Abstract
AIM: To investigate the impact of arachidonic acid (AA) and docosahexaenoic acid (DHA) and their combination on colon cancer cell growth.
METHODS: The LS-174T colon cancer cell line was used to study the role of the prostaglandin precursor AA and the omega-3 polyunsaturated fatty acid DHA on cell growth. Cell viability was assessed in XTT assays. For analysis of cell cycle and cell death, flow cytometry and DAPI staining were applied. Expression of cyclooxygenase-2 (COX-2), p21 and bcl-2 in cells incubated with AA or DHA was examined by real-time RT-PCR. Prostaglandin E2 (PGE2) generation in the presence of AA and DHA was measured using a PGE2-ELISA.
RESULTS: AA increased cell growth, whereas DHA reduced viability of LS 174T cells in a time- and dose-dependent manner. Furthermore, DHA down- regulated mRNA of bcl-2 and up-regulated p21. Interestingly, DHA was able to suppress AA-induced cell proliferation and significantly lowered AA-derived PGE2 formation. DHA also down-regulated COX-2 expression. In addition to the effect on PGE2 formation, DHA directly reduced PGE2-induced cell proliferation in a dose-dependent manner.
CONCLUSION: These results suggest that DHA can inhibit the pro-proliferative effect of abundant AA or PGE2.
Keywords: Colorectal carcinoma, Colon cancer, Omega-3, Omega-6, Polyunsaturated fatty acids, Arachidonic acid, Docosahexaenoic acid, Prostaglandin E2, Cyclooxygenase-2, Apoptosis
INTRODUCTION
Colon cancer is one of the leading causes of death in Western countries[1]. Increased levels of cyclooxygenase-2 (COX-2) were detected in 50% of colorectal adenomas and in up to 85% of colorectal cancers[2–4]. Prostaglandin E2 (PGE2) is generated from the omega-6 polyunsaturated fatty acid (n-6 PUFA) arachidonic acid (AA) via action of the COX-1 and -2. Several studies have established PGE2 as an important factor for proliferation of colon cancer cells in vitro[5–7]. Regular Western diets are highly abundant in n-6 PUFAs[8]. Since AA is the precursor of PGE2, this may contribute to the high prevalence of colon cancer in the Western world[9]. In contrast, diets rich in omega-3 polyunsaturated fatty acids (n-3 PUFAs) such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), which are mainly found in fish oil, might reduce the risk of colon cancer development, and an inverse association of consumption of fish and colon cancer has been observed epidemiologically[10–12]. EPA was found to inhibit colon crypt cell proliferation in vivo[13]. A recent study has demonstrated an inverse association of the n-6/n-3 ratio with colon adenoma formation[14]. In an animal model system with increased amounts of endogenously synthesized n-3 PUFA (the fat-1 mouse), two studies have shown a protective effect against colon tumor development[15,16].
In vitro studies in Caco-2 colon cancer cells with the n-3 PUFA DHA have demonstrated growth-inhibitory effects by induction of apoptosis[17–20]. Other results have shown that PGE2 formation and vascular endothelial growth factor expression are suppressed, while apoptosis is induced by DHA and EPA in the HT-29 colon cancer cell line[21]. In contrast, several other studies in colon cancer cell lines have demonstrated that AA as well as DHA or EPA suppresses growth[22,23]. Other studies have found a pro-apoptotic effect of DHA and AA in HT-29 colon cancer cells[24,25] and in the A549 lung cancer cell line[26]. These in vitro observations have led to uncertainty regarding a differential role of n-3 and n-6 PUFA for growth of tumor cells. Furthermore, they do not address the effect of a changed n-3/n-6 ratio on cell proliferation.
In the present study, we used the LS-174T colon cancer cell line, for which a potent PGE2-triggered activation of proliferation has been demonstrated previously[6,27–29], to test the effect of DHA co-incubation with AA or PGE2 on cell growth, thereby mimicking a change in the ratio of n-3/n-6 fatty acids. We show that DHA suppressed cell growth, while AA increased proliferation, and that DHA co-incubation suppressed AA- and PGE2-induced cell growth.
MATERIALS AND METHODS
Cell culture
Cells were cultured in a saturated atmosphere of 5% CO2 and 95% air at 37°C. LS-174T cells were grown in Dulbecco’s modified Eagle’s medium (Gibco, Carlsbad, CA, USA) without phenol red, which contained 10% heat-inactivated fetal bovine serum (FBS; HyClone, Logan, UT, USA), 2 mmol/L glutamine and 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco, Carlsbad, CA, USA). Medium that contained PUFAs (NuchekPrep, Elysian, MN, USA) or PGE2 (Caymanchem, Ann Arbor, MI, USA) was prepared with 2% FBS and 1 mg/mL fatty-acid-free bovine serum albumin (BSA). All chemicals used were bought from Sigma (St. Louis, MO, USA) except where stated otherwise.
Cell proliferation assay
Cell viability was determined by XTT (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) assay according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA). Briefly, 2500 LS-174T cells per well were seeded into a 96-well plate. After 24 h, medium was removed and replaced by medium that contained the appropriate concentration of respective PUFAs. In order to avoid unspecific toxic effects of free long-chain fatty acids, the maximum total fatty acid concentration used in the long-term incubation cell viability experiments was 100 μmol/L. Cell proliferation was assessed photometrically in dual wave length measurements at different time points after addition of activated XTT assay solution.
Flow cytometry assays
For cell cycle analysis 5 × 105 cells were plated in 10-cm dishes. After 24 h, medium was removed and replaced by 10 mL medium that contained PUFAs. Cells were harvested for flow cytometry after 72 h. For detection of the sub-G1 DNA fraction, cells were stained with 0.1 mg/mL propidium iodide, which contained 0.5 mg/mL RNase and 0.1% NP40 detergent. Afterwards, cells were analyzed on a FACSCalibur (Becton Dickinson, San Jose, CA, USA) flow cytometer.
4',6'-diamidino-2-phenylindole (DAPI) staining
Cells were fixed using 2% paraformaldehyde and permeabilized with 0.1% Triton X 100. Cells were stained with DAPI solution and assessed for cell morphology and apoptotic bodies.
Semi-quantitative real-time RT-PCR
Total RNA was isolated from LS-174T cells using the RNAeasy mini kit (Qiagen, Valencia, CA, USA), following the manufacturer’s instructions. Reverse transcription of mRNA was performed using random primers (Promega, Madison, WI, USA) to generate cDNA. Real-time RT-PCR was carried out using Absolute QPCR SYBR Green Mix (ABgene, Rockford, IL, USA) in an ABI Prism 7000 Sequence detection system (Applied Biosystems, Foster City, CA, USA), following the manufacturer’s protocol. Primers were designed with Primer Select 5.00 Software (DNASTAR Inc., Madison, WI, USA). Primer sequences were: COX-2for CGCTCAGCCATACAGCAAATCCTT, COX-2rev AATCCTGTCCGGGTACAATCGCA; p21for GTGGGGGCATCATCAAAAACTT, p21rev ACCCCACCTTCCCCCTGCCTTCAC; bcl-2for CATGCCAAGGGGGAAACACCAGAA, bcl-2rev CACGGCCCCCAGAGAAAGAAGAGG; GAPDHfor GGTGAAGGTCGGAGTCAAC, GAPDHrev CCATGGGTGGAATCATATTG.
PGE2-ELISA
For PGE2 analysis, cells were treated with PUFAs, as described above. The PGE2-ELISA was then performed according to the manufacturer’s protocol (R&D Systems, Minneapolis, MN, USA).
Statistical analysis
All results are presented as mean ± SE, except where stated otherwise. Student’s t test was used to evaluate the difference between two groups. RT-PCR was analyzed by using the 2ΔCt method. Statistical significance was accepted at the level of P < 0.05, and Prism 4 for Windows Software (GraphPad, La Jolla, CA, USA) was used for calculations.
RESULTS
Opposing effects of AA and n-3 PUFA on colon cancer cell proliferation
LS-174T cells were treated with different concentrations of fatty acids bound to BSA. In XTT assays, DHA significantly diminished cell growth and viability in a time- and dose-dependent manner. At the same time, AA at identical concentrations was found to increase proliferation (Figure 1A and B).
Figure 1.
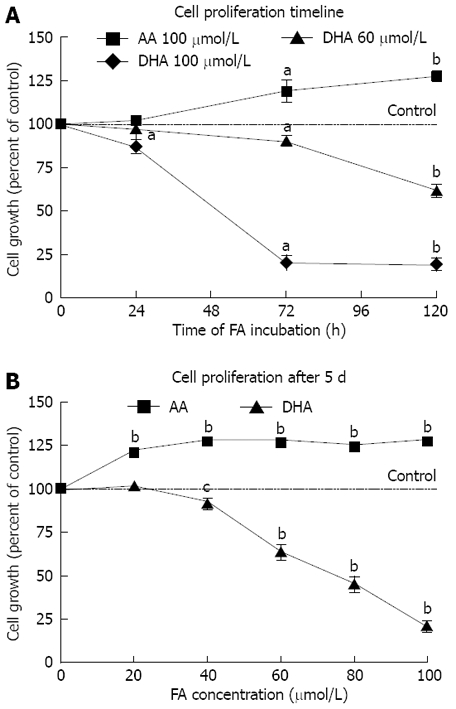
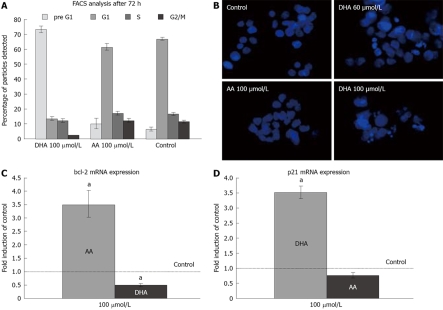
Effects of fatty acids on cellular viability. A: Growth of LS-174T cells during incubation with different concentrations of fatty acids in the medium with 1 mg/mL BSA. Data points represent at least five independent experiments. aP < 0.05 versus control, bP < 0.001 versus control. B: Concentration-dependent effect of DHA and AA on growth of LS-174T colon cancer cells after 5 d incubation. Data points represent at least 13 independent experiments. cP < 0.01 versus control, bP < 0.001 versus control.
In order to further explain the suppression of cell proliferation by DHA, we studied apoptosis by flow cytometry and DAPI staining. DHA increased the pre-G1 fraction (an indicator of apoptosis) in LS-174T cells, while the same concentrations of AA did not significantly alter the pre-G1 fraction compared to untreated cells (Figure 2A). DHA-induced apoptosis was further confirmed by DAPI staining (Figure 2B). We then investigated differential cellular gene expression by means of semi-quantitative RT-PCR. DHA significantly down-regulated anti-apoptotic bcl-2 mRNA, while in contrast, AA up-regulated bcl-2 (Figure 2C). In addition, DHA up-regulated the expression of p21, while AA did not alter the amount of p21 mRNA (Figure 2D).
Figure 2.
Effect of DHA and AA on cell cycle and apoptosis. A: Cell cycle analysis by flow cytometry. Induction of apoptosis is indicated by an increased pre-G1 fraction. Results represent five independent experiments. B: DAPI staining of LS-174T cells incubated with different concentrations of AA and DHA showed a clear increase of apoptotic bodies in cells incubated with DHA. C: RT-PCR demonstrated induction of bcl-2 expression by AA, while DHA suppressed bcl-2 mRNA expression. aP < 0.01 versus control. n = 3 for each group. D: DHA induced transcription of p21, while AA did not alter p21 mRNA formation. aP < 0.01 versus control. n = 3 for each group.
Inhibition of AA- and PGE2-induced cell growth by DHA
LS-174T cells were treated with combinations of fatty acids bound to BSA and proliferation was assessed in XTT assays. DHA co-incubation was able to reverse the proliferation associated with AA (Figure 3A). The anti-proliferative effect of DHA in the context of high AA concentrations was associated with a significant reduction of PGE2 formation from AA (Figure 3B). This may have been caused by decreased presence of COX-2, as DHA incubation significantly reduced COX-2 gene expression in a dose-dependent manner (Figure 3C). However, the effect of DHA-associated growth inhibition was in part independent from a pure blocking effect on PGE2-formation, as co-incubation experiments with DHA and PGE2 revealed that DHA also suppressed the PGE2-induced induction of proliferation (Figure 3D).
Figure 3.
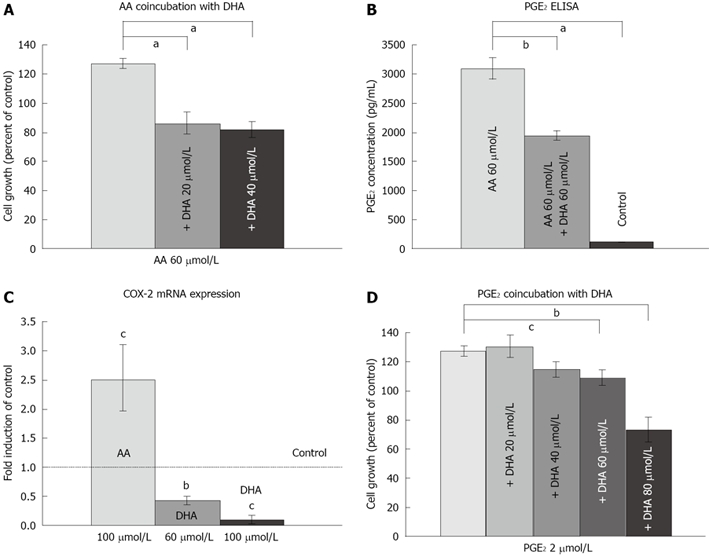
DHA suppresses AA- and PGE2-induced proliferation. A: DHA inhibited AA-induced proliferation, aP < 0.001. Results represent six independent experiments. B: PGE2 formation was induced by AA treatment and was suppressed by concomitant DHA incubation, aP < 0.001, bP < 0.01. Results represent the mean of PGE2 measurements from three independent samples. C: COX-2 transcription was activated by AA, but suppressed by DHA. cP < 0.05 versus control, bP < 0.01 versus control. Bars represent at least three experiments. D: DHA suppressed PGE2-induced cell proliferation, cP < 0.05, bP < 0.01. Results represent five independent experiments.
DISCUSSION
As far as we are aware, our results demonstrate for the first time that DHA can directly suppress AA-induced colon cancer cell growth. Our data confirm that AA is a potent proliferative agent for colon cancer cells that are responsive to PGE2. In contrast, the n-3 PUFA DHA down-regulates anti-apoptotic factors, induces apoptosis and decreases PGE2 formation. This leads to a potent suppression of tumor cell growth by DHA. Our results confirm several previous studies that have shown that DHA is a potent suppressor of colon cancer cell proliferation and stimulates apoptosis[17–19,21,30]. However, previous studies have failed to address the differential effects of n-3 PUFA and n-6 PUFA, and of their combination, on cancer cell growth. In light of several studies that have demonstrated cancer cell growth inhibition by n-3 PUFA or n-6 PUFA[22,23,26], our data help to clarify the issue of differential effects of n-6 and n-3 PUFAs on apoptosis and cell growth.
The most important result presented here is that the proliferation-stimulating effect of high concentrations of AA as a precursor of proliferation-stimulating lipid mediators (most notably PGE2) can be suppressed by increasing the DHA content of the cells. Indeed, DHA can also directly inhibit PGE2-induced proliferation in this context. Although our results are limited to an in vitro setup, they add evidence to the argument that the ratio of n-6/n-3 PUFA (and in particular the ratio of AA versus DHA) may be a critical determinant of proliferation and tumor growth in the colon, and that DHA supplementation can suppress tumor cell growth, even in the presence of high AA- and PGE2-levels.
COMMENTS
Background
Colon cancer is one of the leading causes of death in Western countries. It is known, that prostaglandin E2 (PGE2), generated from the omega-6 polyunsaturated fatty acid (n-6 PUFA) arachidonic acid (AA) is important in the tumorigenesis of colon cancer.
Research frontiers
Several studies with the LS-174T colon cancer cell line have shown an important role of PGE2 for tumor cell growth, but the effect of n-3 and n-6 PUFA has not been examined. Here, we used the LS-174T colon cancer cell line to study the role of the prostaglandin precursor AA and the omega-3 polyunsaturated fatty acid (n-3 PUFA) docosahexaenoic acid (DHA) on cell growth.
Innovations and breakthroughs
The results presented here demonstrate that the n-3 PUFA DHA can directly suppress AA- as well as PGE2-induced colon cancer cell growth. The data add evidence to the argument that the ratio of n-6/n-3 PUFA (and in particular the ratio of AA versus DHA) may be a critical determinant of proliferation and tumor growth in the colon, and that DHA supplementation can suppress tumor cell growth even in the presence of high AA- and PGE2-levels.
Applications
The results suggest that supplementation of DHA may be a powerful tool to counteract AA- and PGE2-promoted colon cancer cell growth that might be associated with the predominant Western diet.
Terminology
PGE2 is generated from the n-6 PUFA AA via action of cyclooxygenases 1 and 2. PGE2 is important for proliferation of colon cancer cells in vitro. In contrast, diets rich in n-3 PUFAs, such as DHA and eicosapentaenoic acid, which are mainly found in fish oil, might reduce the risk of colon cancer development.
Peer review
The authors show that addition of DHA to cell cultures decreased cell proliferation in a dose- and time-dependent manner. Overall, these studies establish the importance of the ratio of n-3 to n-6 PUFA and the beneficial effect of fish oil in neoplastic growth. Although this is a limited in vitro study, its implications are significant.
Supported by Grants from the German National Academic Foundation (to P.H.) and from the American Cancer Society (RSG-03-140-01-CNE) and the NIH (NIH R01 113605) (both to J.X.K.), and the German Research Foundation (DFG) and a Charité Research Grant (both to K.H.W.)
Peer reviewer: Meenakshisundaram Ananthanarayanan, Associated Professor, Department of Pediatrics, Annenberg Bldg, Rm.14-24A, Box 1664, The Mount Sinai Medical Center, One Gustave L. Levy Place, New York, NY, 10029, United States
S- Editor Tian L L- Editor Kerr C E- Editor Zheng XM
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 3.Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995;55:3785–3789. [PubMed] [Google Scholar]
- 4.Kargman SL, O'Neill GP, Vickers PJ, Evans JF, Mancini JA, Jothy S. Expression of prostaglandin G/H synthase-1 and -2 protein in human colon cancer. Cancer Res. 1995;55:2556–2559. [PubMed] [Google Scholar]
- 5.Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 6.Shao J, Jung C, Liu C, Sheng H. Prostaglandin E2 Stimulates the beta-catenin/T cell factor-dependent transcription in colon cancer. J Biol Chem. 2005;280:26565–26572. doi: 10.1074/jbc.M413056200. [DOI] [PubMed] [Google Scholar]
- 7.Yu W, Murray NR, Weems C, Chen L, Guo H, Ethridge R, Ceci JD, Evers BM, Thompson EA, Fields AP. Role of cyclooxygenase 2 in protein kinase C beta II-mediated colon carcinogenesis. J Biol Chem. 2003;278:11167–11174. doi: 10.1074/jbc.M211424200. [DOI] [PubMed] [Google Scholar]
- 8.Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother. 2006;60:502–507. doi: 10.1016/j.biopha.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 9.Backlund MG, Mann JR, Dubois RN. Mechanisms for the prevention of gastrointestinal cancer: the role of prostaglandin E2. Oncology. 2005;69 Suppl 1:28–32. doi: 10.1159/000086629. [DOI] [PubMed] [Google Scholar]
- 10.Caygill CP, Charlett A, Hill MJ. Relationship between the intake of high-fibre foods and energy and the risk of cancer of the large bowel and breast. Eur J Cancer Prev. 1998;7 Suppl 2:S11–S17. doi: 10.1097/00008469-199805000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Hall MN, Chavarro JE, Lee IM, Willett WC, Ma J. A 22-year prospective study of fish, n-3 fatty acid intake, and colorectal cancer risk in men. Cancer Epidemiol Biomarkers Prev. 2008;17:1136–1143. doi: 10.1158/1055-9965.EPI-07-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norat T, Bingham S, Ferrari P, Slimani N, Jenab M, Mazuir M, Overvad K, Olsen A, Tjønneland A, Clavel F, et al. Meat, fish, and colorectal cancer risk: the European Prospective Investigation into cancer and nutrition. J Natl Cancer Inst. 2005;97:906–916. doi: 10.1093/jnci/dji164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Courtney ED, Matthews S, Finlayson C, Di Pierro D, Belluzzi A, Roda E, Kang JY, Leicester RJ. Eicosapentaenoic acid (EPA) reduces crypt cell proliferation and increases apoptosis in normal colonic mucosa in subjects with a history of colorectal adenomas. Int J Colorectal Dis. 2007;22:765–776. doi: 10.1007/s00384-006-0240-4. [DOI] [PubMed] [Google Scholar]
- 14.Pot GK, Geelen A, van Heijningen EM, Siezen CL, van Kranen HJ, Kampman E. Opposing associations of serum n-3 and n-6 polyunsaturated fatty acids with colorectal adenoma risk: an endoscopy-based case-control study. Int J Cancer. 2008;123:1974–1977. doi: 10.1002/ijc.23729. [DOI] [PubMed] [Google Scholar]
- 15.Jia Q, Lupton JR, Smith R, Weeks BR, Callaway E, Davidson LA, Kim W, Fan YY, Yang P, Newman RA, et al. Reduced colitis-associated colon cancer in Fat-1 (n-3 fatty acid desaturase) transgenic mice. Cancer Res. 2008;68:3985–3991. doi: 10.1158/0008-5472.CAN-07-6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nowak J, Weylandt KH, Habbel P, Wang J, Dignass A, Glickman JN, Kang JX. Colitis-associated colon tumorigenesis is suppressed in transgenic mice rich in endogenous n-3 fatty acids. Carcinogenesis. 2007;28:1991–1995. doi: 10.1093/carcin/bgm166. [DOI] [PubMed] [Google Scholar]
- 17.Narayanan BA, Narayanan NK, Reddy BS. Docosahexaenoic acid regulated genes and transcription factors inducing apoptosis in human colon cancer cells. Int J Oncol. 2001;19:1255–1262. doi: 10.3892/ijo.19.6.1255. [DOI] [PubMed] [Google Scholar]
- 18.Narayanan BA, Narayanan NK, Simi B, Reddy BS. Modulation of inducible nitric oxide synthase and related proinflammatory genes by the omega-3 fatty acid docosahexaenoic acid in human colon cancer cells. Cancer Res. 2003;63:972–979. [PubMed] [Google Scholar]
- 19.Narayanan BA, Narayanan NK, Desai D, Pittman B, Reddy BS. Effects of a combination of docosahexaenoic acid and 1,4-phenylene bis(methylene) selenocyanate on cyclooxygenase 2, inducible nitric oxide synthase and beta-catenin pathways in colon cancer cells. Carcinogenesis. 2004;25:2443–2449. doi: 10.1093/carcin/bgh252. [DOI] [PubMed] [Google Scholar]
- 20.Toit-Kohn JL, Louw L, Engelbrecht AM. Docosahexaenoic acid induces apoptosis in colorectal carcinoma cells by modulating the PI3 kinase and p38 MAPK pathways. J Nutr Biochem. 2009;20:106–114. doi: 10.1016/j.jnutbio.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Calviello G, Di Nicuolo F, Gragnoli S, Piccioni E, Serini S, Maggiano N, Tringali G, Navarra P, Ranelletti FO, Palozza P. n-3 PUFAs reduce VEGF expression in human colon cancer cells modulating the COX-2/PGE2 induced ERK-1 and -2 and HIF-1alpha induction pathway. Carcinogenesis. 2004;25:2303–2310. doi: 10.1093/carcin/bgh265. [DOI] [PubMed] [Google Scholar]
- 22.Schønberg SA, Lundemo AG, Fladvad T, Holmgren K, Bremseth H, Nilsen A, Gederaas O, Tvedt KE, Egeberg KW, Krokan HE. Closely related colon cancer cell lines display different sensitivity to polyunsaturated fatty acids, accumulate different lipid classes and downregulate sterol regulatory element-binding protein 1. FEBS J. 2006;273:2749–2765. doi: 10.1111/j.1742-4658.2006.05292.x. [DOI] [PubMed] [Google Scholar]
- 23.Dommels YE, Haring MM, Keestra NG, Alink GM, van Bladeren PJ, van Ommen B. The role of cyclooxygenase in n-6 and n-3 polyunsaturated fatty acid mediated effects on cell proliferation, PGE(2) synthesis and cytotoxicity in human colorectal carcinoma cell lines. Carcinogenesis. 2003;24:385–392. doi: 10.1093/carcin/24.3.385. [DOI] [PubMed] [Google Scholar]
- 24.Hofmanová J, Vaculová A, Kozubík A. Polyunsaturated fatty acids sensitize human colon adenocarcinoma HT-29 cells to death receptor-mediated apoptosis. Cancer Lett. 2005;218:33–41. doi: 10.1016/j.canlet.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 25.Hofmanová J, Vaculová A, Lojek A, Kozubík A. Interaction of polyunsaturated fatty acids and sodium butyrate during apoptosis in HT-29 human colon adenocarcinoma cells. Eur J Nutr. 2005;44:40–51. doi: 10.1007/s00394-004-0490-2. [DOI] [PubMed] [Google Scholar]
- 26.Trombetta A, Maggiora M, Martinasso G, Cotogni P, Canuto RA, Muzio G. Arachidonic and docosahexaenoic acids reduce the growth of A549 human lung-tumor cells increasing lipid peroxidation and PPARs. Chem Biol Interact. 2007;165:239–250. doi: 10.1016/j.cbi.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Sheng H, Shao J, Washington MK, DuBois RN. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J Biol Chem. 2001;276:18075–18081. doi: 10.1074/jbc.M009689200. [DOI] [PubMed] [Google Scholar]
- 28.Shao J, Lee SB, Guo H, Evers BM, Sheng H. Prostaglandin E2 stimulates the growth of colon cancer cells via induction of amphiregulin. Cancer Res. 2003;63:5218–5223. [PubMed] [Google Scholar]
- 29.Shao J, Evers BM, Sheng H. Prostaglandin E2 synergistically enhances receptor tyrosine kinase-dependent signaling system in colon cancer cells. J Biol Chem. 2004;279:14287–14293. doi: 10.1074/jbc.M313276200. [DOI] [PubMed] [Google Scholar]
- 30.Chen ZY, Istfan NW. Docosahexaenoic acid is a potent inducer of apoptosis in HT-29 colon cancer cells. Prostaglandins Leukot Essent Fatty Acids. 2000;63:301–308. doi: 10.1054/plef.2000.0218. [DOI] [PubMed] [Google Scholar]