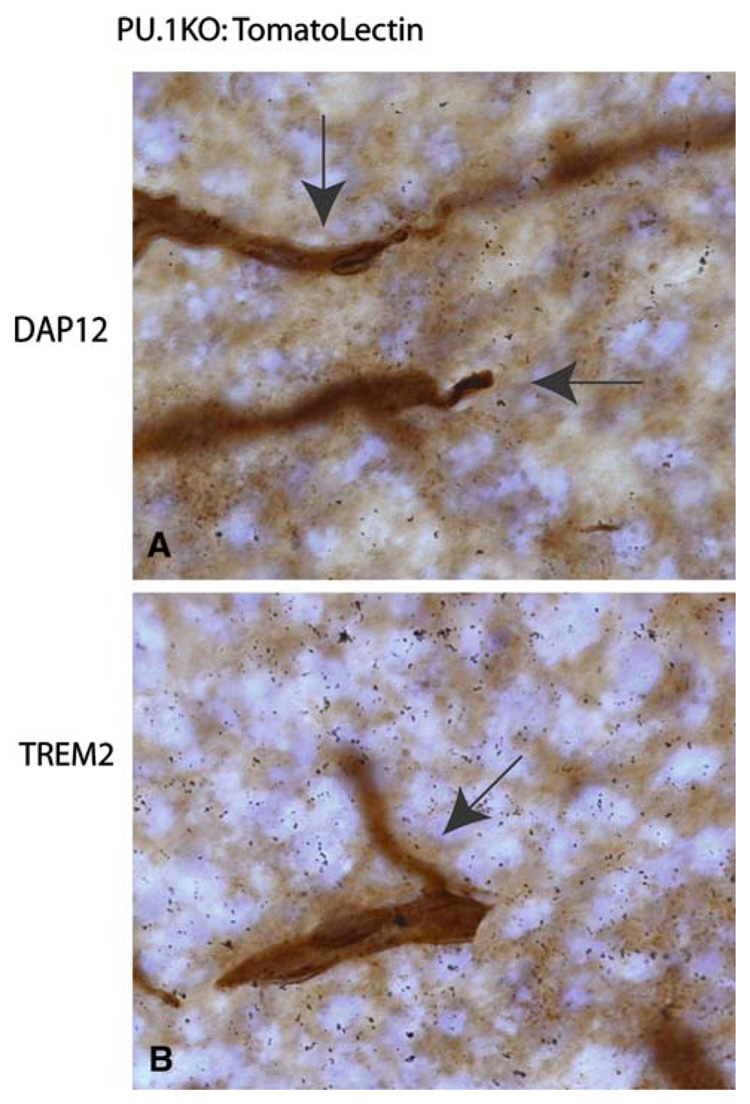
Finally, we characterized Trem2 and DAP12 expression in mice lacking the transcription factor PU.1 [29]. PU.1 mice fail to develop cells of myeloid lineage. While these knock-out mice survive to birth, they rarely survive for more than a day or two post-birth. Here, we confirm that post-natal day 1 PU.1 KO mice do lack microglia in all regions of the CNS (Fig. 5). While blood vessels are strongly labeled (Fig. 5a and b, arrows), no microglia could be detected even when HRP immunoreactivity was allowed to proceed for extended periods (resulting in elevated HRP background labeling of tissue). DAP12 expression is known to be PU.1 dependent [30] and thus the failure to detect DAP12 expression in PU.1 CNS tissue was predicted (Fig. 5a). However, Trem2 expression was also undetectable by in situ hybridization analysis of PU.1 KO CNS (Fig. 5b).
Fig. 5.
Neither DAP12 nor Trem2 are detected in PU.1 mice lacking CNS microglia and macrophages. Blood vessels are visualized in brown using tomato lectin (arrows). As in Fig. 3 and Fig. 4, film emulsion was used to detect 33P labeled DAP12 and Trem2 riboprobe hybridization to tissue. To document the absence of microglia and macrophages, 5-fold longer development times for HRP-detection were used for this figure as compared to Fig. 3 and Fig. 4 (Note: For interpretation of the references to color in this figure legend, the reader is referred to the online version of this article)
Discussion
Seminal studies by Peltonen and colleagues identified loss of function mutations in Trem2 and DAP12, as the genetic cause of the recessive genetic disorder, Nasu-Hakola disease [3–5]. Nasu-Hakola disease is defined by early onset cognitive dementia and bone abnormalities [1, 2]. Therefore, it was logical to postulate that CNS neurons and osteoblasts would be the primary cells expressing these molecules within the CNS and bone respectively. At the time these positional cloning studies were published, the expression of both of these molecules in peripheral (non-CNS) myeloid cells had already been described [6, 8–10]. Subsequently, osteoclasts (myeloid lineage) not osteoblasts were found to express these molecules in bone [8]. Furthermore, we reported that in the healthy adult murine CNS, only subsets of microglia expressed Trem2 mRNA as assayed by in situ hybridization analysis [15]. However, in vitro studies indicated the potential for oligodendrocytes to express Trem2 and DAP12 while DAP12KO mice were observed to display modest myelin abnormalities [19–22]. Here we directly characterize microglial and oligodendrocyte expression of Trem2 and DAP12 by dual immunohistochemistry/in situ hybridization analysis during post-natal period of oligodendrocyte development and myelination.
In our current studies, we find that DAP12 is expressed at very high levels in microglia isolated from the same mixed glial culture systems used to characterize oligodendrocyte development and gene expression. These data do suggest that it is essential to monitor expression of microglial specific molecules in real-time PCR studies using cells purified from mixed glial cultures to quantify the degree of microglial contamination. Simple immunostaining assays often under-report the percentages of microglia in purified cells for the simple reason that the fixation conditions optimal for most macroglial-specific antisera (paraformaldehyde) substantially reduce the immunostaining of microglial/macrophage-specific antisera [24, 26]. In addition, microglia in mixed glial cultures are found not only on the surface of astrocytes, but also narrowly interplexed between other much larger cells [24, 26]. Altogether these factors can contribute to mis-assigning microglial gene expression to the other macroglia present in mixed glial culture systems.
Our in vivo developmental studies do confirm the ability of Trem2 and DAP12 to indirectly modulate CNS development from as early as embryonic day 14. We can never exclude that oligodendrocytes express very low levels of Trem2 or DAP12 below our level of our detection or before post-natal day 1. However, using dual immunohistochemistry/ in situ hybridization analysis, both DAP12 and Trem2 expression was always found to co-localize with tomato lectin, a marker of microglia, macrophages and blood vessels. It is important to note, that in these tomato lectin stained sections, Trem2 or DAP12 expression was never observed on cells negative for tomato lectin. Consistent with these observations, we also fail to detect either Trem2 or DAP12 expression in mouse model that lacks all macrophage populations including microglia: PU.1 knock-out mice. Taken together, these data do suggest that myelin deficits in DAP12 KO mice and in individuals with Nasu-Hakola disease are highly unlikely to be due to loss of a functional Trem2 pathway in oligodendrocytes.
Although the percentage of microglia does increase during post-natal development, we and others have not observed a robust increase in either Trem2 or DAP12 expression with age [19]. This may result in part from an age-correlated increase in heterogeneity of expression, both in the level of expression and percentages of Trem2 and DAP12 expressing microglia. Microglia can be induced to express other DAP12-associated receptors including Trem1 (Thrash and Carson, unpublished data). It is uncertain and unexamined whether these other DAP12 (but not Trem2) associated microglial functions may play any role in CNS disorders including Nasu-Hakola.
In tissue sections labeled with CNP antisera, it was evident that Trem2 and DAP12 positive cells were in frequent close apposition to oligodendrocytes. The very close apposition demonstrated in Fig. 3a was most often detected near oligodendrocytes that had not yet extended myelin to surrounding axons. In vitro studies have suggested that microglia have the potential to play protective or supportive roles in oligodendrocyte development (reviewed in [31, 32]). While, DAP12 KO mice exhibit oligodendrocyte and myelin deficiencies, the data presented here do not prove a oligodendrocyte/myelin-supporting function for Trem2/DAP12 microglia during development. However, Trem2 overexpression and knock-down studies have demonstrated that Trem2 does function to inhibit microglial and macrophage cytokine expression, to limit the severity of anti-CNS pro-inflammatory immune responses, and does function to promote microglial phagocytosis of neuronal cell debris [8, 16–18]. Therefore, it is possible that the myelin deficits observed in humans and mice lacking functional Trem2 pathways are the result of insufficient suppression of microglial activation. While much is still left to define, the data presented here provide continuing support for Nasu-Hakola disease being the first neuropsychological disorder identified as resulting from a primary dysfunction in microglia.
Acknowledgments
MJC was supported by NIH grants NS045735, NS39508 and a PIC grant from the Division of Biomedical Sciences, University of California Riverside.
Contributor Information
J. Cameron Thrash, Division of Biomedical Sciences, Center for Glial-Neuronal Interactions, University of California Riverside, 900 University Ave, Riverside, CA 92521, USA.
Bruce E. Torbett, Department of Molecular and Experimental Medicine, The Scripps Research Institute, La Jolla, USA
Monica J. Carson, Division of Biomedical Sciences, Center for Glial-Neuronal Interactions, University of California Riverside, 900 University Ave, Riverside, CA 92521, USA, e-mail: monica.carson@ucr.edu
References
- 1.Verloes A, Maquet P, Sadzot B, Vivario M, Thiry A, Franck G. Nasu-Hakola syndrome: polycystic lipomembranous osteodysplasia with sclerosing leucoencephalopathy and presenile dementia. J Med Genet. 1997;34:753–757. doi: 10.1136/jmg.34.9.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bianchin MM, Capella HM, Chaves DL, Steindel M, Grisard EC, Ganev GG, da Silva Junior JP, Neto Evaldo S, Poffo MA, Walz R, Carlotti Junior CG, Sakamoto AC. Nasu-Hakola disease (polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy— PLOSL): a dementia associated with bone cystic lesions. From clinical to genetic and molecular aspects. Cell Mol Neurobiol. 2004;24:1–24. doi: 10.1023/B:CEMN.0000012721.08168.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paloneva J, Kestila M, Wu J, Salminen A, Bohling T, Ruotsalainen V, Hakola P, Bakker AB, Phillips JH, Pekkarinen P, Lanier LL, Timonen T, Peltonen L. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat Genet. 2000;25:357–361. doi: 10.1038/77153. [DOI] [PubMed] [Google Scholar]
- 4.Paloneva J, Manninen T, Christman G, Hovanes K, Mandelin J, Adolfsson R, Bianchin M, Bird T, Miranda R, Salmaggi A, Tranebjaerg L, Konttinen Y, Peltonen L. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet. 2002;71:656–662. doi: 10.1086/342259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klunemann HH, Ridha BH, Magy L, Wherrett JR, Hemelsoet DM, Keen RW, De Bleecker JL, Rossor MN, Marienhagen J, Klein HE, Peltonen L, Paloneva J. The genetic causes of basal ganglia calcification, dementia, and bone cysts: DAP12 and TREM2. Neurology. 2005;64:1502–1507. doi: 10.1212/01.WNL.0000160304.00003.CA. [DOI] [PubMed] [Google Scholar]
- 6.Daws MR, Lanier LL, Seaman WE, Ryan JC. Cloning and characterization of a novel mouse myeloid DAP12-associated receptor family. Eur J Immunol. 2001;31:783–791. doi: 10.1002/1521-4141(200103)31:3<783::aid-immu783>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 7.Daws MR, Sullam PM, Niemi EC, Chen TT, Tchao NK, Seaman WE. Pattern recognition by TREM-2: binding of anionic ligands. J Immunol. 2003;171:594–599. doi: 10.4049/jimmunol.171.2.594. [DOI] [PubMed] [Google Scholar]
- 8.Bouchon A, Hernandez-Munain C, Cella M, Colonna M. A dap12-mediated pathway regulates expression of cc chemokine receptor 7 and maturation of human dendritic cells. J Exp Med. 2001;194:1111–1122. doi: 10.1084/jem.194.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164:4991–4995. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- 10.Prada I, Ongania GN, Buonsanti C, Panina-Bordignon P, Meldolesi J. Triggering receptor expressed in myeloid cells 2 (TREM2) trafficking in microglial cells: continuous shuttling to and from the plasma membrane regulated by cell stimulation. Neuroscience. 2006;140:1139–1148. doi: 10.1016/j.neuroscience.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 11.Lanier LL, Corliss B, Wu J, Phillips JH. Association of DAP12 with activating CD94/NKG2C NK cell receptors. Immunity. 1998;8:693–701. doi: 10.1016/s1074-7613(00)80574-9. [DOI] [PubMed] [Google Scholar]
- 12.Lanier LL, Bakker AB. The ITAM-bearing transmembrane adaptor DAP12 in lymphoid and myeloid cell function. Immunol Today. 2000;21:611–614. doi: 10.1016/s0167-5699(00)01745-x. [DOI] [PubMed] [Google Scholar]
- 13.Aoki N, Kimura S, Takiyama Y, Atsuta Y, Abe A, Sato K, Katagiri M. The role of the DAP12 signal in mouse myeloid differentiation. J Immunol. 2000;165:3790–3796. doi: 10.4049/jimmunol.165.7.3790. [DOI] [PubMed] [Google Scholar]
- 14.Humphrey MB, Lanier LL, Nakamura MC. Role of ITAM-containing adapter proteins and their receptors in the immune system and bone. Immunol Rev. 2005;208:50–65. doi: 10.1111/j.0105-2896.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 15.Schmid CD, Sautkulis LN, Danielson PE, Cooper J, Hasel KW, Hilbush BS, Sutcliffe JG, Carson MJ. Heterogeneous expression of the triggering receptor expressed on myeloid cells-2 on adult murine microglia. J Neurochem. 2002;83:1309–1320. doi: 10.1046/j.1471-4159.2002.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neumann H, Takahashi K. Essential role of the microglial triggering receptor expressed on myeloid cells-2 (TREM2) for central nervous tissue immune homeostasis. J Neuroimmunol. 2007;184:92–99. doi: 10.1016/j.jneuroim.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 17.Piccio L, Buonsanti C, Mariani M, Cella M, Gilfillan S, Cross AH, Colonna M, Panina-Bordignon P. Blockade of TREM-2 exacerbates experimental autoimmune encephalomyelitis. Eur J Immunol. 2007;37:1290–1301. doi: 10.1002/eji.200636837. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi K, Prinz M, Stagi M, Chechneva O, Neumann H. TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. PLoS Med. 2007;4:e124. doi: 10.1371/journal.pmed.0040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiialainen A, Hovanes K, Paloneva J, Kopra O, Peltonen L. Dap12 and Trem2, molecules involved in innate immunity and neurodegeneration, are co-expressed in the CNS. Neurobiol Dis. 2005;18:314–322. doi: 10.1016/j.nbd.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Kaifu T, Nakahara J, Inui M, Mishima K, Momiyama T, Kaji M, Sugahara A, Koito H, Ujike-Asai A, Nakamura A, Kanazawa K, Tan-Takeuchi K, Iwasaki K, Yokoyama WM, Kudo A, Fujiwara M, Asou H, Takai T. Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J Clin Invest. 2003;111:323–332. doi: 10.1172/JCI16923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roumier A, Bechade C, Poncer JC, Smalla KH, Tomasello E, Vivier E, Gundelfinger ED, Triller A, Bessis A. Impaired synaptic function in the microglial KARAP/DAP12-deficient mouse. J Neurosci. 2004;24:11421–11428. doi: 10.1523/JNEUROSCI.2251-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nataf S, Anginot A, Vuaillat C, Malaval L, Fodil N, Chereul E, Langlois JB, Dumontel C, Cavillon G, Confavreux C, Mazzorana M, Vico L, Belin MF, Vivier E, Tomasello E, Jurdic P. Brain and bone damage in KARAP/DAP12 loss-of-function mice correlate with alterations in microglia and osteoclast lineages. Am J Pathol. 2005;166:275–286. doi: 10.1016/S0002-9440(10)62251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiialainen A, Veckman V, Saharinen J, Paloneva J, Gentile M, Hakola P, Hemelsoet D, Ridha B, Kopra O, Julkunen I, Peltonen L. Transcript profiles of dendritic cells of PLOSL patients link demyelinating CNS disorders with abnormalities in pathways of actin bundling and immune response. J Mol Med. 2007;85:971–983. doi: 10.1007/s00109-007-0191-4. [DOI] [PubMed] [Google Scholar]
- 24.Melchior B, Puntambekar SS, Carson MJ. Microglia and the control of autoreactive T cell responses. Neurochem Int. 2006;49:145–153. doi: 10.1016/j.neuint.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiacco TA, Agulhon C, Taves SR, Petravicz J, Casper KB, Dong X, Chen J, McCarthy KD. Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity. Neuron. 2007;54:611–626. doi: 10.1016/j.neuron.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 26.Carson MJ, Reilly CR, Sutcliffe JG, Lo D. Mature microglia resemble immature antigen-presenting cells. Glia. 1998;22:72–85. doi: 10.1002/(sici)1098-1136(199801)22:1<72::aid-glia7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 27.Thomas EA, George RC, Danielson PE, Nelson PA, Warren AJ, Lo D, Sutcliffe JG. Antipsychotic drug treatment alters expression of mRNAs encoding lipid metabolism-related proteins. Mol Psychiatry. 2003;8:983–993. 950. doi: 10.1038/sj.mp.4001425. [DOI] [PubMed] [Google Scholar]
- 28.Cella M, Buonsanti C, Strader C, Kondo T, Salmaggi A, Colonna M. Impaired differentiation of osteoclasts in TREM-2-deficient individuals. J Exp Med. 2003;198:645–651. doi: 10.1084/jem.20022220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson KL, Smith KA, Conners K, McKercher SR, Maki RA, Torbett BE. Myeloid development is selectively disrupted in PU.1 null mice. Blood. 1998;91:3702–3710. [PubMed] [Google Scholar]
- 30.Henkel GW, McKercher SR, Maki RA. Identification of three genes up-regulated in PU.1 rescued monocytic precursor cells. Int Immunol. 2001;14:723–732. doi: 10.1093/intimm/dxf040. [DOI] [PubMed] [Google Scholar]
- 31.Streit WJ. Microglia and neuroprotection: implications for Alzheimer’s disease. Brain Res Brain Res Rev. 2005;48:234–239. doi: 10.1016/j.brainresrev.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 32.Carson MJ, Thrash JC, Walters B. The role of leukocytes, astrocytes and microglia in neuroinflammation: implications for neuronal survival and apoptosis. Clin Neurosci Res. 2006;6:237–246. doi: 10.1016/j.cnr.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]