Abstract
AIM: To assess the hepatic changes after induction of different periods of renal ischemia.
METHODS: Rats were subjected to either sham operation or ischemia (30, 45 and 60 min) followed by 60 min reperfusion. Liver and renal functional indices were measured. Hepatic glutathione (GSH) and ferric reducing antioxidant power levels and the concentration of interleukin (IL)-10 and tumor necrosis factor (TNF-α) were evaluated. Portions of liver and kidney tissues were fixed for histological evaluation.
RESULTS: Forty-five minutes renal ischemia followed by 60 min reperfusion caused significant changes in liver structure and a significant reduction in renal function. These rats showed a significant decrease in liver GSH, as well as a significant increase in TNF-α and IL-10 concentrations. These results demonstrated that renal ischemia caused changes in liver histology, function, oxidative stress and inflammatory status, which led to a reduction in hepatic antioxidant capacity. With 30 min ischemia, the magnitude of these changes was less than those with 45 or 60 min ischemia.
CONCLUSION: A minimum of 45 min ischemia is needed to study the effects of renal injury on the liver as a remote organ.
Keywords: Renal ischemia, Liver, Remote organ, Oxidative stress, Inflammation
INTRODUCTION
Liver and kidney are both involved in the regulation of body homeostatic responses, metabolism and excretion of drugs and toxic products. Recent studies have suggested cross-talk between the liver and kidneys. Ischemia/reperfusion (I/R)-induced local response in kidney tissue has been well documented in a number of studies[1,2]. However, remote effects of renal I/R injury on the liver need further investigation. Renal injury associated with liver disease is an extensively encountered clinical problem of varied etiology and high mortality. I/R injury induces an inflammatory response, which results in the formation of reactive oxygen species (ROS) that augments local tissue damage or affects organs remote from the site of I/R. An important function of ROS is the regulation of cytokine gene expression[3].
In 2002, Miyazawa et al[4] showed an influx of neutrophils and lymphocytes, not only in the clamped kidney, but also in the hepatic sinusoids concomitantly with liver dysfunction. These findings indicate that a systemic cellular immune response, including intermediate T cells, affects multiple organs during ischemic acute renal failure (ARF), which may play an important role in the development of multi-organ failure. Since the liver tissue represents one of the vascular beds into which ROS are delivered, it would be likely to manifest a number of toxic effects of these molecules. It has been suggested that dehydroepiandrosterone treatment has a beneficial effect on antioxidant defenses against hepatic injury after renal I/R in rabbits, possibly by augmenting glutathione (GSH) levels and lowering malondialdehyde (MDA) production[5].
Kielar et al[6] have evaluated the extrarenal regulation of ARF. This regulation may be as a result of increased production of cytokines such as tumor necrosis factor (TNF)-α and growth factors such as hepatocyte growth factor (HGF) produced by extrarenal organs[7]. In addition, there is an inflammatory response to renal ischemia that results in secondary injury[8]. Further more, renal ischemia results in increased interleukin (IL)-6 mRNA expression[9], renal production of IL-6, and expression of IL-10 receptors. IL-6 stimulates the production of IL-10 by the liver, which might ameliorate renal injury.
The question remaining is to what extent the renal damage affects the liver, although the liver function itself may enhance or reduce the extent of renal damage. It has been suggested that, while liver disease may alter the course of renal injury, exogenous administration of regulatory factors produced by extrarenal organs may play a therapeutic role in ARF[6,10]. Thus, the aim of the present study was to examine the effects of different periods of renal ischemia on rat liver function, histology, cytokine levels and antioxidant status.
MATERIALS AND METHODS
Surgical procedure
Twenty male Sprague-Dawley rats weighing 250–300 g were included in this study and randomly assigned into one of the four experimental groups (n = 5): (1) sham-operated; (2) 30 min ischemia, 60 min reperfusion; (3) 45 min ischemia, 60 min reperfusion; and (4) 60 min ischemia, 60 min reperfusion.
Rats were placed on a warming pad and anesthetized with pentobarbital sodium (60 mg/kg ip, followed by 6 mg/kg per hour iv). Body temperature was maintained at 37 ± 1°C. A tracheotomy was performed to facilitate free breathing. The right femoral artery was cannulated and connected to a pressure transducer for mean arterial pressure measurement. The tail vein was cannulated for infusion of 0.9% (9 g/L) NaCl solution. A midline laparatomy was performed and the renal arteries were carefully separated from around the tissues. After completion of the surgery, rats were allowed to stabilize for 30 min.
In the I/R groups, renal arteries were occluded by a non-traumatic micro-vascular clips for 30, 45 and 60 min, followed by 1 h reperfusion. Occlusion was approved visually by color change of the kidney to a paler shade and reperfusion by blushing. Sham-operated animals underwent identical surgical treatment, including isolation of both renal arteries. However, artery occlusion was not performed. At the end of the experimental procedure, serum was collected for determination of blood urea nitrogen (BUN) and creatinine. Kidney and liver tissues were removed and prepared for future analysis.
Measurement of arterial blood pressure
Arterial blood pressure and heart rate were continuously monitored via a femoral artery cannula that was connected to a pressure transducer device. The transducer was connected to a PowerLab/4SP data acquisition system (AD Instruments).
Biochemical assay
Blood concentration of creatinine, BUN, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined by commercially available kits.
Histological procedures
After formalin fixation (10% phosphate-buffered) and dehydration, paraffin-embedded renal and hepatic sections (4 μm) were stained by hematoxylin and eosin. Histopathology for all tissues was evaluated per section in at least 10 randomly selected non-overlapping fields at × 400 magnification of the sections. Kidney tissues were evaluated for the presence of congestion. Tubules were evaluated for the presence of degenerative changes (vacuolization), tubular dilatation, luminal debris and cast formation, and loss of brush borders from proximal tubules. Liver sections were evaluated for the presence of congestion, cellular degenerative changes, cytoplasmic vacuolization and leukocyte infiltration.
Tissue homogenization
A portion of each liver was homogenized in KCl buffer (pH 7.4) for ferric reducing antioxidant power (FRAP) assay and in TCA for GSH assay. After centrifugation for 30 min, the supernatants were removed and subjected to analysis.
GSH assay
Liver GSH was assayed according to the Tietz method. 5, 5’-Dithiobis 2-nitrobenzoic acid was used as a chromogen and the absorbance of the reduced chromogen was measured at 412 nm. The value for each sample was extracted from the standard curve.
Measurement of ferric reducing ability of liver (FRAP)
FRAP assay was performed according to the method of Benzie and Strain (1996)[11]. Briefly, 50 μL supernatant was added to 1.5 mL freshly prepared FRAP solution, and the absorbance was measured at 593 nm.
Measurement of cytokine concentrations in liver tissues
TNF-α and IL-10 concentrations were measured in the liver of animals using an ELISA (Rat IL-10 and TNF-α ELISA kit, Diaclone A; Tepnel, Besancon, France). One hundred milligrams of tissue were homogenized in 1 mL PBS that contained antiproteases (0.1 mmol/L phenylmethylsulfonyl fluoride, 0.1 mmol/L benzethonium chloride, 10 mmol/L EDTA, 20 KI aprotinin A and 0.05% Tween 20). The samples were then centrifuged for 10 min at 3000 r/min and the supernatant was used for ELISA.
Enzyme activities and Northern blot analysis
Total cellular RNA was extracted and hybridization was performed according to Church and Gilbert[12]. The expression of spermidine/spermine N-acetyl-transferase (SSAT) and collaborates with alternate reading frame (CARF), two mediators of tissue injury, in normal and experimental groups was evaluated.
Statistical analysis
The results are given as mean ± SE. Statistical analysis was performed by analysis of variance using a post-hoc Duncan test. The null hypothesis was rejected at the 0.05 level of significance. SPSS 11.0 software (Chicago, IL, USA) was used for data analysis.
RESULTS
In all groups, the mean arterial pressure (MAP) during the experimental period was not significantly different from the basal value (110 ± 9.8 mmHg and 109 ± 10.3, respectively). Data on liver function tests are presented in Table 1.
Table 1.
Data on liver function tests in different groups
| Groups | AST (U/L) | ALT (U/L) |
| Sham-operated | 263.7 ± 25.7 | 128 ± 27.5 |
| 30 min ischemia + 1 h reperfusion | 275 ± 52.6 | 119 ± 20.8 |
| 45 min ischemia + 1 h reperfusion | 459.7 ± 17.7a | 128 ± 15.5 |
| 60 min ischemia + 1 h reperfusion | 491.5 ± 74.8a | 444.6 ± 198.6a |
P < 0.05 compared to sham-operated group. The data are presented as mean ± SE (n = 5 in each group).
Effect of renal ischemia on serum biochemical parameters
BUN did not change after 30 min renal artery occlusion followed by 1 h reperfusion, but serum creatinine increased significantly compared to sham-operated animals (9.8 ± 1.1 mg/L vs 5.5 ± 1.5 mg/L, P < 0.05). Both 45 and 60 min ischemia followed by 1 h reperfusion resulted in significant increases in plasma creatinine (11.1 ± 1.7 mg/L and 12.4 ± 0.7 mg/L vs 5.5 ± 1.5 mg/L, P < 0.05) and BUN (340 ± 38.5 mg/L and 350 ± 28.1 mg/L vs 237.5 ± 11.0 mg/L, P < 0.05) compared to the sham-operated group (Figure 1).
Figure 1.
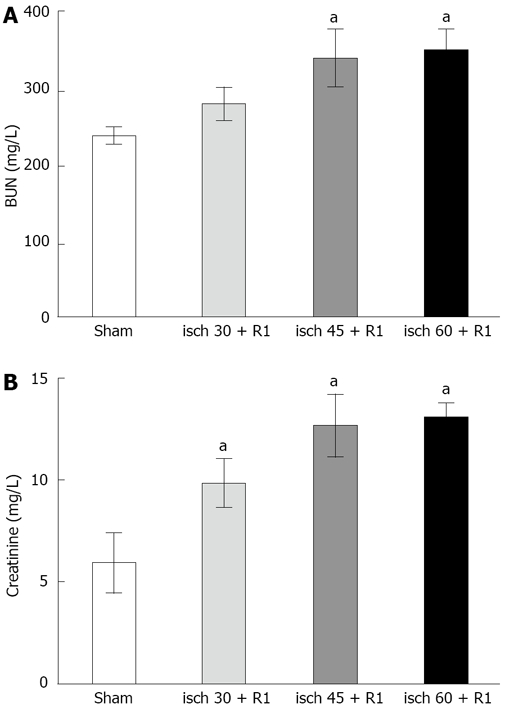
Alterations in renal function during different renal I/R periods. A: BUN; B: Plasma creatinine. The data are presented as mean ± SE. aP < 0.05 vs sham-operated group. All ischemic periods were followed by 1 h reperfusion.
Effect of renal ischemia on liver oxidative parameters
The level of liver GSH did not change significantly after 30 min renal ischemia followed by 1 h reperfusion (Figure 2). Both 45 (19.5 ± 2.7 μmol/g, P < 0.05) and 60 min (25.86 ± 1.71 μmol/g, P < 0.05) ischemia followed by 1 h reperfusion caused a significant reduction in liver GSH compared to the sham-operated group (36.2 ± 2.07 μmol/g, P < 0.05). Figure 2 shows the level of FRAP in liver tissues. There were no significant differences between the groups.
Figure 2.
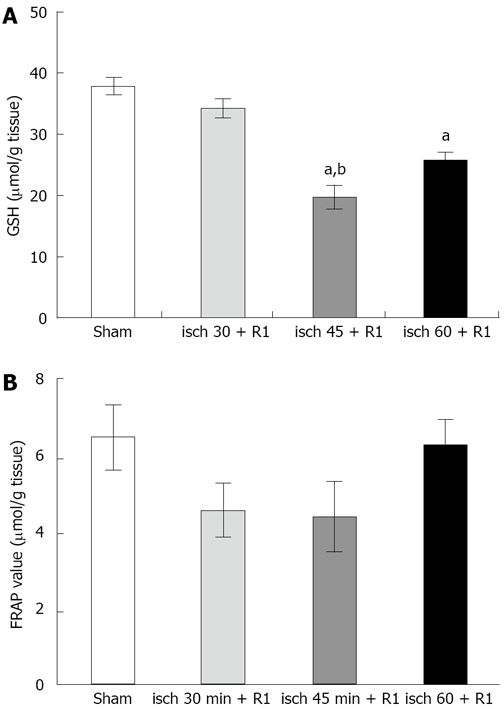
Alterations in liver GSH (A) and FRAP (B) during different renal I/R periods. The data are presented as mean ± SE. aP < 0.05 vs sham-operated group; bP < 0.05 vs 30-min ischemia group. All ischemic periods were followed by 1 h reperfusion.
Effect of renal ischemia on liver TNF-α
The level of liver TNF-α was increased significantly after 30 (466.9 ± 71.7 pg/100 mg, P < 0.05), 45 (507.2 ± 71.7 pg/100 mg, P < 0.05) and 60 min (718.6 ± 68.8 pg/100 mg, P < 0.05) renal ischemia followed by 1 h reperfusion compared to the sham-operated group (210 ± 16.6 pg/100 mg). The increase in liver TNF-α following the induction of 60 min ischemia was significantly higher than the other ischemic periods.
Effect of renal ischemia on liver IL-10
Renal ischemia (30, 45 and 60 min) followed by 1 h reperfusion resulted in a significant increase in liver IL-10 compared to that in the sham-operated group (121 ± 34.5 pg/100 mg, P < 0.05). Induction of 60 min ischemia followed by 1 h reperfusion (667.8 ± 34.5 pg/100 mg, P < 0.05) showed a significant difference from the other groups (Figure 3).
Figure 3.
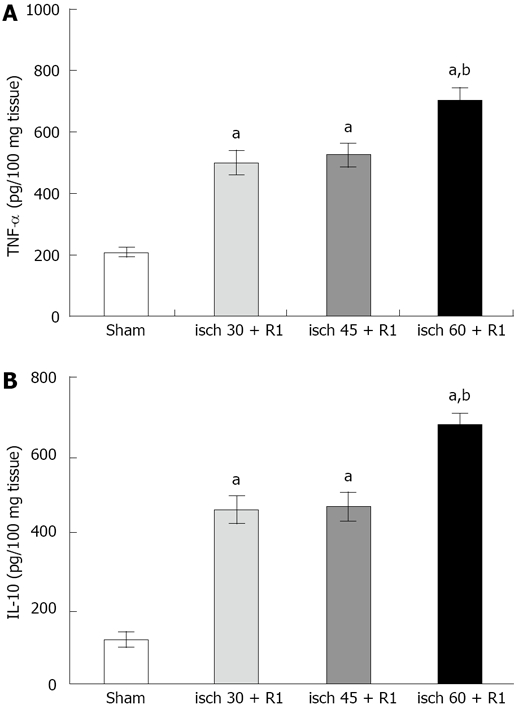
Alterations in liver TNF-α (A) and IL-10 (B) during different renal I/R periods. The data are presented as mean ± SE. aP < 0.05 vs sham-operated group; bP < 0.05 vs other groups. All ischemic periods were followed by 1 h reperfusion.
Effect of renal ischemia on liver histology
Liver sections were evaluated for the presence of congestion, cellular degenerative changes, cytoplasmic vacuolization and leukocyte infiltration. The sections from the sham-operated rats displayed minimal/no changes. In the 30-min ischemia group, congestion was present, but much less than for the 45- or 60-min ischemic-reperfused group. There was no apparent evidence of cellular degenerative changes including cytoplasmic vacuolization. Leukocyte infiltration was almost absent in this group. Vacuolization was frequent in the 45-min ischemic-reperfused tissues. Irregularity, pale (atypical) nuclei, disintegrated cytoplasm, and infiltration of leukocytes were seen. Similar changes were observed in the 60-min ischemic-reperfused group (Figure 4).
Figure 4.
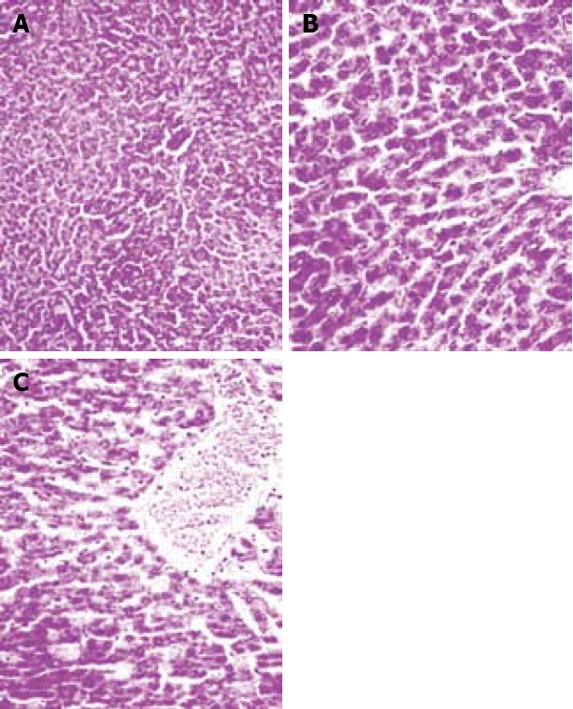
Hematoxylin and eosin-stained sections of rat liver. A: Sham-operated group. B: I/R group, vacuolization was frequent in the 45 min ischemia-reperfused tissues. Irregularity, pale (atypical) nuclei, disintegrated cytoplasm, and infiltration of leukocytes were seen. C: Similar changes were observed in the 60 min ischemia-reperfused group (× 400).
Effect of renal ischemia on SSAT and CARF
RNA isolation and Northern hybridization were performed on some liver samples and the expression of SSAT was examined (Figure 5A). There was an increase in the 60-min renal ischemia compared with the control group, albeit by only about 35%, which was significant. The expression of CARF remained unchanged (Figure 5B).
Figure 5.
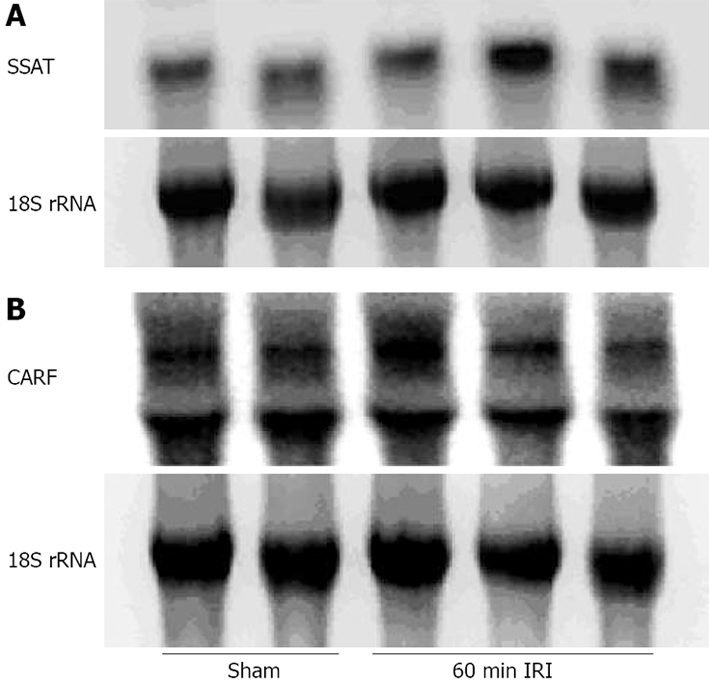
Alterations in liver SSAT and CARF expression during 60 min renal ischemia followed by 1 h reperfusion. A: SSAT mRNA levels in the liver of renal ischemic samples were 139 ± 7% vs the sham-operated group, after adjustment for RNA loading as determined by 18S rRNA. P < 0.05 vs sham-operated group. B: CARF mRNA levels in the liver of renal ischemic samples were 98% ± 8% of levels in sham-operated group, after adjustment for RNA loading as determined by 18S rRNA.
DISCUSSION
Acute renal ischemic injury continues to be associated with a high mortality rate. Renal I/R injury occurs in many clinical situations, such as transplantation, partial nephrectomy, sepsis, hydronephrosis, or elective urological operations. Although most research in this area has focused on the renal response to this injury, recent work has suggested that renal injury affects and is also regulated by the extra-renal organs including the liver[13]. In the present study, the changes in hepatic function, histology, cytokine levels and antioxidant status were examined after induction of various periods of rat renal ischemic injury.
In 2002, Serteser et al[14] demonstrated some changes in hepatic TNF-α levels and oxidation products after renal I/R injury in mice. They have suggested that 30 min ischemia and 60 min reperfusion is sufficient to elicit remote effects of I/R injury. Their study was performed in mice, while we used rats in our study. This may explain why 30 min ischemia was less injurious compared to 45 and 60 min. In 2003, it was suggested that hepatic production of IL-10 and IL-1 receptor antagonists, in response to acute bile duct ligation, ameliorates ischemic ARF[15]. Hoke et al[16] in 2007 demonstrated that acute absence of kidney function results in pulmonary injury independent of renal ischemia, and highlighted the critical role of the kidney in the maintenance of serum cytokine balance and pulmonary homeostasis. In the present study, all three periods of ischemia caused an increase in hepatic TNF-α levels; but, the increase after 60 min was significantly higher than after 30 and 45 min ischemia. This means that, after 45 min ischemia, there are no irreversible changes, namely severe necrotic alterations.
In our study, as expected, I/R caused a reduction in renal function and structural alteration in an ischemia-time-dependent manner. Liver function was almost preserved after 30 min ischemia, partially reduced after 45 min, but showed a significant reduction in the 60-min ischemia group. This indicated that the liver underwent more prominent and severe damage in the 60-min group. Liver histology showed that, after 30 min ischemia, there was no apparent injury; but, 45 and 60 min ischemia elicited histological changes. The cytokines in the liver tissue were significantly increased after 30 and 45 ischemia; but, the increase after 60 min was very high and showed significant differences from the control and other ischemia groups. Although GSH showed a reduction in all of the experimental groups, the reduction in the 45- and 60-min groups was much higher than that in the 30-min ischemia group. The changes in GSH concentration suggested that ROS mediated the biomolecular alterations. On the other hand, FRAP showed a reduction after ischemia (although not significant). The rise in the 60-min group may have resulted from the contribution of uric acid to the overall concentration of FRAP, as reported previously[2].
RNA isolation and Northern hybridization were also conducted on some liver samples. Total cellular RNA was extracted and hybridization was performed according to Church and Gilbert[12]. The expression of SSAT and CARF, two mediators of tissue injury, in normal and experimental groups was evaluated. The expression of SSAT was increased in the 60-min renal ischemia group compared with the controls. The expression of CARF remained unchanged. It is likely that SSAT is a more sensitive marker of injury than CARF.
These data clearly demonstrate that renal ischemia causes detrimental changes in liver histology, function, oxidative stress and inflammatory status, which leads to a reduction in hepatic antioxidant capacity. After 30 min ischemia, the magnitude of these changes is much less than after 45 or 60 min ischemia. A minimum of 45 min ischemia is needed to study the effects of renal injury on liver as a remote organ. Care should be taken to protect other organs remote from I/R sites, especially during renal surgery.
COMMENTS
Background
The effect of locally applied ischemia/reperfusion (I/R) injury to the kidney has been under investigation for many years. However, little is known about the changes in liver function and oxidative stress in renal I/R injury.
Research frontiers
Renal injury associated with liver disease is an extensively encountered clinical problem of varied etiology and high mortality. Recent studies have suggested crosstalk between the liver and kidneys. I/R-induced local response in kidney tissue has been well documented in a number of studies. However, remote effects of renal I/R injury on the liver need further investigation. The aim of the present study was to assess the hepatic changes after induction of different periods of renal ischemia.
Innovations and breakthroughs
Rats were subjected to sham operation or ischemia (30, 45 and 60 min) followed by 60 min reperfusion. This study compared different ischemia times by the use of the following indices: hepatic glutathione and ferric reducing antioxidant power levels, and concentration of interleukin (IL)-10 and tumor necrosis factor-α.
Applications
To study the effects of renal injury on liver as a remote organ, a minimum of 45 min ischemia is needed. Care should be taken to protect organs remote from I/R sites especially during renal surgery.
Peer review
The authors describe the effects of different periods of rat renal ischemia on the liver in a rat model. They observed significant hepatic damage after at least 45-60 min of renal ischemia. This manuscript is well written and provides new information concerning the consequences of renal I/R injury on the liver.
Supported by A grant from Tehran Medical Sciences University
Peer reviewer: Valentin Fuhrmann, MD, Department of Internal Medicine 4, Intensive Care Unit, Medical University Vienna, Waehringer Guertel 18-20, A-1090 Vienna, Austria
S- Editor Li LF L- Editor Kerr C E- Editor Zheng XM
References
- 1.Kadkhodaee M, Hanson GR, Towner RA, Endre ZH. Detection of hydroxyl and carbon-centred radicals by EPR spectroscopy after ischaemia and reperfusion of the rat kidney. Free Radic Res. 1996;25:31–42. doi: 10.3109/10715769609145654. [DOI] [PubMed] [Google Scholar]
- 2.Kadkhodaee M, Hemmati M, Zahmatkesh M, Ghaznavi R, Mirershadi F, Mahdavi-Mazde M, Seifi B. Assessment of plasma antioxidant status in hemodialysis patients. Ther Apher Dial. 2008;12:147–151. doi: 10.1111/j.1744-9987.2008.00561.x. [DOI] [PubMed] [Google Scholar]
- 3.Remick DG, Villarete L. Regulation of cytokine gene expression by reactive oxygen and reactive nitrogen intermediates. J Leukoc Biol. 1996;59:471–475. doi: 10.1002/jlb.59.4.471. [DOI] [PubMed] [Google Scholar]
- 4.Miyazawa S, Watanabe H, Miyaji C, Hotta O, Abo T. Leukocyte accumulation and changes in extra-renal organs during renal ischemia reperfusion in mice. J Lab Clin Med. 2002;139:269–278. doi: 10.1067/mlc.2002.122832. [DOI] [PubMed] [Google Scholar]
- 5.Yildirim A, Gumus M, Dalga S, Sahin YN, Akcay F. Dehydroepiandrosterone improves hepatic antioxidant systems after renal ischemia-reperfusion injury in rabbits. Ann Clin Lab Sci. 2003;33:459–464. [PubMed] [Google Scholar]
- 6.Kielar ML, Rohan Jeyarajah D, Lu CY. The regulation of ischemic acute renal failure by extrarenal organs. Curr Opin Nephrol Hypertens. 2002;11:451–457. doi: 10.1097/00041552-200207000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Nakatani T, Kim T, Uchida J, Kumata N, Kawashima H, Sugimura K. Hepatocyte growth factor ameliorates renal hemodynamic disorder after ischemia/reperfusion. Int J Mol Med. 2002;10:217–219. [PubMed] [Google Scholar]
- 8.Daemen MA, van de Ven MW, Heineman E, Buurman WA. Involvement of endogenous interleukin-10 and tumor necrosis factor-alpha in renal ischemia-reperfusion injury. Transplantation. 1999;67:792–800. doi: 10.1097/00007890-199903270-00003. [DOI] [PubMed] [Google Scholar]
- 9.Lemay S, Rabb H, Postler G, Singh AK. Prominent and sustained up-regulation of gp130-signaling cytokines and the chemokine MIP-2 in murine renal ischemia-reperfusion injury. Transplantation. 2000;69:959–963. doi: 10.1097/00007890-200003150-00049. [DOI] [PubMed] [Google Scholar]
- 10.Gupta S, Verfaillie C, Chmielewski D, Kim Y, Rosenberg ME. A role for extrarenal cells in the regeneration following acute renal failure. Kidney Int. 2002;62:1285–1290. doi: 10.1111/j.1523-1755.2002.kid569.x. [DOI] [PubMed] [Google Scholar]
- 11.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 12.Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol. 2003;14:1549–1558. doi: 10.1097/01.asn.0000064946.94590.46. [DOI] [PubMed] [Google Scholar]
- 14.Serteser M, Koken T, Kahraman A, Yilmaz K, Akbulut G, Dilek ON. Changes in hepatic TNF-alpha levels, antioxidant status, and oxidation products after renal ischemia/reperfusion injury in mice. J Surg Res. 2002;107:234–240. doi: 10.1006/jsre.2002.6513. [DOI] [PubMed] [Google Scholar]
- 15.Jeyarajah DR, Kielar ML, Zhou XJ, Zhang Y, Lu CY. Acute bile duct ligation ameliorates ischemic renal failure. Nephron Physiol. 2003;95:28–35. doi: 10.1159/000073677. [DOI] [PubMed] [Google Scholar]
- 16.Hoke TS, Douglas IS, Klein CL, He Z, Fang W, Thurman JM, Tao Y, Dursun B, Voelkel NF, Edelstein CL, et al. Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. J Am Soc Nephrol. 2007;18:155–164. doi: 10.1681/ASN.2006050494. [DOI] [PubMed] [Google Scholar]


