Abstract
Background
Interferon (IFN)-alpha has been used to study the effects of innate immune cytokines on the brain and behavior in humans. The degree to which peripheral administration of IFN-alpha accesses the brain and is associated with a central nervous system (CNS) inflammatory response is unknown. Moreover, the relationship among IFN-alpha-associated CNS inflammatory responses, neurotransmitter metabolism and behavior has yet to be established.
Methods
Twenty-four patients with hepatitis C underwent lumbar puncture and blood sampling after ~12 weeks of either no treatment (n=12) or treatment with pegylated IFN-alpha 2b (n=12). Cerebrospinal fluid (CSF) and blood samples were analyzed for proinflammatory cytokines and their receptors as well as the chemokine, monocyte chemoattractant protein (MCP)-1, and IFN-alpha. CSF samples were additionally analyzed for monoamine metabolites and corticotropin releasing hormone. Depressive symptoms were assessed using the Montgomery Asberg Depression Rating Scale.
Results
IFN-alpha was detected in the CSF of all IFN-alpha-treated patients and only one control subject. Despite no increases in plasma IL-6, IFN-alpha-treated patients exhibited significant elevations in CSF IL-6 and MCP-1, both of which were highly correlated with CSF IFN-alpha concentrations. Of the immunologic and neurotransmitter variables, log-transformed CSF concentrations of the serotonin metabolite, 5-hydroxyindoleacetic acid (5-HIAA) were the strongest predictor of depressive symptoms. Log-transformed CSF concentrations of IL-6, but not IFN-alpha or MCP-1, were negatively correlated with log-transformed CSF 5-HIAA (r2=−0.25, p<0.05).
Conclusions
These data indicate that a peripherally administered cytokine can activate a CNS inflammatory response in humans that interacts with monoamine (serotonin) metabolism, which is associated with depression.
Keywords: Inflammation, Depression, Cytokines, Monoamines, Cerebrospinal Fluid
Introduction
There has been increasing interest in the role of innate immune cytokines in behavioral disorders including depression in medically ill and medically healthy individuals (1–3). For example, elevations in the innate immune cytokines, interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-alpha as well as their soluble receptors, have been found in peripheral blood and cerebrospinal fluid (CSF) of patients with major depression (1). In addition, innate immune cytokines have been shown to influence virtually every pathophysiologic domain relevant to depression including neurotransmitter metabolism, neuroendocrine function, synaptic plasticity and regional brain activity (1,2).
Because cytokines are relatively large molecules (~15–25 kD) that do not freely pass through the blood-brain-barrier (BBB) (4), much attention has been paid to the pathways by which peripheral cytokine signals reach the brain. Data in laboratory animals indicate that cytokines elaborated or administered peripherally can access the brain through several routes including 1) passage through leaky regions in the BBB, 2) active transport via saturable transport molecules, 3) activation of endothelial cells (as well as other cells lining the cerebral vasculature), which then release inflammatory mediators within the brain parenchyma and 4) binding to cytokine receptors associated with peripheral afferent nerve fibers (e.g. the vagus nerve) which in turn relay signals to relevant brain nuclei (2,5–7). It should be noted, however, that it remains to be established whether peripherally administered cytokines can access the brain and activate central inflammatory pathways in humans. Moreover, it has yet to be determined whether cytokine-induced changes in behavior in humans are related to effects of central cytokines on the metabolism of behaviorally-relevant neurotransmitter systems.
One opportunity to explore the access and action of peripheral blood cytokines on the brain in humans is to study patients undergoing treatment with interferon (IFN)-alpha. IFN-alpha is an innate immune cytokine that has both antiviral and anti-proliferative activities and is therefore used to treat infectious diseases and cancer (8,9). Although an effective therapy, IFN-alpha induces high rates of behavioral disturbance, including depression, which develops to a clinically-significant degree in 30–50% of IFN-alpha-treated patients (10–15). Prior studies have linked development of depression during IFN-alpha therapy to changes in peripheral blood inflammatory markers as well as changes in peripheral availability of monoamine neurotransmitter precursors including tryptophan, the primary precursor for serotonin (16–20). Moreover, exaggerated hypothalamic-pituitary-adrenal (HPA) axis responses have been associated with depression during IFN-alpha therapy, suggesting that sensitized neuroendocrine pathways, including corticotropin releasing hormone (CRH), may represent a vulnerability to IFN-alpha-induced behavioral changes (21).
To date, however, no studies have examined central nervous system (CNS) immune, monoamine or neuropeptide effects of IFN-alpha that might be relevant to depression. Therefore, we sought to determine 1) whether IFN-alpha enters the brain when administered peripherally; 2) whether peripherally-administered IFN-alpha is associated with activation of CNS inflammatory pathways; 3) whether activation of these inflammatory pathways affects metabolism of relevant monoamines or CRH; and 4) whether IFN-alpha-associated changes in CNS inflammatory pathways, monoamine metabolism or CRH are associated with depression.
To accomplish these aims, CSF and plasma concentrations of several inflammatory mediators, monoamine neurotransmitter metabolites and CRH were assessed in patients undergoing treatment with pegylated IFN-alpha plus ribavirin for hepatitis C virus (HCV) infection. HCV-infected patients awaiting IFN-alpha therapy served as controls. Inflammatory mediators included IL-6 and its soluble receptor (sIL-6R), TNF-alpha and its type 2 soluble receptor (sTNF-R2), IL-1-beta, and monocyte chemoattractant protein-1 (MCP-1). Administration of IFN-alpha has been shown to induce IL-6 and TNF-alpha and their soluble receptors as well as IL-1-beta in the peripheral blood of relevant patient populations and/or peripheral blood mononuclear cells and a variety of cell lines (21,22). In addition, peripheral blood concentrations of IL-6 and TNF and their soluble receptors, both at baseline and during IFN-alpha therapy, have been found to predict depression during IFN-alpha therapy (16,20,23,24). MCP-1 has been shown to be elevated in CSF of patients with human immunodeficiency virus (HIV) infection (25–27), a condition associated with increased CSF concentrations of IFN-alpha (28,29). IFN-alpha is also known to induce inflammatory responses through induction of MCP-1 (30), and MCP-1 has been found to prime microglia in the brain following CNS administration of inflammatory stimuli [e.g. lipopolysaccharide (LPS)] (31).
To evaluate the effect of IFN-alpha on CNS neurotransmission, CSF concentrations of the primary metabolites for serotonin (5-hydroxyindoleacetic acid [5-HIAA]), dopamine (homovanillic acid [HVA]) and norepinephrine (3-methoxy-4-hydroxyphenylglycol [MHPG]) were examined. These metabolites were selected based on data that IFN-alpha alters peripheral availability of the serotonin precursor, tryptophan, and on a large literature linking these monoamines and their metabolites to depression and other behavioral disturbances (32–34). Finally, CSF and plasma concentrations of IFN-alpha as well as the neuroregulatory peptide, CRH, were determined.
Methods and Materials
Subjects
24 HCV-positive subjects (15 males, 9 females) were enrolled in the study. Subjects were required to be serum positive for anti-HCV antibodies or HCV-RNA positive by reverse transcription-polymerase chain reaction. Exclusion criteria included decompensated liver disease; liver disease from any cause other than HCV; infection with HIV (as reported by the subjects’ treating physician), unstable cardiovascular, endocrinologic, hematologic, renal or neurologic disease (as determined by physical examination and laboratory testing); history of schizophrenia, bipolar disorder or a diagnosis of major depression (MD) or substance abuse/dependence within six months of study entry [as determined by the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders—Fourth Edition (SCID)](35), and/or a score <24 on the Mini Mental State Exam, indicating more than mild cognitive impairment (36). Patients were required to be off all antidepressant, antipsychotic or mood stabilizer medications for at least 4 weeks prior to study entry (8 weeks for fluoxetine). Subjects were also required to discontinue other agents that might affect study results (i.e. narcotic analgesics, benzodiazepines, and anti-inflammatory agents) at least two weeks prior to undergoing lumbar puncture (LP) or blood collection. The subjects reported on herein represent a subsample of subjects included in previous studies on effects of IFN-alpha on cognitive performance and neuroendocrine function (20,37). All subjects provided written informed consent, and study procedures received a priori approval by the Emory University Institutional Review Board.
Study design
Study participants were enrolled in a longitudinal study examining immune and other neurobiological variables at baseline and after ~12 weeks of either no treatment or treatment with IFN-alpha/ribavirin (see above). Cross-sectional assessments of immune and neurotransmitter variables in CSF and peripheral blood of HCV patients awaiting IFN-alpha/ribavirin therapy (controls, n=12) versus HCV patients treated with IFN-alpha plus ribavirin for ~12 weeks (treatment group, n=12) were evaluated for the purposes of this study. All subjects who underwent IFN-alpha treatment received pegylated IFN-alfa-2b (Pegintron®, Schering Plough) 1.5μg/kg weekly (administered subcutaneously) and ribavirin (800–1400 mg/d). Participation in the treatment versus control group was determined by patients and their physician based on scheduling constraints and personal preferences, and was not based on standardized criteria or controlled by study protocol.
For LP, subjects were admitted to the Emory University General Clinical Research Center (GCRC) at 1pm. LP was performed between 4–5pm by a trained physician. For each subject, ~10cc of CSF was withdrawn, after discarding the initial 1cc to avoid blood contamination. Samples were collected into chilled tubes, aliquoted into 1cc vials, and immediately frozen at −80°C until assay. Subjects were then discharged after an overnight stay.
In order to limit the impact of the stress of CSF sampling on peripheral blood immune parameters and assessments of depression, blood sampling and depression assessments were conducted 6–7 days after LP. For blood sampling, subjects were admitted to the Emory GCRC in the evening with lights out at 10pm. The following morning, subjects were awakened at 7:15am and served breakfast, and neuropsychiatric assessments were conducted. Following lunch at 12pm, blood was withdrawn from an indwelling catheter into chilled EDTA-coated tubes at 4pm, corresponding to the time of the LP. During blood sampling, subjects were asked to rest quietly for 30 minutes prior to blood withdrawal. Following sampling, blood was immediately centrifuged at 1000×g for 10 minutes at 4°C. Plasma was then removed and frozen at −80°C until assay. Because behavioral effects of pegylated IFN-alpha tend to be most pronounced immediately following the weekly injection, both LP and blood sampling were scheduled 4–5 days following each subject’s last injection. Urine drug screens were conducted at all visits to rule out substance abuse. Control subjects participated in all study procedures in parallel with IFN-alpha/ribavirin-treated patients.
Behavioral Assessments
Depression was evaluated using the mood disorders module of the SCID and the Montgomery-Asberg Depression Rating Scale (MADRS) (38). The MADRS is a 10-item, clinician-administered scale that assesses the severity of depressive symptoms.
Assessment of Immune Parameters and CSF Monoamine Metabolites
TNF-alpha, IL-1-beta and IL-6 were measured in duplicate by high sensitivity quantitative enzyme-linked immunosorbent assays (ELISA) (R&D Systems, Minneapolis, MN). Cytokine soluble receptors (sTNF-R2, IL6-sR) and MCP-1 were determined by R&D Quantikine ELISA kits. Plasma IFN-alpha was measured by a high sensitivity quantitative ELISA (Amersham Biosciences, Piscataway, NJ), which was found to identify both recombinant human IFN-alpha as well as the pegylated IFN-alpha preparation. Inter- and intra-assay variability were reliably <12% for TNF-alpha, Il-1 beta and IL-6 and <10% for sTNF-R2, IL-6sR, MCP-1 and IFN-alpha. Assay sensitivities were as follows: TNF-alpha: 0.11pg/ml; IL-1 beta: 0.06pg/ml; IL-6: 0.04pg/ml; sTNFR2: 1.0pg/ml; IL-6sR: 6.5pg/ml; MCP-1: 5.0pg/ml; IFN-alpha: 0.1pg/ml.
C-reactive protein (CRP) was measured using the Beckman Coulter High Sensitivity CRP assay (Beckman Coulter Diagnostics, Brea, CA) on the Synchron LX-20 analyzer (Beckman Coulter, Fullerton, CA).
5-HIAA, HVA, and MHPG were measured in duplicate 20μl CSF aliquots using high performance liquid chromatography as described (39). Electrochemical detection was performed using a dual electrode system (ESA Incorporated, Chelmsford, MA), and peak areas were computed using Turbochrom software (PE-Nelson, San Jose, CA). Sensitivities for all analytes were ~1pg on column.
CRH was measured in quadruplicate 50μl CSF aliquots by non-equilibrium radioimmunoassay using a high affinity antibody (S-2021, raised in rabbit against hCRH) (Bachem Americas Inc., Torrance, CA). Antibody (25 μl) was added to CSF, and the mixture was incubated at 2°C for 24 hours. Trace 125I-tyr0-CRH (25μl) was then added, and the mixture incubated for an additional 16 hours. The CRH-antibody complex was precipitated using goat-anti-rabbit IgG. Assay sensitivity was 2.8pg/ml, and the coefficient of variation between quadruplicates was 7.5%.
All biological samples were analyzed by research staff blinded to the clinical status of study participants.
Statistical Analysis
Descriptive statistics (mean, median, standard deviation and interquartile range) were used to characterize relevant variables in the groups. Differences between groups were assessed using t-tests and Wilcoxon Rank Sum tests for continuous measures, and Chi-square or Fischer tests (as appropriate) for categorical variables. Generalized linear models (GLM) were employed to complement statistical comparisons of immune and neurotransmitter variables between groups, controlling for factors that may have influenced relevant CSF and plasma biomarkers including age, sex, body mass index (BMI), history of MD, and baseline inflammatory status (as reflected by plasma CRP determined at the initial GCRC visit). Despite the robustness of GLM against non-normality, only transformed data were used for these analyses for the sake of consistency across tests and variables and to improve the homogeneity of variances as well as the normality of the residuals. In t tests where the Levene’s test indicated non-homogeneity of variances, Welch’s t tests were employed.
Pearson correlations were computed to evaluate associations between relevant continuous variables, following log transformation to improve normality. Based on the sample size of 24, the study had ~80% power to detect medium effect sizes (r2=0.25) using two-tailed tests of significance and an alpha level of 0.05. Correlations between CSF IFN-alpha and relevant continuous variables were conducted using Spearman tests due to the large number of 0 values for CSF IFN-alpha in controls. Where indicated, Bonferroni correction was used to control for multiple comparisons within and across tissue compartments. To explore the relative contribution of relevant immune and neurotransmitter variables to scores of depression, a stepwise regression analysis was applied using both backward and forward selection. The level of significance for independent predictors in the final model was set at p<0.05.
Results
Sample Characteristics
As shown in Table 1, IFN-alpha/ribavirin-treated subjects and controls did not differ significantly on relevant clinical characteristics including age, race, gender, past history of substance abuse, BMI and baseline CRP. Nevertheless, IFN-alpha-treated subjects were more likely to have a past history of MD. At the time of study, scores of depression were significantly higher in IFN-alpha/ribavirin-treated subjects versus controls. Of note, depression scores were not significantly different in IFN-alpha/ribavirin-treated patients with or without a past history of MD (data not shown). No patients met symptom criteria for MD at the time of CSF or blood sampling.
Table 1.
Characteristics of Study Participants
| Characteristic | Control (n=12) | Interferon-alpha (n=12) | p-value |
|---|---|---|---|
| Age (mean, SD) | 48.3 (7.7) | 48.3 (3.6) | 1.00 |
| Gender (n, %) Males | 6 (50.0) | 9 (75.0) | 0.40 |
| Race (n, %) | |||
| Caucasian | 6 (50.0) | 4 (33.3) | 0.68 |
| Black | 6 (50.0) | 7 (58.3) | |
| Asian | 0 (0.0) | 1 (8.3) | |
| Education (n, %) | |||
| College (1 or more years) | 10 (83.3) | 7 (58.3) | 0.37 |
| Past MDD (n, %) | 0 (0) | 5 (41.7) | 0.04 |
| Past Substance Abuse (n, %) | 6 (50) | 6 (50) | 1.00 |
| MADRS (mean, SD) | 3.2 (3.8) | 8.3 (5.8) | 0.02 |
| BMI (mean, SD) | 29.2 (5.6) | 29.5 (4.5) | 0.88 |
| Baseline CRP (mg/L) (mean, SD) | 0.78 (0.8) | 0.87 (1.0) | 0.80 |
SD-standard deviation; MDD-Major Depressive Disorder; MADRS-Montgomery Asberg Depression Scale; BMI-Body Mass Index; CRP-c-reactive protein
CSF Concentrations of Relevant Immune Biomarkers
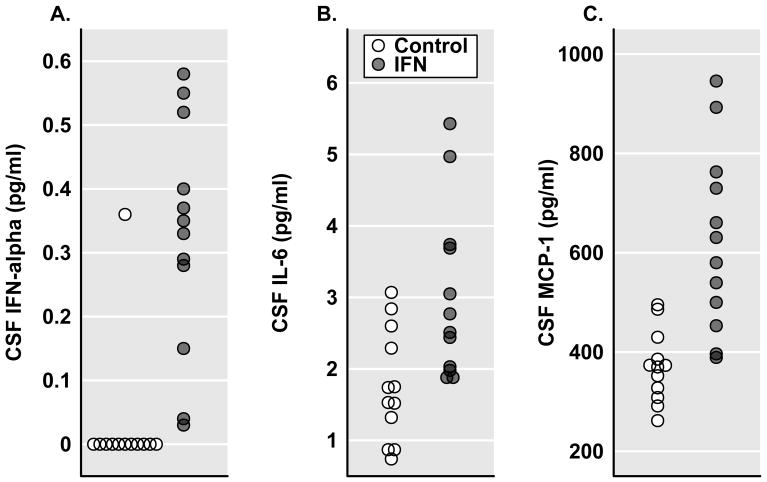
Evaluation of selected immune biomarkers in CSF of study participants revealed that IFN-alpha-treated patients exhibited significantly higher concentrations of CSF IFN-alpha, IL-6, and MCP-1 compared to controls (Figure 1, Table 2). TNF-alpha and IL-1 beta were also assayed. However, in the majority of samples, results were below the limits of assay detection. CSF concentrations of soluble receptors for both IL-6 and TNF were slightly elevated in IFN-alpha/ribavirin-treated subjects, although the differences only reached statistical significance for sIL-6R. To confirm differences in relevant CSF immune variables between groups, analyses were repeated controlling for age, sex, BMI, baseline inflammatory status (CRP), and history of depression. Differences between groups remained significant for all variables. In addition, after adding the noted covariates into the model, the difference in CSF sTNF-R-2 between groups was found to be significant (p<0.01).
Figure 1.
CSF Immunologic Biomarkers in Control versus IFN-alpha/ribavirin-treated patients with HCV. Cerebrospinal fluid (CSF) samples for the assessment of relevant innate immune cytokines including interferon (IFN)-alpha (Panel A), interleukin (IL)-6 (Panel B) as well as the chemokine, monocyte chemoattractant protein 1 (MCP-1) (Panel C), were compared in control (n=12) and interferon (IFN)-alpha/ribavirin-treated (n=12) patients with hepatitis C virus (HCV) infection. IFN-alpha/ribavirin-treated subjects were studied after ~12 weeks on IFN-alpha/ribavirin therapy. CSF concentrations of IFN-alpha, IL-6 and MCP-1 were significantly elevated in IFN-alpha-treated subjects compared to controls (p<0.01).
Table 2.
CSF Concentrations of Immune Biomarkers
| Immune Measure (Median, IQR) | Control (n=12) | Interferon-alpha (n=12) | p-value1 |
|---|---|---|---|
| Cytokines (pg/ml) | |||
| IFN-alpha | 0 (0) | 0.34 (0.25) | 0.0001* |
| IL-6 | 1.64 (1.35) | 2.64 (1.71) | 0.005* |
| TNF-alpha† | -- | -- | |
| IL-1-beta† | -- | -- | |
| Chemokines (ng/ml) | |||
| MCP-1 | 371.55 (89.6) | 605.3 (296.8) | 0.0001* |
| Soluble Receptors (ng/ml) | |||
| sIL-6R | 0.76 (0.12) | 0.98 (0.44) | 0.03 |
| sTNF-R-2 | 0.26 (0.07) | 0.36 (0.19) | 0.07‡ |
IQR – Interquartile range
Exact Wilcoxon Rank Sum test;
indicates significance after adjustment for multiple comparisons as well as controlling for relevant covariates (see text)
TNF-alpha and IL-1 beta were not detectable in the majority of subjects
sTNF-R-II exhhibited a significant difference between groups (p<0.01) after controlling for relevant covariates as well as controlling for multiple comparisons
IFN-interferon; IL-interleukin, TNF-tumor necrosis factor; MCP-1-monocytye chemoattractant protein 1; sIL-6R-soluble IL-6 receptor; sTNF-R-2-soluble TNF-alpha receptor 2
Correlational analyses among immune biomarkers that were significantly elevated in CSF revealed high correlations between IFN-alpha and log-transformed (ln)IL-6 and lnMCP-1 (r2=0.52, df=22, p<0.0001, and r2=0.53, df=22, p<0.0001, respectively), as well as a significant correlation between lnIL-6 and lnMCP-1 (r2=0.23, df=22, p=0.018).
Plasma Concentrations of Relevant Immune Biomarkers
Examination of immune measures in peripheral blood revealed significant elevations in plasma IFN-alpha and MCP-1 in IFN-alpha-treated subjects versus controls, but no differences between groups in IL-1-beta, IL-6 and TNF-alpha or their soluble receptors (Table 3). There was a trend for an increase in plasma sTNF-R-2 in subjects receiving IFN-alpha. Differences between groups in plasma IFN-alpha and MCP-1 remained significant after controlling for relevant clinical and biological variables. Of note, there was no significant correlation between plasma lnIFN-alpha and plasma lnMCP-1 (r2=0.06, df=22, p=0.23) in the study sample.
Table 3.
Plasma Concentrations of Immune Biomarkers
| Immune Measure (Median, IQR) | Control (n=12) | Interferon-alpha (n=12) | p-value1 |
|---|---|---|---|
| Cytokines (pg/ml) | |||
| IFN-alpha | 4.65 (8.35) | 37.85 (40.16) | 0.0001* |
| IL-6 | 3.67 (2.1) | 3.17 (1.5) | 0.48 |
| TNF-alpha | 1.46 (1.22) | 1.77 (1.90) | 0.75 |
| IL-1 beta† | -- | -- | |
| Chemokines (ng/ml) | |||
| MCP-1 | 225.0 (142.5) | 365.5 (132.0) | 0.005* |
| Soluble Receptors (ng/ml) | |||
| sIL-6R | 33.38 (9.7) | 28.95 (10.5) | 0.51 |
| sTNF-R-2 | 2.05 (1.89) | 2.84 (0.96) | 0.09 |
IQR – Interquartile range
Exact Wilcoxon Rank Sum test;
indicates significance after adjustment for multiple comparisons
IL-1 beta were not detectable in the majority of subjects
IFN-interferon; IL-interleukin, TNF-tumor necrosis factor; MCP-1-monocytye chemoattractant protein 1; sIL-6R-soluble IL-6 receptor; sTNF-R-2-soluble TNF-alpha receptor 2
Regarding the relationship between plasma and CSF immune variables, plasma lnIFN-alpha was correlated with CSF IFN-alpha and CSF lnIL-6 (r2=0.26, df=22, p=0.011 and r2=0.20, df=22, p=0.03, respectively). Nevertheless, these correlations were not significant after controlling for the multiple (6) comparisons of relevant variables across these tissue compartments (p corrected=0.066 and p corrected=0.18, respectively). The correlation between plasma lnIFN-alpha and CSF lnMCP-1 (r2=0.13, p=0.08) as well as correlations between plasma and CSF concentrations of lnIL-6 (r2=0.001, p=0.97) and lnMCP-1 (r2=0.12, p=0.10) also did not reach statistical significance, nor did the correlation between plasma lnMCP-1 and CSF lnIL-6 (r2=0.12, p=0.10).
CSF Monoamine Metabolites
No differences were found between groups for the monoamine metabolites, 5-HIAA, HVA and MHPG (Table 4). CSF lnHVA was highly correlated with CSF ln5-HIAA (r2=0.74, p<0.001) as well as CSF lnMHPG (r2=0.24, p=0.014). No significant correlation was found between CSF ln5-HIAA and CSF lnMHPG (r2=0.13, p<0.08).
Table 4.
CSF Concentrations of Neurotransmitters
| Monoamine Metabolite (Median, IQR) | Control (n=12) | Interferon-alpha (n=12) | p-value1 |
|---|---|---|---|
| HVA (ng/ml) | 104.7 (60.15) | 76.8 (63.20) | 0.27 |
| 5-HIAA (ng/ml) | 33.45 (21.55) | 29.60 (21.70) | 0.16 |
| MHPG (ng/ml) | 16.80 (5.55) | 15.65 (5.00) | 0.45 |
IQR – Interquartile range
Exact Wilcoxon Rank Sum test;
indicates significance after adjustment for multiple comparisons
HVA-homovanillic acid; 5-HIAA-5-hydroxyindoleacetic acid; MHPG-3-methoxy-4-hydroxyphenylglycol
CSF CRH
No differences were found in CSF CRH concentrations between controls versus IFN-alpha/ribavirin-treated patients (40.1 SD±16.9 versus 33.3 SD±6.5 pg/ml, respectively, t=1.3, df=14.5, p=0.22). Correlational analyses revealed significant correlations between CSF lnCRH and CSF ln HVA (r2=0.23, p=0.02) and CSF lnMHPG (r2=0.21, p=0.03), but these correlations were not significant after controlling for the multiple (6) comparisons made between CSF CRH and relevant CSF monoamine and immune variables (p corrected=0.12 and p corrected=0.18, respectively). No significant correlations were found between CSF lnCRH and ln5-HIAA or any of the immune variables that were found to be elevated in the CSF (i.e. IFN-alpha, IL-6 or MCP-1).
Stepwise Regression Analysis: Immune, Monoamine and Neuropeptide Predictors of Depression
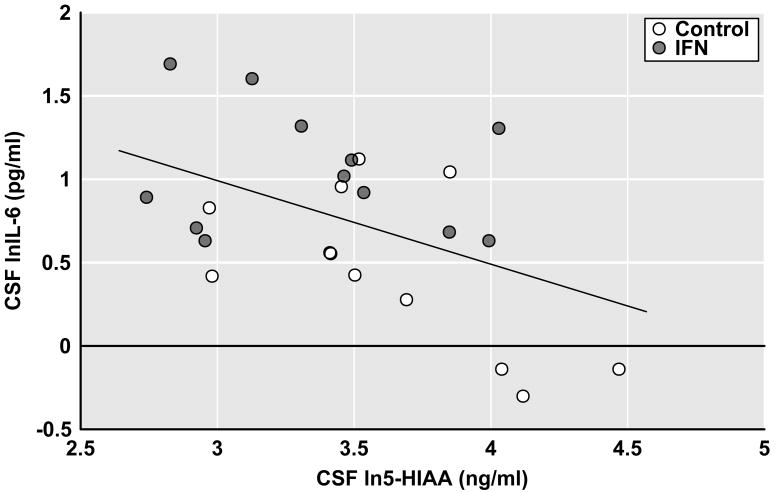
To explore the relative contribution of immune and neurotransmitter variables to depressive symptoms, a stepwise regression analysis using both forward and backward selection was conducted including immune variables that were significantly increased in IFN-alpha-treated subjects versus controls as well as mood relevant monoamine metabolites and CRH. CSF IFN-alpha, plasma lnIFN-alpha, plasma MCP-1, CSF lnMCP-1, CSF lnIL-6, CSF ln5-HIAA, CSF lnHVA, CSF lnMHPG and CSF lnCRH were entered into the model. The final, reduced model using forward or backward selection included only CSF ln5-HIAA (F[1,23]=6.05, p=0.02)(R2=0.21). Of note, plasma lnIFN-alpha approached significance (p= 0.07), but was not included in the final model because the criteria for independent contribution was pre-set at an alpha of 0.05 (see Methods). Of the immune variables that were found to be elevated in the CSF, only CSF lnIL-6 was negatively correlated with CSF ln5-HIAA (r2=−0.25, df=22, p=0.013) (Figure 2). No correlations were found between CSF ln5-HIAA and CSF IFN-alpha or lnMCP-1 (r2=−0.05, df=22, p=0.31 and r2=−0.01, df=22, p=0.66, respectively).
Figure 2.
Correlation between CSF IL-6 and 5-HIAA in Control and IFN-alpha/ribavirin-treated patients with HCV. Cerebrospinal fluid (CSF) samples for the assessment of interleukin (IL)-6 and the serotonin metabolite, 5-hydroxyindoleacetic acid (5-HIAA), were obtained from control (n=12) and interferon (IFN)-alpha/ribavirin-treated (n=12) patients with hepatitis C virus (HCV) infection. IFN-alpha/ribavirin-treated subjects were studied after ~12 weeks on IFN-alpha/ribavirin therapy. CSF immune and monoamine measures were log transformed (ln) to achieve normality. CSF lnIL-6 was found to significantly correlate with CSF ln5-HIAA (r=−0.50, df=24, p=0.013).
Discussion
The data indicate that peripherally administered IFN-alpha is capable of accessing the brain in humans and is associated with an inflammatory response in the CNS as reflected by elevations in CSF IL-6 and MCP-1. In addition, increases in IL-6 were associated with decreases in the serotonin metabolite, 5-HIAA, which in turn were correlated with depression.
The current study provides the first demonstration that pegylated IFN-alpha administered peripherally leads to increases in CSF IFN-alpha in humans. Although it remains to be established whether the IFN-alpha in the CSF is reflective of the pegylated preparation administered peripherally, these data are consistent with studies in rhesus monkeys, where increased CSF IFN-alpha concentrations have been found after acute and chronic (4 weeks) administration of non-pegylated preparations of IFN-alpha (40,41). In addition, studies in mice have found profound CNS induction of IFN-alpha inducible genes following intraperitoneal injection of recombinant mouse IFN-alpha. These studies in laboratory animals demonstrate that IFN-alpha is capable of accessing (and possibly entering) the brain (41,42). Nevertheless, to date, no saturable transport system for IFN-alpha has been described (43). Thus, the appearance of IFN-alpha in the CSF of IFN-alpha-treated patients suggests that IFN-alpha either enters the brain via passage through leaky regions in the BBB or alternatively activates cells at the BBB to induce local IFN-alpha production. Regarding the latter possibility, IFN-alpha has been shown to upregulate multiple immune genes in human endothelial cells including genes for toll-like receptor 3 and interferon regulatory factor 7, both of which play major roles in IFN-alpha production in response to relevant immunologic stimuli (e.g. viruses) (44–46). In addition, recombinant human IFN-alpha has been shown to induce IFN-alpha production from human peripheral blood mononuclear cells (47). It should also be noted that BBB permeability during IFN-alpha therapy may be facilitated by MCP-1, which was found to be elevated in the peripheral blood and CSF of IFN-alpha-treated patients. Previous in vitro and in vivo studies have indicated that prolonged exposure to MCP-1 can significantly increase BBB permeability through effects of MCP-1 on the receptor for C-C motif chemokine receptor–2 (48). In the context of viral infection, IFN-alpha has been shown to induce the production of MCP-1, which in turn plays a key role in recruiting relevant cell types including monocytes to the site of infection (30). Thus, IFN-alpha-induced production of MCP-1 in the periphery may increase BBB permeability to IFN-alpha as well as to MCP-1 itself.
In association with IFN-alpha, CSF IL-6 was also found to be elevated in IFN-alpha-treated patients. However, in contrast to IFN-alpha, plasma IL-6 concentrations were not elevated in IFN-alpha-treated subjects and did not correlate with CSF concentrations of IL-6. These data suggest that IL-6 is being synthesized de novo in the brain as a function of IFN-alpha penetration. Relevant in this regard is that MCP-1 has been shown to play an important role in priming cells within the CNS (e.g. microglia) to produce local proinflammatory cytokines. For example, MCP-1 knock-out mice produce significantly less IL-1 beta and TNF-alpha following intra-parenchymal injection of LPS despite morphological evidence of activation of microglia and astrocytes (31). Moreover, IFN-beta (which binds to the identical receptor as IFN-alpha) has been shown to induce MCP-1 mRNA and protein in human fetal microglia (49). Thus, IFN-alpha-induction of MCP-1 within the CNS may contribute to increases in IL-6 through priming of local glial elements, which in turn produce IL-6 within the brain. Given microglial expression of IFN-alpha receptors (50), direct effects of IFN-alpha on microglial cytokine production including IL-6 may also be involved.
IFN-alpha has been detected in CSF of patients with a number of infectious diseases including viral and bacterial meningitis as well as systemic lupus erythematosis and Aicardi-Goutieres syndrome, where high concentrations of blood and CSF IFN-alpha are associated with diffuse neuropathology (29,51–55). In HIV infection, CSF IFN-alpha was found to be significantly higher in subjects with HIV dementia compared to patients without dementia and controls (28,29). These data indicate that increased CSF IFN-alpha in the context of a number of disorders (as well as IFN-alpha therapy) may contribute to behavioral changes.
Stepwise linear regression analysis found that 5-HIAA was the primary predictor of depression among the relevant immune and neurotransmitter variables entered into the model. These data suggest that IFN-alpha-induced depressive symptoms may be mediated by effects on serotonin metabolism. The ability of serotonin reuptake inhibitors to prevent or reverse depressive symptoms in patients treated with IFN-alpha supports this notion (10,56–59). Moreover, these data are consistent with a recent study indicating that polymorphisms in the serotonin transporter gene interact with IL-6 gene polymorphisms to influence depressive symptoms in IFN-alpha-treated HCV patients (60). There are several mechanisms whereby IFN-alpha may influence serotonin metabolism. For example, IFN-alpha is a potent inducer of p38 mitogen activated protein kinase (MAPK), which has been shown to upregulate expression and function of membrane transporters for serotonin (61,62). Indeed, p38 MAPK activation in peripheral blood mononuclear cells has been associated with decreased CSF 5-HIAA in rhesus monkeys exposed to maternal abuse/neglect in infancy (63). In addition, IFN-alpha and p38 MAPK pathways have been shown to activate the enzyme indoleamine 2,3 dioxygenase (64), which breaks down tryptophan into kynurenine and quinolinic acid. Decreased peripheral blood tryptophan has been found to correlate with IFN-alpha-induced depressive symptoms, and increased peripheral blood kynurenine has been found in IFN-alpha-treated patients with MD (17,18,65). IFN-alpha also has been found to downregulate serotonin 1A receptors in relevant cell lines (66). Of note, IFN-alpha-associated increases in CSF IL-6 may further contribute to alterations in serotonin metabolism. Previous studies have shown that IL-6 administration to laboratory animals can have a profound influence on serotonin metabolism in a number of brain regions (67,68). It should also be mentioned that IL-6 has been associated with impaired growth of neuronal progenitor cells, suggesting that IL-6 may have direct effects on synaptic plasticity and behavior (69).
There are several limitations of the current study that warrant consideration. Because of the relatively small sample of subjects, we only had adequate statistical power (80% or greater) to detect medium effect sizes (r2 =0.25), accounting for ~25% or more of the variance in observed relationships. A larger sample size may have allowed detection of more subtle relationships among variables, notably the relationship between plasma and CSF MCP-1. Another limitation is that blood samples and depression assessments were obtained 6–7 days after LP, and CSF samples were only obtained once during the study. This design strategy was employed to limit the impact of the stress and anxiety of CSF sampling on peripheral blood and behavioral measures, and to limit the risk of drop-out due to repeated LP. However, because blood and behavioral assessments were not obtained concurrently with CSF samples, relationships among these variables may have been less robust. Longitudinal assessments would also have considerably strengthened the study design and allowed greater control of individual differences and assessment of cause and effect relationships. It also should be noted that because the analyses were restricted to depressive symptoms as measured by the MADRS, relationships among immune and neurotransmitter variables and other symptom domains remain to be determined. Finally, because group assignment was not randomized, the IFN-alpha/ribavirin treatment group had a higher rate of past history of MD. Nevertheless, controlling for a past history of depression did not significantly affect observed differences between groups.
Acknowledgments
This study was supported in part by grants from the National Institutes of Health (K05 MH069124, K23 MH064619, R01 MH070553, R01 HL073921, T32 MH020018), an NIH/NCRR General Clinical Research Center grant (M01 RR00039), and the Centers for Disease Control and Prevention.
Footnotes
Financial Disclosures
Charles L. Raison is on the speakers’ bureau for Lilly and Wyeth and Schering-Plough and has served as a consultant or an advisory board member for Schering-Plough, Wyeth, Lilly, and Centocor; Andrey S. Borisov, Matthias Majer, Daniel F. Drake, Giuseppe Pagnoni, Bobbi J Woolwine, Gerald Vogt, and Breanne Massung reported no biomedical financial interests or potential conflicts of interests; Andrew H. Miller has served as a consultant or an advisory board member for Schering-Plough and Centocor, and has received research funding from Janssen/Johnson and Johnson, GlaxoSmithKline, and Schering-Plough.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Raison CL, Capuron LMiller AH. Cytokines sing the blues: inflammation and the pathogenesis of major depression. Trends in Immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dantzer R, O’Connor JC, Freund GG, Johnson RWKelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yirmiya R, Weidenfeld J, Pollak Y, Morag M, Morag A, Avitsur R, et al. Cytokines, “depression due to a general medical condition,” and antidepressant drugs. Adv Exp Med Biol. 1999;461:283–316. doi: 10.1007/978-0-585-37970-8_16. [DOI] [PubMed] [Google Scholar]
- 4.Abbas AK, Lichtman AH. Cellular and Molecular Immunology. 5. Philadelphia: W.B. Saunders Company; 2003. [Google Scholar]
- 5.Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun. 2007;21:727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Ericsson A, Kovacs KJSawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goehler LE, Gaykema RP, Hansen MK, Anderson K, Maier SFWatkins LR. Vagal immune-to-brain communication: a visceral chemosensory pathway. Autonomic Neuroscience-Basic & Clinical. 2000;85:49–59. doi: 10.1016/S1566-0702(00)00219-8. [DOI] [PubMed] [Google Scholar]
- 8.Kirkwood J. Cancer immunotherapy: the interferon-alpha experience. Semin Oncol. 2002;29:18–26. doi: 10.1053/sonc.2002.33078. [DOI] [PubMed] [Google Scholar]
- 9.Dorr RT. Interferon-alpha in malignant and viral diseases. A review. Drugs. 1993;45:177–211. doi: 10.2165/00003495-199345020-00003. [DOI] [PubMed] [Google Scholar]
- 10.Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. [see comments.] N Engl J Med. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 11.Schaefer M, Engelbrecht MA, Gut O, Fiebich BL, Bauer J, Schmidt F, et al. Interferon alpha (IFNa) and psychiatric syndromes: a review. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2002;26:731–746. doi: 10.1016/s0278-5846(01)00324-4. [DOI] [PubMed] [Google Scholar]
- 12.Raison CL, Demetrashvili M, Capuron LMiller AH. Neuropsychiatric side effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19:1–19. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lotrich FE, Rabinovitz M, Gironda PPollock BG. Depression following pegylated interferon-alpha: characteristics and vulnerability. J Psychosom Res. 2007;63:131–135. doi: 10.1016/j.jpsychores.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asnis GM, De La Garza R., 2nd Interferon-induced depression in chronic hepatitis C: a review of its prevalence, risk factors, biology, and treatment approaches.[see comment] Journal of Clinical Gastroenterology. 2006;40:322–335. doi: 10.1097/01.mcg.0000210099.36500.fe. [DOI] [PubMed] [Google Scholar]
- 15.Orru MG, Baita A, Sitzia R, Costa A, Muntoni E, Landau S, et al. [Interferon-alpha-induced psychiatric side effects in patients with chronic viral hepatitis: a prospective, observational, controlled study] Epidemiol Psichiatr Soc. 2005;14:145–153. doi: 10.1017/s1121189x00006394. [DOI] [PubMed] [Google Scholar]
- 16.Wichers MC, Kenis G, Koek GH, Robaeys G, Nicolson NAMaes M. Interferon-alpha-induced depressive symptoms are related to changes in the cytokine network but not to cortisol. J Psychosom Res. 2007;62:207–214. doi: 10.1016/j.jpsychores.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, et al. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J Clin Psychopharmacol. 2002;22:86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes MDantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002;7:468–473. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- 19.Schaefer M, Schwaiger M, Pich M, Lieb KHeinz A. Neurotransmitter changes by interferon-alpha and therapeutic implications. Pharmacopsychiatry. 2003;36(Suppl 3):S203–206. doi: 10.1055/s-2003-45131. [DOI] [PubMed] [Google Scholar]
- 20.Raison CL, Borisov AS, Woolwine BJ, Massung B, Vogt G, Miller AH. Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behavior. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CBMiller AH. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003;160:1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- 22.Taylor JL, Grossberg SE. The effects of interferon-alpha on the production and action of other cytokines. Semin Oncol. 1998;25:23–29. [PubMed] [Google Scholar]
- 23.Wichers MC, Kenis G, Leue C, Koek G, Robaeys GMaes M. Baseline immune activation as a risk factor for the onset of depression during interferon-alpha treatment. Biol Psychiatry. 2006;60:77–79. doi: 10.1016/j.biopsych.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 24.Friebe A, Schwarz MJ, Schmid-Wendtner M, Volkenandt M, Schmidt F, Horn M, et al. Pretreatment levels of sTNF-R1 and sIL-6R are associated with a higher vulnerability for IFN-alpha-induced depressive symptoms in patients with malignant melanoma. Journal of Immunotherapy. 2007;30:333–337. doi: 10.1097/01.cji.0000211346.19330.c9. [DOI] [PubMed] [Google Scholar]
- 25.Monteiro de Almeida S, Letendre S, Zimmerman J, Lazzaretto D, McCutchan AEllis R. Dynamics of monocyte chemoattractant protein type one (MCP-1) and HIV viral load in human cerebrospinal fluid and plasma. J Neuroimmunol. 2005;169:144–152. doi: 10.1016/j.jneuroim.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Chang L, Ernst T, St Hillaire CConant K. Antiretroviral treatment alters relationship between MCP-1 and neurometabolites in HIV patients. Antivir Ther. 2004;9:431–440. doi: 10.1177/135965350400900302. [DOI] [PubMed] [Google Scholar]
- 27.Cinque P, Vago L, Mengozzi M, Torri V, Ceresa D, Vicenzi E, et al. Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. Aids. 1998;12:1327–1332. doi: 10.1097/00002030-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Rho MB, Wesselingh S, Glass JD, McArthur JC, Choi S, Griffin J, et al. A potential role for interferon-alpha in the pathogenesis of HIV-associated dementia. Brain Behav Immun. 1995;9:366–377. doi: 10.1006/brbi.1995.1034. [DOI] [PubMed] [Google Scholar]
- 29.Krivine A, Force G, Servan J, Cabee A, Rozenberg F, Dighiero L, et al. Measuring HIV-1 RNA and interferon-alpha in the cerebrospinal fluid of AIDS patients: insights into the pathogenesis of AIDS Dementia Complex. Journal of Neurovirology. 1999;5:500–506. doi: 10.3109/13550289909045379. [DOI] [PubMed] [Google Scholar]
- 30.Hokeness KL, Kuziel WA, Biron CASalazar-Mather TP. Monocyte chemoattractant protein-1 and CCR2 interactions are required for IFN-alpha/beta-induced inflammatory responses and antiviral defense in liver. J Immunol. 2005;174:1549–1556. doi: 10.4049/jimmunol.174.3.1549. [DOI] [PubMed] [Google Scholar]
- 31.Rankine EL, Hughes PM, Botham MS, Perry VHFelton LM. Brain cytokine synthesis induced by an intraparenchymal injection of LPS is reduced in MCP-1-deficient mice prior to leucocyte recruitment. Eur J Neurosci. 2006;24:77–86. doi: 10.1111/j.1460-9568.2006.04891.x. [DOI] [PubMed] [Google Scholar]
- 32.Mendels J, Frazer A, Fitzgerald RG, Ramsey TAStokes JW. Biogenic amine metabolites in cerebrospinal fluid of depressed and manic patients. Science. 1972;175:1380–1382. doi: 10.1126/science.175.4028.1380. [DOI] [PubMed] [Google Scholar]
- 33.Mendels J, Frazer A. Brain biogenic amine depletion and mood. Arch Gen Psychiatry. 1974;30:447–451. doi: 10.1001/archpsyc.1974.01760100019004. [DOI] [PubMed] [Google Scholar]
- 34.Szabo ST, Gould TD, Manji HK. Neurotransmitters, receptors, signal transduction, and second messengers in psychiatric disorders. In: Schatzberg AF, Nemeroff CB, editors. Textbook of Psychopharmacology. American Psychiatric Publishing; Washington, DC: 2004. pp. 3–52. [Google Scholar]
- 35.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV. Washington DC: American Psychiatric Press; 1997. [Google Scholar]
- 36.Folstein MF, Folstein SEMcHugh PR. Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 37.Majer M, Wellberg LAM, Capuron L, Pagnoni G, Raison CL, Miller AH. IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. Brain Behav Immun. 2008 doi: 10.1016/j.bbi.2007.12.009. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 39.Wightman RM, Plotsky PM, Strope E, Delcore R, Jr, Adams RN. Liquid chromatographic monitoring of CSF metabolites. Brain Res. 1977;131:345–349. doi: 10.1016/0006-8993(77)90526-1. [DOI] [PubMed] [Google Scholar]
- 40.Felger JF, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM, et al. Effects of interferon-alpha on rhesus monkeys: a non-human primate model of cytokine-induced depression. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2007.05.026. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins JM, Riccardi R, Trown P, O’Neill DPoplack DG. Plasma and cerebrospinal fluid pharmacokinetics of recombinant interferon alpha A in monkeys: comparison of intravenous, intramuscular, and intraventricular delivery. Cancer Drug Delivery. 1985;2:247–253. doi: 10.1089/cdd.1985.2.247. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Campbell IL, Zhang H. Systemic interferon-alpha regulates interferon-stimulated genes in the central nervous system. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002013. [DOI] [PubMed] [Google Scholar]
- 43.Pan W, Banks WAKastin AJ. Permeability of the blood-brain and blood-spinal cord barriers to interferons. J Neuroimmunol. 1997;76:105–111. doi: 10.1016/s0165-5728(97)00034-9. [DOI] [PubMed] [Google Scholar]
- 44.Indraccolo S, Pfeffer U, Minuzzo S, Esposito G, Roni V, Mandruzzato S, et al. Identification of genes selectively regulated by IFNs in endothelial cells. J Immunol. 2007;178:1122–1135. doi: 10.4049/jimmunol.178.2.1122. [DOI] [PubMed] [Google Scholar]
- 45.Tissari J, Siren J, Meri S, Julkunen IMatikainen S. IFN-alpha enhances TLR3-mediated antiviral cytokine expression in human endothelial and epithelial cells by up-regulating TLR3 expression. J Immunol. 2005;174:4289–4294. doi: 10.4049/jimmunol.174.7.4289. [DOI] [PubMed] [Google Scholar]
- 46.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 47.Shehata M, Schwarzmeier JD, Nguyen ST, Hilgarth M, Berger R, Hubmann R, et al. Reconstitution of endogenous interferon a by recombinant interferon in hairy cell leukemia. Cancer Res. 2000;60:5420–5426. [PubMed] [Google Scholar]
- 48.Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, et al. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. Journal of Cerebral Blood Flow & Metabolism. 2005;25:593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]
- 49.McManus CM, Liu JS, Hahn MT, Hua LL, Brosnan CF, Berman JW, et al. Differential induction of chemokines in human microglia by type I and II interferons. GLIA. 2000;29:273–280. [PubMed] [Google Scholar]
- 50.Yamada T, Yamanaka I. Microglial localization of alpha-interferon receptor in human brain tissues. Neurosci Lett. 1995;189:73–76. doi: 10.1016/0304-3940(95)11452-3. [DOI] [PubMed] [Google Scholar]
- 51.Barth PG. The neuropathology of Alcardi-Goutieres syndrome. European Journal of Pediatric Neurology. 2002;6:A27–A31. doi: 10.1053/ejpn.2002.0570. [DOI] [PubMed] [Google Scholar]
- 52.Shiozawa S, Kuroki Y, Kim M, Hirohata SOgino T. Interferon-alpha in lupus psychosis. Arthritis Rheum. 1992;35:417–422. doi: 10.1002/art.1780350410. [DOI] [PubMed] [Google Scholar]
- 53.Jonsen A, Bengtsson AA, Nived O, Ryberg B, Truedsson L, Ronnblom L, et al. The heterogeneity of neuropsychiatric systemic lupus erythematosus is reflected in lack of association with cerebrospinal fluid cytokine profiles. Lupus. 2003;12:846–850. doi: 10.1191/0961203303lu472sr. [DOI] [PubMed] [Google Scholar]
- 54.Abbott RJ, Bolderson IGruer PJ. Assessment of an immunoassay for interferon-alpha in cerebrospinal fluid as a diagnostic aid in infections of the central nervous system. Journal of Infection. 1987;15:153–160. doi: 10.1016/s0163-4453(87)93147-1. [DOI] [PubMed] [Google Scholar]
- 55.Ichimura H, Shimase K, Tamura I, Kaneto E, Kurimura O, Aramitsu Y, et al. Neutralizing antibody and interferon-alpha in cerebrospinal fluids and sera of acute aseptic meningitis. Journal of Medical Virology. 1985;15:231–237. doi: 10.1002/jmv.1890150304. [DOI] [PubMed] [Google Scholar]
- 56.Raison CL, Woolwine BJ, Binongo J, Staub J, Weinrieb RM, Rosenblate R, et al. Paroxetine for the prevention of depression in patients undergoing treatment with interferon-alpha plus ribavirin for hepatitis C. Brain Behav Immun. 2006;20:E58. [Google Scholar]
- 57.Schaefer M, Schwaiger M, Garkisch AS, Pich M, Hinzpeter A, Uebelhack R, et al. Prevention of interferon-alpha associated depression in psychiatric risk patients with chronic hepatitis C.[see comment] Journal of Hepatology. 2005;42:793–798. doi: 10.1016/j.jhep.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 58.Hauser P, Khosla J, Aurora H, Laurin J, Kling MA, Hill J, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7:942–947. doi: 10.1038/sj.mp.4001119. [DOI] [PubMed] [Google Scholar]
- 59.Kraus MR, Schafer A, Faller H, Csef HScheurlen M. Paroxetine for the treatment of interferon-alpha-induced depression in chronic hepatitis C. Alimentary Pharmacology & Therapeutics. 2002;16:1091–1099. doi: 10.1046/j.1365-2036.2002.01265.x. [DOI] [PubMed] [Google Scholar]
- 60.Bull SJ, Huezo-Diaz P, Binder EB, Cubells JF, Ranjith G, Maddock C, et al. Functional polymorphisms in the interleukin-6 and serotonin transporter genes, and depression and fatigue induced by interferon-alpha and ribavirin treatment. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu CB, Blakely RDHewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31:2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- 62.Zhu CB, Carneiro AM, Dostmann WR, Hewlett WABlakely RD. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J Biol Chem. 2005;280:15649–15658. doi: 10.1074/jbc.M410858200. [DOI] [PubMed] [Google Scholar]
- 63.Sanchez MM, Alagbe O, Felger JC, Zhang J, Graff AE, Grand AP, et al. Activated p38 MAPK is associated with decreased CSF 5-HIAA and increased maternal rejection during infancy in rhesus monkeys. Mol Psychiatry. 2007;12:895–897. doi: 10.1038/sj.mp.4002025. [DOI] [PubMed] [Google Scholar]
- 64.Fujigaki H, Saito K, Fujigaki S, Takemura M, Sudo K, Ishiguro H, et al. The signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokines. Journal of Biochemistry. 2006;139:655–662. doi: 10.1093/jb/mvj072. [DOI] [PubMed] [Google Scholar]
- 65.Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, et al. Interferon-alpha-induced changes in tryptophan metabolism: relationship to depression and paroxetine treatment. Biol Psychiatry. 2003;54:906–914. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 66.Cai W, Khaoustov VI, Xie Q, Pan T, Le WYoffe B. Interferon-alpha-induced modulation of glucocorticoid and serotonin receptors as a mechanism of depression. J Hepatol. 2005;42:880–887. doi: 10.1016/j.jhep.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 67.Dunn AJ. Effects of cytokines and infections on brain neurochemistry. Clinical Neuroscience Research. 2006;6:52–68. doi: 10.1016/j.cnr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song C, Merali ZAnisman H. Variations of nucleus accumbens dopamine and serotonin following systemic interleukin-1, interleukin-2 or interleukin-6 treatment. Neuroscience. 1999;88:823–836. doi: 10.1016/s0306-4522(98)00271-1. [DOI] [PubMed] [Google Scholar]
- 69.Monje ML, Toda HPalmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]