SUMMARY
In order to serve as enzymes, receptors and ion channels, proteins require structural precision. This is monitored by a cellular quality control system (QCS) that rejects misfolded proteins and thereby protects the cell against aberrant activity. Misfolding can result in protein molecules that retain intrinsic function, yet become misrouted within the cell; these cease to perform normally and result in disease. A therapeutic opportunity exists to correct misrouting and rescue mutants using “pharmacoperones,” (small molecular folding templates, often peptidomimetics, which promote correct folding and rescue) thereby restoring function and potentially curing the underlying disease. Because of its small size, the GnRH (gonadotropin-releasing hormone) receptor (GnRHR) is an excellent model for GPCRs (G protein-coupled receptor) and has allowed elucidation of the precise biochemical mechanism of pharmacoperone action necessary for rational design of new therapeutic agents. This review summarizes what has been learned about the structural requirements of the GnRHR that govern its interaction with the QCS and now presents the potential for the rational design of pharmacoperones. Because of the role of protein processing, this approach is likely to be applicable to other GCPCs and other proteins in general.
Keywords: protein folding, protein targeting, misfolded proteins, pharmacoperone, GnRH receptor, GPCR
Endogenous Chaperones of the Endoplasmic Reticulum Monitor the Precise Folding Needed for Proteins to Perform Properly
As proteins are synthesized, formation of Cys bonds and steric considerations provide higher order structure, as does the formation of ion pairs (salt bridges). This latter event also buries electrostatic interactions and helps satisfy kinetic requirements for protein folding (Radford and Dobson, 1999; Sitia and Braakman, 2003). Ion pairing also increases net lipophilicity, allows movement across membranes (Levinthal, 1968) and provides interactions that limit subsequent conformational choices during the folding process. This restriction is important because of “Levinthal’s Paradox,” which points out that the random number of potential configurations for an “average” protein is high (10143 in the original paper). This number of choices, if approached randomly, is far too many to result in a significant success rate or to explain the observation that most cellular proteins fold “correctly” in a micro-second time frame. The resolution of this paradox is to recognize that proteins do not fold randomly, but are restricted by interactions with endogenous chaperone proteins of the endoplasmic reticulum (ER) forming a quality control system (QCS) that assists in folding and retains misfolded structures in the ER, either allowing them to refold correctly or be degraded through the polyubiquitination/proteasome pathway.
Pharmacoperone Drugs Can Refold Misfolded Mutants, Allow Them to Pass the QCS, and Rescue Proteins that would Otherwise Be Misfolded and Misrouted (i.e. Retained in the ER)
By rejecting misfolded proteins, the QCS protects the cell against aberrant activity (Ellgaard and Helenius, 2001; Sanders and Nagy, 2000; Sitia and Braakman, 2003; Ulloa-Aguirre et al., 2004b) and disease (Aridor, 2007; Nakatsukasa and Brodsky, 2008). The QCS contains a chemically heterogeneous class of endogenous chaperone proteins that promote and facilitate folding and assembly by engaging in association with nascent proteins which display “inappropriate” features. One example of such a feature is the unexpected presentation of a hydrophobic plate in an aqueous environment. Accumulation of such proteins is dangerous since this has the potential to result in unexpected aggregation and/or interactions of misfolded proteins with other molecules in the crowded ER environment (Hartl and Hayer-Hartl, 2002; Horwich, 2002). This is established to lead to potentially toxic intracellular accumulation or even to excessive protein accumulation in the plasma with extracellular amyloid deposition (Chiti and Dobson, 2006; Dobson, 1999; Forloni et al., 2002; Kopito and Ron, 2000). A similar mechanism may explain the formation of cataracts (Sandilands et al., 2002).
A growing list supports the view that mutants of receptors, enzymes, and ion channels frequently result in protein misfolding and subsequent retention by the cellular QCS (Bernier et al., 2004a; Bernier et al., 2004b; Burrows et al., 2000; Conn and Janovick, 2005; Ishii et al., 2004; Janovick et al., 2002; Leanos-Miranda et al., 2002; Loo et al., 2005; Pastores and Barnett, 2005; Suzuki, 2006; Tamarappoo and Verkman, 1998; Ulloa-Aguirre et al., 2003; Ulloa-Aguirre et al., 2004a; Ulloa-Aguirre et al., 2004b; Ulloa-Aguirre et al., 2006; Wang et al., 2006; Yam et al., 2005). This observation contrasts with the prior presumption that mutational inactivation always reflects loss of intrinsic function (i.e., a receptor that either fails to recognize a ligand or does not couple productively to its effector). Recognition of the importance of misrouting of otherwise functional proteins immediately presents the therapeutic opportunity to correct misrouting and rescue mutants using pharmacological chaperones (“pharmacoperone,” low molecular weight drugs that refold, misfolded proteins and cause them to route correctly: http://en.wikipedia.org/wiki/Pharmacoperone).
The GnRHR is a Good Model for Understanding the Folding of GPCRs
The GnRHR-ligand system is a particularly good model to understand cellular trafficking of GPCRs for a number of reasons:
The GnRHR is one of the smallest GPCRs (328 amino acids in the human); it may contain only the essentials required for ligand binding and signal transduction. A small size means that there are fewer domains to consider in identification of important structural motifs. Small proteins require fewer primers for synthesis and for sequencing than do larger GPCRs (typically at least twice the size of the GnRHR), and there is less sequence length that might lead to random mutation during the PCR process. DNA sequencing of mutants is also cheaper than for a larger protein. We have relied on hundreds of mutants (over 200 are reported here, (Janovick et al., 2006; Knollman et al., 2005).
The ligand, GnRH itself, is small (a decapeptide) and has good thermal and chemical stability (no oxidizable Met) and no internal Cys bridges (minimal fixed structure). Its size and stability has led to the availability of thousands of analogs, some of which have been used for preparation of radioligand assays and other markers (colloidal gold, fluorescein, Texas Red), that can be used as cellular markers (Brothers et al., 2003; Cornea et al., 1999; Cornea et al., 2001; Hazum et al., 1980; Jennes et al., 1984; Jennes et al., 1986; Lin et al., 1998a).
The physiology of the system mediated by the GnRHR is well-characterized in many animal models and this information has already led to useful drugs for the treatment of disorders of reproduction and for cancer.
Sequence differences between different species (Conn, 1994) has made it possible to determine how changes in routing have been impacted by sequence changes (i.e. natural mutation, Conn et al., 2006a; Conn et al., 2006b; Janovick et al., 2003a; Janovick et al., 2007a). There are structural changes among particular animals that appear to be explained by reproductive specializations (Janovick et al., 2007a; Janovick et al., 2006; Knollman et al., 2005). The human GnRHR, appears “balanced” in its distribution between the PM and ER (Conn et al., 2006a; Conn et al., 2006b), so much so that about 50% of the human WT GnRHR (in cells transfected with the corresponding sequence) is retained in the ER and can be “rescued” by the approach described above. The strong and convergent evolutionary pressure for this “inefficiency” suggests a regulatory advantage (Conn et al., 2006a;Conn et al., 2006b; Ulloa-Aguirre et al., 2006). This system offers the ability to examine the evolution of the QCS system since these receptors have been cloned from a wide range of animals (fish, birds, reptiles, many mammals and multiple primates).
We have available, substantive information on the mechanism of misfolding (Conn et al., 2006a; Conn et al., 2006b), mutant interactions with pharmacoperones (Ulloa-Aguirre et al., 2003), the molecular basis of the dominant-negative effect (Conn et al., 2006a; Conn et al., 2006b), and access to multiple drug classes of pharmacoperones for the GnRHR and multiple drugs within each class (Janovick et al., 2003b) with sufficient quantities to enable in vivo studies. Recent studies (Janovick et al., 2007b) indicate that the mutant receptor that is already trapped in the ER can be freed by pharmacoperones—a surprising result that increases the potential therapeutic reach of this approach since pharmacoperones do not need to be present at the moment of receptor synthesis.
Structural Features of the GnRHR that Impact on its Level at the Plasma Membrane and Trafficking Through the QCS
A. The Carboxyl-Terminal Cytoplasmic Tail
One feature that contributes to the small size of the human GnRHR is the absence of the long cytoplasmic tail at the carboxyl terminal typical of members of this superfamily (Figure 1, which is provided as a map for this and subsequent sections describing specific amino acids in the human GnRHR). Unlike the mammalian GnRHR, fish, reptile and bird GnRHRs have an extended carboxyl tail that prolongs the presence of the receptor on the plasma membrane (Lin et al., 1998b). This is absent in mammals in which a lower percentage of the total synthesized GnRHR is expressed at the plasma membrane. When a chimera of the rat GnRHR with the carboxyl terminal from the catfish (a 51 amino acid sequence) is created, the result is increased levels of this chimera at the plasma membrane, likely due to alterations in the pattern of down-regulation; the selectivity of effector coupling is also changed (Lin et al., 1998b).
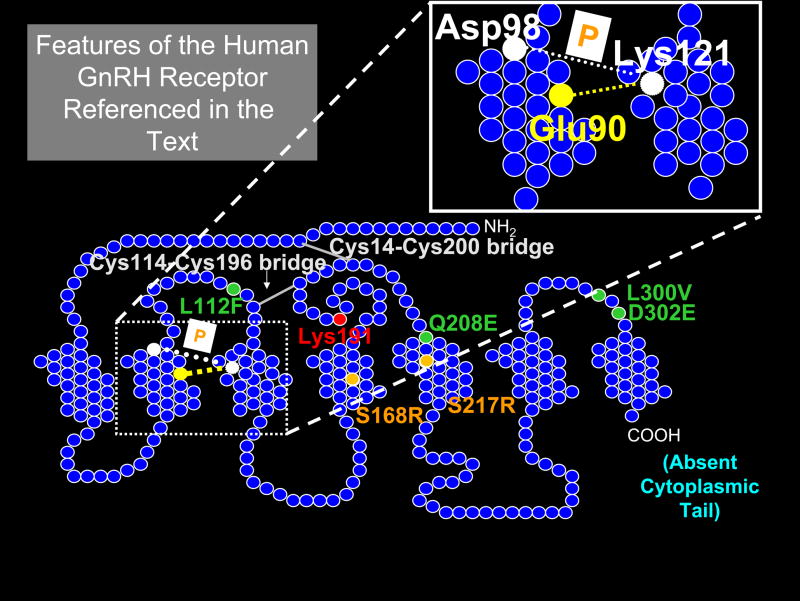
Figure 1. Model of the human GnRH receptor showing residues of interest referred to in the text.
Circles represent amino acids; those colored green form a motif of four non-contiguous residues that are required in the human GnRHR for Lys191 (red) to destabilize the formation of the Cys14-Cys200 (Cys199 in the rat or mouse) bridge (gray). Circles colored orange represent amino acids for the two naturally occurring mutants that cannot be rescued by known pharmacoperones. Thermodynamically unfavored modifications at these sites (both Ser → Arg) cause misfolding due to misalignment of the Cys residues that normally form the Cys14-Cys200 bridge, as described in the text. The site of pharmacoperone (shown as “P” in the white box) action (bridging residues Asp98 to Lys121) is shown, as is the naturally occurring Glu90-Lys121 salt bridge. This portion of the hGnRHR is also shown in the enlarged detail of transmembrane segments 2 and 3. The mammalian GnRHR lacks the long cytoplasmic tail (COOH terminal) that is typical of this super-family and present in piscine, reptilian and avian GnRHRs.
B. The Impact of Amino Acid Differences between Rat, Mouse and Human WT GnRHR
Rat WT retains the ability to oligomerize (since human and mouse mutants exert a dominate negative (DN) effect on rat WT sequence, Knollman et al., 2005), but, unlike human or mouse, escapes the DN effect of GnRHR mutants because rGnRHR mutants route to the plasma membrane with higher efficiency than does mouse or human mutants. These distinct behaviors of mouse and rat GnRHRs (distinguished by only four semi- or non-conservative amino acid differences) led us to assess the role of each amino acid. The difference in both routing and the DN effect appears mediated primarily by Ser216 in the rat GnRHR. The homologous amino acid in the hGnRHR is also Ser (Ser217, the numbering difference due to the absence of Lys191 in the rat), and is compensated for by the primate-unique insertion of Lys191 that, alone, dramatically decreases routing of the receptor (see section C2 below). These studies establish the importance of amino acid 216 in the rodent for the altered DN effect and altered receptor trafficking in the mouse and rat. Both of these GnRHs express with a higher efficiency at the plasma membrane and this explains why hGnRHR is more susceptible to defective trafficking by disease-related point mutations than rodent counterparts; such mutations simply have a more pronounced effect on the plasma membrane expression of the human receptor since it is expressed with low efficiency at the plasma membrane (Knollman et al., 2005). Control of the efficiency is a function of the formation of Cys bridges, discussed in the following section.
C. Cysteine Bridges and Control of Their Formation
1. The Cys114-Cys195/196 Bridge
In a biochemical study comparing the human and the rat GnRHR (Janovick et al., 2006), we examined the two Cys bridges present in the molecule. One bridge (Cys114-Cys195/196) connects the first and second extracellular loops and is so essential for activity that, in rats, mice and humans, conversion of either Cys to Ala (i.e. to break the bridge), results in almost complete loss of activity at the plasma membrane, a likely effect of recognition of the mutant as a misfolded protein by the QCS. Homologous and obligatory bridges are found in almost all GPCRs known and so this observation was not too surprising; the bridge that appears to be a structural feature associated with the fundamental stability of the GPCR motif is in question. Pharmacoperones were unsuccessful at rescuing this mutant.
2. The Cys14-Cys199/200 Bridge
The other bridge (Cys14- Cys199/200), connects the amino terminal with the second extracellular loop and is uncommon in GPCRs. This bridge appears to be required for a correctly folded molecule in the human GnRHR but is less important in the mouse or rat GnRHR (Janovick et al., 2006). Mutations (Cys → Ala) in the rat or mouse had only modest effects on receptor expression at the plasma membrane.
There were three additional observations that were important in understanding the regulation of the formation of the Cys14-Cys200 bridge in the human GnRHR:
First, a pharmacoperone could rescue the human receptor with Cys14Ala or Cys200Ala, suggesting that the bridge was needed for proper folding.
Second, the deletion of Lys191 in the human obviated the need to form the bridge (Janovick et al., 2006), suggesting that this residue was destabilizing the structure required for formation of the bridge. Mammals (other than rats and mice) contain the insertion of an amino acid, Glu191 (in most non-primates) and Lys191 (among primates). This is associated with diminished expression at the plasma membrane. Replacement of the Lys191 normally in humans with Glu191 showed that this amino acid was slightly less effective than the Lysine in inhibiting movement to the plasma membrane. Rats and mice lack this residue (Knollman et al., 2005) and are, accordingly, one amino acid shorter than most other mammalian orthologs.
Third, preparation of the rat homolog in which Lys191 was inserted did not result in destabilization of the rodent receptor. This simply meant there was a more complex difference between the rat and the human that was required for the destabilizing effect of the Lys191.
3. A Four Amino Acid Motif Enables Lys191 to Destabilize the Cys14-Cys200 Bridge in the Human GnRHR
The identification of the amino acid differences between the rat and the human GnRHR that enabled Lys191 to destabilize the Cys14-Cys200 bridge was a daunting task since there were 39 amino acid differences between the rat and human GnRHR sequence and a seemingly endless number of mutants to explore all the combinations. We approached this problem by locating the thermodynamically unfavored changes (Janovick et al., 2006), figuring that these might be the most important. Interestingly, there were only 3 and these were located in close physical proximity to the Lys191 and to the Cys14-Cys200 bridge. It was also interesting that these all involved the loss or gain of a Ser or Pro, both amino acids associated with introducing a bend in the protein backbone and setting the alignment between the second extracellular loop and the amino terminal. Pro forms a five-membered nitrogen-containing ring, a feature that causes it to be found in very tight turns in protein structures (i.e. where the polypeptide chain must change direction). Clearly Nature has tipped her hand: the peptide backbone is being bent to control the relation between Cys14 and Cys200 thereby controlling the probability of formation of the bridge.
The rest of the motif was identified by making guesses about the physical relation between amino acids in the 3-dimensional state. We used this information to create chimeric human receptors that were modified to be rat-like at four (non-contiguous) residues. These expressed at the higher levels associated with the rat receptor and lacked the requirement for the Cys14-Cys200 bridge—another feature of the rat GnRHR.
The rest of the motif that enables the human structure to take advantage of the steric interference by insertion of the “extra” amino acid at position 191 was identified as 4 non-contiguous change residues 112, 208, 300 and 302 (Fig. 1). The mechanism by which these act is not intuitively obvious, since in the structural model we constructed (Janovick et al., 2009) there is no clear relation between these residues and Lys191 or the bridge. Nonetheless, human mutants containing the orthologous rat sequence at those sites expressed higher receptor levels at the plasma membrane associated with the rat sequence and, moreover, lost the requirement for the Cys14-Cys200 bridge.
4. Understanding the Alignment of the Cys14-Cys200 Bridge Explains the Inability of Mutants Ser168Arg and Ser217Arg to Pass the QCS or be Rescued
The spatial alignment needed for formation of the Cys14-Cys200 bridge was quite subtle since the two Cys residues had to be within a distance corresponding to about the size of one water molecule in order for the bridge to form. When the bridge forms, the human GnRHR is recognized by the cellular QCS as correctly folded. When it does not form, it is viewed as defective and retained (then presumably destroyed) in the ER. The cell is exploiting this approach as a means of controlling routing in normal function of healthy cells using this technique to control the efficiency of expression of the protein at the plasma membrane. The nature of the regulation remains unknown but is presumably sufficient to offset the cost of “wasting” some of the receptor and the added burden for mutational disease—mutations are much more significant to the disruption of trafficking of the GnRHR in human than in the rat or mouse.
Mutants Ser168Arg and Ser217Arg (Figure 1) are in a previously reported “zone of death” (Conn et al., 2002) and cannot be rescued by any of several different chemical classes (indoles, quinolines, and erythromycin macrolides) of pharmacoperones that successfully rescued other mutants; a rare circumstance, since the vast majority of mutants are rescuable by all classes (Janovick et al., 2003b).
We had initially considered these sites might be unrescuable because they were important for the ligand-receptor interactions. We now realize that there is a different explanation. This observation and the physical relation between TMS4 and TMS5 to ECL2 make it attractive to consider that (charge altering) mutations in these two residues exert their influence by regulating the position of ECL2 and the intimacy of Cys14 and Cys200. Due to charge considerations, the thermodynamically unfavored exchange of Ser and Arg likely moves the ECL2 into a position from which the formation of a Cys bridge is improbable and the mutant never passes the cellular QCS, even in the presence of pharmacoperones.
The homologous position of amino acid 217 in the human is 216 in the rat (since there is no ortholog to position 191 in the rat or mouse). In addition to being in the “zone of death” this is the very same position that distinguishes the trafficking characteristic of the rat from the mouse. The mouse contains Gly216 while the rat has Ser216. The amino acids which differ very modestly have substantial functional differences. Ser, with a slightly polar nature, small size, and propensity of the side-chain hydroxyl oxygen to H bond with the protein backbone, is often found in association with the tight turns of the protein structure. Gly, on the other hand, is very flexible.
D. The Conserved and Essential Glu90-Lys121 Salt Bridge and the Biochemical Mechanism of Pharmacoperone Action
A mutation of the GnRHR, Glu90Lys, which converts a negatively charged residue to a positively charged one in humans, leads to hypogonadotropic hypogonadism in humans and suggests the importance of this residue in formation of the receptor structure. When expressed in cell cultures, this gene product does not appear at the plasma membrane (assessed by binding or by IP production) and is fully rescued by pharmacoperones.
We have recently (Janovick et al., 2009) modeled the GnRHR and noticed that this mutation obviates the formation of a critical salt bridge (Glu90-Lys121) needed for correct processing in the quality control system. The residues that form the bridge are heavily conserved in the GnRH receptor of virtually all mammals, fish, birds and reptiles and it clearly precedes many of the requirements noted above, from an evolutionary point of view.
The ability of pharmacoperone drugs to rescue this mutant, virtually fully, and the observation that these drugs bind in the same area, led us to examine the relation between the bridge and these drugs. The combination of modeling studies and mutational analysis with confocal microscopy enabled us to reach the conclusion that indole and quinolone pharmacoperones act by forming a surrogate bridge from residue Asp98 to Lys121 that can substitute for the salt bridge that is broken in the human mutation described above.
We were interested to note that Asp98 and Lys121 are also points of contact of the receptor’s ligand, GnRH, and wondered if it was a mere coincidence that Asp98 and Lys121 are points of contact for both rescuing pharmacoperones and GnRH. Given the number of amino acids, it seemed unlikely to be a random event and one could imagine that there would be good reason to recognize GPCRs with defective ligand binding sites that are totally defective and not allow them to traffic to the plasma membrane.
The charged residues in both the Glu90-Lys121 and the ligand-mediated Asp98-Lys121 bridges, although rare among the hydrophobic amino acids of the transmembrane helices 2 and 3, are highly conserved in GnRHRs. Asp98 is absolutely conserved in all of the mammalian, reptilian, avian and piscine GnRHRs sequenced to date. In the fruit fly, a conservative change is made to Glu98. Likewise, Lys121 is maintained in the same groups and in fruit flies, the residue is a conservative change, Arg121. Glu90 is conserved in mammals, but Val90 is present in eels, reptiles, avians and flies and perciform fish and the residue is Met90 in trout and catfish. Another apparent point of contact for quinolone pharmacoperones is the highly conserved Phe313 (Leu313 in canines and equines) which has already been reported to be the basis of the inability of these species to recognize these drugs (Cui et al., 2000). It is certainly possible that this could result in further structural stability to the receptor.
It was initially curious to us that pharmacoperone drugs from different chemical classes all happened to interact identically by creating a surrogate bridge for Glu90-Lys121. While it is conceivable that the correctly formed structure of the ligand binding site is, in some way, related to the configuration of the receptor that is allowed to pass the cellular QCS, we considered other possibilities, including prejudice in the screening process used to select these drugs. In that regard, all the pharmacoperones used in the present study were selected from high throughput screens for antagonism of the natural ligand. Accordingly, as competitors of the natural ligand, it is not surprising that they would interact at (or near) the ligand binding site. This site resides in the lateral plane of the plasma membrane, a region characterized by a high percentage of hydrophobic residues. The linear sequence around Glu90 is, for example, Leu-Leu-Glu90-Thr-Leu-Ile-Val-Met-Pro-Leu-Asp98 and around 121 is Val-Leu-Ser-Tyr-Leu-Lys121-Leu-Phe-Ser-Met. This is a predominantly hydrophobic region with a modest number of ionic or polar groups. Accordingly, the observation of this common ionic site could reflect that the drugs were all selected with the same prejudice for this preferential ion-pair and/or polar interaction with the charged residue sites. Accordingly, our data do not allow the conclusion that stabilization of the ligand binding site is, itself, sufficient for a pharmacoperone to allow a molecule to pass the cellular QCS.
It is clear that pharmacoperones rescue most of the GnRHR mutants (Brothers et al., 2004; Conn et al., 2006a; Janovick et al., 2002; Janovick et al., 2003b; Janovick et al., 2006; Leanos-Miranda et al., 2005), even though mutations appear at many sites in the receptor, both in the transmembrane component and in intra- and extracellular sites. It was initially curious that stabilization of the relation between TM2 and TM3 would successfully rescue such a diverse set of mutations. This may reflect the highly interactive nature of GPCRs, and the critical requirement of this salt bridge for the chaperone system of the cell to recognize the protein as correctly folded.
In order to determine whether the biochemical mechanism of action of the indole and quinolone classes of drugs (i.e. bridging the Asp98-Lys121 residues) was unique to these classes, we examined two additional pharmacoperone classes “A177775” (3′-N-des-methyl-3′-N-cyclopentyl-11-deoxy-11-[carboxy-(3,4-dichlorophenylethylamino)]-6-O-methyl-erythromycin A 11,12-(cyclic carbamate)) (Abbott laboratories, Abbott Park, IL) and “TAK-013” (N-{4-[5-{[benzyl(methyl)amino]methyl}-1-(2,6-difluorobenzyl)-2,4-dioxo-3-phenyl-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl]phenyl}-N′-methoxyurea) (Takeda, Osaka, Japan). The former is an erythromycin macrolide and the latter is a thieno[2,3-b]pyrimidine-2,4-dione; these chemical structures (see Figure 2) are very different from the indoles and quinolone structures described previously (Janovick et al., 2009), although all four are derived from screening approaches that identify GnRHR antagonists and, accordingly, interact with the GnRHR at sites that are identical or adjacent (and provide steric hindrance).
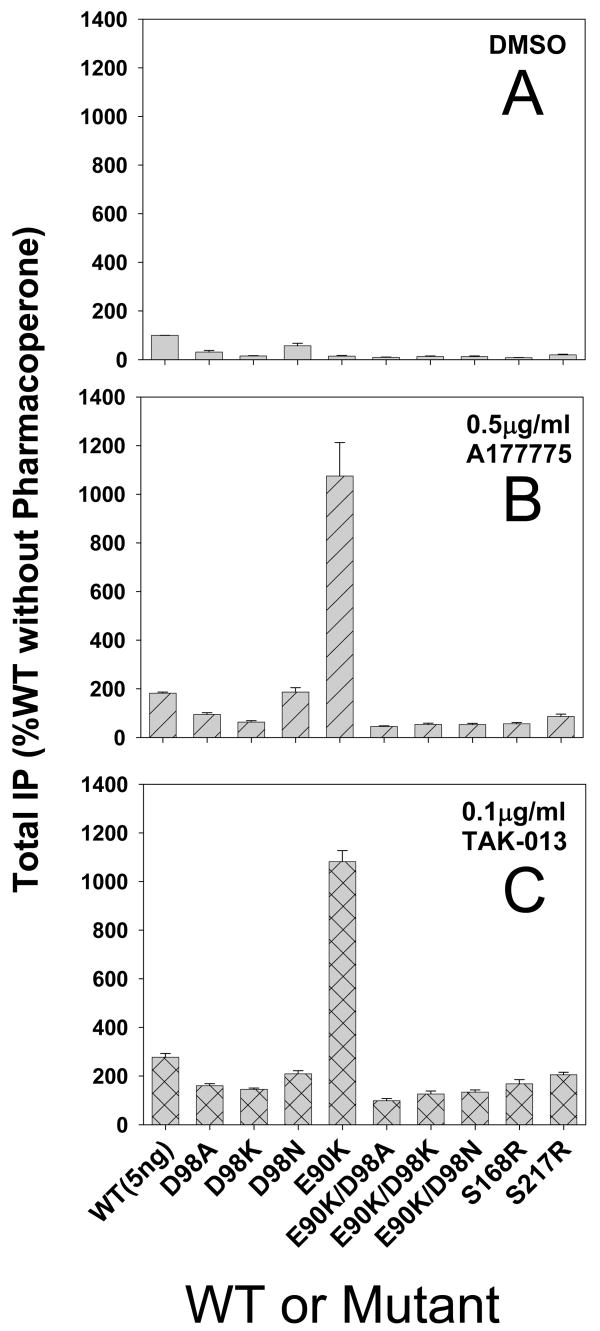
Figure 2. Effect of mutation of residue Asp98 to Ala, Lys, or Asn on rescue with two classes of pharmacoperones.
Total IP production was measured in response to a saturating concentration of Buserelin (10−7 M). Data are expressed as percent of WT without pharmacoperone (panel A) or after preincubation (rescue) with A177775 (panel B) or TAK-013 (panel C). Cells were transfected with 95 ng of WT or mutant cDNA. Mutants Ser168Arg and Ser217Arg were used as controls, since these are grossly misfolded and cannot be rescued from ER retention with pharmacoperone. Mutant Glu90Lys was included as a positive control for rescue with pharmacoperone. Inset illustration shows the dose response curves (log scale) for each class of pharmacoperone rescue. Averages and SEMs of at least 3 independent experiments performed in replicates of 6 are shown. The dose-response for rescue and chemical structures are shown to the right of graphics B–C.
In order to test the postulated role for Asp98 in the biochemical mechanism of action of pharmacoperones, we constructed three single mutants of the hGnRHR, Asp98Ala, Asp98Lys and Asp98Asn and three double mutants Glu90Lys/Asp98Ala, Glu90Lys/Asp98Lys and Glu90Lys/Asp98Asn. Glu90Lys is the naturally occurring mutation that breaks the salt bridge Glu90-Lys121 and results in HH. The three single mutants (transfected at 95 ng) responded at basal levels only to the agonist, Buserelin (10−7 M, Figure 2A). The axes of all images in Figure 2 are identical to allow comparisons. Likewise, none of the three double mutants (also transfected at 95 ng) responded measurably to Buserelin (Figure 2A). As controls, we also included WT hGnRHR, known to be only fractionally routed to the plasma membrane (Janovick et al., 2007b), as well as the mutant Glu90Lys (rescuable by pharmacoperones, Janovick et al., 2002; Janovick et al., 2003b) and two mutants Ser168Arg and Ser217Arg that cannot be rescued by pharmacoperones (Janovick et al., 2006). These two mutations (Glu90 and Asp98) promote loss of the physical relation between the amino terminal and ECL2 that normally allows formation of the essential Cys14-Cys200 bridge. Human GnRHR mutants that cannot form this bridge are recognized as misfolded by the cellular QCS (Janovick et al., 2006) and are retained in the ER. None of these three control mutants produced a measurable response to Buserelin.
95 ng mutant Asp98Asn was modestly rescuable by both pharmacoperones (Figure 2B and 2C, and mutant Asp98Ala showed a slight rescue with TAK-013, but this was not significant and more modestly with A177775 (Figure 2C). For comparison in Figures 2B-C, an inset is shown with the dose response curves for rescue of WT hGnRHR and Glu90Lys hGnRHR with each pharmacoperone used. As expected Glu90Lys, Ser168Arg and Ser217Arg (without pharmacoperone rescue) did not produce a response, although the Glu90Lys was rescuable by each of the two pharmacoperones (Janovick et al., 2002).
Among the three double mutants (Glu90Lys/Asp98Ala, Glu90Lys/Asp98Asn, Glu90Lys/Asp98Lys), further encumbered by the inability to form the Glu90-Lys121 salt bridge, there was no response to Buserelin and no ability to rescue with any of the potential pharmacoperone molecules (Figure 2A–C).
In evaluating the preceding data, it is important to recognize that Asp98 and Lys121 are also believed to be points of contact for GnRH and other GnRHR agonists (Flanagan et al., 2000; Zhou et al., 1995). Accordingly, the inability to observe responsiveness might reflect inability to bind Buserelin or bind pharmacoperone, the retention of the mutant by the QCS, or a combination of these. In order to distinguish whether the loss of responsiveness resulted from the loss of GnRHR agonist binding or from the retention of the receptor by the ER QCS, we took advantage of the dominant-negative effect of GnRHR mutants (Brothers et al., 2004; Leanos-Miranda et al., 2003). Because the movement of the newly synthesized receptor from the ER to the plasma membrane involves oligomerization and the cellular QCS assesses the overall quality of the oligomer (potentially a combination of mutant and WT), the presence of the mutant also results in retention of WT GnRHR. Accordingly, we co-transfected WT hGnRHR (5 ng) in the presence of excess (95 ng) of each of the three single, three double mutants or control mutants described above, then assessed the ability to measure coupling due to WT receptor with or without each potential pharmacoperone (Figure 3A–C). The ratio of 1:19 (WT:mutant) has been shown to be optimally effective (Brothers et al., 2004) since it increases the chances that the individual cells, which receive WT hGnRHR, also receive the mutant. Moreover, this ratio minimizes the formation of WT:WT oligomers which would traffic correctly to the plasma membrane.
Figure 3. Dominant-negative effect of co-transfecting cells with 5 ng WT and 95 ng mutant cDNA (1:19) on control (panel A) or pharmacoperone rescue with A177775 (panel B) or TAK-013 (panel C) as described in the text.
Averages and SEMs of at least 3 independent experiments performed in replicates of 6 are shown.
Co-transfection of WT with each of the Asp98 mutants (Figure 3A) leads to the most retention of WT GnRHR by Asp98Lys, suggesting that this mutant is retained in the ER. These observations suggest that mutants Asp98Ala and Asp98Asn exert a more modest dominant-negative effect on (5 ng) WT hGnRHR.
When the dominant-negative effect on WT hGnRHR due to co-transfection with the double mutants was examined, it resulted in a very modest response, as occurred for Glu90Lys. Glu90Lys however could be rescued by either pharmacoperone, while mutants Ser168Arg and Ser217Arg could not be rescued (Figure 3A–C).
We also examined the impact of mutations at K121 and found that the ability of all four pharmacoperone classes were highly sensitive to changes at this site (Figure 4 and Janovick, et al, 2009). Only the highly conservative change of K121 to R121 resulted in rescue by pharmacoperones. Likewise mutants that had binding activity (Figure 5 and Janovick, et al, 2009) showed that [125I]-Buserelin binding could be displaced by pharmacoperones in a competitive binding paradigm.
Figure 4. Effect of mutation of residue Lys121 to Ala, Asp, Glu, Gly, Asn, Gln or Arg on rescue with two classes of pharmacoperones.
Cells were transiently transfected with 25 ng of WT hGnRHR or mutant cDNA in which residue Lys121 was replaced by Ala, Asp, Glu, Gly, Asn, Gln or Arg. Cells were treated with or without pharmacoperone TAK-013 or A177775 and IP response was measured to a saturating dose of Buserelin (10−7 M). Empty vector (pcDNA3.1) was run as a control and was typically 175 ± 20 cpm. Averages and SEMs were calculated from at least 3 independent experiments performed in replicates of 6.
Figure 5. Displacement of 125I-Buserelin binding to the GnRHR by pharmacoperones TAK-013 or A177775.
Cells were plated, transfected with the indicated vector and incubated in 1.25 × 105 cpm/ml of [125I]-Buserelin in the presence of the indicated concentrations of pharmacoperones A177775 or TAK-013. Binding was determined as described in Janovick et al., 2009.
Creation of Pharmacoperones By a Rational Process
Because of its small size, the GnRHR is a good model for studies of WT and mutant GPCR folding and trafficking to the plasma membrane, as well as rescue by pharmacoperones. In principle, the pharmacoperone rescue approach might apply to a diverse array of human diseases that result from the misfolding of GPRCs and other molecules—among these are cystic fibrosis (Amaral, 2006; Dormer et al., 2001; Galietta et al., 2001; Zhang et al., 2003), hypogonadotropic hypogonadism (HH, (Ulloa-Aguirre et al., 2003), nephrogenic diabetes insipidus (Bernier et al., 2004b; Bichet, 2006; Morello and Bichet, 2001), retinitis pigmentosa (Noorwez et al., 2004), hypercholesterolemia, cataracts (Benedek et al., 1999), neurodegenerative diseases [Huntington’s, Alzheimer’s, Parkinson’s (Forloni et al., 2002; Heiser et al., 2000; Muchowski and Wacker, 2005; Permanne et al., 2002; Soto et al., 2000)] and cancer (Peng et al., 2003). In the case of particular proteins (e.g. the GnRHR, vasopressin type 2 receptor (V2R) and rhodopsin), rescue has succeeded with a striking number of different mutants, supporting the view that pharmacoperones will become powerful ammunition in our therapeutic arsenal (Conn et al., 2007).
In addition to mutants and, as noted above for the GnRHR, it has also become clear that variable (but significant) amounts of other WT GPCRs are misrouted (i.e. retained in the ER), apparently as a result of misfolding (Andersson et al., 2003; Cook et al., 2003; Janovick et al., 2003b; Lu et al., 2003; Lu et al., 2004; Petaja-Repo et al., 2000; Petaja-Repo et al., 2001; Pietila et al., 2005), suggesting that this level of post-translational control may itself provide another level of potential therapeutic intervention (Ulloa-Aguirre et al., 2006).
Since mutants and WT proteins are subjected to scrutiny by the QCS, it is clearly advantageous to be able to rely on pharmacoperones as therapeutic agents to control the plasma membrane expression levels of such molecules. One problem in reducing this to practice is that almost all known pharmacoperones for GPCRs are peptidomimetic antagonists of the native ligand; one agonist has been used for this purpose (Petaja-Repo et al., 2002) and one molecule has been used that does not appear to compete for the agonist or antagonist binding site (Janovick et al., 2008). This “overlap” of the majority of the pharmacoperones with the binding site means that there will likely be competition that will result in issues in vivo that will necessitate episodic administration and washout. From a therapeutic point of view, this presents a problem since it makes oral dosing more difficult (there must be a washout period if the pharmacoperone is competing with the endogenous ligand).
We initially selected antagonists since we knew that they would interact with the receptor; it was this interaction that we were seeking and not the antagonism as such. It has certainly not been established that antagonism is a necessary pre-requisite for pharmacoperone activity and it may not be (Janovick et al., 2008; Janovick et al., 2009). This would be an unexpected requirement since one could imagine pharmacoperones that might stabilize the correctly routed form of the receptor and not show any antagonism. The potential advantage of identifying pharmacoperone drugs that do not compete with the naturally occurring ligand binding site is that these agents may not have to be given in a pulsatile fashion (since they would not have to be removed prior to activation with an agonist).
Accordingly, a detailed understanding of the biochemical mechanism by which stabilization occurs (i.e. at what residues do interactions need to occur?) is valuable in order to understand this process and allow rationale design of pharmacoperones, including those that effect stabilization, without competing for the natural ligand binding site. This information may open the door to the rationale design of pharmacoperones that can stabilize without inhibiting endogenous (or exogenous) agonists. It is, of course, conceivable that it will turn out that all pharmacoperones must occupy sites that overlap with the agonist binding site. This result would be disappointing since it would make drug design more difficult (pharmacoperone would have to be removed in vivo), but it would be essential information. There is no reason, of which we are aware, that this should be the case, but it is conceivable that there might be selective pressure that the ligand binding site has some relation to the overall receptor structure that passes the QCS.
Acknowledgments
This work was supported by NIH grants: HD-19899, RR-00163, and HD-18185. We thank Jo Ann Binkerd for formatting the manuscript and Darren Kafka for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaral MD. Therapy through chaperones: sense or antisense? Cystic fibrosis as a model disease. J Inherit Metab Dis. 2006;29:477–87. doi: 10.1007/s10545-006-0251-x. [DOI] [PubMed] [Google Scholar]
- Andersson H, D’Antona AM, Kendall DA, Von Heijne G, Chin CN. Membrane assembly of the cannabinoid receptor 1: impact of a long N-terminal tail. Mol Pharmacol. 2003;64:570–7. doi: 10.1124/mol.64.3.570. [DOI] [PubMed] [Google Scholar]
- Aridor M. Visiting the ER: the endoplasmic reticulum as a target for therapeutics in traffic related diseases. Adv Drug Deliv Rev. 2007;59:759–81. doi: 10.1016/j.addr.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Benedek GB, Pande J, Thurston GM, Clark JI. Theoretical and experimental basis for the inhibition of cataract. Prog Retin Eye Res. 1999;18:391–402. doi: 10.1016/s1350-9462(98)00023-8. [DOI] [PubMed] [Google Scholar]
- Bernier V, Bichet DG, Bouvier M. Pharmacological chaperone action on G-protein-coupled receptors. Curr Opin Pharmacol. 2004a;4:528–33. doi: 10.1016/j.coph.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Bernier V, Lagace M, Bichet DG, Bouvier M. Pharmacological chaperones: potential treatment for conformational diseases. Trends Endocrinol Metab. 2004b;15:222–8. doi: 10.1016/j.tem.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Bichet DG. Nephrogenic diabetes insipidus. Adv Chronic Kidney Dis. 2006;13:96–104. doi: 10.1053/j.ackd.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Brothers SP, Janovick JA, Conn PM. Unexpected effects of epitope and chimeric tags on gonadotropin-releasing hormone receptors: implications for understanding the molecular etiology of hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2003;88:6107–12. doi: 10.1210/jc.2003-031047. [DOI] [PubMed] [Google Scholar]
- Brothers SP, Cornea A, Janovick JA, Conn PM. Human loss-of-function gonadotropin-releasing hormone receptor mutants retain wild-type receptors in the endoplasmic reticulum: molecular basis of the dominant-negative effect. Mol Endocrinol. 2004;18:1787–97. doi: 10.1210/me.2004-0091. [DOI] [PubMed] [Google Scholar]
- Burrows JA, Willis LK, Perlmutter DH. Chemical chaperones mediate increased secretion of mutant alpha 1-antitrypsin (alpha 1-AT) Z: A potential pharmacological strategy for prevention of liver injury and emphysema in alpha 1-AT deficiency. Proc Natl Acad Sci U S A. 2000;97:1796–801. doi: 10.1073/pnas.97.4.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–66. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- Conn PM. The molecular mechanism of gonadotropin releasing hormone action in the pituitary. In: Knobil E, Neill J, editors. The Physiology of Reproduction. 2. Raven Press; New York: 1994. pp. 1815–1832. [Google Scholar]
- Conn PM, Janovick JA. A New Understanding of Protein Mutation Unfolds. American Scientist. 2005;93:314–321. [Google Scholar]
- Conn PM, Leanos-Miranda A, Janovick JA. Protein origami: therapeutic rescue of misfolded gene products. Mol Interv. 2002;2:308–16. doi: 10.1124/mi.2.5.308. [DOI] [PubMed] [Google Scholar]
- Conn PM, Knollman PE, Brothers SP, Janovick JA. Protein folding as posttranslational regulation: evolution of a mechanism for controlled plasma membrane expression of a G protein-coupled receptor. Mol Endocrinol. 2006a;20:3035–41. doi: 10.1210/me.2006-0066. [DOI] [PubMed] [Google Scholar]
- Conn PM, Janovick JA, Brothers SP, Knollman PE. ‘Effective inefficiency’: cellular control of protein trafficking as a mechanism of post-translational regulation. J Endocrinol. 2006b;190:13–6. doi: 10.1677/joe.1.06771. [DOI] [PubMed] [Google Scholar]
- Conn PM, Ulloa-Aguirre A, Ito J, Janovick JA. G protein-coupled receptor trafficking in health and disease: lessons learned to prepare for therapeutic mutant rescue in vivo. Pharmacol Rev. 2007;59:225–50. doi: 10.1124/pr.59.3.2. [DOI] [PubMed] [Google Scholar]
- Cook LB, Zhu CC, Hinkle PM. Thyrotropin-releasing hormone receptor processing: role of ubiquitination and proteasomal degradation. Mol Endocrinol. 2003;17:1777–91. doi: 10.1210/me.2003-0073. [DOI] [PubMed] [Google Scholar]
- Cornea A, Janovick JA, Lin X, Conn PM. Simultaneous and independent visualization of the gonadotropin-releasing hormone receptor and its ligand: evidence for independent processing and recycling in living cells. Endocrinology. 1999;140:4272–80. doi: 10.1210/endo.140.9.7049. [DOI] [PubMed] [Google Scholar]
- Cornea A, Janovick JA, Maya-Nunez G, Conn PM. Gonadotropin-releasing hormone receptor microaggregation. Rate monitored by fluorescence resonance energy transfer. J Biol Chem. 2001;276:2153–8. doi: 10.1074/jbc.M007850200. [DOI] [PubMed] [Google Scholar]
- Cui J, Smith RG, Mount GR, Lo JL, Yu J, Walsh TF, Singh SB, DeVita RJ, Goulet MT, Schaeffer JM, Cheng K. Identification of Phe313 of the gonadotropin-releasing hormone (GnRH) receptor as a site critical for the binding of nonpeptide GnRH antagonists. Mol Endocrinol. 2000;14:671–81. doi: 10.1210/mend.14.5.0464. [DOI] [PubMed] [Google Scholar]
- Dobson CM. Protein misfolding, evolution and disease. Trends Biochem Sci. 1999;24:329–32. doi: 10.1016/s0968-0004(99)01445-0. [DOI] [PubMed] [Google Scholar]
- Dormer RL, Derand R, McNeilly CM, Mettey Y, Bulteau-Pignoux L, Metaye T, Vierfond JM, Gray MA, Galietta LJ, Morris MR, Pereira MM, Doull IJ, Becq F, McPherson MA. Correction of delF508-CFTR activity with benzo(c)quinolizinium compounds through facilitation of its processing in cystic fibrosis airway cells. J Cell Sci. 2001;114:4073–81. doi: 10.1242/jcs.114.22.4073. [DOI] [PubMed] [Google Scholar]
- Ellgaard L, Helenius A. ER quality control: towards an understanding at the molecular level. Curr Opin Cell Biol. 2001;13:431–7. doi: 10.1016/s0955-0674(00)00233-7. [DOI] [PubMed] [Google Scholar]
- Flanagan CA, Rodic V, Konvicka K, Yuen T, Chi L, Rivier JE, Millar RP, Weinstein H, Sealfon SC. Multiple interactions of the Asp(2.61(98)) side chain of the gonadotropin-releasing hormone receptor contribute differentially to ligand interaction. Biochemistry. 2000;39:8133–41. doi: 10.1021/bi000085g. [DOI] [PubMed] [Google Scholar]
- Forloni G, Terreni L, Bertani I, Fogliarino S, Invernizzi R, Assini A, Ribizzi G, Negro A, Calabrese E, Volonte MA, Mariani C, Franceschi M, Tabaton M, Bertoli A. Protein misfolding in Alzheimer’s and Parkinson’s disease: genetics and molecular mechanisms. Neurobiol Aging. 2002;23:957–76. doi: 10.1016/s0197-4580(02)00076-3. [DOI] [PubMed] [Google Scholar]
- Galietta LJ, Springsteel MF, Eda M, Niedzinski EJ, By K, Haddadin MJ, Kurth MJ, Nantz MH, Verkman AS. Novel CFTR chloride channel activators identified by screening of combinatorial libraries based on flavone and benzoquinolizinium lead compounds. J Biol Chem. 2001;276:19723–8. doi: 10.1074/jbc.M101892200. [DOI] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–8. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- Hazum E, Cuatrecasas P, Marian J, Conn PM. Receptor-mediated internalization of fluorescent gonadotropin-releasing hormone by pituitary gonadotropes. Proc Natl Acad Sci U S A. 1980;77:6692–5. doi: 10.1073/pnas.77.11.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiser V, Scherzinger E, Boeddrich A, Nordhoff E, Lurz R, Schugardt N, Lehrach H, Wanker EE. Inhibition of huntingtin fibrillogenesis by specific antibodies and small molecules: implications for Huntington’s disease therapy. Proc Natl Acad Sci U S A. 2000;97:6739–44. doi: 10.1073/pnas.110138997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwich A. Protein aggregation in disease: a role for folding intermediates forming specific multimeric interactions. J Clin Invest. 2002;110:1221–32. doi: 10.1172/JCI16781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S, Yoshioka H, Mannen K, Kulkarni AB, Fan JQ. Transgenic mouse expressing human mutant alpha-galactosidase A in an endogenous enzyme deficient background: a biochemical animal model for studying active-site specific chaperone therapy for Fabry disease. Biochim Biophys Acta. 2004;1690:250–7. doi: 10.1016/j.bbadis.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Janovick JA, Maya-Nunez G, Conn PM. Rescue of hypogonadotropic hypogonadism-causing and manufactured GnRH receptor mutants by a specific protein-folding template: misrouted proteins as a novel disease etiology and therapeutic target. J Clin Endocrinol Metab. 2002;87:3255–62. doi: 10.1210/jcem.87.7.8582. [DOI] [PubMed] [Google Scholar]
- Janovick JA, Ulloa-Aguirre A, Conn PM. Evolved regulation of gonadotropin-releasing hormone receptor cell surface expression. Endocrine. 2003a;22:317–27. doi: 10.1385/ENDO:22:3:317. [DOI] [PubMed] [Google Scholar]
- Janovick JA, Brothers SP, Knollman PE, Conn PM. Specializations of a G-protein-coupled receptor that appear to aid with detection of frequency-modulated signals from its ligand. FASEB J. 2007a;21:384–92. doi: 10.1096/fj.06-6901com. [DOI] [PubMed] [Google Scholar]
- Janovick JA, Goulet M, Bush E, Greer J, Wettlaufer DG, Conn PM. Structure-activity relations of successful pharmacologic chaperones for rescue of naturally occurring and manufactured mutants of the gonadotropin-releasing hormone receptor. J Pharmacol Exp Ther. 2003b;305:608–14. doi: 10.1124/jpet.102.048454. [DOI] [PubMed] [Google Scholar]
- Janovick JA, Knollman PE, Brothers SP, Ayala-Yanez R, Aziz AS, Conn PM. Regulation of G protein-coupled receptor trafficking by inefficient plasma membrane expression: molecular basis of an evolved strategy. J Biol Chem. 2006;281:8417–25. doi: 10.1074/jbc.M510601200. [DOI] [PubMed] [Google Scholar]
- Janovick JA, Maya-Nunez G, Ulloa-Aguirre A, Ji T, Huhtaniemi I, Dias JA, Verbost P, Conn PM. Increased plasma membrane expression of hFSHR by a small molecule Thienopyr(lm)ldine. Mol Cell Endocrinol. 2008 doi: 10.1016/j.mce.2008.09.015. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janovick JA, Patney A, Mosely R, Goulet M, Altman M, Rush T, Cornea A, Conn PM. Biochemical mechanism of action of pharmacoperones in rescue of misfolded G-protein coupled receptor mutants. 2009 doi: 10.1210/me.2008-0384. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janovick JA, Brothers SP, Cornea A, Bush E, Goulet MT, Ashton WT, Sauer DR, Haviv F, Greer J, Conn PM. Refolding of misfolded mutant GPCR: post-translational pharmacoperone action in vitro. Mol Cell Endocrinol. 2007b;272:77–85. doi: 10.1016/j.mce.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennes L, Stumpf WE, Conn PM. Receptor-mediated binding and uptake of GnRH agonist and antagonist by pituitary cells. Peptides. 1984;5(Suppl 1):215–20. doi: 10.1016/0196-9781(84)90279-1. [DOI] [PubMed] [Google Scholar]
- Jennes L, Conn PM, Stumff WE. Synthesis and use of colloidal gold-coupled receptor ligands. Methods Enzymol. 1986;124:36–47. doi: 10.1016/0076-6879(86)24006-9. [DOI] [PubMed] [Google Scholar]
- Knollman PE, Janovick JA, Brothers SP, Conn PM. Parallel regulation of membrane trafficking and dominant-negative effects by misrouted gonadotropin-releasing hormone receptor mutants. J Biol Chem. 2005;280:24506–14. doi: 10.1074/jbc.M501978200. [DOI] [PubMed] [Google Scholar]
- Kopito RR, Ron D. Conformational disease. Nat Cell Biol. 2000;2:E207–9. doi: 10.1038/35041139. [DOI] [PubMed] [Google Scholar]
- Leanos-Miranda A, Janovick JA, Conn PM. Receptor-misrouting: an unexpectedly prevalent and rescuable etiology in gonadotropin-releasing hormone receptor-mediated hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2002;87:4825–8. doi: 10.1210/jc.2002-020961. [DOI] [PubMed] [Google Scholar]
- Leanos-Miranda A, Ulloa-Aguirre A, Janovick JA, Conn PM. In vitro coexpression and pharmacological rescue of mutant gonadotropin-releasing hormone receptors causing hypogonadotropic hypogonadism in humans expressing compound heterozygous alleles. J Clin Endocrinol Metab. 2005;90:3001–8. doi: 10.1210/jc.2004-2071. [DOI] [PubMed] [Google Scholar]
- Leanos-Miranda A, Ulloa-Aguirre A, Ji TH, Janovick JA, Conn PM. Dominant-negative action of disease-causing gonadotropin-releasing hormone receptor (GnRHR) mutants: a trait that potentially coevolved with decreased plasma membrane expression of GnRHR in humans. J Clin Endocrinol Metab. 2003;88:3360–7. doi: 10.1210/jc.2003-030084. [DOI] [PubMed] [Google Scholar]
- Levinthal C. Are There Pathways for Protein Folding? Extrait du Journal de Chimie Physique. 1968;65:44. [Google Scholar]
- Lin X, Cornea A, Janovick JA, Conn PM. Visualization of unoccupied and occupied gonadotropin-releasing hormone receptors in living cells. Mol Cell Endocrinol. 1998a;146:27–37. doi: 10.1016/s0303-7207(98)00204-4. [DOI] [PubMed] [Google Scholar]
- Lin X, Janovick JA, Brothers S, Blomenrohr M, Bogerd J, Conn PM. Addition of catfish gonadotropin-releasing hormone (GnRH) receptor intracellular carboxyl-terminal tail to rat GnRH receptor alters receptor expression and regulation. Mol Endocrinol. 1998b;12:161–71. doi: 10.1210/mend.12.2.0056. [DOI] [PubMed] [Google Scholar]
- Loo TW, Bartlett MC, Clarke DM. Rescue of folding defects in ABC transporters using pharmacological chaperones. J Bioenerg Biomembr. 2005;37:501–7. doi: 10.1007/s10863-005-9499-3. [DOI] [PubMed] [Google Scholar]
- Lu M, Echeverri F, Moyer BD. Endoplasmic reticulum retention, degradation, and aggregation of olfactory G-protein coupled receptors. Traffic. 2003;4:416–33. doi: 10.1034/j.1600-0854.2003.00097.x. [DOI] [PubMed] [Google Scholar]
- Lu M, Staszewski L, Echeverri F, Xu H, Moyer BD. Endoplasmic reticulum degradation impedes olfactory G-protein coupled receptor functional expression. BMC Cell Biol. 2004;5:34. doi: 10.1186/1471-2121-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello JP, Bichet DG. Nephrogenic diabetes insipidus. Annu Rev Physiol. 2001;63:607–30. doi: 10.1146/annurev.physiol.63.1.607. [DOI] [PubMed] [Google Scholar]
- Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005;6:11–22. doi: 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]
- Nakatsukasa K, Brodsky JL. The recognition and retrotranslocation of misfolded proteins from the endoplasmic reticulum. Traffic. 2008;9:861–70. doi: 10.1111/j.1600-0854.2008.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorwez SM, Malhotra R, McDowell JH, Smith KA, Krebs MP, Kaushal S. Retinoids assist the cellular folding of the autosomal dominant retinitis pigmentosa opsin mutant P23H. J Biol Chem. 2004;279:16278–84. doi: 10.1074/jbc.M312101200. [DOI] [PubMed] [Google Scholar]
- Pastores GM, Barnett NL. Current and emerging therapies for the lysosomal storage disorders. Expert Opin Emerg Drugs. 2005;10:891–902. doi: 10.1517/14728214.10.4.891. [DOI] [PubMed] [Google Scholar]
- Peng Y, Li C, Chen L, Sebti S, Chen J. Rescue of mutant p53 transcription function by ellipticine. Oncogene. 2003;22:4478–87. doi: 10.1038/sj.onc.1206777. [DOI] [PubMed] [Google Scholar]
- Permanne B, Adessi C, Saborio GP, Fraga S, Frossard MJ, Van Dorpe J, Dewachter I, Banks WA, Van Leuven F, Soto C. Reduction of amyloid load and cerebral damage in a transgenic mouse model of Alzheimer’s disease by treatment with a beta-sheet breaker peptide. FASEB J. 2002;16:860–2. doi: 10.1096/fj.01-0841fje. [DOI] [PubMed] [Google Scholar]
- Petaja-Repo UE, Hogue M, Laperriere A, Walker P, Bouvier M. Export from the endoplasmic reticulum represents the limiting step in the maturation and cell surface expression of the human delta opioid receptor. J Biol Chem. 2000;275:13727–36. doi: 10.1074/jbc.275.18.13727. [DOI] [PubMed] [Google Scholar]
- Petaja-Repo UE, Hogue M, Laperriere A, Bhalla S, Walker P, Bouvier M. Newly synthesized human delta opioid receptors retained in the endoplasmic reticulum are retrotranslocated to the cytosol, deglycosylated, ubiquitinated, and degraded by the proteasome. J Biol Chem. 2001;276:4416–23. doi: 10.1074/jbc.M007151200. [DOI] [PubMed] [Google Scholar]
- Petaja-Repo UE, Hogue M, Bhalla S, Laperriere A, Morello JP, Bouvier M. Ligands act as pharmacological chaperones and increase the efficiency of delta opioid receptor maturation. EMBO J. 2002;21:1628–37. doi: 10.1093/emboj/21.7.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietila EM, Tuusa JT, Apaja PM, Aatsinki JT, Hakalahti AE, Rajaniemi HJ, Petaja-Repo UE. Inefficient maturation of the rat luteinizing hormone receptor. A putative way to regulate receptor numbers at the cell surface. J Biol Chem. 2005;280:26622–9. doi: 10.1074/jbc.M413815200. [DOI] [PubMed] [Google Scholar]
- Radford SE, Dobson CM. From computer simulations to human disease: emerging themes in protein folding. Cell. 1999;97:291–8. doi: 10.1016/s0092-8674(00)80739-4. [DOI] [PubMed] [Google Scholar]
- Sanders CR, Nagy JK. Misfolding of membrane proteins in health and disease: the lady or the tiger? Curr Opin Struct Biol. 2000;10:438–42. doi: 10.1016/s0959-440x(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Sandilands A, Hutcheson AM, Long HA, Prescott AR, Vrensen G, Loster J, Klopp N, Lutz RB, Graw J, Masaki S, Dobson CM, MacPhee CE, Quinlan RA. Altered aggregation properties of mutant gamma-crystallins cause inherited cataract. EMBO J. 2002;21:6005–14. doi: 10.1093/emboj/cdf609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia R, Braakman I. Quality control in the endoplasmic reticulum protein factory. Nature. 2003;426:891–4. doi: 10.1038/nature02262. [DOI] [PubMed] [Google Scholar]
- Soto C, Kascsak RJ, Saborio GP, Aucouturier P, Wisniewski T, Prelli F, Kascsak R, Mendez E, Harris DA, Ironside J, Tagliavini F, Carp RI, Frangione B. Reversion of prion protein conformational changes by synthetic beta-sheet breaker peptides. Lancet. 2000;355:192–7. doi: 10.1016/s0140-6736(99)11419-3. [DOI] [PubMed] [Google Scholar]
- Suzuki Y. Beta-galactosidase deficiency: an approach to chaperone therapy. J Inherit Metab Dis. 2006;29:471–6. doi: 10.1007/s10545-006-0287-y. [DOI] [PubMed] [Google Scholar]
- Tamarappoo BK, Verkman AS. Defective aquaporin-2 trafficking in nephrogenic diabetes insipidus and correction by chemical chaperones. J Clin Invest. 1998;101:2257–67. doi: 10.1172/JCI2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Janovick JA, Leanos-Miranda A, Conn PM. Misrouted cell surface receptors as a novel disease aetiology and potential therapeutic target: the case of hypogonadotropic hypogonadism due to gonadotropin-releasing hormone resistance. Expert Opin Ther Targets. 2003;7:175–85. doi: 10.1517/14728222.7.2.175. [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Janovick JA, Leanos-Miranda A, Conn PM. Misrouted cell surface GnRH receptors as a disease aetiology for congenital isolated hypogonadotrophic hypogonadism. Hum Reprod Update. 2004a;10:177–92. doi: 10.1093/humupd/dmh015. [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Janovick JA, Brothers SP, Conn PM. Pharmacologic rescue of conformationally-defective proteins: implications for the treatment of human disease. Traffic. 2004b;5:821–37. doi: 10.1111/j.1600-0854.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Janovick JA, Miranda AL, Conn PM. G-protein-coupled receptor trafficking: understanding the chemical basis of health and disease. ACS Chem Biol. 2006;1:631–8. doi: 10.1021/cb600360h. [DOI] [PubMed] [Google Scholar]
- Wang Y, Bartlett MC, Loo TW, Clarke DM. Specific rescue of cystic fibrosis transmembrane conductance regulator processing mutants using pharmacological chaperones. Mol Pharmacol. 2006;70:297–302. doi: 10.1124/mol.106.023994. [DOI] [PubMed] [Google Scholar]
- Yam GH, Zuber C, Roth J. A synthetic chaperone corrects the trafficking defect and disease phenotype in a protein misfolding disorder. FASEB J. 2005;19:12–8. doi: 10.1096/fj.04-2375com. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Wang XT, Yue H, Leung SW, Thibodeau PH, Thomas PJ, Guggino SE. Organic solutes rescue the functional defect in delta F508 cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2003;278:51232–42. doi: 10.1074/jbc.M309076200. [DOI] [PubMed] [Google Scholar]
- Zhou W, Rodic V, Kitanovic S, Flanagan CA, Chi L, Weinstein H, Maayani S, Millar RP, Sealfon SC. A locus of the gonadotropin-releasing hormone receptor that differentiates agonist and antagonist binding sites. J Biol Chem. 1995;270:18853–7. doi: 10.1074/jbc.270.32.18853. [DOI] [PubMed] [Google Scholar]