Abstract
To evaluate the clinicopathologic features of renal cell carcinoma in younger adults (≤40 years), we retrospectively reviewed 838 consecutive cases of renal cell carcinoma (RCC) occurred in a single tertiary hospital. Forty-four 44 (5.2%) cases occurred in the young adult group (24 to 40 years of age). Clinicopathologic features including tumor size, stage, histologic subtype, lymph node and distant metastasis, and overall survival were compared with that of cases occurred in older age group (>40 years). The tumor size of the young adult group were smaller (5.3 vs 5.9 cm) and presented at less advanced stages (T3/T4 tumors, 18% vs 31%) than those occurring in the older age group (>40 years of age). The incidences of chromophobe RCC (12% vs. 6%) and of collecting duct carcinoma (5% vs 0.5%) were higher in the young adult group. The rate of nodal or distant metastasis was lower in young adult group (5% vs. 8.3%). More patients underwent partial nephrectomy in younger than older age group (30% vs 19%). There was no overall survival difference at 5 years (77% vs 70%), but there was a trend for a favorable survival in young adults at 10 years (77% vs 52%). In conclusion, RCC are relatively infrequent in patients who are younger than 40 years. The tumors in this group appear to be smaller and less advanced at presentation. Chromophobe RCC and collecting duct carcinoma are more frequently seen. More patients undergo partial nephrectomy and overall long term survival appears to be more favorable.
Keywords: Renal cell carcinoma, young adult, partial nephrectomy, chromophobe RCC, collecting duct carcinoma
Introduction
Renal cell carcinoma (RCC) accounts for approximately 3% of adult malignancies, and its incidence increases with age, with a peak in the sixth decade of life and a median age of 55 years [1]. Although most RCCs are sporadic and relatively uncommon in young adults, the incidence of RCC in this age group has steadily increased during the past several decades [2]. Several studies have indicated that clinicopathologic characteristics of RCC in young adults (defined as those between the ages of 14 to 45 years) may be different from those occurring in the older age group [3–7]. However, there are some conflicting results regarding their biologic behavior, and their relationship with histologic subtypes is not well documented.
The understanding of different histologic and clinical features of RCCs occurring in young adults may lead to insights in their biologic behavior and provide information that may be important for therapeutic decisions and follow up-strategies. This study describes the histopathologic characteristics and clinical outcomes of RCC in young adults between the ages of 24 to 40 years in a single adult tertiary hospital and compares them with those of RCC occurring in the older age group.
Materials and Methods
We reviewed the medical records, pathologic slides and reports of 838 patients with RCC treated at The Methodist Hospital, Houston, TX, USA, from 1990 to 2005. Patients treated with either partial or radical nephrectomy were included. Of the 838 patients, 44 (5.2%) patients were between the ages of 24 and 40 years at the time of surgery, and the remaining 794 patients were 41 years or older.
The clinicopathologic features including histologic subtype, tumor size, tumor extension, nuclear grade, tumor stages, nodal and distant metastasis, and survival were evaluated. Histologic subtypes were classified based upon the 2004 WHO classification [8]; tumor staging was based on the 2002 AJCC staging system [9]; and nuclear grading was based on the Fuhrman system [10].
For comparison with this group of RCCs in young adult, we selected all of the remaining 794 RCC patients from our database who were older than 40 years as a control group. Of the 794 patients in this group, 492 (62%) were male and 302 (48%) were female. No patient in this group had history of von Hippel-Lindau disease or other hereditary RCC syndromes.
Statistical differences of the evaluated features were tested using chi-square test or independent student t-test for continuous variables. Survival after diagnosis of RCC was calculated by the Kaplan-Meier method. All hypothesis tests were performed at the 0.05 level of significance. Statistics were calculated with SPSS software version 12.0 (SPSS Inc., Chicago, Illinois)
Results
The clinical features of patients in both age groups are summarized in Table 1. Forty-four cases out of 838 (5.2%) consecutive RCCs were in patients at the ages of 40 years or younger with a mean age of 37. The youngest patient was 24 years old and had a conventional clear cell RCC. The gender distribution was equal in young adults with RCCs (22 each for males or females), contrasting with a typical male predominance in the older patients (62% in men and 38% in women, p=0.07). The frequency of partial nephrectomy was higher in young adult group than the older one (30% vs 19%, p=0.08).
Table 1.
Clinical features of RCC occurring in young (≤40 years) and older adults (>40 years)
| ≤40 years | >40 years | P value | |
|---|---|---|---|
| Number of Cases | 44 | 794 | NA* |
| Age (years) | |||
| Mean | 37 | 63 | NA |
| Range | 24–40 | 41–88 | NA |
| Gender | |||
| Male | 22 | 492 | 0.07 |
| Female | 22 | 302 | |
| Nephrectomy | |||
| Partial | 13 | 151 | 0.08 |
| Radical | 31 | 643 | |
| Follow-up (months) | |||
| Mean | 44 | 45.9 | |
| Range | 2–143 | 0.5–269 | |
NA: not applicable
Comparative pathologic characteristics of both groups are summarized in Table 2. Tumors in the young adult group were slightly smaller than those occurring in the older adult group (mean young adult group than the older adult group (82% vs 69% and 18% vs 31%, respectively, 5.3 vs 5.9 cm, p=0.37). More pT1/pT2 tumors and less pT3/pT4 tumors were noted in the p=0.09). Both groups showed a similar incidence of clear cell RCC (71% vs 79%) and papillary RCCs (14% vs 13%). However, the incidences of chromophobe RCC (12% vs 6%, p=0.02) and collecting duct carcinoma (5% vs 0.5%) in young adult group were higher than in the older adult group. There was no significant difference of the Fuhrman nuclear grade between both groups. Nodal and distant metastasis were less common in the young adult group than older adult group (5% vs 8.3%, p=0.57). Higher stages (stage III and IV) were significantly less frequent in younger adult group than old patient group (18% vs 33%, p=0.047). Four patients in the young adult group had von Hippel-Lindau disease and one had Birt-HoggDube syndrome. In contrast, none of the patients in older adult group had hereditary RCC syndromes.
Table 2.
Pathologic features of RCC occurring in young (≤40 years) and older adults (>40 years)
| ≤40 years (n=44) | >40 years (n=794) | P value | |
|---|---|---|---|
| Mean Tumor Size (cm) | 5.3 | 5.9 | NS* |
| T3 or T4 Tumors | 18% | 31% | 0.08 |
| Stage III/IV | 18% | 33% | 0.047 |
| Grade 3 or 4 Tumors | 32% | 31% | NS |
| Clear cell RCC | 71% | 79% | NS |
| Papillary RCC | 14% | 13% | NS |
| Chromophobe RCC | 12% | 6% | 0.03 |
| Collecting Duct RCC | 5% | 0.50% | 0.03 |
| Nodal or Distant Metastasis | 5% | 8.30% | NS |
NS: no significant statistical difference.
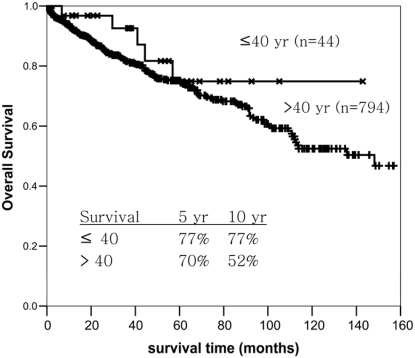
The mean follow-up in the young adult group was 44 months (range 2 to 143 months). The mean follow-up time for the older adult group was 45.9 months (range 0.5 to 269 months). Kaplan-Meier survival analysis suggested that patients in the young adult RCC group had a better survival at 10 years (77% vs 52%) than that of the older adult group, but the difference was not statistically significant (Figure 1.).
Figure 1.
Overall survival of patients with RCC occurring in young (≤40 years) and older adults (>40 years).
Discussion
The incidence of RCC in young adult in our series was 5.4 % (44/838) in an adult tertiary hospital. It was similar to that of previous reports showing that 3.1% to 7.3% of RCCs have occurred in patients younger than 40 years old [2, 5, 7, 11, 12]. The incidence of RCC diagnosed at a younger age has steadily increased during last several decades [2, 13]. This may be partly due to the availability and widespread application of abdominal imaging techniques including ultrasonography.
In our series, the gender distribution of RCC in young adult group was equal, contrasting with a relative male predominance in older adult group. As shown in Table 3, there are some differences among previous reports in the gender distribution of RCC in young adults. Those data suggest that there is no distinct gender predominance in RCC in young adults. However, it is well known that RCC in general is more frequent in males than in females.
Table 3.
Comparison of present study with recently published studies of RCC in young adults
| Goetzl et al [19] | Eggener et al [13] | Sanchez-Ortiz et al [3] | This study | |
|---|---|---|---|---|
| Number of Patients | 34 | 86 | 106 | 44 |
| Age (years) | ||||
| Median | 35 | 37.9 | 37 | 37 |
| Range | 16–40 | 17–45 | 14–40 | 24–40 |
| Gender | ||||
| Male | 14 | 57 (66.3%) | 62 (58%) | 22 (50%) |
| Female | 20 | 29 (33.7%) | 44 (42%) | 22 (50%) |
| Partial nephrectomy | 13 (38.2%) | N/A | 18 (17%) | 13 (29.6%) |
| Mean tumor size (cm) | 3.8 | 6.6 | 6.7 | 5.3 |
| Pathologic T3/T4 | 10 (26.5%) | 10 (11%) | 32 (30.2%) | 9 (18%) |
| Histologic subtype | ||||
| Clear cell | 24 (70.6%) | 69 (75.8%) | 80 (75.5%) | 31 (71%) |
| Papillary | 3 (8.8%) | 9 (9.9%) | 8 (7.5%) | 6 (14%) |
| Chromophobe | 6 (17.7%) | 11 (12.1%) | 5 (4.7%) | 5(12%) |
| Collecting duct | 0 | 1 (1.1%) | 3 (2.9%) | 2(5%) |
| Medullary | 0 | 1 (1.1%) | 1 (0.9%) | 0 |
| Unclassified | 1 (2.9%) | 0 | 5 (4.7%) | 0 |
| Nodal or distant metastasis | 1 (2.9%) | 7 (7.7%) | 25% nodal, 34% distant | 2 (5%) |
| 5-year-survival rate (older patient group) | 73% (80%) | NA | 66% (52%) | 77% (70%) |
NA: not available.
In our series, the majority of RCCs in young adults presented at a lower stages. The mean tumor size was 5.3 cm, which was slightly smaller than that of the older adult group (5.9 cm). The pT stage and staging grouping of RCC in young adult were significantly lower than those of the older adult group (pT3/4, 18% vs 31%, p=0.08; stage III/IV, 18% vs 33%, p=0.047). These observations are similar to previous studies, which showed that RCC in younger adults presented at a lower stages at the time of diagnosis [4, 5, 14]. This may partly explain the higher incidence of partial nephrectomies in the younger adult group than in the older adult group (p=0.08), as shown in Table 2.
The incidence of the different histopathologic subtypes of RCC in young adults compared to that of older patients has not been well-documented and the results are inconsistent in the literature. Some earlier studies showed that the incidence of clear cell RCC in young adults was similar to that of the older age group [17, 18]. However, several recent studies have found that the incidence of clear cell RCC in young adults is significantly lower than that of older adults [5, 6, 14–16]. Results of our study showed that the incidence of clear cell RCC in young adults is similar to that of the older adult group. Likewise, we did not find a statistically significant difference in the incidence of papillary RCC between the younger and older adult groups. However, it was worth noting that chromophobe RCC and collecting duct carcinoma had a significantly higher incidence in young adults compared to the older adult group in our study. This result is similar to the findings published by Eggener et al who reported that the incidence of chromophobe RCC was increased in the young adult group (12.1%) [13].
In our series, 2 of 44 (5%) RCCs in young adults had nodal metastasis, an incidence slightly lower than that of the older adult group (8.3%); this difference, however, had no statistical significance. The incidences of nodal metastasis in patients with RCC in young adults were reported to be from 2.9% to 25% in the literature. Although distant organ metastasis was not found in the younger adult group in our series, an incidence as high as 34% was reported in one study [3]. This discrepancy may be due to more advanced cancers or more aggressive histologic subtypes included in that series. Indeed, 30.2% of the patients in that study presented with local advanced diseases compared to 18% in our series [3].
Five of 44 (11.4%) patients with RCC in the younger adult group in our study had RCC-related hereditary syndromes. This observation strongly indicated the genetic predisposition of renal cancers in those patients who develop renal cancers at a younger age. Family history, genetic testing and consulting are warranted in these patients.
Controversies have persisted on the prognosis of patients with RCC in young adults. A better prognosis for RCC in young adult patients compared to those in older adult group was noted in most studies, but similar or poorer outcomes were also reported in a few other series [11, 18, 19]. In our series, Kaplan-Meier survival analysis showed that patients with RCC in the younger adult group may have a better long-term prognosis than patients in the older adult group (5 years, 77% vs 70%; 10 years, 77% vs 52%), although no statistical significance was detected. It may be partly due to a relatively lower number of younger patients, and/or loss of follow-up. No patients in the younger adult group died after 5 years in the follow up period.
Conclusions
In summary, the results of this study indicate that RCCs in young adults have a higher incidence of chromophobe RCC and collecting duct carcinoma, tend to be of smaller size and lower stage at presentation, and have lower incidences of nodal or distant metastasis; therefore, they are more amenable for partial nephrectomy. These findings may contribute to a favorable long-term survival in young adult RCC patients.
References
- 1.Murrphy WM, Grignon DJ, Perlman EJ. Atlas of Tumor Pathology Fourth Series Fascicle 1. Washington DC: American Registry of Pathology; 2004. Tumors of the Kidney, Bladder, and Related Urinary Structures; pp. 101–240. [Google Scholar]
- 2.Katz DL, Zheng T, Holford TR, Flannery J. Time trends in the incidence of renal carcinoma: analysis of Connecticut Tumor Registry data, 1935–1989. Int J Cancer. 1994;58:57–63. doi: 10.1002/ijc.2910580111. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez-Ortiz RF, Rosser CJ, Madsen LT, Swanson DA, Wood CG. Young age is an independent prognostic factor for survival of sporadic renal cell carcinoma. J Urol. 2004;171:2160–2165. doi: 10.1097/01.ju.0000125487.96469.2e. [DOI] [PubMed] [Google Scholar]
- 4.Yusim I, Mermershtain W, Neulander E, Eidelberg I, Gusakova I, Kaneti J. Influence of age on the prognosis of patients with renal cell carcinoma (RCC) Onkologie. 2002;25:548–550. doi: 10.1159/000068626. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez A, Patard JJ, Lobel B. Renal cell carcinoma in young adults: incidence, disease outcome and review of the literature. Arch Esp Urol. 2002;55:969–975. [PubMed] [Google Scholar]
- 6.Gillett MD, Cheville JC, Karnes RJ, Lohse CM, Kwon ED, Leibovich BC, Zincke H, Blute ML. Comparison of presentation and outcome for patients 18 to 40 and 60 to 70 years old with solid renal masses. J Urol. 2005;173:1893–1896. doi: 10.1097/01.ju.0000158157.57981.80. [DOI] [PubMed] [Google Scholar]
- 7.Schiff M, Jr, Herter G, Lytton B. Renal adenocarcinoma in young adults. Urology. 1985;25:357–359. doi: 10.1016/0090-4295(85)90486-8. [DOI] [PubMed] [Google Scholar]
- 8.Eble JN, Sauter G, Epstein JI, Sesterhenn IA. Health Organization Classification of Tumours Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. France: IARC Press; 2004. pp. 9–87. [Google Scholar]
- 9.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. AJCC Cancer Staging Manual. 6th Ed. New York, NY: Springer; 2002. pp. 355–366. [Google Scholar]
- 10.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–663. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Lieber MM, Tomera FM, Taylor WF, Farrow GM. Renal adenocarcinoma in young adults: survival and variables affecting prognosis. J Urol. 1981;125:164–168. doi: 10.1016/s0022-5347(17)54948-4. [DOI] [PubMed] [Google Scholar]
- 12.Boykin WH, Bright KE, Zeidman EJ, Thompson IM. Renal tumors in young adults. Urology. 1992;40:503–505. doi: 10.1016/0090-4295(92)90402-i. [DOI] [PubMed] [Google Scholar]
- 13.Eggener SE, Rubenstein JN, Smith ND, Nadler RB, Kontak J, Flanigan RC, Waters WB, Picken M, Campbell SC. Renal tumors in young adults. J Urol. 2004;171:106–110. doi: 10.1097/01.ju.0000099028.95679.52. [DOI] [PubMed] [Google Scholar]
- 14.Cao Y, Paner GP, Perry KT, Flanigan RC, Campbell SC, Picken MM. Renal neoplasms in younger adults: analysis of 112 tumors from a single institution according to the new 2004 World Health Organization classification and 2002 American Joint Committee on Cancer Staging System. Arch Pathol Lab Med. 2005;129:487–491. doi: 10.5858/2005-129-487-RNIYAA. [DOI] [PubMed] [Google Scholar]
- 15.Bruder E, Passera O, Harms D, Leuschner I, Ladanyi M, Argani P, Eble JN, Struckmann K, Schraml P, Moch H. Morphologic and molecular characterization of renal cell carcinoma in children and young adults. Am J Surg Pathol. 2004;28:1117–1132. doi: 10.1097/01.pas.0000131558.32412.40. [DOI] [PubMed] [Google Scholar]
- 16.Renshaw AA, Granter SR, Fletcher JA, Kozakewich HP, Corless CL, Perez-Atayde AR. Renal cell carcinomas in children and young adults: increased incidence of papillary architecture and unique subtypes. Am J Surg Pathol. 1999;23:795–802. doi: 10.1097/00000478-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Rainwater LM, Zincke H, Farrow GM, Gonchoroff NJ. Renal cell carcinoma in young and old patients. Comparison of prognostic pathologic variables (cell type, tumor grade and stage, and DNA ploidy pattern) and their impact on disease outcome. Urology. 1991;38:1–5. doi: 10.1016/0090-4295(91)80002-o. [DOI] [PubMed] [Google Scholar]
- 18.Abou El Fettouh HI, Cherullo EE, El-Jack M, Al Maslamani Y, Novick AC. Sporadic renal cell carcinoma in young adults: presentation, treatment, and outcome. Urology. 2002;60:806–810. doi: 10.1016/s0090-4295(02)01884-8. [DOI] [PubMed] [Google Scholar]
- 19.Goetzl MA, Desai M, Mansukhani M, Goluboff ET, Katz AE, Sawczuk IS, Benson MC, Olsson CA, Mckiernan JM. Natural history and clinical outcome of sporadic renal cortical tumors diagnosed in the young adult. Urology. 2004;63:41–45. doi: 10.1016/j.urology.2003.08.020. [DOI] [PubMed] [Google Scholar]