Abstract
OBJECTIVE
To estimate the year-round burden of healthcare visits attributable to bronchiolitis and identify risk factors for bronchiolitis in term, healthy infants.
PATIENTS AND METHODS
We conducted a population-based, retrospective cohort study of 103,670 term, non-low birth weight infants enrolled in Tennessee Medicaid, 1995 to 2003. We followed infants through the first year of life. Risk factors for bronchiolitis during infancy and rates of inpatient, emergency department, and outpatient visits during the study period were calculated using claims data.
RESULTS
Over the 9 study years, rates of bronchiolitis visits per 1000 infant years were: 238 (outpatient), 77 (emergency department), and 71 (hospitalization). Average annual rates of bronchiolitis visits increased 41% from 188 to 265/1000 infant years from 1996-1997 to 2002-2003 (test of trend, p<.001). Analysis of the linear trend in 500 gram increments demonstrated a negative association between increasing birth weight and bronchiolitis diagnosis (p<0.0001). There was a significant, negative trend between maternal age and infant bronchiolitis diagnosis. Compared to infants of mothers aged 20-29 years, infants of mothers aged 15-19 had a small increase in risk of having a bronchiolitis visit (Hazard ratio 1.05, 95% Confidence Interval 1.01-1.09), while infants of older mothers were less likely to have a visit including women aged 30-39 (Hazard ratio 0.76, 95% Confidence Interval 0.72-0.79) and 40-44 (Hazard ratio 0.54, 95% CI 0.43-0.68).
CONCLUSIONS
The disease burden of bronchiolitis is substantial with increasing rates of all types of visits among term, otherwise healthy infants enrolled in Tennessee Medicaid from 1995 to 2003. Protective factors in this cohort of term infants included higher birth weight and older maternal age.
Keywords: bronchiolitis, risk factors, trends
Introduction
Bronchiolitis is a disease of the lower respiratory tract characterized clinically by cough, tachypnea, wheezing and/or rales.1 Yearly, up to 3% of healthy infants in the United States are hospitalized for bronchiolitis resulting in an estimated 120,000 hospitalizations with report of increasing hospitalization rates between1988 and 1996.2;3 Several viruses cause bronchiolitis, including respiratory syncytial virus (RSV), influenza virus, human rhinovirus, and human metapneumovirus.4-6 RSV infects most children in the first year of life, and typically causes yearly epidemics of bronchiolitis between November and April resulting in an estimated 80,000 infant hospitalizations yearly.3;7 Although children with chronic lung disease, cardiac disease, or those born prematurely are at increased risk of developing severe RSV bronchiolitis, the majority of illness occurs in term, other-wise healthy infants. Efforts to develop a vaccine for RSV have been on-going over the past several decades with challenges in developing a safe and effective vaccine.8 Although national estimates for bronchiolitis visits exist,9-12 there have been no population-based studies examining the full spectrum of the health care burden of bronchiolitis in term infants by examining outpatient and inpatient visits for bronchiolitis during both RSV and non-RSV peaks.
Using a large population-based administrative database linked with vital records, we examined outpatient and inpatient visits for bronchiolitis among term infants enrolled in the Tennessee Medicaid Program. To investigate risk factors associated with bronchiolitis, we assembled a cohort of term otherwise healthy infants, to avoid potential confounders such as chronic lung disease and prematurity. The objectives of this study were to estimate the year round burden of healthcare visits attributable to bronchiolitis, determine if rates are continuing to increase since the last report on hospitalization trends (1988 – 1996),3;13 and estimate risk factors for bronchiolitis diagnoses in term, healthy infants. These data are important to establish the burden and trends in disease for both outpatient and inpatient visits, to establish potentially modifiable risk factors for term otherwise healthy infants, and to inform biologic research on the mechanisms of disease.
Patients and Methods
We conducted a population-based retrospective cohort study of over 100,000 term, otherwise healthy infants enrolled in the Tennessee Medicaid Program, during 1995-2003. Approximately 50% of infants born in Tennessee are enrolled in the Tennessee Medicaid Program. Using previously described methods, we obtained study data from linked Tennessee Medicaid administrative data files and Tennessee State vital records.14;15 The protocol was approved by the Institutional Review Boards of Vanderbilt University and the Tennessee Department of Health.
Eligible infants were ≥37 weeks estimated gestational age (EGA), weighed ≥2500 grams at birth, and were born to women who were continuously enrolled in the Tennessee Medicaid program. Continuous maternal enrollment was defined as no more than 45 days of non-enrollment during the year prior to pregnancy (last menstrual period minus 365 days) through delivery. In order to investigate risk factors for bronchiolitis in infants without the confounding of chronic disease, we excluded infants with any of the following during the first three months of life (3.11%): an International Classification of Diseases, Ninth Revision (ICD-9) code for congenital heart disease, chronic lung disease, or congenital anomaly of the airway, a Current Procedural Terminology (CPT) code indicating surgery for congenital heart disease, or receipt of one or more doses of RSV immune globulin. Infant EGA was determined using the date of last menstrual period on the birth certificate (91.7%), calculated based on the median gestational period in weeks for the infant’s race, birth weight, and birth year (8.22%) or assigned last menstrual period as 270 days prior to birth (0.04%).16;17
We determined infant healthcare visits for bronchiolitis using ICD-9 codes for bronchiolitis (466.1) and/or RSV pneumonia (480.1). To investigate the full-spectrum of the health care burden of bronchiolitis in term infants we examined outpatient and inpatient visits for bronchiolitis year-round. During the first few months of life, infant Medicaid visits may be billed to the infant’s mother. Therefore we attributed bronchiolitis visits in the mother’s record to the infant, as bronchiolitis is a rare diagnosis in women of child bearing age.
To illustrate the pattern of the monthly distribution of bronchiolitis diagnoses, we captured all diagnoses by month from 1995 to 2003. All infants in the study cohort were followed until one year of age, until they had more than 21 days of non-enrollment in Tennessee Medicaid, or death. We determined the rate of bronchiolitis associated outpatient visits (not associated with a same day hospitalization or emergency department visit), emergency department visits (not associated with a same day 23 hour observation or hospitalization), and combined 23 hour observations and hospitalizations per 1,000 infant years. To examine trends in bronchiolitis rates, we studied years 1996 to 2003 only. During this time period, we determined the first and all ICD-9 diagnoses for all bronchiolitis visit types. The numerator consisted of bronchiolitis diagnoses. To estimate the denominator for each year we determined the total number of infants who were less than 12 months of age on July 1 for each year 1996 to 2003. We applied Poisson regression to assess temporal trends in the rates.18
In this large cohort of term infants, we estimated the association between bronchiolitis during infancy and available demographic variables, including infant birth weight and maternal age at delivery. 2;19-22 From infant birth certificate data we identified infant birth weight, maternal age at delivery, infant sex, siblings (none, one, two or more based on birth certificate report of number of prior live births), self-reported maternal smoking during pregnancy, maternal education level, and marital status. We identified infant race and region of residence (urban, suburban, rural) from Tennessee Medicaid enrollment files. We identified mothers with asthma by capturing asthma-specific health care visits and medication as previously described.17 To estimate independent predictors of at least one bronchiolitis healthcare visit during infancy, we included the above variables in a Cox proportional hazard model with age as the time dependent variable. As a measure of severe bronchiolitis, we also estimated predictors of a bronchiolitis hospitalization. We calculated linear test of trend of the effects of infant birth weight, maternal age at delivery, number of cigarettes smoked during pregnancy , and number of siblings as ordered continuous variables using Cox proportional hazards models. We tested for interaction between maternal age and infant sex on bronchiolitis incidence.
Results
Among 103,670 term infants there were 23,306 outpatient visits, 7,511 emergency department visits, and 6,936 hospitalizations for bronchiolitis. Overall, 20% of infants had at least one bronchiolitis visit, and 8.8 % of infants had more than one healthcare visit for bronchiolitis during infancy. During the first year of life, 13.3% of infants had a clinic visit, 6.2% had an emergency department visit, and 5.5% of infants had a 23 hour observation or hospitalization. Bronchiolitis visits peaked December through February, which parallels the known epidemiology of RSV (Figure 1), with 80% of visits occurring during the winter virus season between November and April.23 There were 98,080 eligible infant-years during the study with 238 bronchiolitis outpatient visits, 77 emergency department visits, and 71 hospitalizations per 1000 infant years.
Figure 1.
Monthly Cumulative Frequency of Bronchiolitis Diagnoses Among Term Infants Enrolled in Tennessee Medicaid, 1995-2003
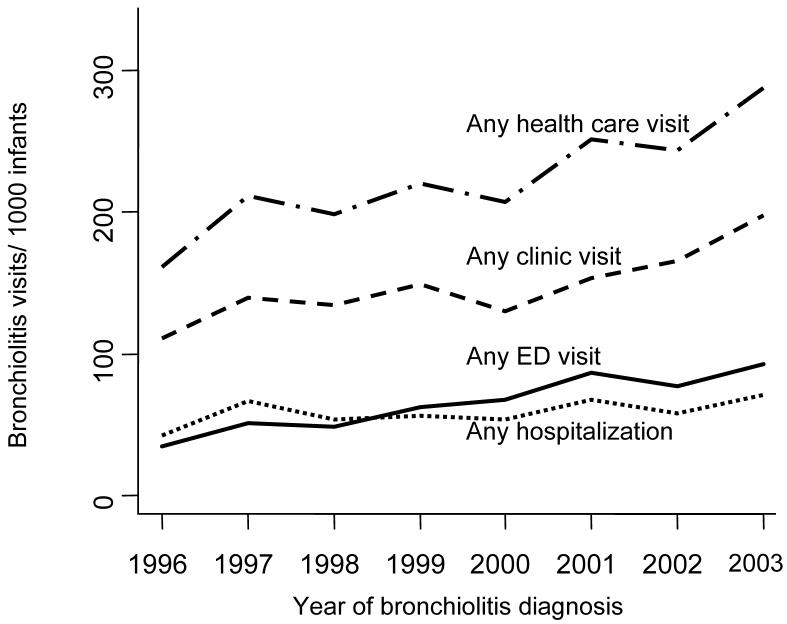
Figure 2, demonstrates an overall increase in yearly bronchiolitis rates, 1996-2003, for infants with at least one visit for bronchiolitis (first visit during infancy, combined inpatient and outpatient), and the rates stratified by visit type. Comparing the average of years 1996 and 1997 to that of years 2002 and 2003, the rates of infants having any bronchiolitis diagnosis increased 41% from 188/1000 to 265/1000 infants. There was a positive trend in increased rates of bronchiolitis 1996 to 2003 (test of trend, p<0.001). The average rates of having at least one hospitalization for bronchiolitis increased from 5.5% to 6.4% comparing the same time periods. We also estimated the total burden of all visits for bronchiolitis (repeat visits included), 1996 through 2003. The average burden of all visits for bronchiolitis increased 48% from 304/1000 infants in 1996-1997 to 449/1000 infants in 2002-2003.
Figure 2.
Trends in Rates of Bronchiolitis Diagnoses Among Term Infants Enrolled in Tennessee Medicaid, 1995-2003
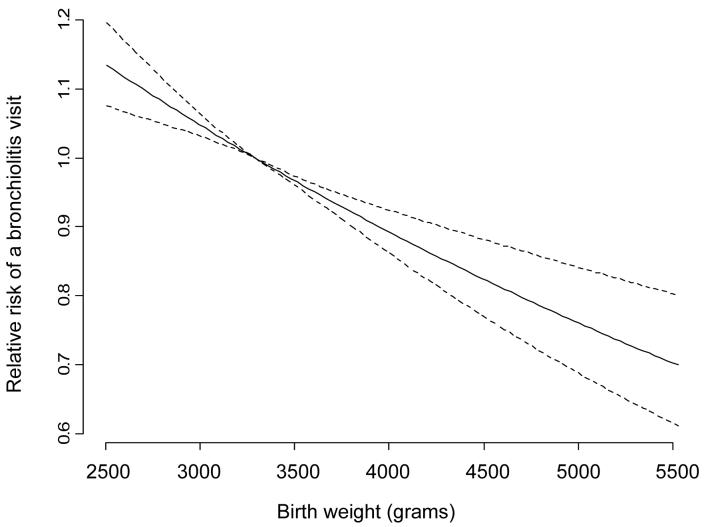
Differences in rates of outpatient visits, emergency department visits, and hospitalizations by sociodemographic factors are listed in Table 1. In a multivariable analysis, we compared the risk of having a visit for bronchiolitis by sociodemographic factors (Table 2). As illustrated in Table 2 and figure 3, analysis of the linear trend in 500 gram increments demonstrated a significant negative association with bronchiolitis risk (p<0.0001). In addition, there was a significant and negative relationship between maternal age and infant bronchiolitis diagnosis (Test of trend, p<0.0001). Compared to infants of mothers who were 20-29 years at the time of delivery, infants of younger mothers had a small increase in risk of bronchiolitis diagnosis while infants of older mothers were much less likely to have a bronchiolitis diagnosis (Table 2). Similar to results for any bronchiolitis health care visit, a negative linear relationship was found between maternal age at delivery and risk of a bronchiolitis hospitalization (data not shown). Adjusted for other factors, females and African-Americans remained less likely to have a visit for bronchiolitis in the first year of life compared to males and whites, respectively (Table 2). Although female infants have a lower bronchiolitis risk than males, increasing maternal age is protective in both sexes (Figure 4). Statistically significant differences in the rate of visits for bronchiolitis were not seen by maternal education level. Infants whose mothers have asthma were more likely to have a bronchiolitis diagnosis than infants whose mothers did not have asthma (HR 1.35, 95% CI 1.28-1.41). There was a significant dose-response relationship between number of cigarettes smoked and bronchiolitis incidence (Test of trend, p<0.001). In analyses stratifying infants by their maternal asthma and smoking history, infants of women with asthma and who smoked 10 or more cigarettes daily had the highest risk of bronchiolitis. In addition, there was a significant graded association with number of siblings and bronchiolitis (Test of trend, p<.0001). Infants with siblings were 20% (one sibling) to 30% (≥2 siblings) more likely to have a bronchiolitis diagnosis than infants without a sibling. Infants in rural and suburban areas were more likely to have a bronchiolitis diagnosis than infants in urban areas. In addition, as indicated in Table 2, factors such as higher birth weight, older maternal age, residing in an urban area, and having no siblings were also associated with a decreased risk, or tended to have a decreased risk, of a bronchiolitis hospitalization in term infants.
Table 1.
Rates of Bronchiolitis Visits Among 103,670 Term Infants Enrolled in Tennessee Medicaid 1995-2003, by Maternal and Infant Characteristics
| Characteristic | Child years |
Outpatient visits |
Outpatient visits/1000 child years |
ED Visits |
ED visits/1000 child years |
Hospital visits |
Hospital visits/1000 child years |
|---|---|---|---|---|---|---|---|
| 98080 | 23306 | 238 | 7511 | 77 | 6936 | 71 | |
| Birth Weight | |||||||
| 2500 to 3000 grams | 23449 | 5725 | 244 | 2013 | 86 | 1883 | 80 |
| 3001 to 3500 grams | 43238 | 10040 | 232 | 3245 | 75 | 3019 | 70 |
| 3501 to 4000 grams | 24749 | 6084 | 246 | 1839 | 74 | 1636 | 66 |
| 4001 to 4500 grams | 5636 | 1248 | 221 | 371 | 66 | 353 | 63 |
| 4501 to 5000 grams | 1008 | 209 | 207 | 43 | 43 | 45 | 45 |
| Maternal Age* | |||||||
| 15-19 | 25137 | 5592 | 222 | 2074 | 83 | 1801 | 72 |
| 20-29 | 61272 | 15310 | 250 | 4804 | 78 | 4550 | 74 |
| 30-39 | 11076 | 2311 | 209 | 608 | 55 | 566 | 51 |
| 40-44 | 594 | 93 | 157 | 25 | 42 | 19 | 32 |
| Infant Sex | |||||||
| Male | 50324 | 13927 | 277 | 4485 | 89 | 4062 | 81 |
| Female | 47756 | 9379 | 196 | 3026 | 63 | 2874 | 60 |
| Infant Race | |||||||
| White | 54048 | 16599 | 307 | 3788 | 70 | 4889 | 90 |
| African-American | 40648 | 5919 | 146 | 3445 | 85 | 1730 | 43 |
| Latino | 978 | 223 | 228 | 91 | 93 | 69 | 71 |
|
Maternal Education |
|||||||
| <12 Years | 41616 | 9809 | 236 | 3609 | 87 | 3372 | 81 |
| 12 Years | 43529 | 10479 | 241 | 3145 | 72 | 2849 | 65 |
| >12 Years | 12727 | 2975 | 234 | 746 | 59 | 698 | 55 |
| Maternal Asthma | |||||||
| No | 91473 | 21179 | 232 | 6708 | 73 | 6224 | 68 |
| Yes | 6607 | 2127 | 322 | 803 | 122 | 712 | 108 |
|
Maternal Smoking† |
|||||||
| Non-smoker | 71107 | 15207 | 214 | 5180 | 73 | 4236 | 60 |
| 1 - 9 cigarettes/day | 6078 | 1518 | 250 | 519 | 85 | 479 | 79 |
| ≥10 cigarettes/day | 20261 | 6404 | 316 | 1772 | 87 | 2167 | 107 |
|
Number of Siblings |
|||||||
| No Siblings | 28419 | 6447 | 227 | 2026 | 71 | 1699 | 60 |
| One sibling | 35830 | 9199 | 257 | 2725 | 76 | 2694 | 75 |
| Two or more siblings |
33751 | 7637 | 226 | 2756 | 82 | 2534 | 75 |
| Residence | |||||||
| Metropolitan | 44009 | 6477 | 147 | 4163 | 95 | 1907 | 43 |
| Suburban | 21945 | 6343 | 289 | 1486 | 68 | 1535 | 70 |
| Rural | 32034 | 10475 | 327 | 1855 | 58 | 3488 | 109 |
maternal age at delivery
maternal smoking during pregnancy
Table 2.
Risk Factors for Any Type of Bronchiolitis Visit or Hospitalization* Among Term Infants Enrolled in Tennessee Medicaid, 1995-2003
| Characteristic | Any Visit HR ( 95% CI) |
Hospitalization HR ( 95% CI) |
|---|---|---|
| Birth Weight | ||
| 2500 to 3000 grams | 1 | 1 |
| 3001 to 3500 grams | 0.92 (0.89-0.96) | 0.86 (0.81-0.92) |
| 3501 to 4000 grams | 0.90 (0.86-0.94) | 0.80 (0.75-0.87) |
| 4001 to 4500 grams | 0.80 (0.75-0.85) | 0.76 (0.67-0.86) |
| 4501 to 5000 grams | 0.72 (0.61-0.83) | 0.57 (0.41-0.78) |
| Maternal Age† | ||
| 15-19 | 1.05 (1.01-1.09) | 1.15 (1.06-1.23) |
| 20-29 | 1 | 1 |
| 30-39 | 0.76 (0.72-0.79) | 0.66 (0.60-0.73) |
| 40-44 | 0.54 (0.43-0.68) | 0.43 (0.26-0.69) |
| Infant Sex | ||
| Female | 1 | 1 |
| Male | 1.35 (1.31-1.39) | 1.33 (1.26-1.41) |
| Infant Race | ||
| White | 1 | 1 |
| African-American | 0.73 (0.70-0.76) | 0.66 (0.61-0.71) |
| Latino | 0.88 (0.77-1.02) | 0.87 (0.67-1.13) |
| Maternal Education | ||
| <12 Years | 1.03 (0.99-1.08) | 1.15 (1.04-1.26) |
| 12 Years | 1.01 (0.97-1.06) | 1.02 (0.93-1.11) |
| >12 Years | 1 | 1 |
| Maternal Asthma | ||
| No | 1 | 1 |
| Yes | 1.35 (1.28-1.41) | 1.45 (1.33-1.59) |
| Maternal Smoking‡ | ||
| Non-smoker | 1 | 1 |
| 1 - 9 cigarettes/day | 1.09 (1.03-1.15) | 1.14 (1.03-1.27) |
| ≥10 cigarettes/day | 1.17 (1.13-1.22) | 1.28 (1.20-1.36) |
| Number of Siblings | ||
| 0 | 1 | 1 |
| 1 | 1.21 (1.17-1.26) | 1.38 (1.29-1.49) |
| ≥ 2 | 1.31 (1.25-1.36) | 1.64 (1.51-1.78) |
| Residence | ||
| Urban | 1 | 1 |
| Suburban | 1.17 (1.12-1.21) | 1.28 (1.18-1.39) |
| Rural | 1.23 (1.19-1.28) | 1.87 (1.74-2.02) |
Includes 23 hour observations
maternal age at delivery
maternal smoking during pregnancy
Figure 3.
Relative Risk, including 95% Confidence Intervals (dashed lines), of Bronchiolitis Diagnoses by Birth Weight Among Term Infants Enrolled in Tennessee Medicaid 1995-2003
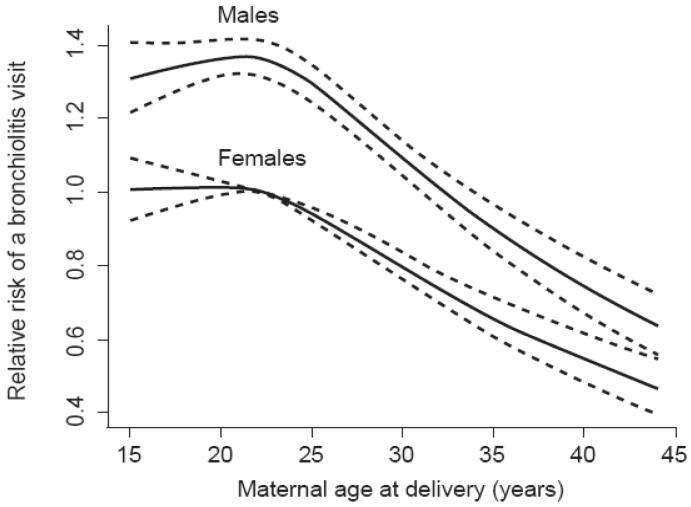
Figure 4.
Relative Risk, including 95% Confidence Intervals (dashed lines), of Bronchiolitis Diagnoses in Males and Females by Maternal Age at Delivery Among Term Infants Enrolled in Tennessee Medicaid 1995-2003
Discussion
This population of term, otherwise healthy, low-income children experienced high rates of bronchiolitis diagnoses with rates for outpatient visits of 238/1000 infant years and hospitalizations of 71/1000 infant years during the first year of life. Overall, 20% had a healthcare claim for bronchiolitis, with 13.3 % having an outpatient visit, 6.2% an emergency department visit, and 5.5% requiring hospitalization. Additionally, overall rates of having at least one bronchiolitis visit have increased by 41% from 1996 – 2003.
Previous studies have reported increases in hospitalizations for bronchiolitis through the 1990s.3 The reasons for the increase in hospitalizations are unclear. Increases in the rates of bronchiolitis visits over time could reflect true increases in disease incidence and severity or non-biologic factors such as improved access to medical care or changes in how physicians code for visits for lower respiratory tract illnesses. It is debated whether increased use of pulse oximetry has influenced hospital admissions for bronchiolitis although several studies have not found increasing rates of hospitalizations during infancy over time for other respiratory illnesses in which pulse oximetry is routinely used.3;24;25 Furthermore, we estimated trends in rates of all bronchiolitis diagnoses, not solely hospitalizations, and found that rates of both outpatient and inpatient bronchiolitis diagnoses are substantial and increasing.
We also determined risk factors for a bronchiolitis healthcare visit in the first year of life. Even among infants who were normal birth weight, higher birth weight was protective for bronchiolitis and severe bronchiolitis. Hoo et al. found that low birth weight for gestational age is associated with lower lung function in infancy and lower lung function during early infancy has been associated with other respiratory illnesses later in life.26-28 There has been an increasing trend in the rates of elective induction of labor prior to full term birth.29;30 This practice may impact the birth weight of infants and therefore decrease the protection that higher birth weight confers on bronchiolitis risk. Elective induction of labor before full term delivery may have important implications particularly for infants with the highest risks of developing asthma, those born to mothers with asthma. Infants whose mothers have asthma are known to have lower birth weights corrected for gestational age, than infants whose mothers do not have asthma.31
Across the age continuum, infants born to younger women are at increased risk of bronchiolitis compared to infants of older women, even after adjustment for number of living siblings, infant birth weight, infant race, region of residence, and maternal education level. Further supporting this association is that older maternal age was also protective against severe bronchiolitis as indicated by the negative linear relationship across the age continuum. The association of younger maternal age and increased risk of wheezing lower respiratory illnesses during infancy, particularly in male infants, has been described in a prospective cohort of middle-class women and their infants.19 In our larger cohort of lower-income families, the increased risk of bronchiolitis in infants of younger women was detected in both male and female infants. It is unclear whether differences in bronchiolitis risk are due to protective factors associated with the in utero environment of older women or due to differences in sociodemographic factors such as health care seeking behaviors, breastfeeding, or day care use.32;33 For example, it is possible that less experienced teenage mothers would be more likely to take their infants in for medical care or that physicians would be more likely to admit infants of teenagers for social reasons. However, as illustrated in Figure 4, the decreased likelihood of a bronchiolitis visit was seen across the continuum of maternal age, not simply when comparing the youngest women to the oldest. For example, when comparing women in the 40 to 44 year age group to the 30 to 39 year age group, women in their 30s had a 40% increased risk of having a bronchiolitis diagnosis than women in their 40s (data not shown). Furthermore, Martinez et al. found decreased risk of wheezing lower respiratory illnesses in infants of older women even after adjustment for day care exposure and infant feeding method.19 Interesting work of others may provide further insights into the association of familial and environmental antenatal exposures and the developing immune system that might explain this association.32;34
Previous findings in the Tennessee Medicaid population suggested that white infants were more likely to be hospitalized for bronchiolitis than African-Americans and we found that white infants were more likely to have a bronchiolitis diagnosis overall.2 The decreased incidence may reflect differences in illness incidence, health seeking behavior, or access to care. In analyses by visit type, African-American and Latino infants were more likely to have emergency department visits for bronchiolitis than white infants, suggesting differential use of services. In a previous investigation, we addressed the primary question of whether there was an the association between a familial predisposition to develop asthma and maternal smoking with the incidence and severity of bronchiolitis during infancy.35 We found that infants with maternal asthma or maternal smoking during pregnancy were more likely to have a bronchiolitis diagnosis than infants without either respective maternal risk factor.35 In this current study, there was a dose-response relationship between maternal smoking during pregnancy and infants having at least one bronchiolitis diagnosis or more severe bronchiolitis. In addition, infants with siblings were 20% to 30% more likely to have a bronchiolitis diagnosis than infants without a sibling likely explained by the greater likelihood for viral exposure and infection among infants with a sibling. Infants in rural and suburban areas were more likely to have a bronchiolitis diagnosis than infants in urban areas, an interesting and unexplained finding. Maternal smoking during pregnancy, having siblings, and living in a rural residence were associated with increased risk of severe bronchiolitis compared to infants without maternal smoking, no siblings, or living in an urban region of the state, respectively.
There are several potential limitations of this work which should be considered. In this retrospective cohort study using existing data to categorize study variables, misclassification of the predictor variables is possible. However, as all predictors were measured before the infant outcome of bronchiolitis, it is likely that any misclassification would be non-differential. If non-differential misclassification occurred this would bias results toward the null which would conservatively lead to an underestimation of the association of the predictor variables and the bronchiolitis outcome. In addition, the cases of bronchiolitis may be over or under-detected. However, ICD-9 diagnoses of bronchiolitis represent objective physician characterized outcomes at the time of illness that would not be influenced by recall bias. Hospitalization for bronchiolitis has been used in epidemiologic investigations as a measure of severity for decades, however it is possible that social factors may have influenced providers’ decisions to hospitalize infants.36-40 Due to the retrospective nature of this cohort it is also possible that study findings were influenced by other unmeasured factors. Therefore, while we determined risk factors for bronchiolitis, we can not conclude that these factors are causal. We conducted our study in the Medicaid population in which approximately half of infants born in Tennessee are enrolled. Population-based cohort investigations in non-Medicaid populations would provide further insight into the disease burden in different socioeconomic populations. Although, results may not be generalizable to the non-Medicaid population, this study cohort represents a substantial portion of children born in the state and in other areas in the United States.
Conclusion
Health care visits for bronchiolitis during infancy are substantial with one in five infants having at least one health care visit for bronchiolitis during infancy. In addition, rates of clinic visits, emergency department visits, and bronchiolitis hospitalizations are all increasing in this otherwise healthy, term, low-income cohort of infants. Protective factors in this cohort of term infants included older maternal age and higher birth weight. These data reinforce the importance of determining why bronchiolitis rates are increasing and acting to prevent or lessen the severity of this cause of significant of infant morbidity.
Acknowledgements
The authors are indebted to the Tennessee Bureau of TennCare of the Department of Finance and Administration, and the Tennessee Department of Health, Office of Policy, Planning & Assessment, for providing the data. The authors are grateful to Fernando Martinez, M.D., The University of Arizona College of Medicine, for his critical review of the manuscript.
This study was supported by grants from the following sources: National Institutes of Health (UO1 HL 72471, MO1 RR00095, KO8 AI01582, K12 RR17697); The Agency for Healthcare Research and Quality, Centers for Education and Research (U18-HS10384); The Geriatric Research Education and Clinical Center, Department of Veterans Affairs; and the Thrasher Research Fund.
Dr. Griffin reports receiving investigator initiated grant support from MedImmune.
Abbreviations
- (RSV)
Respiratory syncytial virus
- (EGA)
Estimated gestational age
- (ICD-9)
International Classification of Diseases, Ninth Revision
- (CPT)
Current Procedural Terminology
- (ED)
Emergency department
- (HR)
Hazard ratio
References
- (1).Smyth RL, Openshaw PJ. Bronchiolitis. Lancet. 2006 Jul 22;368(9532):312–22. doi: 10.1016/S0140-6736(06)69077-6. [DOI] [PubMed] [Google Scholar]
- (2).Boyce TG, Mellen BG, Mitchel EF, Jr., et al. Rates of hospitalization for respiratory syncytial virus infection among children in medicaid. J Pediatr. 2000 Dec;137(6):865–70. doi: 10.1067/mpd.2000.110531. [DOI] [PubMed] [Google Scholar]
- (3).Shay DK, Holman RC, Newman RD, et al. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA. 1999 Oct 20;282(15):1440–6. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- (4).Korppi M, Kotaniemi-Syrjanen A, Waris M, et al. Rhinovirus-associated wheezing in infancy: comparison with respiratory syncytial virus bronchiolitis. Pediatr Infect Dis J. 2004 Nov;23(11):995–9. doi: 10.1097/01.inf.0000143642.72480.53. [DOI] [PubMed] [Google Scholar]
- (5).Williams JV, Harris PA, Tollefson SJ, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004 Jan 29;350(5):443–50. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Rojo JC, Ruiz-Contreras J, Fernandez MB, et al. Influenza-related hospitalizations in children younger than three years of age. Pediatr Infect Dis J. 2006 Jul;25(7):596–601. doi: 10.1097/01.inf.0000220208.59965.95. [DOI] [PubMed] [Google Scholar]
- (7).Glezen WP, Taber LH, Frank AL, et al. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986 Jun;140(6):543–6. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- (8).Karron RA, Wright PF, Belshe RB, et al. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J Infect Dis. 2005 Apr 1;191(7):1093–104. doi: 10.1086/427813. [DOI] [PubMed] [Google Scholar]
- (9).Centers for Disease Control and Prevention (CDC) Bronchiolitis-associated outpatient visits and hospitalizations among American Indian and Alaska Native children--United States, 1990-2000. MMWR Morb Mortal Wkly Rep. 2003 Aug 1;52(30):707–10. [PubMed] [Google Scholar]
- (10).Peck AJ, Holman RC, Curns AT, et al. Lower respiratory tract infections among american Indian and Alaska Native children and the general population of U.S. Children. Pediatr Infect Dis J. 2005 Apr;24(4):342–51. doi: 10.1097/01.inf.0000157250.95880.91. [DOI] [PubMed] [Google Scholar]
- (11).Mansbach JM, Pelletier AJ, Camargo CA., Jr US outpatient office visits for bronchiolitis, 1993-2004. Ambul Pediatr. 2007 Jul;7(4):304–7. doi: 10.1016/j.ambp.2007.03.006. [DOI] [PubMed] [Google Scholar]
- (12).Mansbach JM, Emond JA, Camargo CA., Jr Bronchiolitis in US emergency departments 1992 to 2000: epidemiology and practice variation. Pediatr Emerg Care. 2005 Apr;21(4):242–7. doi: 10.1097/01.pec.0000161469.19841.86. [DOI] [PubMed] [Google Scholar]
- (13).Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003 Nov;143(5 Suppl):S127–S132. doi: 10.1067/s0022-3476(03)00510-9. [DOI] [PubMed] [Google Scholar]
- (14).Neuzil KM, Reed GW, Mitchel EF, et al. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol. 1998 Dec 1;148(11):1094–102. doi: 10.1093/oxfordjournals.aje.a009587. [DOI] [PubMed] [Google Scholar]
- (15).Piper JM, Ray WA, Griffin MR, et al. Methodological issues in evaluating expanded Medicaid coverage for pregnant women. Am J Epidemiol. 1990 Sep;132(3):561–71. doi: 10.1093/oxfordjournals.aje.a115692. [DOI] [PubMed] [Google Scholar]
- (16).Hartert TV, Neuzil KM, Shintani AK, et al. Maternal morbidity and perinatal outcomes among pregnant women with respiratory hospitalizations during influenza season. Am J Obstet Gynecol. 2003 Dec;189(6):1705–12. doi: 10.1016/s0002-9378(03)00857-3. [DOI] [PubMed] [Google Scholar]
- (17).Carroll KN, Griffin MR, Gebretsadik T, et al. Racial differences in asthma morbidity during pregnancy. Obstet Gynecol. 2005 Jul;106(1):66–72. doi: 10.1097/01.AOG.0000164471.87157.4c. [DOI] [PubMed] [Google Scholar]
- (18).Rothman KJ, Greenland S. Modern Epidemiology. 2nd Lippincott Williams & Wilkins; Philadelphia: 1998. [Google Scholar]
- (19).Martinez FD, Wright AL, Holberg CJ, et al. Maternal age as a risk factor for wheezing lower respiratory illnesses in the first year of life. Am J Epidemiol. 1992 Nov 15;136(10):1258–68. doi: 10.1093/oxfordjournals.aje.a116434. [DOI] [PubMed] [Google Scholar]
- (20).Simoes EA. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J Pediatr. 2003 Nov;143(5 Suppl):S118–S126. doi: 10.1067/s0022-3476(03)00511-0. [DOI] [PubMed] [Google Scholar]
- (21).Holman RC, Shay DK, Curns AT, et al. Risk factors for bronchiolitis-associated deaths among infants in the United States. Pediatr Infect Dis J. 2003 Jun;22(6):483–90. doi: 10.1097/01.inf.0000069765.43405.3b. [DOI] [PubMed] [Google Scholar]
- (22).Somech R, Tal G, Gilad E, et al. Epidemiologic, socioeconomic, and clinical factors associated with severity of respiratory syncytial virus infection in previously healthy infants. Clin Pediatr (Phila) 2006 Sep;45(7):621–7. doi: 10.1177/0009922806291012. [DOI] [PubMed] [Google Scholar]
- (23).Kim HW, Arrobio JO, Brandt CD, et al. Epidemiology of respiratory syncytial virus infection in Washington, D.C. I. Importance of the virus in different respiratory tract disease syndromes and temporal distribution of infection. Am J Epidemiol. 1973 Sep;98(3):216–25. doi: 10.1093/oxfordjournals.aje.a121550. [DOI] [PubMed] [Google Scholar]
- (24).Mallory MD, Shay DK, Garrett J, et al. Bronchiolitis management preferences and the influence of pulse oximetry and respiratory rate on the decision to admit. Pediatrics. 2003 Jan;111(1):e45–e51. doi: 10.1542/peds.111.1.e45. [DOI] [PubMed] [Google Scholar]
- (25).van Woensel JB, van Aalderen WM, Kneyber MC, et al. Bronchiolitis hospitalisations in the Netherlands from 1991 to 1999. Arch Dis Child. 2002 May;86(5):370–1. doi: 10.1136/adc.86.5.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Hoo AF, Stocks J, Lum S, et al. Development of lung function in early life: influence of birth weight in infants of nonsmokers. Am J Respir Crit Care Med. 2004 Sep 1;170(5):527–33. doi: 10.1164/rccm.200311-1552OC. [DOI] [PubMed] [Google Scholar]
- (27).Haland G, Carlsen KC, Sandvik L, et al. Reduced lung function at birth and the risk of asthma at 10 years of age. N Engl J Med. 2006 Oct 19;355(16):1682–9. doi: 10.1056/NEJMoa052885. [DOI] [PubMed] [Google Scholar]
- (28).Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995 Jan;332(3):133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- (29).Martin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2003. Natl Vital Stat Rep. 2005 Sep 8;54(2):1–116. [PubMed] [Google Scholar]
- (30).Ramsey PS, Ramin KD, Ramin SM. Labor induction. Curr Opin Obstet Gynecol. 2000 Dec;12(6):463–73. doi: 10.1097/00001703-200012000-00002. [DOI] [PubMed] [Google Scholar]
- (31).Enriquez R, Griffin MR, Carroll KN, et al. Effect of maternal asthma and asthma control on pregnancy and perinatal outcomes. J Allergy Clin Immunol. 2007 Sep;120(3):625–30. doi: 10.1016/j.jaci.2007.05.044. [DOI] [PubMed] [Google Scholar]
- (32).Devereux G, Barker RN, Seaton A. Antenatal determinants of neonatal immune responses to allergens. Clin Exp Allergy. 2002 Jan;32(1):43–50. doi: 10.1046/j.0022-0477.2001.01267.x. [DOI] [PubMed] [Google Scholar]
- (33).Ryan AS, Zhou W, Gaston MH. Regional and sociodemographic variation of breastfeeding in the United States, 2002. Clin Pediatr (Phila) 2004 Nov;43(9):815–24. doi: 10.1177/000992280404300905. [DOI] [PubMed] [Google Scholar]
- (34).Scirica CV, Gold DR, Ryan L, et al. Predictors of cord blood IgE levels in children at risk for asthma and atopy. J Allergy Clin Immunol. 2007 Jan;119(1):81–8. doi: 10.1016/j.jaci.2006.09.002. [DOI] [PubMed] [Google Scholar]
- (35).Carroll KN, Gebretsadik T, Griffin MR, et al. Maternal asthma and maternal smoking are associated with increased risk of bronchiolitis during infancy. Pediatrics. 2007 Jun;119(6):1104–12. doi: 10.1542/peds.2006-2837. [DOI] [PubMed] [Google Scholar]
- (36).Noble V, Murray M, Webb MS, et al. Respiratory status and allergy nine to 10 years after acute bronchiolitis. Arch Dis Child. 1997 Apr;76(4):315–9. doi: 10.1136/adc.76.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Trefny P, Stricker T, Baerlocher C, et al. Family history of atopy and clinical course of RSV infection in ambulatory and hospitalized infants. Pediatr Pulmonol. 2000 Oct;30(4):302–6. doi: 10.1002/1099-0496(200010)30:4<302::aid-ppul5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- (38).Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005 Jan 15;171(2):137–41. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- (39).Henderson J, Hilliard TN, Sherriff A, et al. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol. 2005 Aug;16(5):386–92. doi: 10.1111/j.1399-3038.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- (40).Larouch V, Rivard G, Deschesnes F, et al. Asthma and airway hyperresponsiveness in adults who required hospital admission for bronchiolitis in early childhood. Respir Med. 2000 Mar;94(3):288–94. doi: 10.1053/rmed.1999.0748. [DOI] [PubMed] [Google Scholar]