Abstract
AIM: To investigate the efficacy and toxicity of systemic chemotherapy in a retrospective study of patients with hepatocellular carcinoma (HCC) occurring in normal or fibrotic liver without cirrhosis.
METHODS: Twenty-four patients with metastatic or locally advanced HCC in a normal or a fibrotic liver were given systemic chemotherapy (epirubicin, cisplatin and 5-fluorouracil or epirubicin, cisplatin and capecitabine regimens). Tumor response, time to progression, survival, and toxicity were evaluated.
RESULTS: There were 7 women and 17 men, mean age 54 ± 10 years; 18 patients had a normal liver and 6 had a fibrotic liver (F1/F2 on biopsy). Mean tumor size was 14 cm, 5 patients had portal vein thrombosis and 7 had metastasis. Patients received a median of 4 chemotherapy sessions. Overall tolerance was good. There were 5 partial responses (objective response rate = 22%), and tumor control rate was 52%. Second line surgical resection was possible in two patients. Median survival was 11 mo, and 1- and 2-year overall survival rates were 50% ± 10% and 32% ± 11%, respectively.
CONCLUSION: In patients with HCC in a non-cirrhotic liver, chemotherapy was well tolerated and associated with an objective response rate of 22%, including two patients who underwent secondary surgical resection.
Keywords: Antineoplastic protocols, Chemotherapy, Hepatocellular carcinoma
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most frequent primary liver cancer, the fifth most common malignancy worldwide, and the third most common cause of cancer deaths[1]. Unfortunately, most patients are seen when the disease has reached a stage beyond curative treatment (surgery or percutaneous ablation), leaving palliative care as the only alternative. Based on the Barcelona-Clinic Liver Cancer (BCLC) staging classification[1] and treatment schedule, chemoembolization is the best option for intermediate stage patients, while for advanced stage patients, no standard treatment was established until 2007. Fortunately, after the positive results of the SHARP trial[2], a new treatment, sorafenib, was approved for advanced stage patients because of major improvements in overall survival and time to progression in comparison with placebo. In contrast to studies of most other malignancies, the efficacy of systemic chemotherapy has never been demonstrated in HCC. Despite the lack of demonstrated efficacy, doxorubicin is accepted by some physicians as a possible treatment for advanced HCC. In some recent trials using doxorubicin as a control arm, the investigational arms, a combination of platinum, doxorubicin, 5-fluorouracil and interferon in one trial[3] or nolatrexed[4], a novel thymidylate synthase inhibitor in another trial, did not demonstrate any advantage or were associated with worse overall survival than doxorubicin alone. In one trial, this could have been partly due to toxicity, particularly because of hepatitis B virus reactivation[5]. Most likely, systemic chemotherapy lacks efficacy because of the frequently observed multidrug tumor resistance[6–8] (P-glycoprotein overexpression, p53 gene mutations), although greater drug toxicity related to the underlying cirrhosis might certainly have had an effect. In a search for clues to resolve this question, we retrospectively analyzed our records of cirrhosis-free HCC patients who received a standard chemotherapy regimen {ECF [epirubicin, cisplatin (CDDP) and 5-fluorouracil (5FU)][9] or ECC (epirubicin, CDDP and capecitabine)[10]}. Our goal was to determine whether chemotherapy was more effective in these patients than in HCC patients with cirrhosis.
MATERIALS AND METHODS
Between July 1999 and June 2006, we delivered standard chemotherapy regimens to 30 patients with HCC occurring in a non-cirrhotic liver. There was no indication for curative surgery or palliative treatment in these patients who had good performance status (ECOG PS 0 or 1), preserved liver function, normal blood cell counts (neutrophils > 15 000/mm3, platelets > 100 × 109/L), normal renal function (serum creatinine < 110 μmol/L), and a measurable tumor target. Biopsy specimens, from the tumor and unaffected liver tissue, provided the diagnosis of HCC in non-cirrhotic liver in all 30 patients. None had a fibrolamellar cancer. The diagnosis of a normal liver was, however, less than certain in one patient since tumor cells had invaded most of the “normal liver” biopsy specimen. Five of the 30 patients had received a chemotherapy regimen other than ECF or ECC: capecitabine plus oxaliplatin (n = 3), and gemcitabine plus oxaliplatin (n = 2). Excluding these 6 patients, we thus retrospectively analyzed the records of 24 patients who received an ECF or ECC regimen for HCC occurring in a non-cirrhotic liver (normal liver or F1-F2 fibrosis); the ECF regimen was given from 1999 to 2002 and then we used the ECC regimen, which was much more convenient because it could be given orally.
The ECF treatment schedule was: epirubicin 60 mg/m2 on day 1, CDDP 50 mg/m2 on day 1, and 5FU 200 mg/m2 administered in a continuous infusion from day 1 to day 21; in the ECC regimen, 5FU was replaced by capecitabine 1000 mg/m2 twice a day from day 1 to day 14 followed by a 7-d off period. Courses were repeated every 21 d.
Tumor response was assessed with computed tomography performed before treatment onset and then every 9 wk (three chemotherapy courses). The tumor response (World Health Organization criteria) was considered as complete in the case of total disappearance of all tumors with normalization of α-fetoprotein (AFP) level, as partial for a decrease of tumor size less than 50%, and as stable disease with absence of progression and a decrease in tumor size less than 50%. Progression was an increase in tumor size greater than 25%. An objective response was defined by achievement of a complete or partial response. Patient tolerance was assessed using NCI-CTC AE version 2.0. In some patients with an excellent general status, second line therapy was proposed in the event of disease progression. Patient survival could be precisely evaluated for all patients and was calculated using the Kaplan-Meier method. The cause of death was recorded when available.
RESULTS
The ECF protocol was given to 10 patients, and the ECC protocol to 14. There was no difference in disease status between these two groups. The population was composed of 17 men and 7 women, mean age 54.3 ± 10.7 years (range: 23-77 years). The liver tumors were revealed in all patients by tumor-related complications (pain, fever, constitutional syndrome). The tumor developed in a non-cirrhotic liver in 18 patients and in a fibrotic liver in 6 (classified as F1 or F2). The etiological investigation revealed that 6 patients drank more than 4 units of alcohol per day, one had serological markers of hepatitis C, 2 had steatosis and were overweight, and one had genetic hemochromatosis treated by phlebotomy and was without iron liver overload on liver biopsy; no contributory factor was recognized in 14 patients. Among the 6 patients with minor fibrosis, 3 were drinkers, one had hepatitis C virus infection, one had genetic hemochromatosis and one had steatosis.
There was a unique tumor in 14 patients, 2-4 lesions in 6 and multiple or diffuse tumors in 4. Mean tumor size was huge: 14 ± 7 cm (range: 6-29 cm). Portal vein thrombosis was found in 5 patients and metastases in 7 (bones, lungs, lymph nodes). In the BCLC staging, all these patients could be classified as having an “advanced HCC”, and in cases of unique tumor, tumor size was > 75% of the liver volume and surgery or transarterial chemoembolization could not be considered. Following Child-Pugh classification, and despite the absence of cirrhosis, all these patients were considered as Child class A and, using the CLIP system, 8 patients had 4 points, 10 had 3 points, 5 had 2 points and only one had 1 point (but this patient was metastatic).
None of these patients had previously received treatment for HCC. The patients received from one to 12 courses of the ECF/ECC regimen (median = 4). As the best response, 5 patients had a partial response (objective response rate 22%; 95% CI: 6-38%), 8 patients had stable disease (30%) and 11 had tumor progression; the disease control rate was then 52%. The median time to progression was 6 mo. At the cut-off date of March 15, 2007, 18 patients had died due to progression of their tumor and 6 patients were still alive; median survival time was 11 mo (range 3-90 mo). Actuarial overall survival rates (mean ± SD) at 6 mo, 1 year and 2 years were 71% ± 9%, 50% ± 10% and 32% ± 11%, respectively (Figure 1). The 5 patients with an objective response achieved prolonged survival: 3 died 13, 27 and 48 mo after treatment onset and 2 were still alive at the cut-off date. Surgical resection was undertaken in one patient 10 mo after treatment onset as a result of an objective response. This patient was alive at 90 mo with progression (the same chemotherapy was successfully resumed; she was subsequently treated with sorafenib). The other prolonged survivor had a 22-cm tumor; she underwent surgical resection when a minor response was observed after 9 courses of ECC. She was still alive and disease-free 36 mo after surgery. Initially, these 2 patients had a unique tumor involving more than 75% of the liver without portal vein thrombosis or extrahepatic metastases; both had an AFP level above 500 ng/mL; they were considered as CLIP 3.
Figure 1.
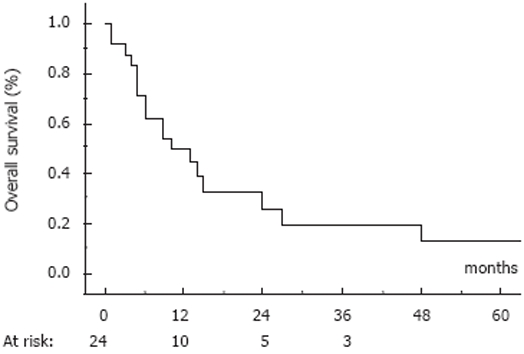
Overall survival in the population of 24 HCC patients with non-cirrhotic liver treated by an ECC/ECF regimen.
Toxicity was graded using NCI-CTC criteria version 2.0. The main toxic effects are summarized in Table 1. Overall tolerance was good. Signs of grade 3-4 toxicity developed in 7 patients (29%), neutropenia in 4 (no cases of febrile neutropenia), gastrointestinal disorders (nausea, vomiting) in 2; one patient died suddenly after the first cycle and one presented with mild thoracic pain assumed to be related to 5FU. Among the other patients, 8 developed alopecia and renal function worsened in one, leading to discontinuation of the treatment.
Table 1.
Adverse effects in this series of 24 patients with HCC in non-cirrhotic liver treated by chemotherapy (ECC regimen)
| Adverse effets | Grade 1-2 | Grade 3-4 (%) |
| Alopecia | 8 | - |
| Neutropenia | 4 | 4 (17) |
| Mucositis | 4 | 0 |
| Diarrhea | 2 | 0 |
| Renal failure | 1 | 0 |
| Asthenia | 2 | 0 |
| Hand-foot syndrome | 5 | 0 |
| Nausea, vomiting | 12 | 2 (4) |
| Anemia | 2 | 0 |
| Coronary spasm | 0 | 1 |
| Percentages not given in this column |
One patient died suddenly during the first course of chemotherapy.
DISCUSSION
Despite the lack of proof of efficacy, doxorubicin is commonly used as a first line therapy for HCC. Recent randomized studies with doxorubicin[3,4] have confirmed the minimal efficacy of this drug in HCC. Results obtained in phase II studies with different regimens using new cytotoxic drugs have not been very impressive. Thus, systemic chemotherapy cannot be considered as the standard of care for HCC patients. This situation could be related to a combination of poor efficacy and increased toxicity. Poor efficacy might result from intrinsic resistance caused by overexpression of multidrug resistance genes observed in most tumors. Obviously the underlying liver cirrhosis increases the risk of severe adverse events as many chemotherapeutic drugs are metabolized or eliminated via the liver. Moreover severe complications are certainly more likely if a cytotoxicity-related side effect occurs on a cirrhotic liver. Certain causes of the underlying cirrhosis, e.g. hepatitis B virus infection[5], may be reactivated after chemotherapy-induced immunodepression, producing an additive toxic effect.
In this particular retrospective series of HCC developed in normal or fibrotic livers, we attempted to estimate the raw efficacy and toxicity of systemic chemotherapy, regardless of liver status. The objective response rate in these patients given the ECF/ECC regimen was 22%, with a disease control rate (objective response plus stable disease) of 52%. The median time to progression was 6 mo, that is to say, quite similar to that observed using sorafenib[2], In addition, despite the fact that most tumors were huge, the reduction in tumor size was sufficient to allow surgical resection in 2 patients having only one huge tumor. Toxicity was mild and most side effects were manageable; one patient died suddenly between two courses. These two regimens (ECF and ECC) are very similar: capecitabine is the oral form of 5FU and, in a randomized 2 × 2 study conducted in advanced esophagogastric cancers[11], these two regimens were demonstrated to be effective. Such results with objective response rates approaching 20% have been reported in some other phase II studies[12–14], including patients with or without cirrhosis: a similar ECC regimen gave, in a Korean series of 29 patients, an objective response rate of 24%[15]. Similar findings have been reported with the PIAF regimen (combination of CDDP, doxorubicin, 5FU and interferon)[16], where, in a series of 50 patients, 26% had a partial response including 9 who underwent surgical resection, and in 4, there were no viable cancer cells on resected specimens. Unfortunately, this did not translate into any difference in overall survival versus doxorubicin alone in a randomized phase III trial despite the fact that less than half of the patients had underlying cirrhosis (but 17% of the patients had Child B class cirrhosis). The response rate of 22% we have observed in this series is a little bit higher than that observed in a retrospective series of 21 patients we have previously published[17] (16 having underlying cirrhosis). Comparing the results of these two series, we suggest that efficacy was better in the non-cirrhotic group. In our first series of 21 HCC patients we observed 3 responders (objective response rate of 14.5%) including one non-cirrhotic patient (among cirrhotic patients, 2/16 were responders); median survival was 10 mo and surgery was possible in one patient. Toxicity appeared to be more frequent in the whole population, as we described 18 cases of grade 3-4 toxicities versus 7 cases in the current 24 non-cirrhotic patients. This might suggest that an ECF/ECC chemotherapy regimen (particularly the ECC regimen which is more convenient, especially in cirrhotic patients) could be delivered to non-cirrhotic patients with a hope of the possibility of secondary resection.
As a result of the recent SHARP trial, which demon-strated the efficacy of sorafenib in advanced-stage HCC, sorafenib is now the new standard of care in that setting. Combinations of sorafenib plus doxorubicin[18] seemed to be well tolerated and have yielded promising results in a randomized phase II trial. A combination of sorafenib with the ECC regimen deserves a phase II trial particularly in patients with HCC in a non-cirrhotic liver.
COMMENTS
Background
Systemic chemotherapy is not usually considered as a standard treatment in hepatocellular carcinoma (HCC) developed in cirrhotic patients.
Research frontiers
New targeted agents, like sorafenib, are efficient in advanced HCC and could be associated with systemic chemotherapy.
Innovations and breakthroughs
When systemic chemotherapy is given to patients who develop HCC without liver cirrhosis, the tolerance and efficacy are better and can allow curative surgery in some cases.
Applications
In selected cases of HCC without liver cirrhosis, systemic chemotherapy can be safely given with promising results.
Peer review
The peer reviewer emphasized the differences between these two regimens; one (ECF) requires a continuous infusion of chemotherapeutic drug (5FU) while the other is given orally and is easier to use.
Footnotes
Peer reviewer: Saùl Villa-Trevio, MD, PhD, Departamento de Biologia Celular, Centro de Investigacion y de Estudios Avanzados del IPN (Cinvestav), Ave, IPN n° 2508, Col. San Pedro, Zacatenco, CP 07360, México, DF, Mexico
S- Editor Tian L L- Editor Cant MR E- Editor Ma WH
References
- 1.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 3.Yeo W, Mok TS, Zee B, Leung TW, Lai PB, Lau WY, Koh J, Mo FK, Yu SC, Chan AT, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532–1538. doi: 10.1093/jnci/dji315. [DOI] [PubMed] [Google Scholar]
- 4.Gish RG, Porta C, Lazar L, Ruff P, Feld R, Croitoru A, Feun L, Jeziorski K, Leighton J, Gallo J, et al. Phase III randomized controlled trial comparing the survival of patients with unresectable hepatocellular carcinoma treated with nolatrexed or doxorubicin. J Clin Oncol. 2007;25:3069–3075. doi: 10.1200/JCO.2006.08.4046. [DOI] [PubMed] [Google Scholar]
- 5.Yeo W, Lam KC, Zee B, Chan PS, Mo FK, Ho WM, Wong WL, Leung TW, Chan AT, Ma B, et al. Hepatitis B reactivation in patients with hepatocellular carcinoma undergoing systemic chemotherapy. Ann Oncol. 2004;15:1661–1666. doi: 10.1093/annonc/mdh430. [DOI] [PubMed] [Google Scholar]
- 6.Fardel O, Loyer P, Lecureur V, Glaise D, Guillouzo A. Constitutive expression of functional P-glycoprotein in rat hepatoma cells. Eur J Biochem. 1994;219:521–528. doi: 10.1111/j.1432-1033.1994.tb19967.x. [DOI] [PubMed] [Google Scholar]
- 7.Chan KT, Lung ML. Mutant p53 expression enhances drug resistance in a hepatocellular carcinoma cell line. Cancer Chemother Pharmacol. 2004;53:519–526. doi: 10.1007/s00280-004-0767-4. [DOI] [PubMed] [Google Scholar]
- 8.Endo T, Yoshikawa M, Ebara M, Kato K, Sunaga M, Fukuda H, Hayasaka A, Kondo F, Sugiura N, Saisho H. Immunohistochemical metallothionein expression in hepatocellular carcinoma: relation to tumor progression and chemoresistance to platinum agents. J Gastroenterol. 2004;39:1196–1201. doi: 10.1007/s00535-004-1471-1. [DOI] [PubMed] [Google Scholar]
- 9.Bamias A, Hill ME, Cunningham D, Norman AR, Ahmed FY, Webb A, Watson M, Hill AS, Nicolson MC, O’Brien ME, et al. Epirubicin, cisplatin, and protracted venous infusion of 5-fluorouracil for esophagogastric adenocarcinoma: response, toxicity, quality of life, and survival. Cancer. 1996;77:1978–1985. doi: 10.1002/(SICI)1097-0142(19960515)77:10<1978::AID-CNCR3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 10.Cho EK, Lee WK, Im SA, Lee SN, Park SH, Bang SM, Park DK, Park YH, Shin DB, Lee JH. A phase II study of epirubicin, cisplatin and capecitabine combination chemotherapy in patients with metastatic or advanced gastric cancer. Oncology. 2005;68:333–340. doi: 10.1159/000086972. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 12.Louafi S, Boige V, Ducreux M, Bonyhay L, Mansourbakht T, de Baere T, Asnacios A, Hannoun L, Poynard T, Taieb J. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC): results of a phase II study. Cancer. 2007;109:1384–1390. doi: 10.1002/cncr.22532. [DOI] [PubMed] [Google Scholar]
- 13.Boige V, Raoul JL, Pignon JP, Bouche O, Blanc JF, Dahan L, Jouve JL, Dupouy N, Ducreux M. Multicentre phase II trial of capecitabine plus oxaliplatin (XELOX) in patients with advanced hepatocellular carcinoma: FFCD 03-03 trial. Br J Cancer. 2007;97:862–867. doi: 10.1038/sj.bjc.6603956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pastorelli D, Cartei G, Zustovich F, Marchese F, Artioli G, Zovato S, Binato S, Ceravolo R, Cingarlini S, Salmaso F, et al. Gemcitabine and liposomal doxorubicin in biliary and hepatic carcinoma (HCC) chemotherapy: preliminary results and review of the literature. Ann Oncol. 2006;17 Suppl 5:v153–v157. doi: 10.1093/annonc/mdj972. [DOI] [PubMed] [Google Scholar]
- 15.Park SH, Lee Y, Han SH, Kwon SY, Kwon OS, Kim SS, Kim JH, Park YH, Lee JN, Bang SM, et al. Systemic chemotherapy with doxorubicin, cisplatin and capecitabine for metastatic hepatocellular carcinoma. BMC Cancer. 2006;6:3. doi: 10.1186/1471-2407-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung TW, Patt YZ, Lau WY, Ho SK, Yu SC, Chan AT, Mok TS, Yeo W, Liew CT, Leung NW, et al. Complete pathological remission is possible with systemic combination chemotherapy for inoperable hepatocellular carcinoma. Clin Cancer Res. 1999;5:1676–1681. [PubMed] [Google Scholar]
- 17.Boucher E, Corbinais S, Brissot P, Boudjema K, Raoul JL. Treatment of hepatocellular carcinoma (HCC) with systemic chemotherapy combining epirubicin, cisplatinum and infusional 5-fluorouracil (ECF regimen) Cancer Chemother Pharmacol. 2002;50:305–308. doi: 10.1007/s00280-002-0503-x. [DOI] [PubMed] [Google Scholar]
- 18.Abou-Alfa GK, Johnson P, Knox J, Lacava J, Leung T, Mori A. Preliminary results from a phase II, randomized, double-blind study of sorafenib plus doxorubicin versus placebo plus doxorubicin in patients with advanced hepatocellular carcinoma.; 23-27 September 2007; ECCO 14, Abst 3500, Barcelona. [Google Scholar]


