Abstract
Objectives
This study investigates the factors associated with frailty and the association of frailty with mortality in a national sample of adults aged 65–109 in China.
Methods
Using the 2002 wave of the Chinese Longitudinal Healthy Longevity Survey, we construct a frailty index (FI) based on 39 measures available in the data set. We use ordinal logistic regressions to examine the factors associated with the FI and use Weibull hazard regression to examine the association between frailty and 3-year mortality from 2002 to 2005.
Results
Age, sex, ethnicity, urban–rural residence, economic condition, religious involvement, and daily exercise are significantly associated with levels of frailty. Hazard analyses further reveal that the FI is a robust predictor of mortality at advanced ages and that the relationship between frailty and mortality is independent of various covariates.
Discussion
The measurement and analysis of frailty have broad implications for public health initiatives designed to target individuals with the diminished capacity to effectively compensate for external stressors and to prevent further declines associated with aging and mortality. A key to healthy longevity is the prevention, postponement, and potential recovery from physical and cognitive deficits at advanced ages through enhanced medical interventions and treatments.
Keywords: China, Old adults, Frailty index, Mortality
GERIATRICIANS and gerontologists generally agree that frailty is a physiological state of nonspecific vulnerability to stressors resulting from decreased physiological reserves and the deregulation of multiple physiological systems associated with advancing age (Bortz, 2002; Campbell & Buchner, 1997; Cohen, 2000; Fried, Ferrucci, Darer, Williamson, & Anderson, 2004; Kulminski et al., 2006; Kulminski, Ukraintseva et al., 2007; Markle-Reid, 2003; Morley, Perry, & Miller, 2002; Rockwood, Mogilner, & Mitnitski, 2004; Yashin et al., 2007). Conceptually, frailty is more than an association with specific diseases or disabilities, but rather a systemic manifestation of physical and cognitive deficits—including signs, symptoms, illnesses, and impairments—that accumulate over the life course (Fried et al., 2004; Kulminski et al., 2006; Kulminski, Ukraintseva et al., 2007; Markle-Reid, 2003; Morley, Perry, & Miller, 2002; Rockwood, Mogilner, & Mitnitski, 2004; Yashin et al., 2007). Empirically, a variety of methods have been used to operationalize frailty, although the most common applications are perhaps the phenotypic approach and the frailty index (FI) (Bergman et al., 2007; Kulminski et al., 2008; Levers, Estabrooks, & Ross Kerr, 2006; Rockwood, Andrew, & Mitnitski, 2007). The phenotypic approach defines frailty based on several items, such as weight loss, exhaustion, weakness, slowness, or low physical activity, and considers any three conditions as an indication of frailty (see Fried et al., 2001). Alternatively, the FI focuses less on the specific deficits of individuals and focuses instead on the cumulative number of health deficiencies (Kulminski et al., 2006; Mitnitski et al., 2005). Despite the similarities between these two approaches, the choice of measurement is often dictated by the outcome under investigation.
Accordingly, recent research shows that FI is more applicable for predicting mortality than is the phenotypic method (Kulminski et al., 2006, 2008; Rockwood et al., 2007). In practice, most studies compute FI as the proportion of cumulative health deficits to all possible deficits for a given individual (Rockwood, 2005). Thus, FI quantifies the general concept of frailty by incorporating a variety of psychological, physiological, and functional conditions and abilities that represent an individual's balance of health assets to deficits (Fisher, 2005; Rockwood, Fox, Stolee, Robertson, & Beattie, 1994). In other words, FIs characterize basic human functioning by emphasizing the aggregate (or systemic) deterioration in psychophysiological performance (Kulminski et al., 2006) rather than focusing on the substance of the specific conditions that define the index. The validity of the FI has been demonstrated in various populations as a proxy for biological age, as a robust predictor of health change, health care utilization, and death, and as an effective tool among geriatricians and others for studying the determinants of aging and its implications for public health monitoring and intervention (Goggins, Woo, Sham, & Ho, 2005; Janssen, Shepard, Katzmarzyk, & Roubenoff, 2004; Kulminski et al., 2006; Mitnitski, Graham, Mogilner, & Rockwood, 2002; Mitnitski, Mogilner, & Rockwood, 2001; Mitnitski et al., 2005; Puts, Lips, & Deeg, 2005; Song, Mitnitski, MacKnight, & Rockwood, 2004; Yashin et al., 2007). We argue that FIs also provide unique insight into age-related processes such as mortality because frailty is independent of chronological age and incorporates so-called “mild-effect traits” that are typically ignored due to their limited individual effects (Kulminski, Yashin, et al., 2007).
Although there is a growing body of literature on frailty in developed countries, research is scarce in developing countries. Perhaps the most apparent lack of research on frailty is in mainland China (hereafter China), which has the world's largest oldest-old population. Compared with many Western nations, China has a rapidly aging population and a burgeoning health care system that has recently weakened despite the nation's economic growth (Yip & Hsiao, 2008). In the coming decades, China will face dramatic population aging; it remains unknown how China's unique social and cultural makeup will respond to the challenges of caring for frail elders and enhancing healthy longevity.
To our knowledge, the associations among frailty, its risk factors, and mortality have not been examined in China. Also unclear is how mortality risks are associated with levels of frailty across age. This study uses the 2002 and 2005 waves of the Chinese Longitudinal Healthy Longevity Survey (CLHLS) to examine the factors associated with age-related frailty and its association with prospective mortality. Unlike most studies, which lack adequate samples of older adults (for exceptions, see Kulminski et al., 2006; Yashin et al., 2007), we employ longitudinal data from a large national sample of adults aged 65 to 109 that includes more than 4,200 octogenarians, 3,700 nonagenarians, and over 3,000 centenarians. We conclude by discussing the implications of our results and what they mean for China's unique sociopolitical landscape and the promotion of exceptional aging for all populations.
DATA AND MEASUREMENT
Data
This study utilizes the 2002 and 2005 waves of the CLHLS. Initiated in 1998 as a multidisciplinary study, the CLHLS is the first nationwide longitudinal survey on the determinants of healthy longevity with the largest sample of oldest old from a developing country. The survey was conducted in half of the randomly selected counties/cities in 22 out of 31 provinces in China and interviewed all known centenarians in the sampled counties/cities with informed consent. For every centenarian with a predesignated random code, interviews were conducted for nearby adults with a predesignated age and sex who were randomly selected from the following age ranges: 65–79, 80–89, and 90–99. The term “nearby” refers to the same village or street or the same town, county, or city, where applicable. The sampling strategy is designed to include comparable numbers of randomly selected men and women at ages 65–99. Our analyses are restricted to the third and fourth waves of the CLHLS (2002 and 2005) because the first two waves of the CLHLS (1998 and 2000) recruited only adults ages 80 and older. Of the 15,919 participants sampled in 2002, 4,845 were ages 65–79 and 11,074 were ages 80–109, with 3,747 nonagenarians and 3,088 centenarians. Of the total participants (N = 15,919), 8,108 (50.9%) were reinterviewed in the 2005 wave, 5,753 (36.1%) died before 2005, and 2,058 (12.9%) were lost to follow-up.
Extensive data were collected on demographic characteristics, family and household characteristics, lifestyle, diet, psychological characteristics, economic resources, family support, self-reported health, self-reported life satisfaction, lower and upper extremities performance, instrumental activities of daily living (IADL), activities of daily (ADL), cognitive functioning, and chronic diseases suffered and their impacts on daily life. All information was obtained through in-home interviews. The dates of death for deceased respondents were collected from various sources including death certificates, next of kin, and neighborhood committees. All dates were validated, and the dates reported on death certificates were ultimately used when available—otherwise the next of kin's report was used, followed by neighborhood registries. Systematic assessments of the CLHLS regarding the accuracy of age reporting, the randomness of attrition, and the reliability, validity, and consistency of numerous measures show that data quality in the CLHLS is high (Gu, 2008; Gu & Dupre, 2008; Zeng & Gu, 2008).
Frailty Index and Covariates
To capture the cumulative health deficits of an individual (Cohen, 2000; Markle-Reid, 2003; Mitnitski et al., 2005), most studies calculate FI using multiple variables that encompass various dimensions of health and limitations (Kulminski, Ukraintseva et al., 2007; Kulminski et al.2008; Mitnitski et al., 2005). Although studies using this approach often do not include the same number or type of indicators to estimate frailty (Rockwood et al., 2007, p. 742), it is shown that a random selection of variables yields comparable results (Rockwood, Mitnitski, Song, Steen, & Skoog, 2006); however, it is necessary to include the same indicators over time to evaluate individual change (Mitnitski et al., 2005; Rockwood et al., 2007).
Following established research (Kulminski, Yashin, et al., 2007; Mitnitski et al., 2001), we defined FI as an unweighted count of the number of deficits divided by the total number of possible deficits for a given person. We used 39 indicators of various dimensions of self-reported health status, cognitive functioning, disability, auditory and visual ability, depression, heart rhythm, and numerous chronic diseases that were collected in the 2002 CLHLS (see Appendix for all items). The items comprising our FI are similar to those used in studies from Canada (Mitnitski et al., 2005), the United States (Kulminski et al., 2006), and Hong Kong (Goggins et al., 2005). Individual items were dichotomized and coded 1 when a deficit is present. Consistent with prior research (Goggins et al., 2005), we assigned a score of 2 if the respondent had a serious illness that caused him/her to be hospitalized or bedridden two or more times. The FI was then computed by summing all deficits and then dividing by the total number of possible deficits (range = 0–1). To assess the validity and sensitivity of our FI, we also analyzed FIs based on different combinations of the individual indicators. We found that the results are consistent as long as the major domains of health are included in the index (i.e., ADLs, IADLs, chronic diseases, and cognitive functioning). For analytical purposes, the FI is categorized into quartiles to minimize skewness.
Table 1 lists the covariates and their frequency distributions. Based on the well-established literature on the health and mortality of older adults, we include covariates for basic demographic factors (ethnicity and urbanicity), socioeconomic status (SES) (education, primary lifetime occupation, economic independence, and family economic condition), family/social support (marital status, proximity to children, and religious participation), and health practices (regular exercise and smoking in the past five years) (see Ferrucci et al., 2003; Liang, Bennett, Sugisawa, Kobayashi, & Fukaya, 2003; Strawbridge, Shema, Cohen, & Kaplan, 2001; Stuck et al., 1999). All covariates are coded dichotomously and include non-Han ethnicity (vs. Han), urban residence (vs. rural), one or more years of education (vs. no schooling), white-collar occupation (vs. all other occupations), economic independence (coded 1 if the primary financial source for daily expenses, excluding medical costs, comes from the respondent's own work income or pension, as opposed to children, government subsidies, or other sources), good family economic standing (coded 1 if the familial economic status is reported to be “rich” or “very rich” compared with others in their community), currently married (vs. no), high proximity to children (coded 1 if coresiding or in the same neighborhood, town, or village), religious involvement (vs. no), regularly exercise (vs. no), and whether the respondent smoked in the past five years (vs. no). Although associations between these covariates and health are well established in the literature, there is the possibility of endogeneity between some covariates and the FI. For example, frailty can lead to reduced economic independence, exercise, and religious participation; therefore, interpretations of these associations should be cautious.
Table 1.
Relative Frequency Distributions of the 2002 Chinese Longitudinal Healthy Longevity Survey Variables
| Age |
||||
| 65–79 | 80–89 | 90–99 | 100+ | |
| Men | ||||
| Total % (N) | 100% (2,438) | 100% (2,128) | 100% (1,584) | 100% (655) |
| FI (mean) | 0.10 | 0.19 | 0.26 | 0.32 |
| % Non-Han ethnicity | 5.3 | 4.8 | 5.1 | 4.4 |
| % Urban residence | 44.5 | 49.6 | 46.0 | 44.6 |
| % 1+ years education | 72.8 | 68.0 | 59.2 | 45.0 |
| % White collar occupation | 21.0 | 15.8 | 11.1 | 8.7 |
| % Economic independence | 61.6 | 38.5 | 28.0 | 19.5 |
| % Good family economic standing | 18.3 | 19.2 | 18.5 | 18.9 |
| % Currently married | 72.9 | 43.9 | 24.4 | 12.2 |
| % High proximity to children | 81.9 | 78.7 | 79.7 | 79.5 |
| % Religious involvement | 13.7 | 11.7 | 10.8 | 9.8 |
| % Regularly exercise | 44.9 | 43.9 | 35.7 | 30.2 |
| % Smoked in the past 5 years | 51.9 | 43.5 | 34.3 | 26.1 |
| Women | ||||
| Total % (N) | 100% (2,407) | 100% (2,111) | 100% (2,163) | 100% (2,433) |
| FI (mean) | 0.12 | 0.23 | 0.32 | 0.38 |
| % Non-Han ethnicity | 6.0 | 6.4 | 6.3 | 5.2 |
| % Urban residence | 43.8 | 52.1 | 43.1 | 44.9 |
| % 1+ years education | 30.0 | 18.0 | 13.7 | 9.0 |
| % White collar occupation | 5.5 | 3.5 | 2.5 | 1.1 |
| % Economic independence | 33.0 | 12.7 | 5.7 | 2.6 |
| % Good family economic standing | 15.6 | 15.5 | 17.4 | 16.4 |
| % Currently married | 46.7 | 14.5 | 3.2 | 0.7 |
| % High proximity to children | 86.8 | 80.7 | 82.5 | 82.2 |
| % Religious involvement | 30.4 | 26.1 | 18.2 | 12.1 |
| % Regularly exercise | 35.6 | 29.1 | 20.5 | 13.4 |
| % Smoked in the past 5 years | 11.6 | 10.7 | 9.9 | 7.7 |
All measures come from the 2002 interview, and less than 2% of data are missing for all variables. Following recommendations by Landerman, Land, & Pieper (1997), we use modal and mean values to impute missing data for the categorical and continuous variables, respectively. Further details of the variable coding are available upon request.
METHODS
The analyses involve two sets and are stratified by age and sex due to the well-documented sex differences in disability and mortality (e.g., Crimmins, Hayward, & Saito, 1996; Lamb, 1997) and recent evidence of sex differences in frailty (Yashin et al., 2007). First, we estimate ordinal logistic models to examine the factors associated with frailty. A test of the proportional odds assumption—that the odds ratios are proportional across the FI quartiles—indicated a violation for age and exercise variables. Although these two variables had small p values in the sensitivity testing, there was evidence to suggest that the proportional odds assumption was not necessarily invalid (Contractor & Kundu, 1998; Peterson & Harrell, 1990). In practice, the assumption is frequently violated, and alternative models often induce more stringent assumptions (Long & Freese, 2006). To be sure, we used quintile regression methods (Buchinsky, 1998) to compare estimates from various quintile intervals, and we found similar results; we also found consistent results from binary logistic models (see Bender & Grouven, 1998).
In the second set of analyses, we use hazard regression models to examine the relationship between frailty and mortality. A Weibull model is selected for the hazard analyses based on model fit and because the estimates are consistent with other full- and semiparametric hazard functions (e.g., Gompertz and Cox). To account for nonlinearity between the FI and mortality (Yashin et al., 2007), we again use FI quartiles and include two groups of covariate adjustments. Because all analyses are stratified by age group and sex, the first group of purely demographic controls includes single year of age and ethnicity (Model I); the second group further adds the remaining covariates described above (Model II). Survival time in the regression analyses is measured in days from the 2002 interview until death or the time of the 2005 interview. Additional sensitivity analyses using other percentile cut-points of the FI were conducted and the results were essentially identical. Those who were lost to follow-up in the 2005 survey were dropped from the hazard analyses because their survival status (dead or alive) was not known. Although persons lost to follow-up are more likely to be women, urban residents, have higher SES, live alone, and be in poor health (Gu, 2007), the inclusion of these individuals’ estimated survival status using multiple imputation—based on their characteristics—yielded minor differences and suggested that the exclusion of those lost to follow-up introduced little bias in the estimates. Therefore, the analytic sample consists of 13,861 respondents (7,929 women and 5,932 men) with 8,108 survivors and 5,753 decedents.
All analyses are performed using Stata version 10.1 (StataCorp, 2007). The sampling weight in the publicly released CLHLS data set is calculated based on the age–sex–urban/rural residence-specific distribution of the population and does not capture other important compositional variables (e.g., marital status, economic status); therefore, we do not use weights in our multivariate analysis. Previous research shows that results from unweighted regression models produce unbiased coefficients when including variables related to sample selection (i.e., age, sex, and urbanicity) (Winship & Radbill, 1994) and that weighted regressions will unnecessarily increase standard errors (see http://www.sociology.ohio-state.edu/people/ptv/faq/weights.htm). Preliminary analyses confirmed that the overall patterns and conclusions were similar between the weighted and unweighted data.
RESULTS
Table 1 presents sample distributions by age and sex. Several distinguishing features are evident; for instance, frailty increases with age for both women and men, and women are frailer than men at all ages. Men tend to have higher SES than women, and with the exception of family economic condition, SES declines steadily with age. As expected, the proportion of respondents who are currently married is higher for men than women but decreases dramatically with age for both sexes. The proportion of respondents with a high proximity to children is relatively stable across age, and the difference between older men and women is marginal. More women are involved in religious activities than men, although religious activity and the sex difference decrease with age. Not surprisingly, the prevalence of regular exercise and smoking in the past five years is much higher among men than women and declines steadily with age.
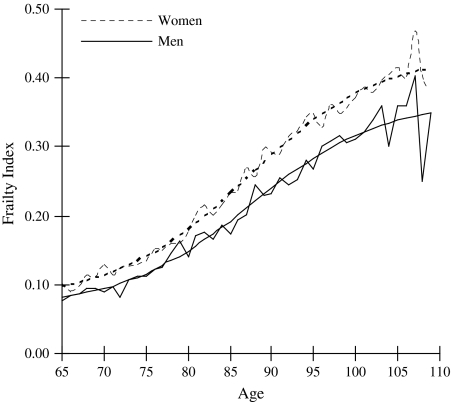
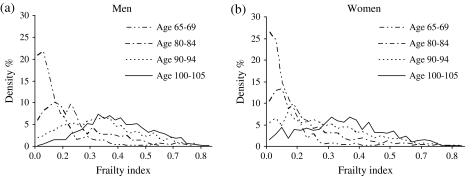
Figure 1 presents the observed and fitted logistic curves of the mean FI by age from 65 to 109 for men and women in 2002. Analyses (not shown) indicated that a logistic distribution describes the data better than exponential, linear, or quadratic specifications. The calculated R2s for the fitted and observed FIs in Figure 1 are 0.95 for men and 0.98 for women, which are comparable to results among older Americans (Kulminski et al., 2006) and from grouped data from Australia, Canada, Sweden, and the United States (Mitnitski et al., 2005, p. 2187). The mean FI value for women is approximately 0.10 at age 65, 0.15 at age 80, and about 0.40 by ages 100 and older. Men have lower overall levels of frailty compared with women, although they exhibit a similar pattern across age. Figure 2 illustrates the density distributions of the FI by sex and shows that frailty levels become less skewed across age. This suggests that the absolute heterogeneity in frailty (i.e., standard deviation of the FI) increases with age, and the relative heterogeneity in frailty (i.e., the inversion of the square root of the shape parameter of the curve) decreases with age. This age pattern is not unexpected given that younger elders on average are healthier than their older counterparts—a pattern similar to that observed in the U.S. and Canada (Kulminski, Ukraintseva et al., 2007; Rockwood, Mogilner, & Mitnitski, 2004).
Figure 1.
Observed and fitted mean frailty levels by age and sex.
Figure 2.
Observed density distributions of frailty by selected ages and sex.
Table 2 presents the results from the ordinal logistic models. Consistent with Table 1, age is strongly related with increased levels of frailty for both men and women. Non-Han ethnic minorities have less frailty than the Han majority, and this finding is more pronounced among women across age. Urban residents have higher frailty levels than rural residents, particularly among women. With few exceptions, the effects of education and occupation on frailty are generally weak; however, economic independence and good family economic conditions reduce frailty for men and even more so for women. The effects of having a spouse and high proximity to children on the FI also are marginal, whereas the association between religious participation and frailty is negative and significant. We also find a robust negative association between regular exercise and frailty across age for both women and men. The relationship between smoking and frailty is not significant.
Table 2.
Odds Ratios of the Factors Associated With Frailty by Age and Sex: Chinese Longitudinal Healthy Longevity Survey, 2002
| Age groups |
||||
| 65–79 | 80–89 | 90–99 | 100+ | |
| Men | ||||
| Age | 1.08 (0.01)*** | 1.12 (0.02)*** | 1.12 (0.02)*** | 1.10 (0.06) |
| Non-Han (ref.: Han) | 0.53 (0.10)** | 0.73 (0.14) | 0.45 (0.10)*** | 0.60 (0.27) |
| Urban (ref.: rural) | 1.04 (0.10) | 1.17 (0.12) | 1.42 (0.17)** | 0.82 (0.15) |
| 1+ years education (ref.: 0) | 0.68 (0.07)*** | 0.92 (0.09) | 0.94 (0.10) | 1.02 (0.18) |
| White collar occupation (ref.: others) | 1.24 (0.15)† | 1.36 (0.18)* | 1.19 (0.24) | 1.71 (0.75) |
| Economic independence (ref.: dependence) | 0.99 (0.10) | 0.80 (0.08)* | 0.86 (0.13) | 1.32 (0.35) |
| Good family economic standing (ref.: no) | 0.89 (0.10) | 0.76 (0.08)** | 0.65 (0.09)** | 0.80 (0.18) |
| Currently married (ref.: no) | 0.90 (0.09) | 1.04 (0.09) | 0.81 (0.10)* | 0.69 (0.19) |
| High proximity to children (ref.: no) | 0.87 (0.10) | 0.94 (0.10) | 0.95 (0.13) | 1.13 (0.27) |
| Religious involvement (ref.: no) | 0.79 (0.10)† | 0.66 (0.09)** | 0.56 (0.09)*** | 0.39 (0.10)*** |
| Regularly exercise (ref.: no) | 0.70 (0.06)*** | 0.37 (0.03)*** | 0.25 (0.03)*** | 0.23 (0.04)*** |
| Smoked in the past 5 years (ref.: no) | 0.87 (0.07) | 1.00 (0.09) | 0.84 (0.09) | 0.69 (0.13)† |
| N | 2,438 | 2,128 | 1,584 | 655 |
| -Log pseudo-likelihood | 2416.8 | 2437.4 | 1603.1 | 563.8 |
| Women | ||||
| Age | 1.10 (0.01)*** | 1.12 (0.02)*** | 1.06 (0.02)*** | 1.08 (0.03)** |
| Non-Han (ref.: Han) | 0.51 (0.10)** | 0.68 (0.13)* | 0.50 (0.08)*** | 0.45 (0.10)*** |
| Urban (ref.: rural) | 1.11 (0.11) | 1.31 (0.12)* | 1.23 (0.11)* | 1.21 (0.11)* |
| 1+ years education (ref.: 0) | 0.98 (0.11) | 1.03 (0.13) | 0.98 (0.13) | 1.22 (0.21) |
| White collar occupation (ref.: others) | 1.11 (0.26) | 1.09 (0.27) | 1.51 (0.39) | 3.41 (1.85)* |
| Economic independence (ref.: dependence) | 0.77 (0.08)* | 0.69 (0.11)* | 0.76 (0.18) | 0.42 (0.13)** |
| Good family economic standing (ref.: no) | 0.52 (0.07)*** | 0.66 (0.08)** | 0.59 (0.07)*** | 0.65 (0.08)*** |
| Currently married (ref.: no) | 0.87 (0.08) | 0.78 (0.10)† | 0.68 (0.16) | 0.35 (0.14)* |
| High proximity to children (ref.: no) | 1.05 (0.16) | 1.07 (0.12) | 0.84 (0.10) | 0.80 (0.09)* |
| Religious involvement (ref.: no) | 0.80 (0.08)* | 0.63 (0.06)*** | 0.54 (0.06)*** | 0.52 (0.07)*** |
| Regularly exercise (ref.: no) | 0.66 (0.07)*** | 0.36 (0.04)*** | 0.28 (0.03)*** | 0.28 (0.03)*** |
| Smoked in the past 5 years (ref.: no) | 1.10 (0.16) | 0.99 (0.14) | 1.03 (0.14) | 0.92 (0.15) |
| N | 2,407 | 2,111 | 2,163 | 2,433 |
| -Log pseudo-likelihood | 1906.7 | 2395.9 | 2331.7 | 2185.0 |
Notes: Odds ratios are from ordinal regression models predicting frailty quartiles. Numbers in the parentheses are standard errors.
†p < 0.1; *p < 0.05; **p < 0.01; ***p < 0.001.
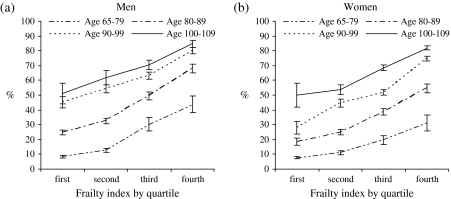
Figure 3 presents the observed proportion of deaths over three years (2002–2005) by frailty, age, and sex in 2002. As expected, the oldest old have a much higher rate of death than their younger counterparts, and elderly men are more likely to die than women at comparable levels of frailty. However, the difference in mortality across frailty quartiles is especially large within the same age groups for men and women. For example, persons aged 65–79 in the third and fourth FI quartiles have the same (or higher) proportions of death than those aged 80–89 in the first or second quartiles. In other words, healthy individuals aged 80–89 appear to exhibit mortality risks as low as persons less than age 80. The age-graded findings between frailty and mortality, though not surprising, are consistent with previous evidence (Rockwood et al., 2006).
Figure 3.
Observed proportion of deaths and 95% confidence intervals over a 3-year period by frailty, sex, and age.
Table 3 presents the relative risks of mortality from the Weibull hazard models. For both men and women, Model I shows that frailty levels in the upper two quartiles are related to higher mortality across all age groups. This pattern is also found in the lower two FI quartiles, with exceptions among young elders aged 65–79 and a few cases for the oldest old. Although the differences in relative hazards across frailty levels vary by sex and age, these differences increase substantially across each quartile. For example, the relative risks for the fourth FI quartile are about five times greater among men and four times greater among women compared with the first quartile. Model II shows that controlling for SES, family/social support, and health practices reduces the relative risks only slightly and suggests only a modest mediating effect of the covariates on the associations between frailty and mortality. Thus, Models I and II both show that the FI is a strong predictor of mortality at late ages and is independent of chronological age and other covariates.
Table 3.
Relative Hazards of Mortality by Frailty, Sex, and Age: Chinese Longitudinal Healthy Longevity Survey, 2002–2005
| Men |
Women |
|||
| Model I | Model II | Model I | Model II | |
| Age 65–79 | ||||
| FI first quartile | 1.00 | 1.00 | 1.00 | 1.00 |
| FI second quartile | 1.18 (0.19) | 1.18 (0.20) | 1.37 (0.22)† | 1.34 (0.22† |
| FI third quartile | 2.05 (0.37)*** | 2.01 (0.36)*** | 2.56 (0.50)*** | 2.34 (0.47)*** |
| FI fourth quartile | 5.17 (1.03)*** | 4.56 (0.96)*** | 4.16 (1.10)*** | 3.84 (1.01)*** |
| Age 80–89 | ||||
| FI first quartile | 1.00 | 1.00 | 1.00 | 1.00 |
| FI second quartile | 1.40 (0.19)* | 1.39 (0.19)* | 1.38 (0.18)* | 1.38 (0.18)* |
| FI third quartile | 1.99 (0.26)*** | 1.94 (0.26)*** | 2.33 (0.30)*** | 2.20 (0.29)*** |
| FI fourth quartile | 4.14 (0.54)*** | 3.99 (0.53)*** | 3.72 (0.49)*** | 3.52 (0.48)*** |
| Age 90–99 | ||||
| FI first quartile | 1.00 | 1.00 | 1.00 | 1.00 |
| FI second quartile | 1.41 (0.23)* | 1.35 (0.22)† | 1.84 (0.29)*** | 1.84 (0.29)*** |
| FI third quartile | 1.69 (0.25)*** | 1.55 (0.23)** | 2.37 (0.36)*** | 2.38 (0.36)*** |
| FI fourth quartile | 2.75 (0.39)*** | 2.41 (0.36)*** | 4.47 (0.67)*** | 4.44 (0.69)*** |
| Age 100+ | ||||
| FI first quartile | 1.00 | 1.00 | 1.00 | 1.00 |
| FI second quartile | 2.45 (0.96)* | 2.12 (0.84)+ | 1.50 (0.30)* | 1.44 (0.29)† |
| FI third quartile | 2.66 (0.99)** | 2.28 (0.85)* | 2.11 (.40)*** | 2.01 (0.38)*** |
| FI fourth quartile | 4.62 (1.68)*** | 3.86 (1.41)*** | 3.17 (.60)*** | 2.94 (.56)*** |
Notes: Relative hazards are from Weibull hazard models. Model I controls for age and ethnicity. Model II further controls for urban–rural residence, SES, family/social connection and support, and health practices. Numbers in the parentheses are standard errors.
†p < 0.1; *p < 0.05; **p < 0.01; ***p < 0.001.
DISCUSSION
Frailty is an important concept for both scholars and health practitioners studying morbidity and mortality at advanced ages. However, there are few studies of the distributional patterns of frailty among the oldest old, particularly in developing countries. The lack of research is primarily due to limited data representing older populations and comprehensive measures of psychophysiological functioning. Drawing from the world's largest aging population, we use data from a nationwide longitudinal survey in China to examine a 39-item index of frailty and its associations with age- and sex-specific mortality. Consistent with studies from other countries (Kulminski et al., 2006; Mitnitski et al., 2005), our data show that the nonlinear relationship between frailty and chronological age also is observed among older adults in China. Overall, we find that these nonlinear changes in frailty across age mark the inherent value of a valid and systemic indicator of the cumulative aspects of aging (Kulminski et al., 2006).
Results from the density distributions of the FI demonstrate significantly increasing levels of frailty across age that generally plateau at later ages among men and women. Although intuitive, these findings also shed light on the heterogeneity of health in older populations who face an accumulation of functional deficits and organ decline with advancing age (Kulminski et al., 2006). For example, our findings suggest that chronological age does not necessarily lead to an accumulation of deficits and that low FI values are not exclusive to younger elders. Rather, we find evidence that concurs with existing research showing the diversity of individuals within and across age groups (Kulminski, Yashin, et al., 2007), suggesting that frailty and bodily damage are not inevitable features of aging and that healthy longevity is an achievable goal for many older adults (Evert, Lawler, Bogan, & Perls, 2003). In fact, a recent study by Yashin et al. (2007) showed that U.S. life expectancy at age 65 would increase by nearly 10 years for men and women respectively if every individual were given the same fixed level (i.e., average population level) of frailty and spent the rest of their lives with that fixed frailty level.
We show significant differences in frailty according to ethnicity, urban–rural residence, SES, participation in religious activities, and regular exercise. Ethnic minorities exhibit relatively lower FI levels compared with the Han majority. We speculate that this finding is due to higher mortality among minorities before reaching older ages (i.e., selection), leaving more robust minorities among those surviving to advanced ages. According to Chinese census data (National Bureau of Statistics of China [NBSC], 2003), the mortality rate of minorities is higher than Han adults before age 80 but reverses thereafter.
The finding that urban elders have more frailty than rural elders also can be attributed to mortality selection, as well as different lifestyles, support networks, and other environmental factors (see Dupre, Liu, & Gu, 2008; Gu & Zeng, 2004). Indeed, research suggests that older adults in developing countries—who predominantly live in rural areas—have increased levels of ADL functioning compared with older adults in developed nations (Lamb, 1999). We also identify other psychosocial factors that are associated with frailty that are presumably not due to mortality selection. Congruent with the previous findings related to physical functioning and self-reported health, we find that the association between economic condition and frailty is stronger than the associations between education and occupation and frailty (Nordstrom, Diez Roux, Jackson, & Gardin, 2004; von dem Knesebeck, Lüschen, Cockerham, & Siegrist, 2000). This suggests that older adults with greater economic resources can purchase treatments and other medical services that benefit health and delay or alleviate symptoms of frailty. We also find that religious participation appears to protect against frailty perhaps because of reduced psychological distress and improved spiritual coping, social support, or a more generalized and positive belief system (see Maselko & Kubzansky, 2006). Regular exercise likely maintains good physical mobility and organ functioning that also help postpone or relieve health deficits (Bortz, 2002). However, these speculations, as well as issues of endogeneity and causal order, should be evaluated with longer follow-up data. For example, it is possible that increased frailty reduces individuals’ economic independence and keeps them from exercising or participating in religious activities, whereas low levels of frailty help maintain and encourage economic independence, exercise, and religious participation. Nonetheless, we believe that endogeneity is likely minor given the context of our measures. For example, economic independence as measured in this study (i.e., the primary financial source for daily expenses comes from the respondent's own work income or pension) is less likely to be influenced by health conditions than medical costs or family economic conditions. Furthermore, many religious Chinese worship at home (Fowler, 2005, p. 246) and thus religious involvement is not necessarily determined by health conditions.
Proximate mechanisms notwithstanding, our findings demonstrate that psychosocial factors play some significant role in the severity of frailty and should remain an important consideration in medical practice and public health interventions. Moreover, this knowledge will become especially useful for initiating and evaluating public health efforts to promote healthy longevity and ameliorate frailty in China, a country which is facing dramatic population aging in a context of fewer family caregivers, greater mobility, rising health care expenditures, and lack of a national social security system (Yip & Hsiao, 2008).
Our analyses corroborate the finding that frailty is highly correlated with the risk of death. Not surprisingly, frail older adults are more likely to die, and the patterns are similar for men and women across age. These findings are consistent with the general argument that frailty represents accumulated deficits that deplete redundant systems and make individuals more vulnerable to disability and death (Gavrilov & Gavrilova, 2001; Mitnitski et al., 2002; Rockwood et al., 2004). Overall, the FI is a strong predictor of mortality; the relationship between frailty and mortality is independent of chronological age and differs little when adjusting for various covariates. This suggests that the association between adaptive regulation (e.g., exercise, lifestyle change) and mortality at advanced ages is largely reduced at higher frailty levels and eventually reaches levels that are clinically inconsequential. To some extent, these results also reinforce the validity and utility of our frailty measure based on the assessments of accumulated health deficits among the Chinese elderly. Our findings also replicate the age- and sex-graded associations between frailty and mortality found in previous research (Mitnitski et al., 2005; Rockwood et al., 2006; Yashin et al., 2007).
An interesting finding is that although women exhibit higher FI levels than men at all ages, women have lower age-specific mortality than men for every given FI level. In other words, women appear to accumulate more deficits over their life course than men of the same age, but men are also dying at a higher rate. We suspect that the more pronounced levels of frailty among women might be due to a combination of higher incidence, longer durations (i.e., low recovery), and lower severity of illnesses (Hardy, Allore, Guo, & Gill, 2008). This seemingly contradictory finding is similar to findings in previous studies from other populations (Kulminski, Ukraintseva et al., 2007; Mitnitski et al., 2002; Puts et al., 2005). The aging literature has long recognized a gender paradox in health and mortality, and it is argued that gender differences in genetic and acquired risks, immune system responses, hormones, disease patterns and prevention, and health-reporting behaviors may explain the lower mortality of women (Bath, 2003; Deeg & Kriegsman, 2003; Idler, 2003; Oksuzyan, Juel, Vaupel, Christensen, 2008; Spiers, Jagger, Clarke, & Arthur, 2003). For example, some studies show that men exhibit higher baseline levels of muscle mass and neuroendocrine and hormonal measurements (testosterone) that may delay the onset and/or accumulation of frailty (Walston & Fried, 1999). Similarly, men are shown to possess greater positive affect than women (Nolen-Hoeksema, Larson, & Grayson, 1999), which also is shown to significantly lower the risks of frailty (Ostir, Ottenbacher, & Markides, 2004). Moreover, research shows that men are more likely to die suddenly, whereas women are more likely to experience a gradual progression of physical degeneration (Puts et al., 2005). Taken together, our findings provide further evidence of the fundamental processes associated with sex differences in frailty and its association with mortality (Kulminski, Yashin et al., 2007).
Our study identifies several avenues for future research and highlights some important implications. Limited samples of the very old are a critical obstacle for researchers studying healthy longevity. To date, there are few surveys with samples of centenarians that exceed 1,000 individuals (Koenig, 2001). Our study overcomes this limitation by utilizing a large-scale sample of oldest-old adults to investigate patterns of frailty across age for men and women. We believe that our analysis provides some of the strongest evidence of the distribution of health deficits among exceptionally old adults. However, we also acknowledge that more research on this topic from other population-based studies is clearly warranted. Undoubtedly, as our understanding of physiological pathways increases, future studies will continue to refine measures of frailty and move beyond the limitations of the current research that assumes equal item weight and excludes important indicators of immune function and biomarkers.
In addition to determining risks of mortality, frailty also reflects individual risks of functional loss and vulnerability (Campbell & Buchner, 1997) and is a useful indicator of the general health burden within the elderly population (Goggins et al., 2005). Furthermore, significant differences in frailty across a variety of sociodemographic characteristics indicate that numerous factors play a role in determining cumulative deficits at old ages. Therefore, the measurement and analysis of frailty have broad implications for public health initiatives designed to target individuals with diminished capacity to effectively compensate for external stressors and prevent further declines associated with aging and mortality (Goggins et al., 2005; Yashin et al., 2007). It is plausible that individuals may live to age 100 and older and remain relatively healthy by adhering to healthy lifestyles and avoiding identifiable risks (Zeng, Crimmins, Carriere, & Robine, 2006).
The remarkable differences in frailty among octogenarians, nonagenarians, and centenarians suggest that the oldest old—especially centenarians—are not a homogeneous population with comparable health reserves. Considering such heterogeneity in frailty and the ongoing dynamics of health change in late ages (Gill, Robison, & Tinetti, 1997; Gu & Zeng, 2004), one key to healthy longevity is the prevention or delay of frailty and the facilitation of recovery from frailty via medical interventions or treatments.
FUNDING
The data used in this study are from the 2002 and 2005 waves of the Chinese Longitudinal Healthy Longevity Survey, funded by the National Institute on Aging (NIA) (R01 AG023627, Principle Investigator: Z.Y.) awarded to Duke University, the China Natural Science Foundation, China Social Science Foundation, the United Nation Population Funds (UNFPA), and Hong Kong Research Grant Council. D.G.’s work was supported by NIA grant R01 AG023627 when he was at Duke. J.S. was supported by an NIA T32 Traineeship in the Social, Economic, and Medical Demography of Aging. Work of Z.Y. and Y.L. was supported by NIA grant R01 AG023627. Conflict of interest: None.
Acknowledgments
D.G. initiated and designed the study, drafted the paper, prepared and analysed the data, and revised the paper. M.E.D. was involved in the analysis design, revised the manuscript, and assisted in interpreting the results. J.S. and H.Z. drafted parts of the discussion, revised the paper, and assisted in interpreting the results. Y.L. assisted in preparing the data. Z.Y. raised funding for the data collection and revised the paper.
Appendix
List of Items Included in the Frailty Index
| No. | Items |
| 1 | IADLs: Unable to visit neighbors by oneself |
| 2 | IADLs: Unable to shop by oneself if necessary |
| 3 | IADLs: Unable to cook meals by oneself if necessary |
| 4 | IADLs: Unable to wash clothing by oneself |
| 5 | IADLs: Unable to walk continuously for 1 kilometer |
| 6 | IADLs: Unable to lift a weight of 5 kg (such as a heavy bag of groceries) |
| 7 | IADLs: Unable to continuously crouch and stand up three times |
| 8 | IADLs: Unable to use public transportation |
| 9 | Functional limitations: Unable to put hand behind neck |
| 10 | Functional limitations: Unable to put hand behind lower back |
| 11 | Functional limitations: Unable to raise arm upright |
| 12 | Functional limitations: Unable to stand up from sitting in a chair |
| 13 | Functional limitations: Unable to pick up a book from the floor |
| 14 | ADLs: Needs assistance bathing |
| 15 | ADLs: Needs assistance dressing |
| 16 | ADLs: Needs assistance toileting |
| 17 | ADLs: Needs assistance in indoor transferring |
| 18 | ADLs: Needs assistance eating |
| 19 | ADLs: Incontinence |
| 20 | Cognitively impaired (based on the Mini Mental State Examination) |
| 21 | Poor self-rated health |
| 22 | Health worsened in the past year |
| 23 | Poor interviewer-rated health |
| 24 | Hearing loss |
| 25 | Vision loss |
| 26 | Abnormal heart rhythm |
| 27 | Symptom of psychological distress (based on loneliness, usefulness, and fearfulness) |
| 28 | Number of serious illnesses in the past 2 yearsa |
| 29 | Suffering from hypertension |
| 30 | Suffering from diabetes |
| 31 | Suffering from tuberculosis |
| 32 | Suffering from heart disease |
| 33 | Suffering from stroke/cerebrovascular disease |
| 34 | Suffering from bronchitis, emphysema, asthma, or pneumonia |
| 35 | Suffering from cancer |
| 36 | Suffering from arthritis |
| 37 | Suffering from bedsores |
| 38 | Suffering from gastric or duodenal ulcers |
| 39 | Suffering from Parkinson's disease |
Notes: IADLs = instrumental activities of daily living; ADLs = activities of daily.
Persons reporting two or more illnesses are assigned a value of 2.
References
- Bath PA. Differences between older men and women in the self-rated health–mortality relationship. The Gerontologist. 2003;43:387–395. doi: 10.1093/geront/43.3.387. [DOI] [PubMed] [Google Scholar]
- Bender R, Grouven U. Using binary logistic regression models for ordinal data with non-proportional odds. Journal of Clinical Epidemiology. 1998;51:809–816. doi: 10.1016/s0895-4356(98)00066-3. [DOI] [PubMed] [Google Scholar]
- Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, Wolfson C. Frailty: An emerging research and clinical paradigm–issues and controversies. Journal of Gerontology: Medical Sciences. 2007;62A(7):731–737. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortz WM. A conceptual framework of frailty: A review. Journals of Gerontology: Medical Sciences. 2002;57A:M283–M288. doi: 10.1093/gerona/57.5.m283. [DOI] [PubMed] [Google Scholar]
- Buchinsky M. Recent advances in quantile regression models: A practical guideline for empirical research. Journal of Human Resources. 1998;33(1):88–126. [Google Scholar]
- Campbell AJ, Buchner DM. Unstable disability and the fluctuations of frailty. Age and Ageing. 1997;26:315–318. doi: 10.1093/ageing/26.4.315. [DOI] [PubMed] [Google Scholar]
- Cohen HJ. In search of the underlying mechanisms of frailty. Journals of Gerontology: Medical Sciences. 2000;55A:M706–M708. doi: 10.1093/gerona/55.12.m706. [DOI] [PubMed] [Google Scholar]
- Contractor FJ, Kundu SK. Model choice in a world of alliances: Analyzing organizational forms in the international hotel sector. Journal of International Business Studies. 1998;29(2):325–358. [Google Scholar]
- Crimmins EM, Hayward MD, Saito Y. Differentials in active life expectancy in the older population of the United States. Journals of Gerontology: Social Sciences. 1996;51B:S111–S120. doi: 10.1093/geronb/51b.3.s111. [DOI] [PubMed] [Google Scholar]
- Deeg DJH, Kriegsman DMW. Concepts of self-rated health: Specifying the gender difference in mortality risk. The Gerontologist. 2003;43:376–386. doi: 10.1093/geront/43.3.376. [DOI] [PubMed] [Google Scholar]
- Dupre ME, Liu G, Gu D. Predictors of longevity: Evidence from the oldest old in China. American Journal of Public Health. 2008;98(7):1203–1208. doi: 10.2105/AJPH.2007.113886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evert J, Lawler E, Bogan H, Perls T. Morbidity profiles of centenarians: Survivors, delayers, and escapers. Journals of Gerontology: Medical Sciences. 2003;58(A):M232–M237. doi: 10.1093/gerona/58.3.m232. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Turchi A, Fumagalli S, Di Bari M, Silvestrini G, Zacchei S, et al. Sex-related differences in the length of disability prior to death in older persons. Aging Clinical and Experimental Research. 2003;15:310–314. doi: 10.1007/BF03324515. [DOI] [PubMed] [Google Scholar]
- Fisher AL. Just what defines frailty? Journal of the American Geriatrics Society. 2005;53:2229–2230. doi: 10.1111/j.1532-5415.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- Fowler J. An introduction to the philosophy and religion of Taoism: Pathways to immortality. Portland, OR: Sussex Academic Press; 2005. [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: Evidence for a phenotype. Journal of Gerontology: Medical Sciences. 2001;56A:M146–M157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Fried LP, Ferrucci L, Darer J, Williamson J, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. Journals of Gerontology: Medical Sciences. 2004;59:M255–M263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- Gavrilov LA, Gavrilova NS. The reliability theory of aging and longevity. Journal of Theoretical Biology. 2001;213:527–545. doi: 10.1006/jtbi.2001.2430. [DOI] [PubMed] [Google Scholar]
- Gill TM, Robison JT, Tinetti ME. Predictors of recovery in activities of daily living among disabled older persons living in the community. Journal of General Internal Medicine. 1997;12:757–762. doi: 10.1046/j.1525-1497.1997.07161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goggins WB, Woo J, Sham A, Ho SC. Frailty index as a measure of biological age in a Chinese population. Journals of Gerontology: Medical Sciences. 2005;60A:M1046–M1051. doi: 10.1093/gerona/60.8.1046. [DOI] [PubMed] [Google Scholar]
- Gu D. General data quality assessment for the 2005 CLHLS wave. The CLHLS Technical Report 2007-No.1. Durham, NC: Duke University; 2007. [Google Scholar]
- Gu D. General data assessment of the Chinese Longitudinal Healthy Longevity Survey in 2002. In: Zeng Y, Poston D, Vlosky DA, Gu D, editors. Healthy longevity in China: Demographic, socioeconomic, and psychological dimensions (pp. 39–59) Dordrecht, The Netherlands: Springer; 2008. [Google Scholar]
- Gu D, Dupre ME. Assessment of reliability of mortality and morbidity in the 1998–2002 CLHLS waves. In: Zeng Y, Poston D, Vlosky DA, Gu D, editors. Healthy longevity in China: Demographic, socioeconomic, and psychological dimensions (pp. 99–115) Dordrecht, The Netherlands: Springer; 2008. [Google Scholar]
- Gu D, Zeng Y. Sociodemographic effects on the onset and recovery of ADL disability among Chinese oldest-old. Demographic Research. 2004;11:1–42. [Google Scholar]
- Hardy SE, Allore HG, Guo Z, Gill TM. Explaining the effect of gender on functional transitions in older persons. Gerontology. 2008;54:79–86. doi: 10.1159/000115004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idler EL. Discussion: Gender differences in self-rated health, in mortality, and in the relationship between the two. The Gerontologist, 2003;43:372–375. [Google Scholar]
- Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. Journal of the American Geriatrics Society. 2004;52:80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- Koenig R. An island of ‘genetic parks’. Science. 2001;291:2075–2076. doi: 10.1126/science.291.5511.2075. [DOI] [PubMed] [Google Scholar]
- Kulminski A, Ukraintseva S, Akushevich I, Arbeev K, Yashin A. Cumulative index of health deficiencies as a characteristic of long life. Journal of the American Geriatrics Society. 2007;55:935–940. doi: 10.1111/j.1532-5415.2007.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulminski A, Ukraintseva S, Kulminskaya IV, Arbeev K, Land K, Yashin A. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: Lessons from the Cardiovascular Health Study. Journal of the American Geriatrics Society, 2008;56:898–903. doi: 10.1111/j.1532-5415.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulminski A, Yashin A, Arbeev K, Akushevich I, Ukraintseva S, Land K, Manton K, et al. Accumulation of heath disorders as an indicator of aging-associated processes in the elderly: Results from analyses of the National Long-term Care Survey. Mechanisms of Ageing and Development, 2007;128:250–258. doi: 10.1016/j.mad.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulminski A, Yashin A, Ukraintseva S, Akushevich I, Arbeev K, Land K, Manton K. Accumulation of heath disorders as a systemic measure of aging: Findings from the NLTCS data. Mechanisms of Ageing and Development, 2006;127:840–848. doi: 10.1016/j.mad.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb VL. Gender differences in correlates of disablement among the elderly in Egypt. Social Science and Medicine. 1997;45(1):127–136. doi: 10.1016/s0277-9536(96)00326-7. [DOI] [PubMed] [Google Scholar]
- Lamb VL. Active life expectancy of the elderly in selected Asian countries. Nihon University; 1999. [Google Scholar]
- Landerman LR, Land KC, Pieper CF. An empirical evaluation of the predictive mean matching method for imputing missing values. Sociological Methods and Research. 1997;26(1):3–33. [Google Scholar]
- Levers MJ, Estabrooks CA, Ross Kerr JC. Factors contributing to frailty: Literature review. Journal of Advanced Nursing. 2006;56:282–291. doi: 10.1111/j.1365-2648.2006.04021.x. [DOI] [PubMed] [Google Scholar]
- Liang J, Bennett JM, Sugisawa H, Kobayashi E, Fukaya T. Gender differences in old age mortality: Roles of health behavior and baseline health status. Journal of Clinical Epidemiology. 2003;56:572–582. doi: 10.1016/s0895-4356(03)00060-x. [DOI] [PubMed] [Google Scholar]
- Long JS, Freese J. Regression models for categorical dependent variables using Stata. 2nd ed. College Station, TX: Stata Press; 2006. [Google Scholar]
- Markle-Reid M. Conceptualizations of frailty in relation to older adults. Journal of Advanced Nursing. 2003;44:58–68. doi: 10.1046/j.1365-2648.2003.02767.x. [DOI] [PubMed] [Google Scholar]
- Maselko J, Kubzansky LD. Gender differences in religious practices, spiritual experiences and health: Results from the US General Social Survey. Social Science & Medicine. 2006;62:2848–2860. doi: 10.1016/j.socscimed.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatrics. 2002;2:1. doi: 10.1186/1471-2318-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Scientific World. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitnitski AB, Song X, Skoog I, Broe GA, Cox JL, Grunfeld E, Rockwood K. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. Journal of the American Geriatrics Society. 2005;35:2184–2189. doi: 10.1111/j.1532-5415.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- Morley JE, Perry HM, III, Miller DK. Something about frailty. Journals of Gerontology: Medical Sciences. 2002;57A:M698–M704. doi: 10.1093/gerona/57.11.m698. [DOI] [PubMed] [Google Scholar]
- National Bureau of Statistics of China. The electric data for the 2000 census (CD) Beijing: National Bureau of Statistics of China; 2003. [Google Scholar]
- Nolen-Hoeksema S, Larson J, Grayson C. Explaining the gender difference in depressive symptoms. Journal of Personality and Social Psychology. 1999;77:1061–1072. doi: 10.1037//0022-3514.77.5.1061. [DOI] [PubMed] [Google Scholar]
- Nordstrom CK, Diez Roux AV, Jackson SA, Gardin JM. The association of personal and neighborhood socioeconomic indicators with subclinical cardiovascular disease in an elderly cohort: The Cardiovascular Health Study. Social Science & Medicine. 2004;59:2139–2147. doi: 10.1016/j.socscimed.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Oksuzyan A, Juel K, Vaupel JW, Christensen K. Men: Good health and high mortality—Sex differences in health and aging. Aging Clinical and Experimental Research. 2008;20(2):91–102. doi: 10.1007/bf03324754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostir GV, Ottenbacher KJ, Markides KS. Onset of frailty in older adults and the protective role of positive affect. Psychology and Aging. 2004;19:402–408. doi: 10.1037/0882-7974.19.3.402. [DOI] [PubMed] [Google Scholar]
- Peterson B, Harrell F. Partial proportional odds models for ordinal response variables. Applied Statistics. 1990;39:205–217. [Google Scholar]
- Puts MT, Lips P, Deeg DJ. Sex differences in the risk of frailty for mortality independent of disability and chronic diseases. Journal of the American Geriatrics Society. 2005;53:40–47. doi: 10.1111/j.1532-5415.2005.53008.x. [DOI] [PubMed] [Google Scholar]
- Rockwood K. Frailty and its definition: A worthy challenge. Journal of the American Geriatrics Society. 2005;53:1069–1070. doi: 10.1111/j.1532-5415.2005.53312.x. [DOI] [PubMed] [Google Scholar]
- Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. Journals of Gerontology: Medical Sciences. 2007;62A(7):M738–M743. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- Rockwood K, Fox RA, Stolee P, Robertson D, Beattie BL. Frailty in elderly people: An evolving concept. Canadian Medical Association Journal. 1994;150:489–495. [PMC free article] [PubMed] [Google Scholar]
- Rockwood K, Mitnitski AB, Song X, Steen B, Skoog I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. Journal of the American Geriatrics Society. 2006;54:975–979. doi: 10.1111/j.1532-5415.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- Rockwood K, Mogilner A, Mitnitski AB. Changes with age in the distribution of a frailty index. Mechanisms of Ageing and Development. 2004;125:517–519. doi: 10.1016/j.mad.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Song X, Mitnitski A, MacKnight C, Rockwood K. Assessment of individual risk of death using self-report data: An artificial neural network compared with a frailty index. Journal of the American Geriatrics Society. 2004;52:1180–1184. doi: 10.1111/j.1532-5415.2004.52319.x. [DOI] [PubMed] [Google Scholar]
- Spiers N, Jagger C, Clarke M, Arthur A. Are gender differences in the relationship between self-rated health and mortality enduring? Results from three birth cohorts in Melton Mowbray, United Kingdom. The Gerontologist. 2003;43:406–411. doi: 10.1093/geront/43.3.406. [DOI] [PubMed] [Google Scholar]
- StataCorp. STATA 10.1. College Station, TX: StataCorp, Inc: 2007. [Google Scholar]
- Strawbridge WJ, Shema SJ, Cohen RD, Kaplan GA. Religious attendance increases survival by improving and maintaining good health behaviors, mental health, and social relationships. Annals of Behavioral Medicine. 2001;23:68–74. doi: 10.1207/s15324796abm2301_10. [DOI] [PubMed] [Google Scholar]
- Stuck AE, Walthert JM, Nikolaus T, Büla CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community-living elderly people: A systematic literature review. Social Science & Medicine. 1999;48:445–489. doi: 10.1016/s0277-9536(98)00370-0. [DOI] [PubMed] [Google Scholar]
- von dem Knesebeck O, Lüschen G, Cockerham WC, Siegrist J. Socioeconomic status and health among the aged in the United States and Germany: A comparative cross-sectional study. Social Science & Medicine. 2000;57:1643–1652. doi: 10.1016/s0277-9536(03)00020-0. [DOI] [PubMed] [Google Scholar]
- Walston J, Fried LP. Frailty and the older man. The Medical Clinics of North America. 1999;3:1173–1194. doi: 10.1016/s0025-7125(05)70157-7. [DOI] [PubMed] [Google Scholar]
- Winship C, Radbill L. Sampling weights and regression analysis. Sociological Methods & Research. 1994;23:230–257. [Google Scholar]
- Yashin AI, Arbeev KG, Kulminski A, Akushevich I, Akushevich L, Ukraintseva SV. Cumulative index of elderly disorders and its dynamic contribution to mortality and longevity. Rejuvenation Research. 2007;10(1):75–86. doi: 10.1089/rej.2006.0500. [DOI] [PubMed] [Google Scholar]
- Yip W, Hsiao WC. The Chinese health system at a crossroads: A new infusion of government funds has sparked debate in China about how best to transform money into effective services. Health Affairs. 2008;27(2):460–468. doi: 10.1377/hlthaff.27.2.460. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Crimmins EM, Carriere Y, Robine JM, editors. Longer life and healthy aging. Dordrecht, The Netherlands: Springer; 2006. [Google Scholar]
- Zeng Y, Gu D. Reliability of age reporting among the Chinese oldest-old in the CLHLS data sets. In: Zeng Y, Poston D, Vlosky DA, Gu D, editors. Healthy longevity in China: Demographic, socioeconomic, and psychological dimensions (pp. 61–78) Dordrecht, The Netherlands: Springer; 2008. [Google Scholar]