Abstract
The regulation of IL-2 production is central to our understanding of the immune system. Key during T cell activation, it also plays an essential role in the regulation of the immune response. This review discusses the function of recently described factors that modulate transcription and chromatin remodeling at the IL2 promoter. Also, it addresses the role of FoxP3 as a transcriptional regulator in conventional T cells and regulatory T cells, and the mechanisms whereby CD28 stabilizes IL2 transcription and translation. Finally, the alterations that prevent T cells from SLE patients from producing normal amounts of IL-2 upon stimulation are described.
Keywords: Chromatin remodeling, FoxP3, IL-2, Systemic Lupus Erythematosus, Transcriptional regulation
The importance of IL-2 as a key cytokine for T cell activation and immune function has extensive experimental support [1]. It plays a role as an auto- and paracrine growth factor during the first 48 to 72 hours of T cell activation. Paradoxically, its absence has been linked to development of lethal autoimmunity in mice [1], and failure to produce normal amounts of IL-2 upon activation is considered a hallmark of T cells from patients with systemic lupus erythematosus (SLE), a chronic autoimmune disease [2]. This apparent contradiction –the development of autoimmune disease caused by the absence of a cytokine believed to be central in T cell activation– has been partially explained by the fact that conditions in which IL-2 is absent lack regulatory T cells [3]. In humans, where the deficiency is not absolute, regulatory T cell function has been reported to be abnormal [4], however the significance of such defect within the complex immune deregulation of patients with SLE remains to be determined. Abnormal control of IL-2 production is expected to impact a number of immune cell functions besides those directly related to regulatory T cells. Accordingly, several T cell defects known to be present in patients with SLE (e.g. defective activation-induced cell death and cytotoxic activity), are probably the result of faulty IL-2 production [2]. The knowledge of the mechanisms that regulate IL-2 production in normal and diseased T cells is central to our understanding of complex autoimmune diseases such as SLE. Furthermore, it will allow us to better comprehend the relationship between the effector and regulatory arms of the immune response. The aim of this review is to analyze recent developments that have modified current perspectives of il2 transcriptional regulation.
New factors and chromatin remodeling in the IL2 promoter
The study of the transcriptional regulation of IL2, has traditionally focused on the analysis of a minimal enhancer region that spans ~300 bp upstream from the transcription start site (TSS) of the IL2 gene. This region defines a high density of binding sites for transcription factors whose importance in the promotion of IL2 transcription has been extensively documented (reviewed in ref. [5]). During the last years, interesting work has revealed the existence of new factors able to regulate IL-2 production, and highlighted the importance of local histone modification in the control of the transcriptional rate of this gene (Table 1).
Table 1.
Recently described factors that alter IL-2 transcription
| Factor | IL-2 | Mechanism | Ref. |
|---|---|---|---|
| RhoA | ↓ | Decreases H3 acetylation | 6 |
| BOB.1/OBF.1 | ↑ | Forms ternary complexes with Oct-1/2 | 8 |
| BCL11B | ↑ | Binds to the IL-2 promoter; recruits p300 | 9 |
| Ikaros | ↓ | Maintains hypoacetylated histones | 12, 13 |
| Egr-1 | ↑ | Couples with NAB2 and binds to the IL-2 promoter | 19 |
| Egr-2, Egr-3 | ↓ | Induce expression of the E3-ubiquitin ligase Cbl-b | 17 |
| NF45, NF90, Ku80, Ku70 | ↑ | Form part of a purine-box regulator that binds to the IL-2 promoter | 16 |
| AML1 | ↑ | Binds to the promoter; induces transcription in a NFAT-dependent fashion | 22 |
| ↓ | When associated with FoxP3, it inhibits IL-2 transcription |
RhoA, a GTP-ase known by its role in cytoskeletal rearrangement, was shown to inhibit IL-2 production in Jurkat and primary T cells by interfering with NFAT activity. Intriguingly, the effect did not affect NFAT nuclear translocation or DNA binding, but its presence caused a decrease in histone-3 acetylation at the IL2 promoter. Therefore, RhoA activation may affect local chromatin remodeling and the ability of NFAT to transactivate DNA transcription [6]. Further work is needed to establish if this molecule plays a role in the physiologic regulation of IL-2.
Originally described as a B cell-specific transcriptional coactivator, BOB.1/OBF.1 is now known to be induced in T cells following activation. BOB.1/OBF.1 deficient mice exhibit skewed cytokine production (towards type-2 cytokines) and produce reduced levels of IL-2. BOB.1/OBF.1 is able to form ternary complexes with Octamer binding proteins (Oct-1 and Oct-2). Oct-1 is a ubiquitous protein and Oct-2 is a lymphocyte-specific factor known to bind to the IL2 promoter in close association with AP-1 [7]. The importance of the BOB.1/OBF.1/Oct-1/2 complexes is suggested by the fact that they have been found bound to the IL2 promoter in activated T cells and their absence in BOB.1/OBF.1-deficient mice, leads to impaired IL-2 production [8].
BCL11B is a zinc finger protein expressed in CD4+ T cells. Over-expression of BCL11B results in increased IL-2 production following T cell activation; its silencing with siRNA has the opposite effect. BCL11B binds to the IL2 promoter of activated T cells, in the region between −243 and −190 bp. There, it associates with the p300 coactivator probably facilitating gene transcription [9].
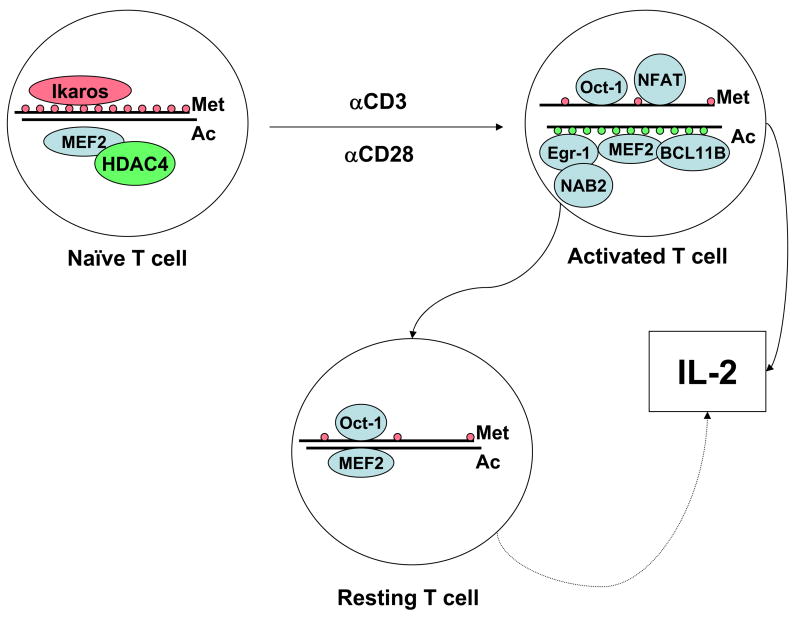
Posttranslational histone modifications regulate the accessibility of DNA to transcription factors and proteins associated with gene transcription (Figure 1). Histone methylation and acetylation have been shown to play a role in the regulation of IL2 transcription. The region comprised within the 1.3 kb segment upstream of the TSS of the human IL2 gene contains six CpG sites. These sites are highly methylated in naïve CD4+ T cells restricting accessibility to the IL2 promoter. Activation-induced demethylation is associated with IL-2 production. As expected, methylation abrogates IL2 transcription as well as NFAT and Oct-1 binding. Interestingly, in cells rested after activation, the site remains unmethylated and Oct-1 remains bound to the site. This phenomenon may be responsible for the fact that T cells produce IL-2 more quickly after repeated stimulations [10].
Figure 1. Transcription factor occupancy and histone methylation and acetylation are entirely different in the IL2 promoter of naïve and activated T cells.
Ikaros, present in naïve T cells, maintains a low level of histone acetylation. This, added to the high methylation status of histones restricts accessibility to the promoter such that key transcription factors (NF-AT, Oct-1) are unable to bind. T cell activation is associated with dramatic changes in histone methylation and acetylation, as well as in transcription factor binding. After cell activation has concluded, most transcription factors disengage from the IL2 promoter, but some (as Oct-1) remain attached. Likewise, although histone acetylation is lost, the locus is not re-methylated. This allows the cell a more rapid response upon re-activation.
The IL2 promoter has two consensus binding sites for Ikaros, a zinc finger DNA-binding protein, component of the nucleosome remodeling and DNA methylation complex. Nucleosomes positioned across the IL2 promoter are hypoacetylated in naïve CD4+ T cells. Cell activation induces strong H3 and H4 acetylation throughout a large region (−4.6 to +0.25 kb) adjacent to the IL2 gene [11]. In the absence of CD28 costimulation, however, they remain hypoacetylated. Only the combination of TCR and costimulation is able to induce histone acetylation at this locus. This phenotype depends on Ikaros: cells that express a mutated form of Ikaros unable to bind DNA lose the hypoacetylated status that characterizes naïve cells. Further, they become CD28-autonomous and produce IL-2 in the absence of costimulation [12]. Interestingly, Ikaros-mediated hypoacetylation of the IL-2 promoter is an important mechanism that contributes to the restriction in IL-2 production during T cell anergy [13].
Kao and coworkers described the purification of an inducible nuclear purine-box regulator from stimulated Jurkat T-cells. It binds specifically to the antigen receptor responsive element (ARRE)/NFAT target DNA sequence in the IL2 promoter. Two of its subunits, NF45 and NF90, are zinc-finger DNA- and RNA-binding proteins, respectively. NF90 contains two double-stranded RNA-binding domains that bind structured RNAs including IL-2 [14]. NF45 and NF90 regulate transcriptional activation, mRNA stabilization, and nuclear export of IL-2 and other mRNAs [15]. SLE autoantigens, Ku80 and Ku70, associate with NF90 and NF45, and contribute to specific binding of the purine-box regulator to the il2 promoter [16].
The levels of the proteins Egr (early growth response)-1, Egr-2, and Egr-3 increase after T cell activation. Egr-1 facilitates the expression of IL-2, whereas Egr-2 and 3 have an inhibitory function over T cell activation and IL-2 production [17]. Egr-1 has a binding site within the IL2 promoter [18]; its capacity to promote IL-2 transcription depends on its coupling with NAB2, a protein expressed in T cells following stimulation through CD28 [19].
MEF2 is bound to the IL2 promoter. In non-stimulated thymocytes, it associates with Cabin-1 and HDAC4. The latter two proteins dissociate following stimulation with PMA and Ionomycin. Decreased binding of MEF2 to the promoter causes a decrease in promoter activity; over expression of Cabin-1 or HDAC4 has the opposite effect [20]. However, a recent study found that inhibition of the enzymatic activity of HDAC4 by a specific inhibitor, or diminution of its protein levels by siRNA transfection decreased PMA and Ionomycin induced IL-2 production in Jurkat cells conferring HDAC4 a positive role in IL-2 transcriptional regulation. Moreover, such effect was dependent on the capacity of HDAC4 to associate with N-CoR [21]. Thus, depending on the experimental setting, HDAC4 has shown to posses both positive and negative influence over IL-2 transcription. These seemingly contradictory results are probably explained by the fact that HDAC4 associates with a number of different proteins that may alter its function. Nevertheless, it is important to emphasize that the experimental evidence collected at the IL2 locus indicates that histone acetylation has a positive influence over transcriptional activity.
Ono and coworkers examined a 2 kb region upstream of the minimal IL2 promoter and found 3 potential AML1-DNA binding consensus sites [22]. The most proximal site (−370) is highly conserved among humans and mice and has been shown to be rapidly demethylated following T cell activation [23]. AML1 was shown to bind to the consensus sites and to stimulate IL-2 production in a NFAT-dependent fashion. Moreover, AML1 knockdown led to a decrease in IL-2 production in mouse primary T cells suggesting a physiological role in the induction of IL-2 production [22]. Interestingly, AML1 was found to associate with FoxP3, and the capacity of the latter of inhibiting IL-2 transcription was highly dependent on the simultaneous presence of AML1 and their ability to form molecular associations.
FoxP3 and the regulation of IL-2 production
Two of the most characteristic features of regulatory T cells are their constitutive expression of the transcriptional regulator FoxP3 and their failure to produce IL-2 upon TCR mediated stimulation [24]. This association has stimulated intense research aimed to understand the mechanisms by which FoxP3 regulates transcription of IL2 and several other genes. Interestingly, FoxP3 expression has been documented in activated non-regulatory CD4+ T cells [25]. Its up-regulation in non-regulatory T cells is dependent on NFAT and AP-1 [25]. Interestingly, its presence in such context does not induce suppression of IL-2 and IFN-γ production [26].
The presence of FoxP3 modifies the expression of a large number of genes. In order to accomplish it, FoxP3 associates with transcription factors, such as NFAT and AML1 [22,27]. The N-terminal region of FoxP3 is necessary for the transcriptional repression and for the inhibition of NFAT-mediated transcriptional activation [27]. In fact, the repressive activity of FoxP3 targets the cooperative NFAT:AP-1 complex rather than other configurations of NFAT. This way, FoxP3 represses NFAT:AP-1 activity by forming a cooperative NFAT:FOXP3:DNA complex that resembles the cooperative NFAT:Fos:Jun:DNA complex [27].
FoxP3 binds to the IL2 promoter [28]. Chen and coworkers identified six forkhead transcription factor consensus elements within the 1 kb region upstream of the murine IL2 locus. One of these sites is located adjacent to the ARRE-2 (NFAT/AP-1/Oct-1), at -263 bp. They confirmed, by ChIP analysis that FoxP3 binds to these sites in FoxP3-transfected Jurkat T cells and in TGF-β-induced FoxP3+ regulatory T cells. Interestingly, FoxP3 binding was only observed after T cell activation through TCR and CD28. Moreover, it was inhibited by cyclosporine A, implying that signaling through calcineurin is important in the process. Importantly, FoxP3 expression was associated with a strong reduction in histone acetylation in the IL2 promoter [28]. In a related study, FoxP3 was shown to be associated with histone acetyltranferase TIP60 and class II histone deacetylases HDAC7 and HDAC9 in vivo. The observed interaction was dynamic and modulated by the activation status of the cell. Interestingly, co-transfection of FoxP3, TIP60, and HDAC7 suppressed IL-2 production in Jurkat T cells [29]. Suppression was dependent on the simultaneous presence of the three molecules.
An interesting study analyzed the expression of histone deacetylases in regulatory and effector T cells. The authors found that HDAC9 is highly expressed in regulatory T cells. This enzyme exits the nucleus following TCR-mediated stimulation. Moreover, treatment of regulatory T cells with trichostatin (a histone deacetylase inhibitor) increased the expression of key molecules (e.g. FoxP3, CTLA-4, GITR), caused FoxP3 acetylation, and enhanced the suppressive capacity. Acetylation of FoxP3 increased its capacity to bind to the IL2 promoter and inhibit IL-2 transcription [30].
A recent publication suggested that FoxP3 may not be the only factor responsible for the IL-2 production defect of regulatory T cells [31]. Using a mouse that expresses a null allele of FoxP3 as well as GFP (through an in-frame insertion of GFP into a stop-codon-disrupted FoxP3 locus), Gavin et al were able to study cells that were selected as regulatory (in the thymus), but lacked a functional FoxP3 protein. These cells exhibited an intermediate phenotype (between effector cells and authentic regulatory T cells), but did not gain the capacity to produce IL-2 (in spite of their lack of FoxP3) [31].
The role of CD28 costimulation
CD28 costimulation is critical for the induction of IL-2 production in T cells. It promotes the translocation of p300/CBP molecules to the fos promoter. At the site, p300/CBP promote histone-4 acetylation and RNA polymerase II binding [32]. Through the induction of fos transcription (which is an immediate-early gene in T cell activation), CD28 costimulation leads to the coupling of Fos and Jun, which form AP-1, a transcription factor that associates with NFAT and induces IL-2 production, rescuing the T cell from anergy induction [33]. Further, signals mediated through CD28 lead to histone acetylation and chromatin remodeling at the IL2 promoter, enabling the binding of transcription factors [34]. Additionally, CD28 costimulation increases the binding of CREB with CBP (CREB-binding protein) and thus its transactivation. Such effect depends on p38 and calcium/calmodulin-dependent kinase IV (CaMKIV) [35].
An additional mechanism by which CD28 increases the production of IL-2 is by favoring the activation of NF90. This protein, an AU-rich element (ARE)-binding protein, binds to the five AREs located in the 3′ UTR of IL-2 mRNA and stabilizes it. In quiescent T cells NF90 is localized in the nucleus. In response to CD28 stimulation, AKT phosphorylates NF90 promoting its translocation to the cytoplasm and enabling it to stabilize IL-2 mRNA [15].
IL-2 regulation in T cells from SLE patients
As mentioned earlier, a phenotypic hallmark of the lupus T cell is a failure to produce normal amounts of IL-2 upon activation. The search for the responsible mechanisms has revealed a number of alterations in transcription factor occupancy at the level of the IL2 promoter of T cells obtained from patients with SLE (reviewed in ref. [2]).
Interestingly, the −180 site has proven to be especially important in the deregulation of IL2 transcription in SLE patients. It comprises a binding site for CREB/CREM. When CREB is phosphorylated, it acts as a positive factor enhancing transcription. On the other hand, when CREM is activated, it displaces pCREB and acts as a transcriptional repressor by preventing the binding of p300 and CBP. Moreover, it promotes local histone acetylation. Accordingly, antisense CREM was able to increase the accessibility of the IL2 promoter to endonucleases [36] and restored IL-2 production in T cells from SLE patients [37].
A consistent finding in the IL2 promoter of T cells obtained from patients with lupus has been an imbalance in the CREM/CREB ratio found at the mentioned site. Intriguingly, numerous factors have been identified that directly or indirectly affect the balance between CREB and CREM in SLE T cells. Some of these aberrations appear to be T cell intrinsic whereas the activity of others resides in the sera of patients. A mechanism by which SLE sera affect CREM levels resides in its abnormal capacity of activating calcium/calmodulin-dependent kinase IV (CaMKIV) [38]. Levels of this kinase are higher in T cells derived from SLE patients. Such alteration is a direct consequence of factors present in sera, particularly in those in which anti-CD3/TCR activity is detected. By a still unknown mechanism CaMKIV migrates into the nuclei of normal T cells when these are incubated in sera derived from SLE patients. In the nucleus, it activates CREM increasing its binding to the −180 site. The former leads to a reduced pCREB/CREM ratio and to a reduction in il2 transcription. The importance of this phenomenon is highlighted by the fact that inhibition of CaMKIV activity by overexpression of a dominant negative CaMKIV isoform abolishes the IL-2 inhibiting effect of SLE sera [38].
PP2A (protein phosphatase 2A) is an enzyme responsible for the dephosphorylation (and thus inactivation) of CREB in T lymphocytes. Its levels, measured as protein or mRNA, are abnormally elevated in T cells from SLE patients [39]. Likewise, its activity is augmented in lupus T cells and contributes to the defect in IL-2 production by altering the pCREB/CREM ratio. The inhibition of PP2A in lupus T cells (by siRNA or by the expression of dominant negative isoforms) increases pCREB binding to the promoters of fos and il2 and, by doing so, corrects the IL-2 production defect [39].
As expected, CREB and CREM are involved in the regulation of other genes and imbalances of their levels alter the expression of several proteins besides IL-2. fos is one of the genes whose transcription is hampered by excessive amounts of CREM. Thus a diminution in AP-1 levels is a second factor that underlies the IL2 transcription deficiency of SLE patients [40].
Concluding remarks
IL-2 is a cytokine that is essential for the correct function of the immune system. Recent work has revealed the existence of novel factors capable of influencing IL-2 production, by either directly stimulating transcription, or by modifying local chromatin conditions. Further work will establish the physiological role of each of these factors and will address their behaviour during disease states. Better understanding of the mechanisms that regulate IL-2 production will allow us to better understand immune function and the relationship between regulatory and effector T cells.
Take-home messages
The rate of il2 transcription is determined by a complex interplay of a large number of transcription factors.
Chromatin remodeling plays an essential role in the regulation of il2 transcription.
The role of FoxP3 as a transcriptional regulator is not confined to regulatory T cells.
Transcriptional factor occupancy is altered in SLE T cells, especially due to an imbalance in CREB/CREM and a decrease in AP-1.
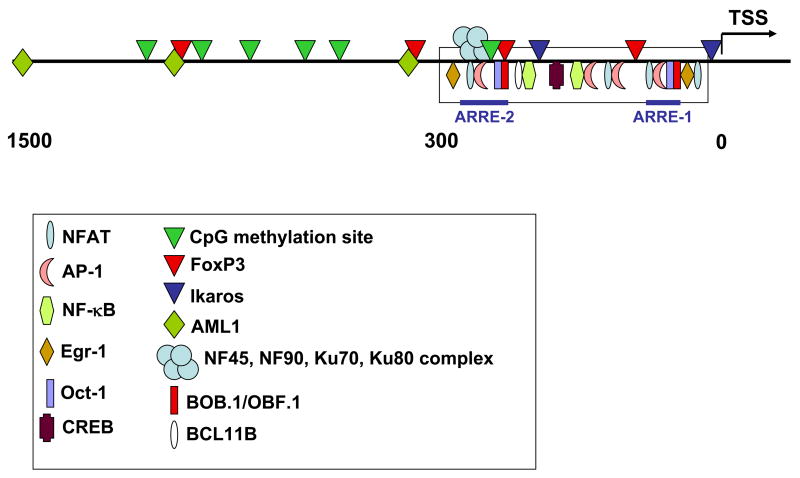
Figure 2. IL2 gene promoter.
Even though most transcription factors bind to a densely packed minimal enhancer region that spans ~300 bp upstream of the TSS of the IL2 gene, sites that bind transcriptional regulators, as well as CpG methylation sites have been recently described in a longer region that includes approximately 1500 bp upstream from the TSS.
Acknowledgments
Work was supported by PHS grant R01 AI 49954.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 2.Crispin JC, Tsokos GC. Novel molecular targets in the treatment of systemic lupus erythematosus. Autoimmun Rev. 2008;7:256–261. doi: 10.1016/j.autrev.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 5.Kim HP, Imbert J, Leonard WJ. Both integrated and differential regulation of components of the IL-2/IL-2 receptor system. Cytokine Growth Factor Rev. 2006;17:349–366. doi: 10.1016/j.cytogfr.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Helms WS, Jeffrey JL, Holmes DA, Townsend MB, Clipstone NA, Su L. Modulation of NFAT-dependent gene expression by the RhoA signaling pathway in T cells. J Leukoc Biol. 2007;82:361–369. doi: 10.1189/jlb.0206120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfeuffer I, Klein-Hessling S, Heinfling A, Chuvpilo S, Escher C, Brabletz T, Hentsch B, Schwarzenbach H, Matthias P, Serfling E. Octamer factors exert a dual effect on the IL-2 and IL-4 promoters. J Immunol. 1994;153:5572–5585. [PubMed] [Google Scholar]
- 8.Brunner C, Sindrilaru A, Girkontaite I, Fischer KD, Sunderkotter C, Wirth T. BOB.1/OBF. 1 controls the balance of TH1 and TH2 immune responses. EMBO J. 2007;26:3191–3202. doi: 10.1038/sj.emboj.7601742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cismasiu VB, Ghanta S, Duque J, Albu DI, Chen HM, Kasturi R, Avram D. BCL11B participates in the activation of IL2 gene expression in CD4+ T lymphocytes. Blood. 2006;108:2695–2702. doi: 10.1182/blood-2006-05-021790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murayama A, Sakura K, Nakama M, Yasuzawa-Tanaka K, Fujita E, Tateishi Y, Wang Y, Ushijima T, Baba T, Shibuya K, Shibuya A, Kawabe Y, Yanagisawa J. A specific CpG site demethylation in the human interleukin 2 gene promoter is an epigenetic memory. EMBO J. 2006;25:1081–1092. doi: 10.1038/sj.emboj.7601012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adachi S, Rothenberg EV. Cell-type-specific epigenetic marking of the IL2 gene at a distal cis-regulatory region in competent, nontranscribing T-cells. Nucleic Acids Res. 2005;33:3200–3210. doi: 10.1093/nar/gki637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas RM, Chunder N, Chen C, Umetsu SE, Winandy S, Wells AD. Ikaros Enforces the Costimulatory Requirement for IL2 Gene Expression and Is Required for Anergy Induction in CD4+ T Lymphocytes. J Immunol. 2007;179:7305–7315. doi: 10.4049/jimmunol.179.11.7305. [DOI] [PubMed] [Google Scholar]
- 13.Bandyopadhyay S, Dure M, Paroder M, Soto-Nieves N, Puga I, Macian F. Interleukin 2 gene transcription is regulated by Ikaros-induced changes in histone acetylation in anergic T cells. Blood. 2007;109:2878–2886. doi: 10.1182/blood-2006-07-037754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi L, Zhao G, Qiu D, Godfrey WR, Vogel H, Rando TA, Hu H, Kao PN. NF90 regulates cell cycle exit and terminal myogenic differentiation by direct binding to the 3′-untranslated region of MyoD and p21WAF1/CIP1 mRNAs. J Biol Chem. 2005;280:18981–18989. doi: 10.1074/jbc.M411034200. [DOI] [PubMed] [Google Scholar]
- 15.Pei Y, Zhu P, Dang Y, Wu J, Yang X, Wan B, Liu JO, Yi Q, Yu L. Nuclear Export of NF90 to Stabilize IL-2 mRNA Is Mediated by AKT-Dependent Phosphorylation at Ser647 in Response to CD28 Costimulation. J Immunol. 2008;180:222–229. doi: 10.4049/jimmunol.180.1.222. [DOI] [PubMed] [Google Scholar]
- 16.Shi L, Qiu D, Zhao G, Corthesy B, Lees-Miller S, Reeves WH, Kao PN. Dynamic binding of Ku80, Ku70 and NF90 to the IL-2 promoter in vivo in activated T-cells. Nucleic Acids Res. 2007;35:2302–2310. doi: 10.1093/nar/gkm117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Safford M, Collins S, Lutz MA, Allen A, Huang CT, Kowalski J, Blackford A, Horton MR, Drake C, Schwartz RH, Powell JD. Egr-2 and Egr-3 are negative regulators of T cell activation. Nat Immunol. 2005;6:472–480. doi: 10.1038/ni1193. [DOI] [PubMed] [Google Scholar]
- 18.Skerka C, Decker EL, Zipfel PF. A regulatory element in the human interleukin 2 gene promoter is a binding site for the zinc finger proteins Sp1 and EGR-1. J Biol Chem. 1995;270:22500–22506. doi: 10.1074/jbc.270.38.22500. [DOI] [PubMed] [Google Scholar]
- 19.Collins S, Wolfraim LA, Drake CG, Horton MR, Powell JD. Cutting Edge: TCR-induced NAB2 enhances T cell function by coactivating IL-2 transcription. J Immunol. 2006;177:8301–8305. doi: 10.4049/jimmunol.177.12.8301. [DOI] [PubMed] [Google Scholar]
- 20.Pan F, Ye Z, Cheng L, Liu JO. Myocyte enhancer factor 2 mediates calcium-dependent transcription of the interleukin-2 gene in T lymphocytes: a calcium signaling module that is distinct from but collaborates with the nuclear factor of activated T cells (NFAT) J Biol Chem. 2004;279:14477–14480. doi: 10.1074/jbc.C300487200. [DOI] [PubMed] [Google Scholar]
- 21.Matsuoka H, Fujimura T, Hayashi M, Matsuda K, Ishii Y, Aramori I, Mutoh S. Disruption of HDAC4/N-CoR complex by histone deacetylase inhibitors leads to inhibition of IL-2 gene expression. Biochem Pharmacol. 2007;74:465–476. doi: 10.1016/j.bcp.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Ono M, Yaguchi H, Ohkura N, Kitabayashi I, Nagamura Y, Nomura T, Miyachi Y, Tsukada T, Sakaguchi S. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature. 2007;446:685–689. doi: 10.1038/nature05673. [DOI] [PubMed] [Google Scholar]
- 23.Bruniquel D, Schwartz RH. Selective, stable demethylation of the interleukin-2 gene enhances transcription by an active process. Nat Immunol. 2003;4:235–240. doi: 10.1038/ni887. [DOI] [PubMed] [Google Scholar]
- 24.Shevach EM. Regulatory T cells in autoimmmunity*. Annu Rev Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 25.Mantel PY, Ouaked N, Ruckert B, Karagiannidis C, Welz R, Blaser K, Schmidt-Weber CB. Molecular mechanisms underlying FOXP3 induction in human T cells. J Immunol. 2006;176:3593–3602. doi: 10.4049/jimmunol.176.6.3593. [DOI] [PubMed] [Google Scholar]
- 26.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 27.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, Mathis D, Benoist C, Chen L, Rao A. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 28.Chen C, Rowell EA, Thomas RM, Hancock WW, Wells AD. Transcriptional regulation by Foxp3 is associated with direct promoter occupancy and modulation of histone acetylation. J Biol Chem. 2006;281:36828–36834. doi: 10.1074/jbc.M608848200. [DOI] [PubMed] [Google Scholar]
- 29.Li B, Samanta A, Song X, Iacono KT, Bembas K, Tao R, Basu S, Riley JL, Hancock WW, Shen Y, Saouaf SJ, Greene MI. FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc Natl Acad Sci U S A. 2007;104:4571–4576. doi: 10.1073/pnas.0700298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, Li B, Turka LA, Olson EN, Greene MI, Wells AD, Hancock WW. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–1307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 31.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 32.Nandiwada SL, Li W, Zhang R, Mueller DL. p300/Cyclic AMP-responsive element binding-binding protein mediates transcriptional coactivation by the CD28 T cell costimulatory receptor. J Immunol. 2006;177:401–413. doi: 10.4049/jimmunol.177.1.401. [DOI] [PubMed] [Google Scholar]
- 33.Macian F, Garcia-Cozar F, Im SH, Horton HF, Byrne MC, Rao A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 34.Thomas RM, Gao L, Wells AD. Signals from CD28 induce stable epigenetic modification of the IL-2 promoter. J Immunol. 2005;174:4639–4646. doi: 10.4049/jimmunol.174.8.4639. [DOI] [PubMed] [Google Scholar]
- 35.Hsueh YP, Liang HE, Ng SY, Lai MZ. CD28-costimulation activates cyclic AMP-responsive element-binding protein in T lymphocytes. J Immunol. 1997;158:85–93. [PubMed] [Google Scholar]
- 36.Tenbrock K, Juang YT, Tolnay M, Tsokos GC. The cyclic adenosine 5′-monophosphate response element modulator suppresses IL-2 production in stimulated T cells by a chromatin-dependent mechanism. J Immunol. 2003;170:2971–2976. doi: 10.4049/jimmunol.170.6.2971. [DOI] [PubMed] [Google Scholar]
- 37.Tenbrock K, Juang YT, Gourley MF, Nambiar MP, Tsokos GC. Antisense cyclic adenosine 5′-monophosphate response element modulator up-regulates IL-2 in T cells from patients with systemic lupus erythematosus. J Immunol. 2002;169:4147–4152. doi: 10.4049/jimmunol.169.8.4147. [DOI] [PubMed] [Google Scholar]
- 38.Juang YT, Wang Y, Solomou EE, Li Y, Mawrin C, Tenbrock K, Kyttaris VC, Tsokos GC. Systemic lupus erythematosus serum IgG increases CREM binding to the IL-2 promoter and suppresses IL-2 production through CaMKIV. J Clin Invest. 2005;115:996–1005. doi: 10.1172/JCI200522854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katsiari CG, Kyttaris VC, Juang YT, Tsokos GC. Protein phosphatase 2A is a negative regulator of IL-2 production in patients with systemic lupus erythematosus. J Clin Invest. 2005;115:3193–3204. doi: 10.1172/JCI24895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kyttaris VC, Juang YT, Tenbrock K, Weinstein A, Tsokos GC. Cyclic adenosine 5′-monophosphate response element modulator is responsible for the decreased expression of c-fos and activator protein-1 binding in T cells from patients with systemic lupus erythematosus. J Immunol. 2004;173:3557–3563. doi: 10.4049/jimmunol.173.5.3557. [DOI] [PubMed] [Google Scholar]