Abstract
A number of experiments have evaluated self-administration of the combination of a stimulant and an opioid. Less is known about the combination of a stimulant and a CNS depressant. The present experiment was designed to examine self-administration of the mixture of cocaine and pentobarbital (PB). Rhesus monkeys (n=4) prepared with i.v. catheters were allowed to self-administer cocaine or saline under a progressive-ratio schedule. When responding was stable, doses of cocaine and PB, alone or in combination, were made available in test sessions. Cocaine functioned as a positive reinforcer in a dose-related manner in all monkeys. PB functioned as a relatively weaker reinforcer in one of four monkeys. Self-administration of intermediate doses of cocaine (0.025 – 0.1 mg/kg/injection) was decreased when mixed with PB (0.05 – 0.2 mg/kg/injection); full maximum responding was reestablished when cocaine dose was increased. The magnitude of the shift to the right in the cocaine dose-response function was directly related to PB dose. When PB was given as an i.v. pretreatment there was no effect on cocaine self-administration up to a sedative dose of PB (5.6 mg/kg), suggesting that responding was not non-specifically suppressed by PB. Thus, simultaneous self-administration of PB diminished the potency but not the strength of cocaine as a reinforcer, potentially encouraging self-administration of larger doses of cocaine.
Keywords: Drug abuse, self-administration, monkey, cocaine, pentobarbital, drug mixture
1. Introduction
Abuse of drug mixtures is common. It has been suggested that drug interactions that enhance positive effects or diminish negative effects of one or more of the combined drugs contribute to this phenomenon (Kosten et al. 1987; Kreek 1987). Laboratory research using self-administration assays can help us to better understand these sorts of mechanisms. Several drug combinations have been studied in the laboratory in humans and non-humans under a variety of conditions (e.g., Foltin and Fischman 1990; Meisch and Lemaire 1990). Nevertheless, it is not always clear whether drugs delivered in combination interact and, if so, what the mechanisms underlying an interaction might be. For example, several studies have assessed the stimulant/opioid mixture. In some cases, there was little evidence for an interaction (Hemby et al. 1996; Mattox et al. 1997; Mello et al. 1995). In studies that quantified drug interactions, evidence for additivity (Negus, 2005; Smith et al., 2006) or, under some conditions, sub- or super-additivity (Negus, 2005; Woolverton et al., 2008b) of opioids and stimulants has been reported.
We have recently conducted a series of studies of self-administration of drug mixtures by monkeys (Ranaldi and Woolverton, 2002; Wang and Woolverton, 2007; Wee and Woolverton, 2006; Woolverton et al. 2008a, b). One conclusion that can be supported by these and other experiments is that the nature of the interaction between self-administered drugs of abuse depends not only upon the specific drugs that are combined but also upon the proportions of each. It has also been proposed that super-additivity as reinforcers is confined to drugs with different mechanisms of action (Woolverton et al., 2008a). We have previously examined in monkeys responding under a progressive-ratio (PR) schedule the self-administration of drug mixtures with comparable mechanisms of action and found these to be additive (Woolverton et al., 2008a). We have also studied the combination cocaine and the μ opioid agonist remifentanil, or the antihistamine diphenhydramine (Wang and Woolverton, 2007) and found additivity or super-additive interactions. Clearly, super-additivity is not always observed as both additivity and sub-additive interactions have been reported for self-administration of mixtures of cocaine and drugs with different mechanisms of action (Negus 2005; Rowlett et al. 2007; Smith et al. 2006; see also Winger et al., 2007). Additionally, combining cocaine with drugs that have different mechanisms of action and do not function as positive reinforcers can suppress cocaine self-administration (Ranaldi and Woolverton, 2002; Wee and Woolverton, 2006). Therefore, different mechanisms of action may be a necessary but not sufficient condition for super-additive interactions to occur. The present study was designed to extend our analysis of the self-administration of mixtures of cocaine and other abused drugs to another pharmacological class, CNS depressants. Under conditions that were comparable to those used in previous experiments, cocaine and the barbiturate pentobarbital (PB) were made available for self-administration alone and in combination by rhesus monkeys.
2. Methods
All animal use procedures were approved by the University of Mississippi Medical Center’s Animal Care and Use Committee and were in accordance with US National Institutes of Health guidelines.
2.1 Animals and Apparatus
The subjects were four male rhesus monkeys (Macaca mulatta) weighing between 8.8 and 11.9 kg at the beginning of the study. All monkeys had extensive histories of drug self-administration. Most recently, all monkeys had participated in a study of self-administration of cocaine and remifentanil under the schedule of reinforcement used in the present study (Woolverton et al. 2008). All monkeys were provided with sufficient food (160–240 g/day, Teklad 25% Monkey Diet, Harlan/Teklad, Madison, WI) to maintain stable body weight and had unlimited access to water. Fresh fruit and a vitamin supplement were provided daily and three times a week, respectively. Lighting was cycled to maintain 16 hours of light and 8 hours of dark, with lights on at 06:00 hours.
The monkeys were individually housed in the experimental cubicles (1.0 m3, PlasLabs, Lansing, MI). Each monkey was fitted with a stainless-steel harness attached by a tether to the rear wall of the cubicle. The front door of the cubicle was made of transparent plastic and the other walls were opaque. Two response levers (PRL-001, BRS/LVE, Beltsville, MD) were mounted on the inside of the door. Four jeweled stimulus lights, two red and two white, were mounted above each lever. Drug injections were delivered by a peristaltic infusion pump (Cole-Parmer Co., Chicago, IL). A Macintosh computer with custom interface and software controlled all events in an experimental session.
2.2 Procedure
Each monkey was implanted with a single-lumen silastic catheter (0.26 cm o.d. × 0.076 cm i.d.; Cole-Parmer Co., Chicago, IL) into a jugular (internal or external) or femoral vein. Brachial veins were implanted with a silicone catheter (0.17 cm o.d. × 0.07 cm i.d.; Access Technologies, Skokie, IL). For catheter implantation, the monkey was injected with a combination of atropine sulfate (0.04 mg/kg, i.m.) and ketamine hydrochloride (10 mg/kg, i.m.) followed 20–30 min later by inhaled isoflurane under isoflurane anesthesia. The proximal end of the catheter was inserted into the vein and terminated in the vena cava near the right atrium. The distal end was threaded subcutaneously to exit the back of the monkey, threaded through the spring arm, out the rear of the cubicle and connected to the peristaltic pump. In the event of catheter failure, surgery was repeated using another vein, after the veterinarian confirmed the health of the monkey.
Experimental sessions began at 11:00 hours each day and were conducted seven days per week. Thirty minutes before each session started, each monkey's catheter was filled with the solution for the session without infusing the solution into the monkey. At the start of a session, the white lights were illuminated above both levers and pressing the right lever resulted in the delivery of a drug injection for 10 seconds. During the injection, the white lights were extinguished and the red lights were illuminated. Pressing the left lever was counted but had no other programmed consequence. After the session, catheters were filled with 0.9% saline containing heparin (40 units/ml).
Lever pressing was maintained under a progressive-ratio (PR) schedule of reinforcement comparable to that described by Wilcox et al. (2000). The PR schedule consisted of five components, each made up of four trials, for a total of 20 trials. The response requirement was fixed for the four trials within a component. For all monkeys, the response requirement started at 100 responses per injection and doubled in successive components. A subject had 30 minutes to complete a trial (limited hold 30 min: LH 30'). A trial ended with a 10-sec drug injection or the expiration of the LH. There was a 5 minute-timeout (TO 5') after each trial. If the response requirement was not completed for two consecutive trials (i.e., the LH expired), or the animal self-administered all 20 injections, the session ended.
In baseline sessions, cocaine or saline was available for injection. The baseline dose of cocaine or saline was initially available under a double-alternation schedule. That is, two consecutive cocaine sessions were followed by two consecutive saline sessions. When responding was stable (running mean for each type of baseline session within ± 2 injections, and four or fewer injections/session in saline sessions) for at least two consecutive double-alternation sequences (i.e., eight sessions), test sessions were inserted into the daily sequence between two saline and two cocaine sessions. Additionally, to prevent monkeys from learning this session sequence, a randomly determined saline or cocaine baseline session was inserted after every other test session. Thus, the daily sequence of sessions was C, S, T, S, C, T, R, C, S, T, S, C, T, R, where “C”, “S”, “R” and “T”, respectively, represent a cocaine, a saline, a randomly determined cocaine/saline and a test session. The baseline dose of cocaine was the lowest dose that maintained the maximum injections in all monkeys, 0.2 mg/kg/injection. During test sessions, one of various doses of cocaine (0.006–0.4 mg/kg/injection) or PB (0.2–3.2 mg/kg/injection) was available for self-administration under conditions identical to baseline sessions. All doses were tested at least twice in each monkey, once with a saline session the day before and once with a cocaine session the day before. When the two test sessions of a dose showed high variability (the number of injections exceeded ± 3 injections of the mean), the dose was tested twice as before, once after saline session and once after cocaine baseline session. This occurred once each in two of the monkeys. If the redetermined effects were less variable and comparable to one of the original test sessions, then those three sessions of four were used for data analysis. If the redetermined effects were variable like the initially determined effects, all four sessions were used to calculate the mean. In all monkeys, PB was tested first up to 1.6 mg/kg/injection, cocaine was tested second, and then a high dose of PB (3.2mg/kg/injection) was tested. After testing drugs alone, mixtures of cocaine and PB were tested. For mixtures, monkeys were tested with combinations of various doses of cocaine with PB doses of 0.05, 0.1 and 0.2 mg/kg/injection. In all cases, doses of drugs alone and mixtures were available in an irregular order across monkeys.
After studying self-administration of mixtures of cocaine and PB, baseline sessions proceeded as usual. In test sessions monkeys were treated with PB before sessions of self-administration of a low (0.05 mg/kg/injection) or a high (0.4 mg/kg/injection) reinforcing dose of cocaine. PB doses (0.05, 0.1, 0.2, 0.4, 0.8, 1.6, 3.2 and 5.6 mg/kg) were given i.v. via the catheter five minutes before the start of the test session. The dose of PB was increased until ataxia and sedation were observed shortly after the injection.
After PB pretreatment tests were completed, the PR schedule was changed from a PR schedule that began at 100 and doubled in each component, to PR schedule that began at 10 and doubled in each component. The goal of this manipulation was to determine whether responding was maintained with a lower response requirement. When responding in cocaine and saline sessions was again stable, as previously defined, self-administration test sessions were conducted with saline, cocaine 0.05 mg/kg/injection alone, PB 0.05mg/kg/injection alone, and combinations of cocaine 0.05 mg/kg/injection with PB 0.05 mg/kg/injection. The cocaine dose was chosen because it was the lowest, maximally effective doses of cocaine under the PR 100 and, presumably, the most sensitive to modification by PB. The PB dose was chosen because it was the lowest dose that fully decreased the self-administration of 0.05 mg/kg/injection cocaine under the PR 100 schedule.
2.3 Data analysis
The mean number of injections per session was calculated individually from the test sessions as a function of dose (see dePoortere et al. 1993; Rowlett et al. 1996). The range of injections served as a measure of variability in individual subjects. A dose of a drug was considered to function as a reinforcer if the mean number of injections was above levels seen with saline and the ranges did not overlap. Means and standard errors were calculated for all doses and combinations.
Individual ED50 values were calculated from log dose-response functions using the ascending limb of the dose-response function and non-linear regression analysis with level of saline self-administration in baseline sessions and the maximum number of injections of the test drug serving as minimum and maximum values, respectively, for the analysis (GraphPad Prism 4.0). Group mean ED50s were calculated from average individual ED50s. Group mean ED50s of cocaine and the combinations of cocaine with three different doses of PB were compared using one-way analysis of variance (ANOVA) for repeated measures for the cocaine alone and the combinations of cocaine and three different dose of PB. Post-hoc comparisons were made using Newman-Keuls Multiple Comparison test.
Additionally, the maximum number of injections, regardless of dose, was calculated for cocaine and the combinations of cocaine and PB to serve as a measure of reinforcing strength. Statistical significance of differences was analyzed using one-way analysis of variance (ANOVA) for repeated measures for the cocaine alone and the combinations of cocaine with the three different doses of PB.
For PB pretreatment sessions, the number of injections of cocaine at 0.05 or 0.4 mg/kg/injection alone and these same doses of cocaine with various doses of PB as pretreatment were compared using one-way analysis of variance (ANOVA) for repeated measures. The mean number of injections per session of cocaine and the combinations under different PR schedules (PR 10 and PR 100) were compared using a paired t-test.
2.4 Drugs
Cocaine HCl was provided by the National Institute on Drug Abuse. PB sodium was purchased as Nembutal from Abbott Laboratories (North Chicago, IL). All drugs were prepared in 0.9% saline and were used within a week of preparation. Drug mixtures were prepared in appropriate concentrations, mixed in the same solution. Doses are expressed as the salt forms of the drugs. Injections were approximately 1.5 ml delivered over 10 seconds.
3. Results
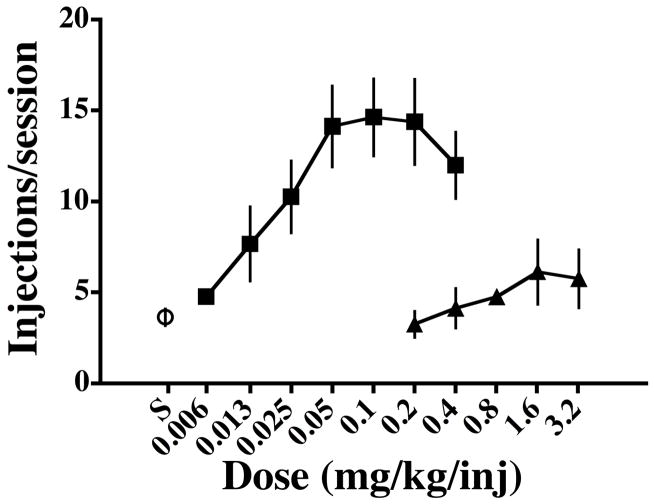
Cocaine functioned as a positive reinforcer in a dose-dependent manner in all monkeys with a mean ED50 of 0.018 (± 0.003 s.e.m.) mg/kg/injection (Figure 1). The dose of 0.025 mg/kg/injection cocaine was a reinforcer in two of the monkeys and higher doses were reinforcers in all monkeys. Individual dose-response functions were asymptotic in two monkeys (R0209 and M1389), and biphasic in the other two monkeys (M1388 and CK47). PB failed to maintain responding in three of four monkeys (Figure 1). In the fourth monkey (CK47), PB served as a reinforcer at both 1.6 and 3.2 mg/kg/injection, maintaining means of 11 (10 and 12) and 9 (8 and 10) injections/session, respectively.
Figure 1.
Dose-response functions for self-administration of cocaine (squares) and PB (triangles) by monkeys responding under a PR schedule of reinforcement. Point above S represents self-administration of saline in test sessions. Symbols represent the mean values for four monkeys and vertical lines are s.e.m.
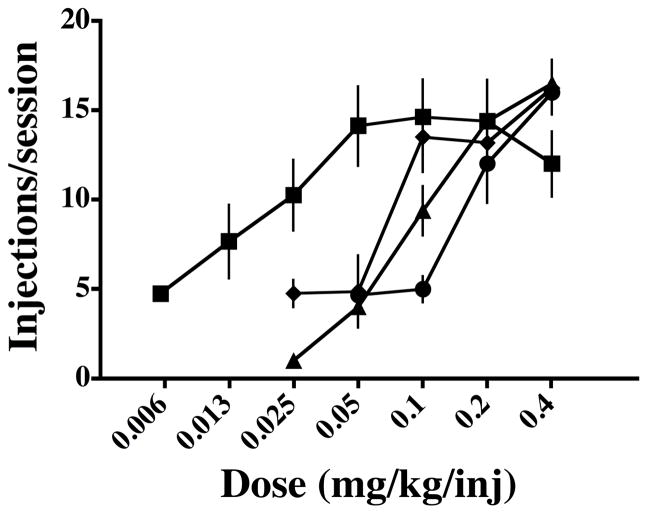
When PB was combined with cocaine there was a dose-related shift to the right in the cocaine dose-response function in all monkeys [Figure 2; F (3,12) = 27.92, p < 0.0001]. The ED50s of cocaine were 0.07 ( ± 0.01 s.e.m.), 0.09 (± 0.01 s.e.m.), and 0.13 (±0.003 s.e.m.), in combination with 0.05, 0.1 and 0.2 mg/kg/injection PB, respectively. The ED50 of cocaine alone was significantly lower than all cocaine and PB combinations (P < 0.05). The ED50s of the combinations of cocaine with PB 0.05 and cocaine with PB 0.1 mg/kg/injection were significantly lower than the combination of cocaine with PB 0.2 mg/kg/injection (P < 0.05). There was no significant difference in ED50s between the combination of cocaine with PB 0.05 and cocaine with PB 0.1 mg/kg/injection (P > 0.05). There was no significant difference in the maximum responding maintained by cocaine and the combinations of cocaine and PB (P > 0.05).
Figure 2.
Dose-response curves for self-administration of cocaine and cocaine:PB mixtures under a PR schedule of reinforcement. Squares represent cocaine alone; diamonds represent cocaine with PB 0.05 mg/kg/injection; triangles represent cocaine with PB 0.1 mg/kg/injection; circles represent cocaine with PB 0.2 mg/kg/injection. Symbols represent the mean values for four monkeys and vertical line are s.e.m.
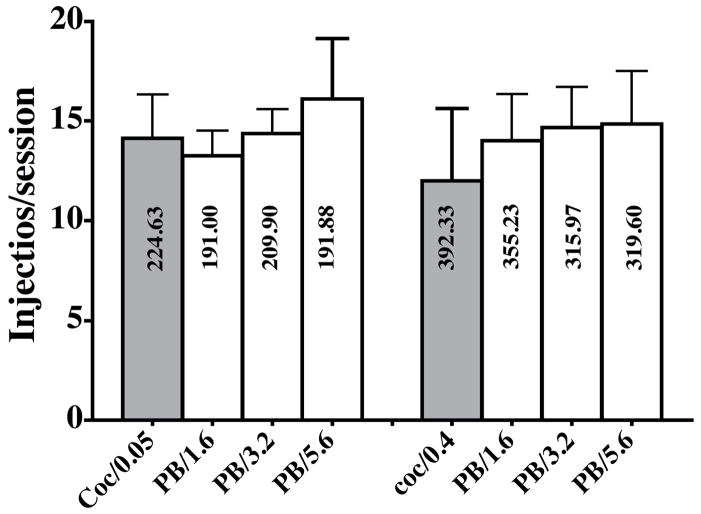
Pretreatment with PB had no effect on the self-administration of 0.05 and 0.4 mg/kg/injection cocaine up to doses of 5.6 mg/kg/injection (Figure 3). The effects of lower doses were no different from those of 1.6 mg/kg and are not shown. Session duration was not affected by PB pretreatment. Animals were ataxic and sedated shortly after doses of 3.2 and 5.6 mg/kg PB.
Figure 3.
Self-administration of 0.05 and 0.4 mg/kg/injection cocaine alone (shaded bars) and after PB pretreatment by monkeys responding under a PR schedule of reinforcement. The labels on the X-axis are the self-administered dose of cocaine and the pretreatment doses of PB in mg/kg/injection. Each bar is the mean value for four monkeys and vertical lines are s.e.m. Numbers in bars are mean session duration in minutes.
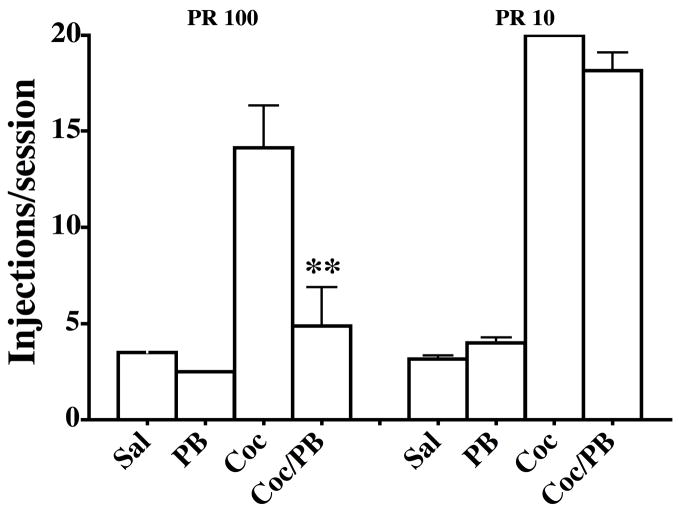
As noted in Figure 1, when the PR schedule began with a response requirement of 100, 0.05 mg/kg/injection cocaine alone functioned as a positive reinforcer while the same dose of PB and the combination of cocaine and pentobarbital did not (Figure 4, left bars). Similarly, when the PR schedule began with a response requirement of 10, 0.05 mg/kg/injection cocaine alone functioned as a positive reinforcer and the same dose of PB did not (Figure 4, right bars). However, under these conditions, the self-administration of the combination of cocaine and pentobarbital was not different from the self-administration of cocaine alone (p > 0.05; Figure 4).
Figure 4.
Self-administration of saline, cocaine 0.05 mg/kg/injection, and PB 0.05 mg/kg/injection alone and in combinations of cocaine 0.05 mg/kg/injection and PB 0.05mg/kg/injection by monkeys responding under PR 100 (left) and PR 10 (right) schedule of reinforcement. The data for the PR 100 are the same as in Figure 2. Each bar is the mean value for four monkeys and vertical lines are s.e.m. ** Indicates statistically significant different compared to cocaine alone group (P < 0.05).
4. Discussion
As has been found previously, cocaine served as an i.v. positive reinforcer in monkeys responding under a PR schedule of reinforcement (Rowlett et al., 1996; Wilcox et al., 2000). PB was, at best, a weak positive reinforcer in one of four monkeys when the PR schedule began with a response requirement of 100 but functioned as a positive reinforcer in all four monkeys when the initial response requirement was decreased to 10. Previous self-administration results with PB have been mixed, with behavior well maintained under some conditions (Griffiths et al., 1981; DeNoble et al., 1982) and less well maintained under other conditions (Goldberg et al., 1971; Johanson, 1982). Under the present conditions, PB was clearly a weaker positive reinforcer than cocaine.
As noted, we have previously reported the self-administration of mixtures of cocaine with another DAT blocker (RTI 117; Woolverton et al., 2008a) and with a μ opioid agonist (remifentanil; Woolverton et al., 2008b). RTI 117 was additive with cocaine while remifentanil was additive or super-additive, depending upon the ratio of the drugs in the mixture. The goal of the present study was to extend those findings to the combination cocaine and a CNS depressant, PB. Surprisingly, self-administration of low-to-intermediate doses of cocaine was decreased by the addition of PB. The effect was surmounted by increasing the dose of cocaine so that the cocaine dose-response function was shifted parallel to the right. Moreover, the magnitude of the shift was directly related to PB dose. Thus, our hypothesis that cocaine and PB would be super-additive was not supported.
One possible account of the data is that PB suppressed cocaine self-administration of intermediate doses of cocaine via direct effects on rate of responding that could be overcome by increasing the cocaine dose. However, when PB was given i.v. before a session of self-administration of 0.05 mg/kg/injection cocaine, responding was unaffected up to doses that were obviously sedating. Although it might be argued that insufficiently high doses of PB were tested, this seems untenable. The highest dose given as a pretreatment (5.6 mg/kg) is approximately 28-fold higher than the total dose of 0.05 mg/kg/injection PB self-administered in combination with 0.05 mg/kg/injection cocaine (approximately 0.2 mg/kg), and clearly made the animals ataxic. Conceivably, responding could have been completely suppressed by PB for the first part of the session and recovered as the drug was metabolized, an effect that would be difficult to detect with the measure of injections/session. Even with no responding, sessions would continue to advance, driven by the LH, and the animals could take normal levels of drug after recovery. The fact that session duration was, if anything, shortened by the PB pretreatments, obviates this concern. A similar lack of effect of PB at doses up to 10 mg/kg (i.m.) on responding maintained by cocaine in monkeys has been reported previously (Herling et al., 1979). On the other hand, Barrett et al. (2005) reported decreases in self-administration of low to intermediate doses of cocaine by rats pretreated with PB. Although it is unclear why effects differed across experiments, species may play a role. It is also possible that behavioral conditions contributed. Barrett et al. (2005) used a fixed-ratio five schedule of reinforcement with a relatively brief (20-second) time-out after injections. It would be possible to accumulate more cocaine under these conditions than with the five-minute TO used here and, thereby, directly decrease the rate of lever pressing with the combination of cocaine and PB. In any case, it should be noted that the conclusion of Barrett et al (2005) that PB decreased self-administration of low-to-intermediate doses of cocaine, but not higher doses, is the same as the conclusion of the present study.
The absence of direct effects of experimenter-administered PB on responding maintained by cocaine in the present study suggests that the contingency relationship played a role in the decrease in self-administration seen with the combination. This is in contrast to the finding by Spealman and Kelleher (1979) that the behavioral effects of cocaine in squirrel monkeys were independent of whether the drug was self- or experimenter-administered, limiting the generality of that conclusion. If lever pressing was not directly suppressed by PB, it is possible that the reinforcing effect of cocaine was either decreased or somehow antagonized by the addition of PB. It is also possible that the cocaine/PB combination was aversive. However, when we decreased the response requirement to a PR that began with 10, responding was well maintained by the combination of 0.05 mg/kg/injection of both drugs, a combination that did not maintain responding under a higher response requirement. This result is consistent with the conclusion that the reinforcing effect of cocaine was reduced but not eliminated, and that the combination was not aversive. Since the PR 10 manipulation was undertaken at the end of the experiment, the possibility should be considered that tolerance had developed to direct rate suppressant effects of PB. However, there was no evidence that 0.05 mg/kg/injection PB had rate suppressant effects. Our results are also consistent with the suggestion of Barrett et al. (2005) that PB pretreatment attenuated the reinforcing effect of cocaine and similar to the recent report by Winger et al. (2007) that the addition of ethanol to cocaine diminished the reinforcing effect of cocaine. Moreover, the results can be contrasted with previous reports of self-administration of combinations of cocaine and scopolamine (Ranaldi and Woolverton, 2002) or d-amphetamine and fenfluramine (Wee and Woolverton, 2006). In those studies, under conditions similar to those used here, the cocaine or d-amphetamine dose-response function was shifted to the right in a non-parallel manner with the addition of scopolamine or fenfluramine, suggesting that the combinations were aversive. Taken together, these studies suggest that the combination of a CNS depressant and cocaine has diminished potency as a reinforcer relative to cocaine alone. It is important to note, however, that the effect of PB was reversible by increasing the dose of cocaine in the combination. This result implies that the addition of PB can encourage the self-administration of higher doses of cocaine.
The mechanism underlying this effect of PB is speculative. There is much evidence to support the conclusion that the reinforcing effect of cocaine involves an increase in DA neurotransmission via blockade of neuronal uptake (e.g., Johanson and Fischman, 1989; Kuhar et al., 1991;Woolverton and Johnson, 1993). Although the reinforcing effects of PB have not been studied in comparable detail, PB is an allosteric modulator of GABA-A receptors and GABA-A agonists can modulate the activity of DA neurons (Sieghart, 1995). This has been suggested to be the mechanism that underlies the apparent ability of GABA-A agonists to diminish the abuse-related effects of cocaine (Barrett et al., 2005; Goeders et al., 1989, 1993; Negus et al., 2000) and may be the mechanism involved in the present results. If this is the case, it is not obvious why the effects of PB would vary with the contingency relationship. Although the effects of PB on mesolimbic DA neurons might depend upon whether it is self-or experimenter-administered, it remains for future research to examine this possibility. The possibility should also be considered that PB diminished the effects of cocaine via a pharmacologically non-specific form of perceptual masking (Overton, 1984). In perceptual masking drug A (in this case PB) masks the Drug B (in this case cocaine) stimulus when the drugs are mixed. Increasing the amount of drug B can overcome the masking effects. Examples of this phenomenon have been reported in the drug discrimination literature (e.g., Gauvin and Young, 1989). Although such a phenomenon is certainly conceivable, additional research will be required to explore this mechanism as well.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barrett AC, Negus SS, Mello NK, Caine SB. Effect of GABA agonists and GABA-A receptor modulators on cocaine- and food-maintained responding and cocaine discrimination in rats. J Pharmacol Exp Ther. 2005;315:858–871. doi: 10.1124/jpet.105.086033. [DOI] [PubMed] [Google Scholar]
- deNoble VJ, Svikis DS, Meisch RA. Orally delivered pentobarbital as a reinforcer for rhesus monkeys with concurrent access to water: effects of concentration, fixed-ratio size, and liquid positions. Pharmacol Biochem Behav. 1982;16:113–117. doi: 10.1016/0091-3057(82)90021-1. [DOI] [PubMed] [Google Scholar]
- dePoortere RY, Li DH, Lane JD, Emmett-Oglesby MW. Parameters of self-administration of cocaine in rats under a progressive-ratio schedule. Pharmacol Biochem Behav. 1993;45:539–548. doi: 10.1016/0091-3057(93)90503-l. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. The effects of combinations of intranasal cocaine, smoked marijuana, and task performance on heart rate and blood pressure. Pharmacol Biochem Behav. 1990;36:311–315. doi: 10.1016/0091-3057(90)90409-b. [DOI] [PubMed] [Google Scholar]
- Gauvin DV, Young AM. Evidence for perceptual masking of the discriminative morphine stimulus. Psychopharmacology. 1989;98:212–221. doi: 10.1007/BF00444694. [DOI] [PubMed] [Google Scholar]
- Goeders NE, McNulty MA, Guerin GF. Effects of alprazolam on intravenous cocaine self-administration in rats. Pharmacol Biochem Behav. 1993;44:471–474. doi: 10.1016/0091-3057(93)90493-d. [DOI] [PubMed] [Google Scholar]
- Goeders NE, McNulty MA, Mirkis S, McAllister KH. Chlordiazepoxide alters intravenous cocaine self-administration in rats. Pharmacol Biochem Behav. 1989;33:859–866. doi: 10.1016/0091-3057(89)90483-8. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Hoffmeister F, Schlichting UU, Wuttke W. A comparison of pentobarbital and cocaine self-administration in rhesus monkeys: effects of dose and fixed-ratio parameter. J Pharmacol Exp Ther. 1971;179:277–283. [PubMed] [Google Scholar]
- Griffiths RR, Lukas SE, Bradford LD, Brady JV, Snell JD. Self-injection of barbiturates and benzodiazepines in baboons. Psychopharmacology. 1981;75:101–109. doi: 10.1007/BF00432169. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Smith JE, Dworkin SI. The effects of eticlopride and naltrexone on responding maintained by food, cocaine, heroin and cocaine/heroin combinations in rats. J Pharmacol Exp Ther. 1996;277:1247–1258. [PubMed] [Google Scholar]
- Herling S, Downs DA, Woods JH. Cocaine, d-amphetamine and pentobarbital effects on responding maintained by food or cocaine in rhesus monkeys. Psychopharmacology. 1979;64:261–269. doi: 10.1007/BF00427508. [DOI] [PubMed] [Google Scholar]
- Johanson CE. Behavior maintained under fixed-interval and second-order schedules of cocaine or pentobarbital in rhesus monkeys. J Pharmacol Exp Ther. 1982;221:384–393. [PubMed] [Google Scholar]
- Johanson CE, Fischman MW. The pharmacology of cocaine related to its abuse. Pharmacol Rev. 1989;41:3–52. [PubMed] [Google Scholar]
- Kosten TR, Rounsaville BJ, Kleber HD. A 2.5 year follow-up of cocaine use among treated opioid addicts. Arch Gen Psychiat. 1987;44:281–284. doi: 10.1001/archpsyc.1987.01800150101012. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Multiple drug abuse patterns and medical consequences. In: Meltzer H, editor. Psychopharmacology, The Third Generation of Progress. New York: Raven Press; 1987. pp. 1597–1604. [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- Mattox AJ, Thompson SS, Carroll ME. Smoked heroin and cocaine base (speedball) combinations in rhesus monkeys. Exp Clin Psychopharmacol. 1997;5:113–118. doi: 10.1037//1064-1297.5.2.113. [DOI] [PubMed] [Google Scholar]
- Meisch RA, Lemaire GA. Reinforcing effects of pentobarbital-ethanol combination relative to each drug alone. Pharmacol Biochem Behav. 1990;35:443–450. doi: 10.1016/0091-3057(90)90182-h. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS, Lukas SE, Mendelson JH, Sholar JW, Driese J. A primate model of polydrug abuse: cocaine and heroin combinations. J Pharmacol Exp Ther. 1995;274:1325–1337. [PubMed] [Google Scholar]
- Negus SS. Interactions between the reinforcing effects of cocaine and heroin in a drug-vs-food choice procedure in rhesus monkeys: a dose-addition analysis. Psychopharmacology. 2005;180:115–124. doi: 10.1007/s00213-004-2133-y. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Fivel PA. Effects of GABA agonists and GABA-A receptor modulators on cocaine discrimination in rhesus monkeys. Psychopharmacology. 2000;152:398–407. doi: 10.1007/s002130000543. [DOI] [PubMed] [Google Scholar]
- Overton DA. State dependent learning and drug discrimination. In: Iverson LL, Iverson SD, Snyder SH, editors. Handbook of Psychopharmacology. Vol. 18. New York: Plenum Press; 1987. pp. 59–127. [Google Scholar]
- Ranaldi R, Woolverton WL. Self-administration of cocaine:scopolamine combinations by rhesus monkeys. Psychopharmacology. 2002;161:442–448. doi: 10.1007/s00213-002-1069-3. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Massey BW, Kleven MS, Woolverton WL. Parametric analysis of cocaine self-administration under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology. 1996;125:361–370. doi: 10.1007/BF02246019. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Platt DM, Wei-Dong Y, Spealman RD. Modulation of heroin and cocaine self-administration by dopamine D1- and D2-like receptor agonists in rhesus monkeys. J Pharmacol Exp Ther. 2007;321:1135–1143. doi: 10.1124/jpet.107.120766. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of gamma-aminobutyric acid A receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Smith JE, Conchita C, Coller MD, Hemby SE, Martin TJ. Self-administered heroin and cocaine combinations in the rat: Additive reinforcing effects-supra-additive effects on nucleus accumbens extracellular dopamine. Neuropsychopharmacology. 2006;31:139–150. doi: 10.1038/sj.npp.1300786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spealman RD, Kelleher RT. Behavioral effects of self-administered cocaine: responding maintained alternately by cocaine and electric shock in squirrel monkeys. J Pharmacol Exp Ther. 1979;210:206–214. [PubMed] [Google Scholar]
- Wang Z, Woolverton WL. Self-administration of cocaine-antihistamine combinations: Super-additive reinforcing effects. Eur J Pharmacol. 2007;557:159–160. doi: 10.1016/j.ejphar.2006.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Woolverton WL. Self-administration of mixtures of fenfluramine and amphetamine by rhesus monkeys. Pharmacol Biochem Behav. 2006;84:337–343. doi: 10.1016/j.pbb.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Rowlett JK, Paul IA, Ordway GA, Woolverton WL. On the relationship between the dopamine transporter and the reinforcing effects of local anesthetics in rhesus monkeys: Practical and theoretical concerns. Psychopharmacology. 2000;153:139–147. doi: 10.1007/s002130000457. [DOI] [PubMed] [Google Scholar]
- Winger G, Galuska CM, Hursh SR. Modification of ethanol's reinforcing effectiveness in rhesus monkeys by cocaine, flunitrazepam, or gamma-hydroxybutyrate. Psychopharmacology. 2007;193:587–598. doi: 10.1007/s00213-007-0809-9. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Johnson KM. Neurobiology of cocaine abuse. Trends Pharmacol Sci. 1992;13:193–200. doi: 10.1016/0165-6147(92)90063-c. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Wang Z, Vasterling T, Carroll FI, Tallarida R. Self-administration of drug mixtures with monkeys: combining drugs with comparable mechanisms of action. Psychopharmacology. 2008a;196:575–582. doi: 10.1007/s00213-007-0991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Wang Z, Vasterling T, Carroll FI, Tallarida R. Self-administration of cocaine-remifentanil mixtures by monkeys: an isobolographic analysis. Psychopharmacology. 2008b;198:387–394. doi: 10.1007/s00213-008-1152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]