Abstract
AIM: To study the action of aminoguanidine on pancreatic cancer xenografts in relation to cell proliferation, apoptosis, redox status and vascularization.
METHODS: Xenografts of PANC-1 cells were developed in nude mice. The animals were separated into two groups: control and aminoguanidine treated. Tumor growth, survival and appearance of metastases were determined in vivo in both groups. Tumors were excised and ex vivo histochemical studies were performed. Cell growth was assessed by Ki-67 expression. Apoptosis was studied by intratumoral expression of B cell lymphoma-2 protein (Bcl-2) family proteins and Terminal deoxynucleotidyl transferase biotin-dUTP Nick End Labeling (Tunel). Redox status was evaluated by the expression of endothelial nitric oxide synthase (eNOS), catalase, copper-zinc superoxide dismutase (CuZnSOD), manganese superoxide dismutase (MnSOD) and glutathione peroxidase (GPx). Finally, vascularization was determined by Massons trichromic staining, and by VEGF and CD34 expression.
RESULTS: Tumor volumes after 32 d of treatment by aminoguanidine (AG) were significantly lower than in control mice (P < 0.01). Median survival of AG mice was significantly greater than control animals (P < 0.01). The appearance of both homolateral and contralateral palpable metastases was significantly delayed in AG group. Apoptotic cells, intratumoral vascularization (trichromic stain) and the expression of Ki-67, Bax, eNOS, CD34, VEGF, catalase, CuZnSOD and MnSOD were diminished in AG treated mice (P < 0.01), while the expression of Bcl-2 and GPx did not change.
CONCLUSION: The antitumoral action of aminoguanidine is associated with decreased cell proliferation, reduced angiogenesis, and reduced expression of antioxidant enzymes.
Keywords: Aminoguanidine, Pancreatic ductal carcinoma, Tumor growth, Metastasis, Apoptosis
INTRODUCTION
Pancreatic cancer is an aggressive carcinoma usually diagnosed at an advanced stage and shows a median survival time of only three months. Approximately half of the cases are metastatic at the time of diagnosis, while the remainder have locally advanced unresectable disease. To date, the only effective treatment is surgical therapy. Adjuvant chemo- and radiotherapy have not led to significant improvements in outcome[1].
Nitric oxide synthase (NOS) produce nitric oxide (NO) by the oxidation of L-arginine. There are three known isoenzymes of NOS: two constitutive forms [neuronal NOS (nNOS) and endothelial NOS (eNOS)] and one inducible form, inducible NOS (iNOS). Constitutive isoforms nNOS and eNOS respond to a calcium influx with a transient release of NO[2]. On the other hand, iNOS always generates high quantities of NO over a prolonged period[3]. This isoform is not only expressed in activated macrophages, usually infiltrating tumors, but also in various types of malignant cells.
Nitric oxide is a highly diffusible, lipophilic free radical. Under certain pathological conditions, NO can combine with the superoxide anion (O2-) to form peroxynitrite (ONOO-), a potent reactive nitric oxide species that nitrates tyrosine residues in proteins and induces DNA damage and lipid peroxidation, leading to cell damage and often cell death. NO in cancer exhibits both a cytotoxic and a cytoprotective effect according to its concentration within the tumor microenvironment. Low levels of NO produced by tumor cells themselves aid tumor progression, while the high level of NO produced by tumor adjacent macrophages function as a tumor suppressor agent through the induction of apoptosis[4–7].
Aminoguanidine, a nucleophilic hydrazine compound first synthesized more than 100 years ago, is an irreversible inhibitor of iNOS, which also inhibits eNOS and nNOS at higher concentrations[8]. It has been shown in vivo to prevent disease states characterized by the pathological overproduction of NO, such as diabetic complications[9], age-related arterial stiffening, cardiac hypertrophy[10] and also tumors (including cholangiocarcinoma[11] and gastric cancer[12]). These effects of AG are exerted by modulating proliferation[12], apoptosis[12], angiogenesis[12], by the production of free radicals[12] and by preventing the formation of advanced glycation end products (AGEs)[13,14].
The human pancreatic ductal carcinoma, PANC-1, cells express constitutive eNOS. However, though it is the most commonly tumor associated synthase isoform, PANC-1 cells do not express iNOS[15].
Although the in vitro action of NOS inhibitors has been extensively studied, little research has been undertaken as regards their in vivo effects on cancer growth. Therefore, the aim of this work was to study the action of the NOS inhibitor AG in PANC-1 human pancreatic cancer xenografts in nude mice in relation to tumor growth, angiogenesis and the expression of antioxidant enzymes.
MATERIALS AND METHODS
Xenografts in nude mice
PANC-1 cells (7 × 106) were collected by centrifugation and resuspended in 100 μL RPMI-1640 (GIBCO, Grand Island, New York, USA) to be inoculated in the dorsal flank of each nude mouse (cepa N:NIH(S)-nu). When the tumors that developed reached a volume of 500 mm3 they were excised, cut into 27 mm3 pieces and grafted into the dorsal flank of another nude mouse. Xenografted mice were separated in four groups (n = 7) and received daily doses of AG (aminoguanidine hydrochloride, Sigma, Saint Louis, Missouri, USA) of 1, 2 or 4 mg/mL in drinking water. In vivo treatments began when the graft volumes reached 100-150 mm3. The control group was left without treatment. Tumor size was measured with a caliper three times a week and volume was calculated as [(mayor diameter + minor diameter)/4]3 × π4/3. Treatments lasted 32 d. Two-way ANOVA, Bonferroni post test and non linear fit of tumor growth data were carried out by GraphPad Prism version 5.00™. All the experiments using mice were performed according to the NRC [National Research Council] Guide for the Care and Use of Laboratory Animals, 7th ed, Washington DC, National Academy Press, 1996.
Survival
Mice bearing xenografts were divided into two groups (n = 7), AG (2 mg/mL in drinking water) and control, and followed until spontaneous death. Kaplan-Meier survival curves, median survival time of each group and P value were obtained by GraphPad Prism™. Development of palpable metastases was checked three times a week in both groups and the metastases were distinguished according to their location as either homolateral (those that appeared in the same flank as the xenograft) or contralateral (those that appeared in the opposite flank).
Histochemistry
At the end of treatments, tumors were excised, fixed in 40 g/L formaldehyde in PBS (formalin), paraffin embedded and sliced into 3-μm thick sections for: (1) Tunel, using TdT-FragEL DNA Fragmentation Detection Kit (Calbiochem, a brand of EMD Biosciences, La Jolla, California, USA) according to the manufacturer’s instructions. 3,3'-Diaminobenzidine tablets (DAB; Sigma) were used for staining and methyl green for counterstaining; (2) Immunohistochemical detection using antibodies against Ki-67 (1/50, Dako Cytomation, Carpinteria, California, USA), PCNA (1/100, Dako Cytomation), Bcl-2 (1/50, Santa Cruz Biotechnology, Santa Cruz, California, USA), Bax (1/50, Santa Cruz Biotechnology), eNOS (1/30 Sigma), VEGF (1/20, R&D Systems, USA), catalase (1/50, Sigma), CuZnSOD (1/50, Calbiochem), MnSOD (1/50, Calbiochem) and GPx (1/125, Stressgen, Ann Arbor, Michigan, USA). The appropriate secondary HRP-conjugated antiserum was employed in each case. DAB tablets were used for staining and hematoxylin for counterstaining; (3) Tumor vascularization was assayed by means of Masson trichrome and CD34 (1/50, Santa Cruz Biotechnology) staining; and (4) hematoxylin and eosin staining of tumors and metastatic lymph nodes.
Microscopic observations were performed using an Axiolab Karl Zeiss microscope by two independent observers. Photographs were taken with a Canon Power Shot G5 digital camera and processed with Remote Capture 2.7 software. Metastatic lymph nodes, Tunel, trichrome stain and CD34 of control mice were observed at 400 × and the remaining determinations at 630 ×. Microscopic observations were done in ten random fields and graded as percent of positive cells. Positive nuclei were considered as positive cells for Tunel, Ki-67 and PCNA, whereas diffuse positive cytoplasms were considered as positive cells for cytoplasmic proteins. Non parametric Mann Whitney tests on the percent of positive cells in control and AG groups were performed by GraphPad PrismTM. Peritumoral vascularity was assessed by screening trichrome stained sections at 50 × magnification to identify the largest vascular areas around the tumor. In these hot spots, individual vessel count was evaluated at 400 × magnification using an ocular grid. Intratumoral vascularity was evaluated on trichrome and CD34 stained slices, counting vessels inside the tumor at 400 × magnification in ten random fields (in the identified hot spots).
RESULTS
In vivo studies
Tumor growth: Animals were separated into four groups (control and 1, 2 and 4 mg/mL AG) to evaluate the effect of AG on tumor growth. Mice were treated for 32 d when grafts volume reached 100-150 mm3. Tumor volumes were determined three times a week and finally referred to the initial volume for each treatment (Figure 1). AG (2 and 4 mg/mL) treatment significantly diminished the tumor growth rate. Tumor volumes of mice treated with AG 2 and 4 mg/mL from day 28 of treatment on were significantly different from tumors of control animals (P < 0.01 for AG 2 and 4 mg/mL vs control, two way ANOVA and Bonferroni post test).
Figure 1.
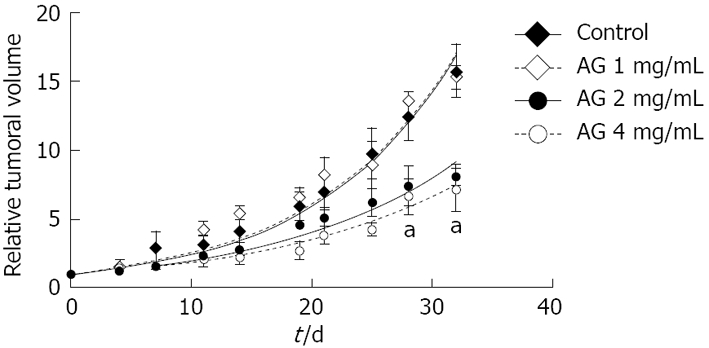
Antitumor effect of AG in PANC-1 xenografted mice. Tumor volumes were determined and referred to the initial volume for each treatment. aP < 0.01 for AG 2 and 4 mg/mL vs control, two way ANOVA and Bonferroni post test.
In the same way, tumor volume doubling time of mice treated with AG 2 or 4 mg/mL was significantly greater than the other groups (Table 1; P < 0.01 vs control and AG 1 mg/mL, Anova one way with Bonferroni post test).
Table 1.
AG enlarges tumor volume doubling time (mean ± SD)
| Treatment | Doubling time (d) |
| Control | 7.8 ± 0.3 |
| AG 1 mg/mL | 7.8 ± 0.3 |
| AG 2 mg/mL | 10.0 ± 0.5a |
| AG 4 mg/mL | 11.1 ± 0.7a |
Tumor volume doubling times were obtained by non linear fit of tumor growth rate (shown in Figure 1) to an exponential growth equation.
P < 0.01 vs control and AG 1 mg/mL, ANOVA one way with Bonferroni post test.
Tumor histology: After excision of tumors, xenografts usually showed macroscopic infiltration of the dermis and abdominal muscular wall. Under microscopic observation, tumors presented undifferentiated adenocarcinoma cells, with high grade of atypia, marked anisokaryosis and anisocytosis. Multinucleated cells and atypical mitoses (often tri- and tetrapolar mitoses) were frequently observed.
Survival and metastases: Two extra groups of PANC-1 xenografted animals (control and AG 2 mg/mL) were followed three times a week until spontaneous death. Kaplan-Meier survival curves (Figure 2A) were obtained for AG 2 mg/mL (dotted line) and control (solid line) groups. Median survival, i.e. the time at which survival percent equals 50%, of AG treated animals (95 d) was significantly greater than that of control mice (74 d; P < 0.01 for AG vs control, log-rank test).
Figure 2.
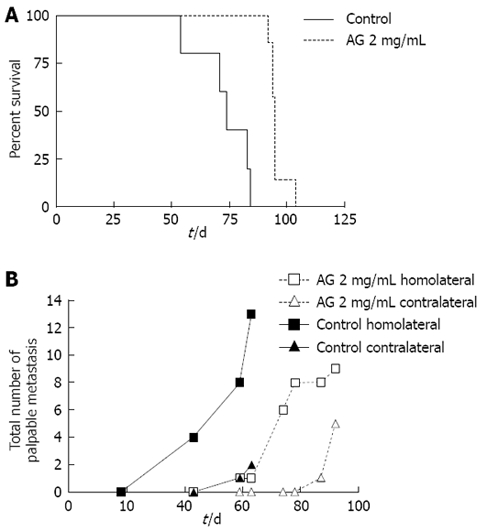
Action of AG on survival and metastases in PANC-1 xenografted mice. A: Time of spontaneous death of mice was determined, Kaplan-Meier survival curves were plotted and median survival was calculated. P < 0.01 for AG vs control, log-rank test; B: The development of palpable homolateral and contralateral metastases, which appeared mainly in lymph nodes, was checked until the death of the animals. Total number of metastases developed in both groups (n = 7 each group) and medians of appearance were determined. Curves were compared by logrank test, P < 0.01.
The same groups of animals were checked for the development of palpable metastases (concomitantly with survival studies) until the death of the animals. The total number of both homolateral and contralateral palpable metastases developed in each group (n = 7 each group) was determined (Figure 2B). Appearance of metastases was significantly delayed in AG treated mice. The medians of appearance were 59 d for control homolateral metastases, 78 d for AG homolateral metastases and 92 d for AG contralateral metastases. The median for control contralateral metastases could not be determined since only two metastases appeared by the time of death. Curves were significantly different.
Also, the number of homolateral metastases per animal was lower in the AG group. The first control mouse died at 64 d; therefore, the mean number of metastases per animal at 63 d was obtained and compared for each of the four groups. Means, expressed as mean ± SD, were significantly different. The number of homolateral metastases in the control (1.8 ± 0.4 per mouse) was significantly greater than in the other groups (0.3 ± 0.3 per mouse for control contralateral, 0.1 ± 0.1 for AG homolateral and 0 for AG contralateral).
The metastases that developed were analyzed by microscopic observation after the mice died (Figure 3A). Both homolateral and contralateral metastases appeared mainly in the lymph nodes. The node architecture was only preserved in a few peripheral sections due to the considerable extent of tumor infiltration. Infiltrating tumor cells showed the same histological characteristics as xenograft neoplastic cells (Figure 3B). This finding confirms the metastatic character of the multiple masses found.
Figure 3.
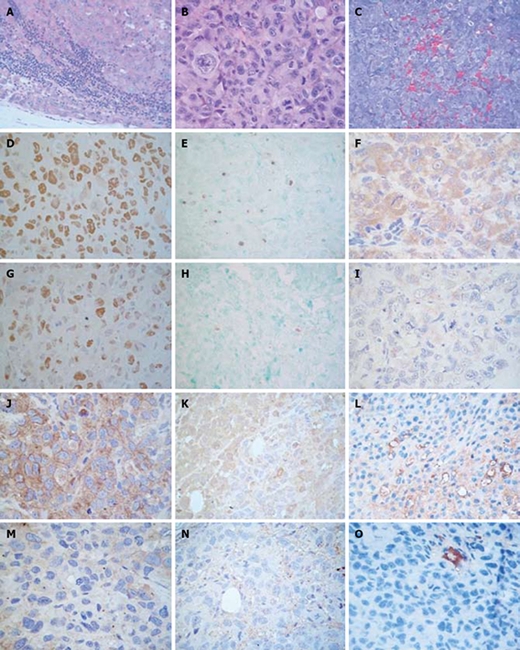
Histopathology and immunohistochemistry of PANC-1 xenografted mice. Formalin fixed paraffin embedded tissue sections of control mice were stained with HE. A: Metastatic lymph node (× 400); B: Xenografts (× 630). Immunohistochemistry in control and AG treated mice. Tissue sections were stained with DAB and counterstained with hematoxylin; C: Formalin fixed paraffin embedded tissue sections of control mice were stained with trichromic solution (× 400); D, G: Ki-67 (× 630); E, H: Tunel (× 400); F, I: Bax (× 630); J, M: VEGF (× 630); K, N: eNOS (× 630); L, O: CD34 in control (× 400) and AG treated (× 630) mice. Formalin fixed paraffin embedded tissue sections of control mice were stained with trichrome solution.
Ex vivo studies
Apoptosis and cell proliferation: Cell proliferation in xenografts was evaluated through the expression of Ki-67[16] (Figure 3C and F). Apoptosis was studied in tumors by the Tunel assay (Figure 3D and G) and by the expression of antiapoptotic protein Bcl-2 and proapoptotic Bax (Figure 3E and H).
Ki-67 expression, evaluated as percent of positive cells (mean ± SD), was lower in tumors of AG treated mice than in the control group (19 ± 11 vs 78 ± 10, P < 0.01, Mann Whitney test). Similar results were obtained by PCNA immunodetection, another proliferation marker.
The Tunel test showed a lower proportion of apoptotic cells in grafts of animals which had received AG than the control group, (1 ± 1 vs 5 ± 1, P < 0.01, Mann Whitney test), evaluated as percent of positive cells (mean ± SD). In the same way, the expression of Bax, evaluated as percent of positive cells (mean ± SD), was significantly lower in tumors of AG treated animals than in control animals (17 ± 13 vs 88 ± 11, P < 0.01, Mann Whitney test). Lastly, Bcl-2 expression was not altered by AG treatment (20 ± 10 vs 15 ± 9, AG vs control). These results, therefore, indicate an antiapoptotic effect of AG in xenografts.
Angiogenesis: Angiogenesis is essential for the growth, invasion and metastasis of a tumor. eNOS has been shown to participate in tumor progression by promoting angiogenesis[17,18]. Vascular endothelial growth factor (VEGF), a potent endothelial cell mitogen and vascular permeability factor, is mainly implicated in tumor growth via the stimulation of NO production[6,18]. To evaluate tumor angiogenesis in PANC-1 xenografts, eNOS and VEGF expressions were assayed, as well as intra and peritumoral vascularity (by the trichrome stain and CD34 expression; a marker of endothelial cells).
The expression of VEGF (Figure 3I and L) and eNOS (Figure 3J and M) was determined as percent of positive cells (mean ± SD). Grafts of AG treated animals expressed lower levels of eNOS and VEGF than tumors of control mice (9 ± 9 vs 75 ± 9, P < 0.01 and 25 ± 17 vs 84 ± 9, P < 0.01, Mann Whitney test, respectively).
Vascularity evaluated by the trichromic stain (Figure 3N) in xenografts of untreated mice showed capillaries and medium size vessels in intratumoral areas, while medium and large size vessels were observed in peritumoral tissues. AG treatment did not modify peritumoral vascularity; however, a diminished level of intratumoral vascularity was detected. As shown in Figure 3K and N, the intratumoral observations were confirmed by CD34 staining (6 ± 3 vs 21 ± 6, AG vs control, P < 0.05, Mann Whitney test), indicating an antiangiogenic effect of AG.
Expression of antioxidant enzymes: Reactive oxygen species (ROS), reactive nitrogen species (RNS) and redox state modulate cell proliferation, apoptosis and angiogenesis. Cells defend themselves against ROS mainly by antioxidant enzymes. In this way, superoxide dismutases convert superoxide radicals into hydrogen peroxide, which is in turn scavenged by catalase and GPx. According to their intracellular distribution, cytosolic CuZnSOD protects against cytosolic superoxide, while mitochondrial MnSOD decomposes mitochondrial-generated superoxide[19]. The four antioxidant enzymes were assessed in PANC-1 xenografts. Tumors of AG treated mice showed a lower expression (evaluated as percent of positive cells; mean ± SD) than grafts of control animals, for CuZnSOD (14 ± 10 vs 80 ± 15), MnSOD (21 ± 12 vs 77 ± 17) and catalase (9 ± 6 vs 49 ± 12) (P < 0.01, Mann Whitney test). Meanwhile, GPx expression remained unchanged (70 ± 11 vs 81 ± 9, AG vs control).
DISCUSSION
The effect of the NOS inhibitor aminoguanidine was studied in xenografts obtained by inoculation of PANC-1 human pancreatic ductal carcinoma cells into nude mice.
In vivo results showed an antitumor effect of AG, including suppression of tumor growth, enhanced survival, delayed appearance of metastases and a lower number of homolateral metastases per animal. Similarly, tumor development diminished in a hamster model of cholangiocarcinoma[11]. However, AG displayed an antimetastatic action on a model of inflammation-based murine fibrosarcoma progression, altering neither tumor incidence nor tumor growth[20]. Metastatic cell behavior could be positively or negatively regulated by nitric oxide, accordingly to iNOS and eNOS expression in endothelial cells, macrophages, stromal fibroblasts and cancer cells[21]. In an attempt to explain the observed in vivo antitumor action of AG, multiple ex vivo experiments were performed.
Tissues, both normal or abnormal, grow mostly by increasing the number of cells. In turn, the cell number of a given population is regulated by the balance between proliferation and death[22]. Altered indices of proliferation and/or apoptosis could explain the diminished tumor growth observed in AG treated mice xenografts. The proliferation index, assessed by Ki-67 and PCNA expression, indicated that AG was antiproliferative in PANC-1 xenografts. Moreover, the apoptotic pathway state (evaluated by Bcl-2/Bax expression ratio) and apoptotic death (assayed by the Tunel assay) revealed an antiapoptotic action of AG in pancreatic tumors. It has been hypothesized that Bcl-2 binds proapoptotic Bax to counteract its effects. Thus, the relative expression of Bcl-2 and Bax are involved in the regulation of the cell death program. In this sense, AG treatment enhanced the Bcl-2/Bax ratio while diminishing the rate of apoptosis. Therefore, AG did not lead to increased tumor cells apoptosis, but caused them not to proliferate.
Many articles support the proapoptotic action of NO associated with iNOS induction and high NO levels[5], while others report a positive association between iNOS induction and tumor cell growth[11,23]. In a recent report[24], the in vivo and in vitro inhibition of hepatocellular carcinoma growth by AG was associated with a proapoptotic effect of this drug. The cross-talk between NO and RAS/ERK and IKK/NF-κB pathways was determined to be crucial to this action. Conversely, our data show that the inhibition of PANC-1 xenografts’ growth induced by AG is coupled to an antiapoptotic effect. It is well known that NO levels are quite different depending on the NOS isoform (eNOS or iNOS) involved in NO production. Although different NO concentrations regulate pathways related to either survival or cell death, the final effect on cell fate also depends on such factors as cell type, signaling pathways involved, genetic background and NO concentration in the microenvironment. Our in vitro experiments using PANC-1 cell line showed that the NO scavenger 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO) prevents cell proliferation (unpublished results). We have also demonstrated that activated ERK 1/2 down-modulation is related to the in vitro inhibition of the proliferation of PANC-1 cells[25]. Thus, even though a direct action of the low levels of NO produced by eNOS on this signal transduction pathway was not assessed in our work, it cannot be ruled out.
Another potential contribution to in vivo tumor growth might be determined by interactions with stroma. Fibroblasts and inflammatory cells express iNOS and NO is related to cytokines and growth factors secretion. The iNOS inhibition in stromal cells by AG treatment might modulate neoplastic cells’ survival and death[26,27]. In addition, vascularity modulation is also involved in tumor growth. Locally, new blood vessel formation is essential for supplying oxygen and other nutrients to tumor cells and is one of the altered manifestations that dictate metastatic tumor growth. It is well known that the VEGF-mediated angiogenesis requires NO production via eNOS in endothelial cells. Growing evidence supports the hypothesis that reciprocal relations between NO and VEGF might contribute to drive angiogenesis in pathophysiological conditions, depending on the amount of produced NO[28]. Our results showed that AG reduced tumor vascularization and VEGF and eNOS expression in PANC-1 grafts. Small amounts of NO synthesized by eNOS are required for VEGF up-regulation[28]. The low level of NO produced by the AG-inhibited eNOS enzyme might induce the down-modulation of VEGF. In turn, VEGF down-regulation might hinder eNOS up-regulation, consistent with the reduced eNOS expression detected in PANC-1 grafts. Therefore, the antiangiogenic action of AG could account for the reduced tumor growth rate, the lower rate of metastasis and the delay in its appearance.
Lastly, the expression of CuZnSOD, MnSOD and catalase antioxidant enzymes was diminished by AG. The same effect on these enzymes was observed in diabetic rats[29] and on superoxide dismutases in doxorubicin treated rats[30]. GPx activity was slightly decreased after AG treatment in a model of liver injury in rat[31]. Chronic treatment with AG might cause a disruption of the cellular redox balance due to its capacity to scavenge oxidant reactive species[32] and to inhibit NO synthesis. This could explain the reduced expression of antioxidant enzymes as a compensatory mechanism. Accordingly, our preliminary in vitro experiments in PANC-1 cells showed that hydrogen peroxide and NO intracellular levels were modified by AG, while cell proliferation was reduced. Cell cycle progression is modified by cellular redox status and the magnitude of its imbalance might lead to cell proliferation, differentiation, growth arrest, apoptosis or necrosis[33]. Different cell lines require the pro-oxidant status to persist beyond the G1-S restriction point in the cell cycle[34,35]. The AG driven impaired production of NO could hinder that oxidant level and, in turn, could prevent proliferation.
In conclusion, aminoguanidine, by exerting an antiapoptotic effect, shows an antitumoral action evidenced by a lower tumor volume at the end of treatment, a delayed appearance of palpable metastases and an extended life span. The antiproliferative action is associated with a lower intratumoral Ki-67 content, an antiangiogenic effect, a reduced NO production and reduced expression of antioxidant enzymes.
COMMENTS
Background
Pancreatic cancer is a devastating disease because of its high mortality rate. Chemotherapy alone or combined with radiotherapy are poor attempts to overcome the illness. In cancer, the free radical nitric oxide exhibits both a cytotoxic and a cytoprotective effect according to its concentration within the tumor microenvironment. Although the in vitro action of nitric oxide synthase inhibitors (such as aminoguanidine) has been extensively studied in tumor cells, little research has been undertaken as regards their in vivo effects on cancer growth.
Research frontiers
Nitric oxide synthases catalyze the production of nitric oxide (NO), which, depending on its concentration, could act both as tumor promoter or suppressor. Compounds that modify expression and/or activity of these enzymes may be considered useful tools in cancer research.
Innovations and breakthroughs
The nitric oxide synthase (NOS) inhibitor aminoguanidine, by exerting an antiapoptotic effect, shows an antitumoral action evidenced by a lower tumor volume at the end of treatment, a delayed appearance of palpable metastases and an extended life span. The antiproliferative action is associated with a lower expression of endothelial NOS (eNOS) and antioxidant enzymes.
Applications
Chemoresistance is still the major problem of anticancer drug treatment of malignant diseases such as pancreatic carcinoma. Aminoguanidine could provide an attractive line of investigation as a multi-modal avenue due to its different effects on tumor biology.
Terminology
Nitric oxide is synthesized by a group of enzymes: the nitric oxide synthases. At least three isoforms have been described: eNOS, neuronal NOS (nNOS) and inducible NOS (iNOS). Most cancer cells express these enzymes and the NO produced is involved in biological processes associated with both survival and cell death.
Peer review
In this work, the authors have studied the action of the nitric oxide synthase inhibitor aminoguanidine (AG) in PANC-1 cells xenografts in relation to cell proliferation, apoptosis, angiogenesis and redox status. Interestingly, authors indicate that AG has strong effects on tumor progression, through inhibiting growth, apoptosis, vascularization and metastasis. This study will be of great interest to the carcinogenesis field, particularly in the design of new therapeutic drugs for pancreatic cancer.
Supported by Grants from University of Buenos Aires (B098 and B112)
Peer reviewers: Isabel Fabregat, PhD, Associate Professor, Laboratori d’Oncologia Molecular, Institut d’Investigación Biomèdica de Bellvitge , Gran Via, Km 2,7, L’Hospitalet, 08907 Barcelona, Spain; Francesco Feo, Professor, Dipartimento di Scienze Biomediche, Sezione di Patologia Sperimentale e Oncologia, Università di Sassari, Via P, Manzella 4, 07100 Sassari, Italy
S- Editor Li LF L- Editor Stewart GJ E- Editor Lin YP
References
- 1.Rosenberg L. Pancreatic cancer: a review of emerging therapies. Drugs. 2000;59:1071–1089. doi: 10.2165/00003495-200059050-00004. [DOI] [PubMed] [Google Scholar]
- 2.Stuehr DJ, Santolini J, Wang ZQ, Wei CC, Adak S. Update on mechanism and catalytic regulation in the NO synthases. J Biol Chem. 2004;279:36167–36170. doi: 10.1074/jbc.R400017200. [DOI] [PubMed] [Google Scholar]
- 3.Fulton D, Fontana J, Sowa G, Gratton JP, Lin M, Li KX, Michell B, Kemp BE, Rodman D, Sessa WC. Localization of endothelial nitric-oxide synthase phosphorylated on serine 1179 and nitric oxide in Golgi and plasma membrane defines the existence of two pools of active enzyme. J Biol Chem. 2002;277:4277–4284. doi: 10.1074/jbc.M106302200. [DOI] [PubMed] [Google Scholar]
- 4.Kim PK, Zamora R, Petrosko P, Billiar TR. The regulatory role of nitric oxide in apoptosis. Int Immunopharmacol. 2001;1:1421–1441. doi: 10.1016/s1567-5769(01)00088-1. [DOI] [PubMed] [Google Scholar]
- 5.Lechner M, Lirk P, Rieder J. Inducible nitric oxide synthase (iNOS) in tumor biology: the two sides of the same coin. Semin Cancer Biol. 2005;15:277–289. doi: 10.1016/j.semcancer.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Fukumura D, Jain RK. Role of nitric oxide in angiogenesis and microcirculation in tumors. Cancer Metastasis Rev. 1998;17:77–89. doi: 10.1023/a:1005908805527. [DOI] [PubMed] [Google Scholar]
- 7.Lala PK, Orucevic A. Role of nitric oxide in tumor progression: lessons from experimental tumors. Cancer Metastasis Rev. 1998;17:91–106. doi: 10.1023/a:1005960822365. [DOI] [PubMed] [Google Scholar]
- 8.Misko TP, Moore WM, Kasten TP, Nickols GA, Corbett JA, Tilton RG, McDaniel ML, Williamson JR, Currie MG. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1993;233:119–125. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- 9.Tilton RG, Chang K, Hasan KS, Smith SR, Petrash JM, Misko TP, Moore WM, Currie MG, Corbett JA, McDaniel ML. Prevention of diabetic vascular dysfunction by guanidines. Inhibition of nitric oxide synthase versus advanced glycation end-product formation. Diabetes. 1993;42:221–232. doi: 10.2337/diab.42.2.221. [DOI] [PubMed] [Google Scholar]
- 10.Corman B, Duriez M, Poitevin P, Heudes D, Bruneval P, Tedgui A, Levy BI. Aminoguanidine prevents age-related arterial stiffening and cardiac hypertrophy. Proc Natl Acad Sci USA. 1998;95:1301–1306. doi: 10.1073/pnas.95.3.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nam KT, Kim DY, Park MS, Jang DD, Yang KH, Han JH, Yoon BI. Suppression of cholangiocarcinoma development by aminoguanidine in the liver fluke-infested hamster. J Toxicol Pathol. 2005;18:65–68. [Google Scholar]
- 12.Wang GY, Ji B, Wang X, Gu JH. Anti-cancer effect of iNOS inhibitor and its correlation with angiogenesis in gastric cancer. World J Gastroenterol. 2005;11:3830–3833. doi: 10.3748/wjg.v11.i25.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nilsson BO. Biological effects of aminoguanidine: an update. Inflamm Res. 1999;48:509–515. doi: 10.1007/s000110050495. [DOI] [PubMed] [Google Scholar]
- 14.Thornalley PJ. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch Biochem Biophys. 2003;419:31–40. doi: 10.1016/j.abb.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Cricco G, Medina V, Núñez M, Mohamad N, Gutiérrez A, Bergoc R, Rivera E, Martín G. Nitric oxide involvement in histamine-mediated PANC-1 cells growth. Inflamm Res. 2007;56 Suppl 1:S39–S40. doi: 10.1007/s00011-006-0519-5. [DOI] [PubMed] [Google Scholar]
- 16.Muskhelishvili L, Latendresse JR, Kodell RL, Henderson EB. Evaluation of cell proliferation in rat tissues with BrdU, PCNA, Ki-67(MIB-5) immunohistochemistry and in situ hybridization for histone mRNA. J Histochem Cytochem. 2003;51:1681–1688. doi: 10.1177/002215540305101212. [DOI] [PubMed] [Google Scholar]
- 17.Ridnour LA, Thomas DD, Donzelli S, Espey MG, Roberts DD, Wink DA, Isenberg JS. The biphasic nature of nitric oxide responses in tumor biology. Antioxid Redox Signal. 2006;8:1329–1337. doi: 10.1089/ars.2006.8.1329. [DOI] [PubMed] [Google Scholar]
- 18.Gupta MK, Qin RY. Mechanism and its regulation of tumor-induced angiogenesis. World J Gastroenterol. 2003;9:1144–1155. doi: 10.3748/wjg.v9.i6.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinnula VL, Crapo JD. Superoxide dismutases in malignant cells and human tumors. Free Radic Biol Med. 2004;36:718–744. doi: 10.1016/j.freeradbiomed.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Okada F, Tazawa H, Kobayashi T, Kobayashi M, Hosokawa M. Involvement of reactive nitrogen oxides for acquisition of metastatic properties of benign tumors in a model of inflammation-based tumor progression. Nitric Oxide. 2006;14:122–129. doi: 10.1016/j.niox.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Williams EL, Djamgoz MB. Nitric oxide and metastatic cell behaviour. Bioessays. 2005;27:1228–1238. doi: 10.1002/bies.20324. [DOI] [PubMed] [Google Scholar]
- 22.Baserga R. The contradictions of the insulin-like growth factor 1 receptor. Oncogene. 2000;19:5574–5581. doi: 10.1038/sj.onc.1203854. [DOI] [PubMed] [Google Scholar]
- 23.Salvucci O, Carsana M, Bersani I, Tragni G, Anichini A. Antiapoptotic role of endogenous nitric oxide in human melanoma cells. Cancer Res. 2001;61:318–326. [PubMed] [Google Scholar]
- 24.Calvisi DF, Pinna F, Ladu S, Pellegrino R, Muroni MR, Simile MM, Frau M, Tomasi ML, De Miglio MR, Seddaiu MA, et al. Aberrant iNOS signaling is under genetic control in rodent liver cancer and potentially prognostic for the human disease. Carcinogenesis. 2008;29:1639–1647. doi: 10.1093/carcin/bgn155. [DOI] [PubMed] [Google Scholar]
- 25.Cricco G, Martín G, Medina V, Núñez M, Gutiérrez A, Cocca C, Bergoc R, Rivera E. Histamine regulates the MAPK pathway via the H(2) receptor in PANC-1 human cells. Inflamm Res. 2004;53 Suppl 1:S65–S66. doi: 10.1007/s00011-003-0331-4. [DOI] [PubMed] [Google Scholar]
- 26.Müerköster S, Wegehenkel K, Arlt A, Witt M, Sipos B, Kruse ML, Sebens T, Klöppel G, Kalthoff H, Fölsch UR, et al. Tumor stroma interactions induce chemoresistance in pancreatic ductal carcinoma cells involving increased secretion and paracrine effects of nitric oxide and interleukin-1beta. Cancer Res. 2004;64:1331–1337. doi: 10.1158/0008-5472.can-03-1860. [DOI] [PubMed] [Google Scholar]
- 27.Tse GM, Wong FC, Tsang AK, Lee CS, Lui PC, Lo AW, Law BK, Scolyer RA, Karim RZ, Putti TC. Stromal nitric oxide synthase (NOS) expression correlates with the grade of mammary phyllodes tumour. J Clin Pathol. 2005;58:600–604. doi: 10.1136/jcp.2004.023028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimura H, Esumi H. Reciprocal regulation between nitric oxide and vascular endothelial growth factor in angiogenesis. Acta Biochim Pol. 2003;50:49–59. [PubMed] [Google Scholar]
- 29.Kedziora-Kornatowska KZ, Luciak M, Blaszczyk J, Pawlak W. Effect of aminoguanidine on erythrocyte lipid peroxidation and activities of antioxidant enzymes in experimental diabetes. Clin Chem Lab Med. 1998;36:771–775. doi: 10.1515/CCLM.1998.137. [DOI] [PubMed] [Google Scholar]
- 30.Abd El-Gawad HM, El-Sawalhi MM. Nitric oxide and oxidative stress in brain and heart of normal rats treated with doxorubicin: role of aminoguanidine. J Biochem Mol Toxicol. 2004;18:69–77. doi: 10.1002/jbt.20013. [DOI] [PubMed] [Google Scholar]
- 31.Díez-Fernández C, Sanz N, Alvarez AM, Zaragoza A, Cascales M. Influence of aminoguanidine on parameters of liver injury and regeneration induced in rats by a necrogenic dose of thioacetamide. Br J Pharmacol. 1998;125:102–108. doi: 10.1038/sj.bjp.0702014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yildiz G, Demiryürek AT, Sahin-Erdemli I, Kanzik I. Comparison of antioxidant activities of aminoguanidine, methylguanidine and guanidine by luminol-enhanced chemiluminescence. Br J Pharmacol. 1998;124:905–910. doi: 10.1038/sj.bjp.0701924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noda T, Iwakiri R, Fujimoto K, Aw TY. Induction of mild intracellular redox imbalance inhibits proliferation of CaCo-2 cells. FASEB J. 2001;15:2131–2139. doi: 10.1096/fj.01-0131com. [DOI] [PubMed] [Google Scholar]
- 34.Menon SG, Goswami PC. A redox cycle within the cell cycle: ring in the old with the new. Oncogene. 2007;26:1101–1109. doi: 10.1038/sj.onc.1209895. [DOI] [PubMed] [Google Scholar]
- 35.Aw TY. Cellular redox: a modulator of intestinal epithelial cell proliferation. News Physiol Sci. 2003;18:201–204. doi: 10.1152/nips.01448.2003. [DOI] [PubMed] [Google Scholar]


