Summary
Adult hippocampal neurogenesis is stimulated by chronic administration of antidepressants (ADs) and by voluntary exercise. Neural progenitor cells in the dentate gyrus (DG) that are capable of continuous proliferation and neuronal differentiation are the source of such structural plasticity. Here we report that mice lacking the receptor tyrosine kinase TrkB in hippocampal NPCs have impaired proliferation and neurogenesis. When exposed to chronic AD or wheel running, no increase in proliferation or neurogenesis is observed. Ablation of TrkB also renders these mice behaviorally insensitive to antidepressive treatment in depression and anxiety-like paradigms. In contrast, mice lacking TrkB only in differentiated DG neurons display typical neurogenesis and respond normally to chronic AD. Thus, our data establish an essential cell autonomous role for TrkB in regulating hippocampal neurogenesis, behavioral sensitivity to antidepressive treatments, and support the notion that impairment of the neurogenic niche is an etiological factor for refractory responses to antidepressive regimen.
Keywords: TrkB, BDNF, hippocampus, neural progenitor cell, antidepressant, running
Introduction
Depression is a significant public health problem due to both its high prevalence and its devastating impact on individuals and society. Despite much excitement generated by recent advances in the knowledge of brain development and function, the mechanisms underlying the pathogenesis of depression, as well as its amelioration by antidepressant (AD) treatment remain poorly understood (Manji et al., 2001; Nestler et al., 2002). Clinical studies demonstrate that 40% of depressed patients fail to respond to first-line ADs and 70% do not undergo full remission (Fagiolini and Kupfer, 2003; Thase and Rush, 1995). Such variable range of effectiveness underscores the heterogeneity of the illness and the urgent need for thorough delineation of the cellular and molecular process involved in the action of ADs.
As predicted by their pharmacological characteristics, most ADs appear to act by increasing the activity of serotonergic and noradrenergic circuits (Morilak and Frazer, 2004). However, these medications usually require chronic administration for weeks to months before clinically appreciable effects are achieved. This represents an extended delay compared to the rapid increase of serotonin and noradrenaline elicited by these drugs (Frazer, 1997). Therefore, gradual, yet essential changes to the brain that occur during the period of delay appear to be required for the response to ADs. Animal studies indicate one such delayed response is the increased production of new neurons in the dentate gyrus (DG) (Malberg et al., 2000; Perera et al., 2007). Physical activity such as running, which is considered beneficial for mental health (Salmon, 2001), also induces increased production of neurons (van Praag et al., 1999). Studies in rodents have shown that upon exposure to chronic ADs or exercise, neural progenitor cells (NPCs) in the sub-granular zone (SGZ) of the DG undergo enhanced proliferation, which drives the increase in neurogenesis (Encinas et al., 2006). Although the functional significance remains to be proven, the positive association of neurogenesis with ADs and exercise, as well as other beneficial stimulations such as diet, learning and environmental enrichment (Alvarez-Buylla, 1992; Kempermann et al., 1997; Lee et al., 2000), implicates it as a physiologically relevant phenomenon. Indeed, irradiation-mediated ablation of proliferating cells in the hippocampus compromises the ability of certain strains of rodents to display a behavioral response to ADs, suggesting that neurogenesis is an intrinsic requirement (Airan et al., 2007; Holick et al., 2008; Santarelli et al., 2003; Wang et al., 2008a). The genetic mechanism underlying this observation remains unclear.
Coinciding with the elevation of neurogenesis, chronic AD exposure elicits a variety of molecular adaptations (Tardito et al., 2006). The mechanisms that link these molecular changes to the biological effects of ADs are only beginning to be unveiled. One of the molecules implicated is brain-derived neurotrophic factor (BDNF), whose level in the hippocampus is increased by chronic but not acute AD exposure (Nibuya et al., 1995). Infusion of BDNF into the DG produces AD-like responses in several behavioral paradigms (Shirayama et al., 2002). Mutant animals with a global reduction of BDNF have a blunted response to acute AD induced behavioral changes (Monteggia et al., 2004; Saarelainen et al., 2003). In human, a single nucleotide polymorphism of the bdnf gene (val66met) that interferes with the activity-dependent secretion of BDNF protein has been associated with cognitive and structural abnormalities of the CNS (Egan et al., 2003; Pezawas et al., 2004), though consensus is lacking on its influence on susceptibility to mood disorders (Choi et al., 2006; Jiang et al., 2005; Schule et al., 2006). Interestingly, recent generation of mutant mice carrying the same polymorphism indicates a causative link between the genetic change and elevated anxiety-like behaviors that are not normalized by AD (Chen et al., 2006). These observations, coupled with evidence that exogenous BDNF promotes proliferation of hippocampal NPCs (Scharfman et al., 2005), suggest an important role for BDNF in mediating the biological response to chronic AD treatments (Wang et al., 2008b). Whether BDNF acts directly on NPCs in vivo, and if its effects on NPCs then contribute to the overall influence of BDNF on AD response is unresolved.
In the present study, we conditionally ablated the gene encoding TrkB, the high affinity receptor for BDNF, in a regional and cell-type specific manner. We show that NPC deletion of trkB, both in embryos or in the adult, results in impairment of hippocampal neurogenesis and prevents behavioral improvements induced by chronic AD administration or by wheel running. In contrast, deleting trkB in differentiated neurons of the same brain regions does not affect neurogenesis or the behavioral responses to ADs. Our findings provide genetic evidence of a functional, cell autonomous requirement of TrkB in the neurogenic and behavioral responses to antidepressive treatments. Furthermore, our results support the notion that DG NPCs are a required component in the amelioration of depression (Zhao et al., 2008).
Results
TrkB is expressed by hippocampal neural progenitor cells
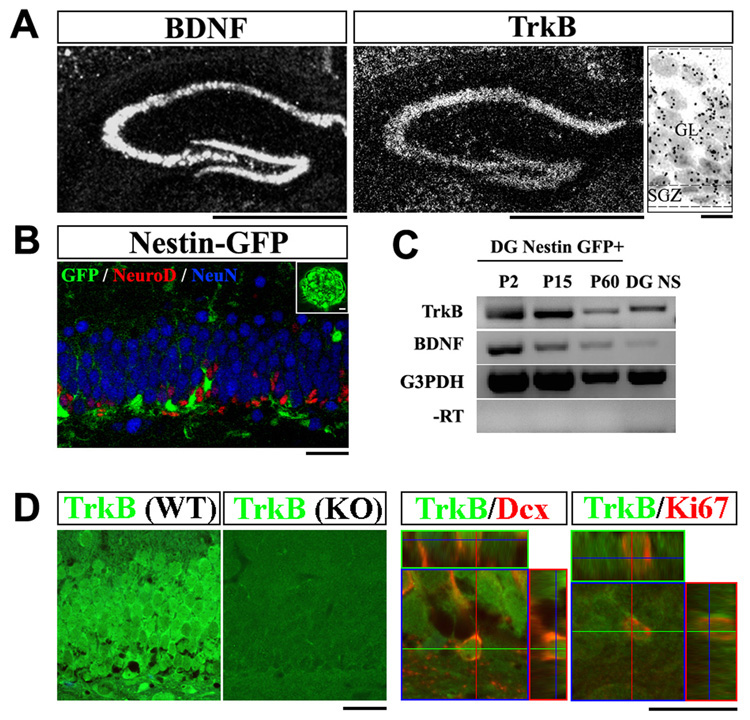
We, and others have previously demonstrated the presence of TrkB transcripts and protein in the hippocampus (Klein et al., 1990; Zhou et al., 1993). To determine whether transcripts are present in the neurogenic zone, we first examined TrkB expression in the postnatal and adult DG. In situ hybridization analysis showed prominent expression of both BDNF and TrkB in the granular layer and SGZ of the DG (Figure 1A). In particular, TrkB mRNA, represented by silver bromide grains, was detectable throughout the cellular layers of the DG (Figure 1A, right panel). To further examine whether the DG NPCs express TrkB, we utilized Nestin-GFP transgenic mice, in which GFP expression is confined to NPCs (Figure 1B) (Yu et al., 2005). Using fluorescent-activated cell sorting (FACS), GFP positive cells from the DG of transgenic mice at various ages were isolated and analysis of these cells by RT-PCR demonstrated the presence of NPCs markers and the absence of markers from the differentiated lineages (Supplemental Figure 1). TrkB and BDNF mRNAs were detected in the GFP positive cells at all ages tested (postnatal day (P) 2, 15 and 60, n=3 animals for each, Figure 1C). Similarly, TrkB and BDNF mRNAs were also detected in neurospheres derived from the DG of adult wild-type mice (n=3, Figure 1C). We further analyzed the distribution of TrkB protein in the progenitor population by co-immunostaining. Consistent with its mRNA distribution, TrkB protein was detected in all layers of the adult DG; confocal microscopy revealed its presence in both proliferating progenitors that are Ki67 positive (86.2%, n=160 cells from 3 animals), and immature neurons that express Doublecortin (94.9%, n=177 cells from 3 animals) (Figure 1D). Taken together, our results demonstrate the presence of TrkB mRNA and protein in hippocampal NPCs.
Figure 1. TrkB is expressed by hippocampal NPCs.
(A) In situ hybridization analysis of TrkB and BDNF mRNA in the adult dentate gyrus (DG). In the high magnification image (right), note the distribution of silver gram (black spheres) in all cells. Scale bars, 1mm (low-magnification) and 10um (high-magnification). GL, granular layer; SGZ, sub-granular zone.
(B) Confocal image of the DG of an adult Nestin-GFP transgenic mouse, co-immunostained for GFP (green), NeuroD (red) and NeuN (blue). GFP expression was restricted to NPCs and did not colocalize with immature (NeuroD+) or mature (NeuN+) neurons. Insert showed a DG derived neurosphere that expresses GFP. Scale bars, 10um.
(C) RT-PCR detection of TrkB and BDNF transcripts in FACS sorted Nestin-GFP positive cells and DG derived neurospheres. NS, neurosphere.
(D) Immuno-staining for TrkB (green) on adult DG sections from wild-type and TrkBhGFAP mice (left panels). Co-staining for TrkB (green) and Ki67 (red), or Doublecortin (red) demonstrated co-localization of TrkB with proliferating (Ki67+) and differentiating (Doublecortin+) cells. Dcx, doublecortin. Scale bars, 10um and 5um. WT, wild-type.
hGFAP-Cre activity in hippocampal progenitor cells
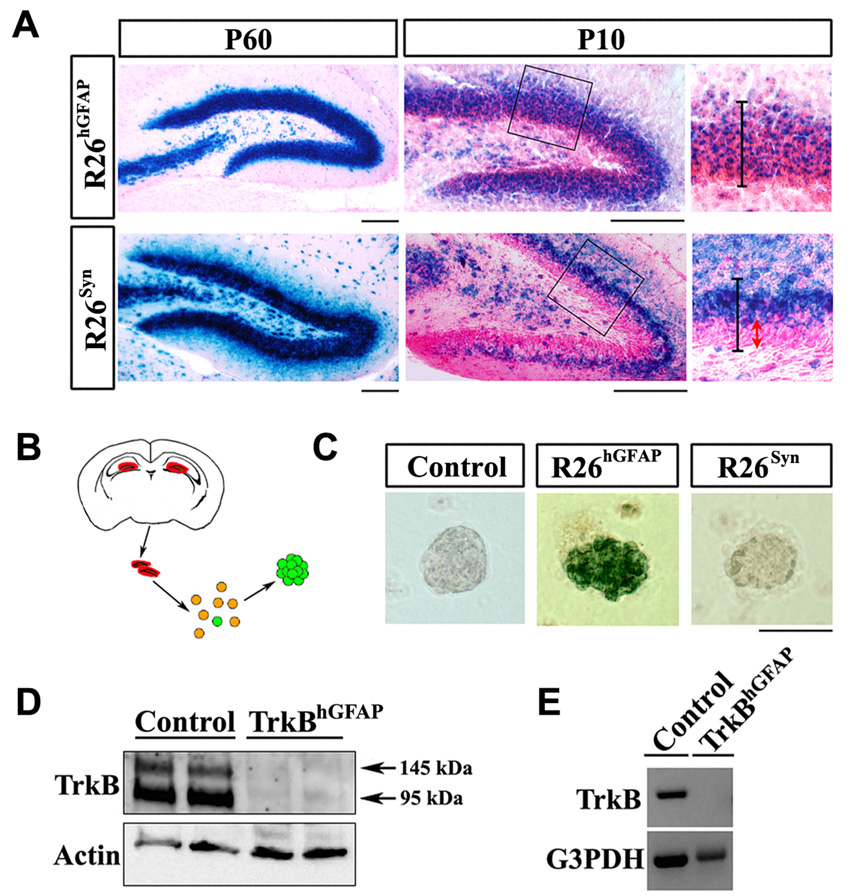
Germline homozygous TrkB knockout animals die shortly after birth. To investigate the role of TrkB in postnatal stages, conditional knockout animals were generated by crossing mice harboring the trkB flox alleles to transgenic mice expressing the Cre recombinase either under the human GFAP (hGFAP) promoter or the Synapsin I (Syn) promoter (He et al., 2004; Luikart et al., 2005). In both the hGFAP-Cre and the Syn-Cre transgenic animals, the pattern of Cre expression allows recombination throughout the forebrain, including cerebral cortex, hippocampus and olfactory bulb. In other regions of the brain, such as the midbrain (dopaminergic neurons) and brainstem (serotonergic neurons), Cre expression is minimal (Supplemental Figure 4 and not shown). When interbred with Rosa26-stop-lacZ reporter mice (R26) (Soriano, 1999) and analyzed at the age of 2 months, a majority of the neurons in these regions appeared to express functional beta-galactosidase (β-gal) (Figure 2A and not shown). However, when such animals were analyzed at a younger age (P10), a distinct anatomical difference in the recombination patterns of the two Cre lines was observed in the DG. In the R26hGFAP animal most cells throughout the DG expressed β-gal; whereas in the R26Syn animal β-gal expression was confined to the outer layers of the DG, while the SGZ and inner granule layer, where NPCs and immature neurons reside, were essentially spared of recombination (Figure 2A).
Figure 2. hGFAP-Cre, but not Syn-Cre, mediated recombination in hippocampal NPCs.
(A) X-gal staining on DG sections of R26RhGFAP and R26Syn mice at P10 and P60. Higher magnification views of the circled areas are shown in the right panels, in which black lines outline the granular layer, while the red arrow highlights the SGZ and inner granular layer where recombination was spared. Scale bars, 100um.
(B) Schematic diagram of the procedure to generate neurospheres from adult DG.
(C) X-gal staining on primary neurospheres derived from the DG of adult control, R26RhGFAP and R26Syn mice. Blue staining in the neurosphere from R26RhGFAP mice indicated the occurrence of recombination. Scale bar, 100um.
(D) Western blots of lysates from the hippocampus of control and TrkBhGFAP mice, probed for TrkB and actin. Note the absence of TrkB in the TrkBhGFAP mice.
(E) RT-PCR detection of TrkB and G3PDH transcripts in FACS sorted Nestin-GFP positive cells from the DG of Control and TrkBhGFAP mice. Note the absence of TrkB in the TrkBhGFAP mice.
We next enriched NPCs from 2-month old mice by culturing primary cells from the DG in neurosphere forming media (Figure 2B). Upon X-gal staining, only neurospheres isolated from the DG of R26hGFAP, but not those from the R26Syn or control mice stained positive for β-gal activity, indicating that recombination only occurred in the hippocampal NPCs from the R26hGFAP mice (Figure 2C). Thus, the masking of X-gal staining difference in the adult DG (Figure 2A, left panels) was likely the result of the considerably reduced numbers of NPCs and immature neuron populations at this age compared to P10. Nonetheless, we demonstrate that in adult mice the Syn-Cre transgene can only elicit recombination in differentiated neurons, while the hGFAP-Cre affects both NPCs and neurons. The effective ablation of TrkB with the hGFAP-Cre was further demonstrated by immuno-blotting for TrkB in the hippocampus of adult mice (Figure 2D), and by RT-PCR detection of TrkB mRNA in FACS isolated Nestin-GFP positive DG NPCs (Figure 2E). Therefore by utilizing these two different Cre transgenic lines, differences between genetic ablation of the TrkB gene in the granule layer of the DG versus additional ablation in the SGZ where progenitors reside can be studied.
Ablation of TrkB in early postnatal NPCs impairs DG morphogenesis
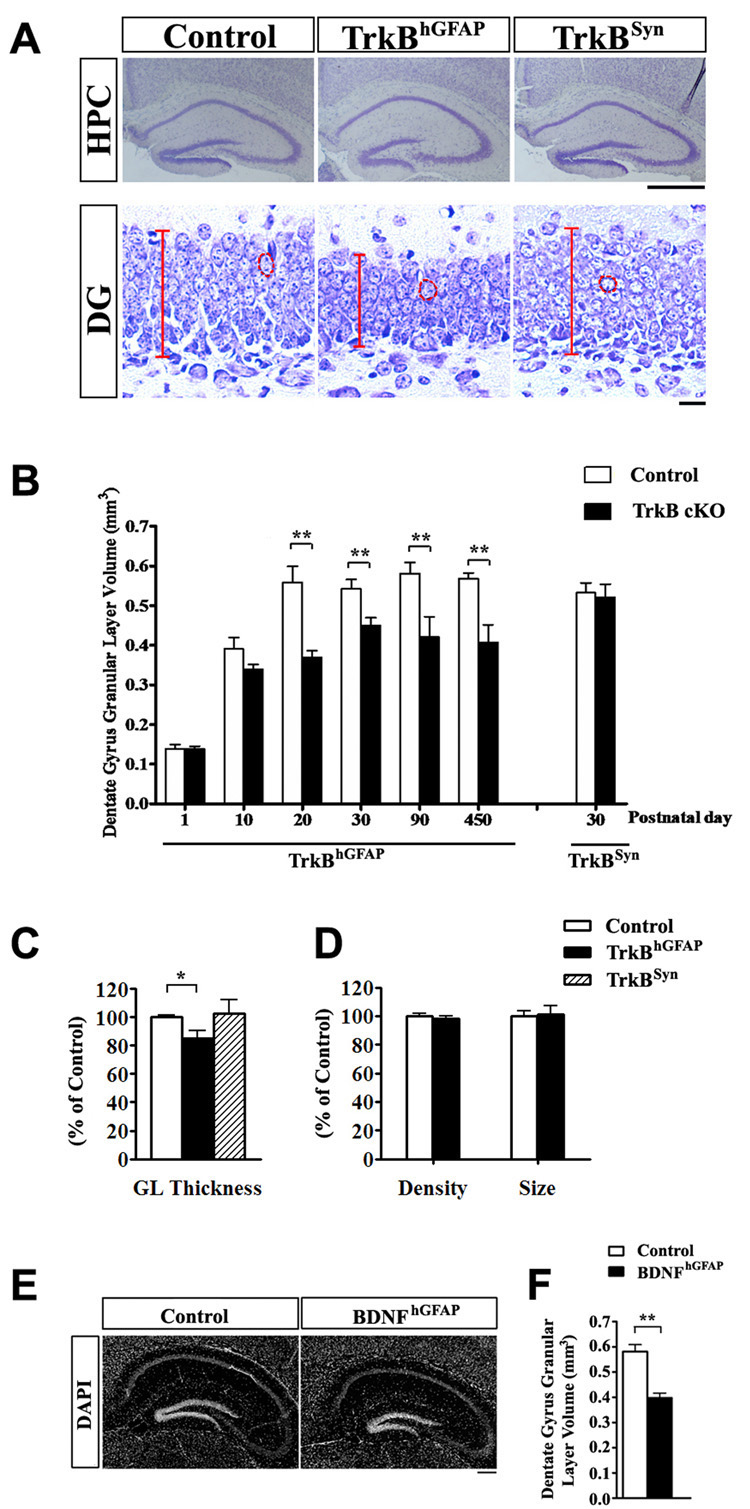
The conditional knockout animals, either carrying the hGFAP-Cre (TrkBhGFAP) or the Syn-Cre (TrkBSyn) are viable at birth and have normal survival rates (up to 15 months recorded) (Luikart et al., 2005). The TrkBhGFAP mice displayed a significant reduction in the volume of the DG granular layer (Figure 3A and 3B) that first became measurable at P10. The volume reduction stabilized at around 30% after the initial postnatal weeks and persisted throughout adulthood. This abnormality was not caused by changes in cell density or cell size (Figure 3D; p>0.2), but rather was a result of decrease in the number of granule neurons, evidenced by a significant reduction in the thickness of DG granular layer (Figure 3C; n=6 for each, F2,15=5.477, p=0.0164). The number and morphology of glial cells were not appreciably affected in the TrkBhGFAP mice (Supplemental Figure 3 and not shown). The reduction of volume was most prominently observed in the hippocampus and DG, and did not appear to be a secondary result of broader developmental defect, as the TrkBhGFAP mice had normal body and brain size at all ages examined (Supplemental Figure 2). We also measured the volumes of other anatomical regions, such as the striatum, in 2-month old TrkBhGFAP mice and found normal volume (23.62 ± 0.94 mm3 in controls, 23.32 ± 1.06 mm3 in TrkBhGFAP, n=6 for each, p>0.2). The TrkBSyn animals, contrary to the TrkBhGFAP mice, displayed normal development of the DG granular layer (Figure 3A to 3C). These observations demonstrate that TrkB expression in the SGZ is required for the normal structural development of the DG. The phenotypic difference between the TrkBhGFAP and TrkBSyn mice suggests a cell autonomous function of TrkB in the DG NPCs. We have previously reported that conditional deletion of BDNF using the hGFAP-Cre (BDNFhGFAP) resulted in 80% reduction of BDNF protein level in the hippocampus (Monteggia et al., 2007), we note that these mice also displayed significantly reduced DG granular layer volume, indicating a ligand-receptor coincidence (Figure 3E and 3F, n=8–10 for each genotype, p<0.005).
Figure 3. Ablation of TrkB in postnatal NPCs impaired DG morphogenesis.
(A) Representative images of Nissl stained DG sections from control, TrkBhGFAP and TrkBSyn mice at P15. The decreases in DG size and granular layer thickness were only observed in TrkBhGFAP mice. Red lines and circles highlight the length of the granular layer, and the size of single cell, respectively. Scale bars, 1mm (hippocampus) and 10um (DG).
(B) Quantitative analysis revealed that the reduction in the DG volume of TrkBhGFAP mice first became measurable at P10 days and persisted in adulthood. Results are mean + SEM here and in subsequent figures; n>7 for each.
(C) At P15 days, TrkBhGFAP, but not TrkBSyn mice had thinner granular layer, demonstrated a decrease in neuronal number. Data was shown as percentage of control. N=6 for each. F2,15=5.477, p=0.0164. GL, granular layer.
(D) Comparative analysis on the size and density of DG granular neurons from control and TrkBhGFAP mice. Note the absence of difference in either category.
(E) DAPI staining of hippocampus sections from control and BDNFhGFAP mice at the age of 2 month. Note the decrease in DG size in the BDNFhGFAP mice. Scale bar, 200um.
(F) Quantitative analysis revealed a 30% reduction in DG volume in the BDNFhGFAP mice at the age of 2 months. N=8–10 for each.
* p<0.05; ** p<0.01
Ablation of TrkB impairs proliferation and neurogenesis
Given the apparent reduction in the number of granule neurons in TrkBhGFAP mice, but not TrkBSyn mice, we examined whether lack of TrkB in the NPCs affected the neurogenic capacity of the SGZ. Control and TrkB mutant animals were evaluated at P15, during a period in which the DG undergoes dynamic morphogenesis (Altman and Bayer, 1990). The number of newly generated neurons (NeuroD positive) was drastically reduced in the TrkBhGFAP mice, whereas the TrkBSyn mice were comparable to the control mice (Figure 4A). Thus TrkB ablation in the NPCs but not in differentiated neurons impairs hippocampal neurogenesis.
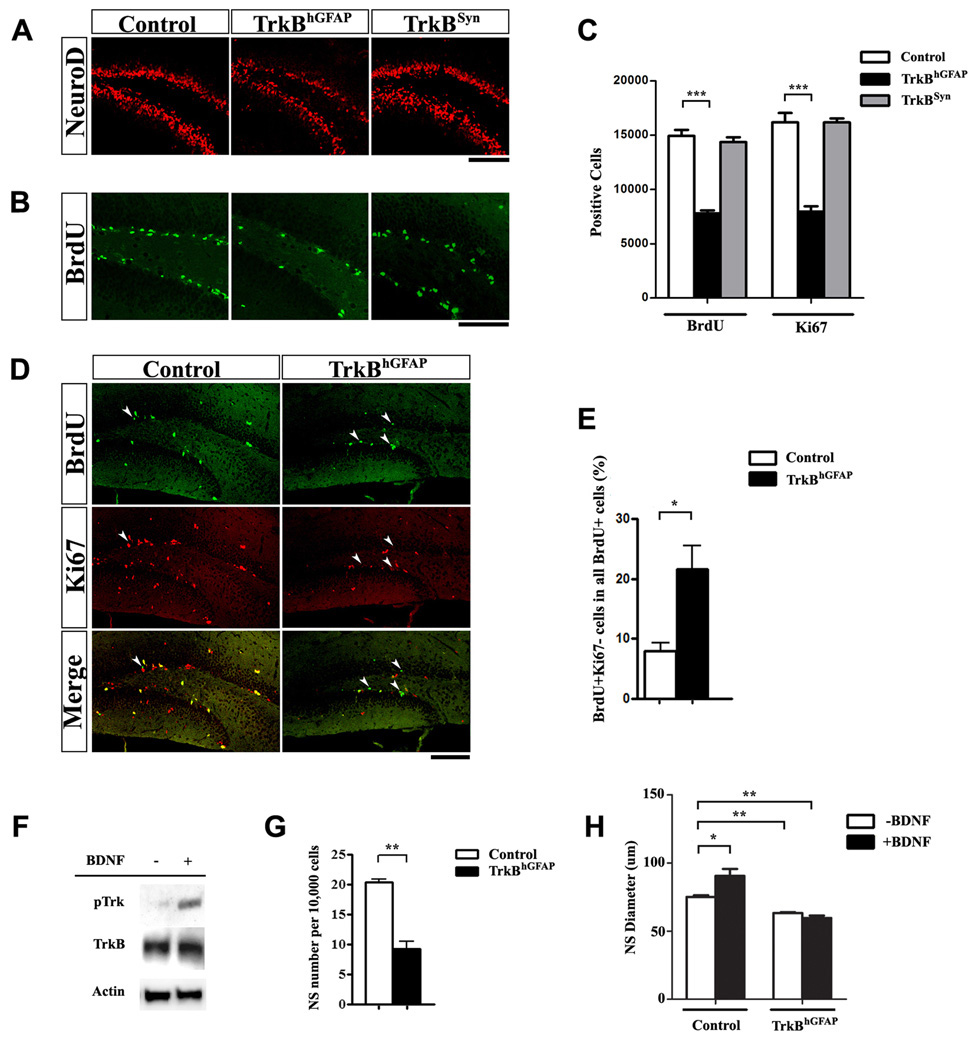
Figure 4. Lack of TrkB in NPCs impaired neurogenesis and proliferation in vivo and in vitro.
(A) Representative confocal images of the DG immunostained for NeuroD (red). Note the reduction of NeuroD positive cells, representing immature neurons, in TrkBhGFAP, but not TrkBSyn mice (P15). Scale bar, 100um.
(B–C) Proliferation in the DG was decreased in TrkBhGFAP, but not TrkBSyn mice, evidence by a reduction in Ki67 positive (C), or BrdU positive cells (B and C). Scale bar, 100um. N=7–9 for each. F2,21=78.39, p<0.0001 (BrdU); F2,21=51.64, p<0.0001 (Ki67).
(D–E) Cell cycle analysis using BrdU pulsing and co-immunostaining for BrdU (green) and Ki67 (red) showed increase in the ratio of BrdU labeled cells that have exited the cell cycle (BrdU+Ki67−) in the DG of TrkBhGFAP mice. Arrowheads indicate BrdU+Ki67− cells. Scale bar, 100um. Data represents the ratio of (BrdU+Ki67−) /(All BrdU+).
(F) Western blots of lysates from DG derived neurospheres probed for phosphor-Trk490, TrkB and actin, with and without BDNF stimulation. Note the abundance of TrkB expression and the increase of phospho-Trk in the presence of BDNF.
(G) Cells from the DG of adult mice were plated at equal density and allowed to proliferate in the presence of EGF and bFGF. The frequency of neurosphere formation was lower in the TrkBhGFAP mice, indicating a decrease in NPC population.
(H) Addition of BDNF facilitated the growth of primary neurospheres derived from the DG of control mice, but not the TrkBhGFAP mice. TrkBhGFAP neurospheres grown without BDNF were also smaller than control, indicated impaired proliferation. N=4 for each. ANOVA revealed significant effects of BDNF (F1,12=6.994, p=0.0214), genotype (F1,12=102.2, p<0.0001) and an interaction between the two (F1,12=19.3, p=0.0009).
*p<0.05; **p<0.01; ***p<0.001.
Reduced neurogenesis in the absence of TrkB could be caused by increased cell death, decreased proliferation, or a combination of both processes. We first examined programmed cell death using TdT-mediated dUTP nick end labeling (TUNEL) assay and immunohistochemistry for activated caspase 3. At all time points examined, the number of apoptotic cells detected with either method was uniformly low and no measurable increase was observed in the TrkBhGFAP mice, indicating that lack of TrkB did not appreciably affect survival in vivo (Supplemental Figure 3; n=6 for each, p>0.2).
We next assessed proliferation by measuring the number of cells that incorporate the DNA synthesis marker 5-bromo-2’-deoxyuridine (BrdU). P15 mice were treated with pulses of BrdU and sacrificed 24 hours after the first injection. Within the area encompassing the inner granular layer and SGZ, the number of BrdU positive cells in the TrkBhGFAP mice displayed a 48% reduction compared to control mice (Figure 4B and 4C; n=7–9 for each; F2,21=78.39, p<0.0001; p<0.001 for post hoc test of control to TrkBhGFAP comparison). Similarly, the number of cells positive for Ki67, an endogenous marker for actively cycling cells, was decreased by 51% in the SGZ of the TrkBhGFAP mice (Figure 4B and 4C; n=7–9 for each; F2,21=51.64, p<0.0001; p<0.001 for post hoc test of control to TrkBhGFAP comparison). Again, the numbers of BrdU or Ki67 positive cells were unaffected in the DG of the TrkBSyn mice (Figure 4B and 4C; p>0.2 for control to TrkBSyn comparisons).
To further investigate the cellular abnormality that lead to the significant decrease of proliferation in the TrkBhGFAP mice, we evaluated cell cycle exit of BrdU incorporating cells by examining their expression of Ki67 after a 2-hour chase period, at which point cells that were labeled with BrdU during the S-phase but have subsequently left the cycle would lose their Ki67 expression (BrdU+; Ki67−), whereas the ones that remained in active cell cycle would be double positive (BrdU+; Ki67+). In the TrkBhGFAP mice 23.1 ± 3.9 % of all BrdU+ cells were Ki67−, displaying a 208% increase in cell cycle exit over the control mice, where only 7.5 ± 1.3 % are Ki67− (Figure 4D and 4E, 1053 BrdU+ cells from 5 control, and 772 from 5 TrkBhGFAP, p<0.05). Collectively, these observations demonstrate that TrkB is required for normal precursor proliferation in the hippocampus.
Activation of TrkB in response to BDNF facilitates proliferation in vitro
Our finding that the TrkBhGFAP but not the TrkBSyn mice displayed impaired hippocampal neurogenesis suggested a cell autonomous requirement for TrkB in NPCs. To further examine their intrinsic properties, we cultured NPCs from the DG in serum-free conditions. In the presence of epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF), DG NPCs formed neurospheres, which expressed TrkB receptor that could be activated by exogenous BDNF (Figure 4F). Although maintaining adult DG-derived primary neurospheres in media containing BDNF (50 ng/ml) for 7–10 days did not increase the frequency of primary neurosphere formation (not shown), there was a significant increase in the size of the neurospheres, suggesting that activation of TrkB facilitates the expansion of neurosphere-forming cells (Figure 4H; n=4 for each group, p<0.05 for post hoc test of with to without BDNF comparison in control cells). Primary neurospheres derived from the DG of adult TrkBhGFAP mice, when plated at equal density, displayed reduction in both the number (Figure 4G; n=4 for each group, p<0.005) and size (Figure 4H; p<0.005 for post hoc test of control to TrkBhGFAP comparison without BDNF; F1,12=102.2, p<0.0001 for genotype; F1,12=6.994, p=0.0214 for BDNF). The ability of primary neurospheres to form secondary neurospheres was also impaired in the TrkBhGFAP mice (secondary-to-primary neurosphere ratio: 0.322 ± 0.058 in TrkBhGFAP and 0.843 ± 0.096 in control, n=4 for each, p<0.005). In addition, deletion of TrkB abolished neurosphere sensitivity to BDNF stimulation (Figure 4H, p>0.2). Using an Annexin V labeling assay, we examined the percentage of apoptotic cells in the neurosphere cultures, and did not observe significant differences between control and TrkBhGFAP cells with or without BDNF treatment (n=4 for each; with 50 ng/ml BDNF: 14.95 ± 1.38% in controls, 16.25 ± 2.65% in TrkBhGFAP; without BDNF: 14.46 ± 1.62% in controls, 17.69 ± 2.56% in TrkBhGFAP; F1,8=1.136, p=0.3177 for genotype; F1,8=0.0498, p=0.8290 for BDNF), suggesting that the reduction in the size of neurosphere derived-from TrkBhGFAP mice was not due to survival deficits. Thus, BDNF facilitates proliferation by acting directly on NPCs and the activation of TrkB is solely responsible for this effect.
TrkB is required for induced proliferation and neurogenesis by antidepressants and voluntary exercise
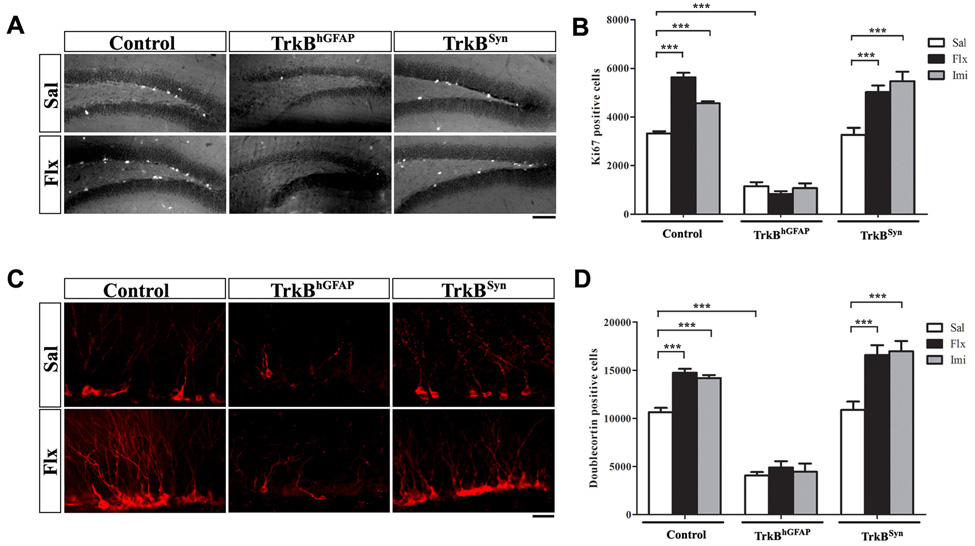
Reduction in hippocampal volume has been observed in animal models of stress (Coe et al., 2003; Czeh et al., 2001), which may be reversed or prevented by chronic AD treatment (Sheline et al., 2003). Similarly, reduction of hippocampal volume has been reported in some studies of human patients with major depression (Bremner et al., 2000; MacQueen et al., 2003) and post-traumatic stress disorder (Gilbertson et al., 2002; Karl et al., 2006; Smith, 2005). Although the cellular mechanism is unclear in humans, animal studies have demonstrated that chronic exposure to various types of ADs induces DG proliferation and neurogenesis (Malberg et al., 2000), thereby potentially contributing to the recovery of volume loss. In this context, we examined whether chronic AD treatment can restore neurogenesis in TrkBhGFAP mice. We thus treated control, TrkBhGFAP and TrkBSyn mice with daily injections of the serotonin reuptake inhibitor fluoxetine (10 µg/g) or the tricyclic imipramine (20 µg/g) for 21 days (n=7–29 for each group, Supplemental Table 1). As previously established, compared with mice receiving daily saline injections, both drugs induced substantial Ki67 immuno-reactivity in the DG of control mice (Figure 5A and 5B; F2,98=40.48, p<0.0001 for treatment; p<0.001 for post hoc test of both AD-to-saline comparisons). The induction of proliferation was echoed by an increase in the number of newly generated neurons expressing Doublecortin and NeuroD (Figure 5C, 5D and not shown; F2,98=30.79, p<0.0001 for treatment; p<0.001 for post hoc test of both AD-to-saline comparisons). In contrast, TrkBhGFAP mice treated with the same ADs did not show increase in the number of proliferating cells or immature neurons (Figure 5A to 5D; F2,98=332.3, p<0.0001 for the effect of genotype on Ki67; F2,98=211.6, p<0.0001 on Doublecortin). This treatment, or an extended six week treatment, also failed to restore the DG volume deficit in the TrkBhGFAP mice (not shown). The TrkBSyn mice responded normally to both ADs (Figure 5A to 5D; p<0.001 for post hoc test of both AD-to-saline comparisons).
Figure 5. TrkB expression in NPCs was required for chronic ADs induced proliferation and neurogenesis.
(A) Representative confocal images of immunostaining for Ki67 in the DG of saline or fluoxetine treated mice. Chronic treatment with fluoxetine increased the number of proliferating cells (Ki67+) in the DG of control, TrkBSyn, but not TrkBhGFAP mice. Scale bar, 100um. Sal, saline; Flx, fluoxetine.
(B) Quantitative analysis of Ki67 positive cells demonstrated lack of increases in the TrkBhGFAP mice after fluoxetine or imipramine treatment. Note the lower number of Ki67 positive cells in the TrkBhGFAP mice with either saline or AD, indicating impairment in proliferation at both basal and stimulated level. N=7–25 for each. ANOVA (GLM) found significant effects of AD treatment (F2.98=40.48, p<0.0001), genotype (F2,98=332.3, p<0.0001) and the interaction of both (F2,98=19.64, p<0.0001). Imi, imipramine.
(C) Chronic fluoxetine treatment increased the number of Doublecortin positive cells (red) in the DG of control and TrkBSyn but not TrkBhGFAP mice. Scale bar, 10um.
(D) Fluoxetine and imipramine failed to elicit an increase in the number of immature neurons (Doublecortin+) in the TrkBhGFAP mice. N=7–25 for each. ANOVA (GLM) revealed significant effects of AD treatment (F2,98=30.79, p<0.0001), genotype (F2,98=211.6, p<0.0001) and interaction between the two (F2,98=5.471, p=0.0005).
***p<0.001.
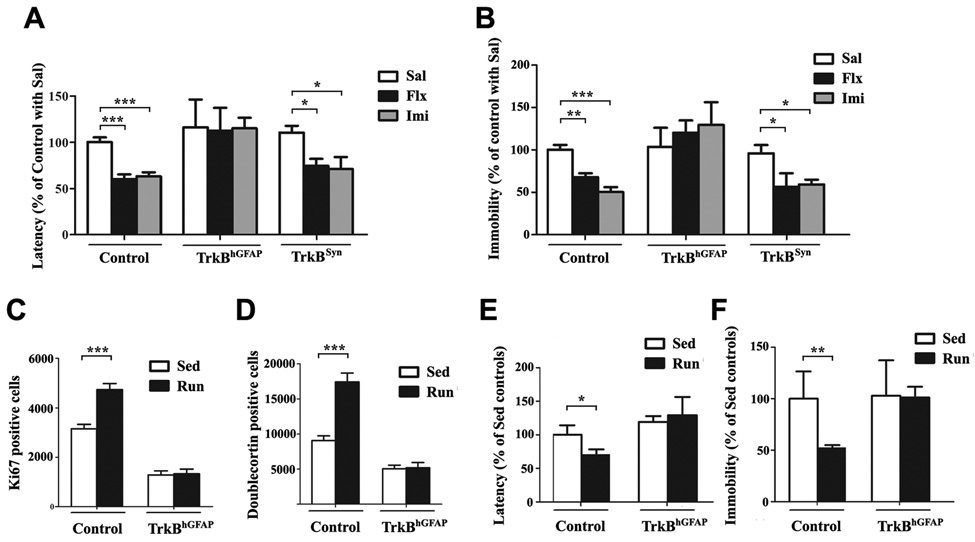
In rodents, voluntary exercise, such as wheel-running behavior has been demonstrated to robustly induce neurogenesis, much in the same fashion as AD treatment (van Praag et al., 1999). Less is known, however, about the underlying mechanism of this AD-like effect of exercise. To determine whether TrkB is also required for this process, we subjected control and TrkBhGFAP mice to 6 weeks of wheel-running (n=8–16 for each group, Supplemental Table 2). Both the numbers of Ki67 positive and Doublecortin positive cells increased in control runners, compared to control sedentary animals (Figure 6C: F1,36=13.64, p=0.0007 for exercise; Figure 6D: F1,36=16.01, p=0.0003; p<0.001 for post hoc test of both comparisons). A significant increase of BDNF protein level was also observed in the hippocampus of runners (66.44 ± 3.65 pg/mg in runners; 45.83 ± 2.99 pg/mg in sedentary controls; n=3 for each; p<0.05). The TrkBhGFAP mice, despite normal running distance (5.96 ± 0.41 km/day in controls, 5.48 ± 0.34 km/day in TrkBhGFAP, p>0.2) as well as elevation of BDNF level in runners (60.33 ± 4.43 pg/mg in runners; 40.94 ± 1.37 pg/ml in sedentary controls; n=3 for each; p<0.05), did not show any increase in proliferation and neurogenesis (Figure 6C and 6D, p>0.2 for both). Since we tested male and female mice in the voluntary exercise paradigm, we further separated the results into gender and genotype-specific groups and found no statistically significant difference between males and females of the same genotype (not shown).
Figure 6. TrkBhGFAP mice were insensitive to chronic AD and exercise induced improvement in depression and anxiety-like behaviors.
(A) In the novelty-suppressed feeding test (NSFT), chronic treatments with fluoxetine or imipramine shortened the latency to feed (indicating reduced anxiety) in control, TrkBSyn but not TrkBhGFAP mice. Data were shown as percentage of control with saline injection. N=6–29 for each group. ANOVA (GLM) found significant effects of AD treatment (F2,100=8.022, p=0.0006) and genotype (F2,100=10.49, p<0.0001).
(B) The tail-suspension test (TST) measured total duration of immobility (“behavior despair”), which could be reduced by chronic fluoxetine or imipramine in control, TrkBSyn but not TrkBhGFAP mice. Data were shown as percentage of control with saline injection. N=7–28 for each. ANOVA (GLM) revealed significant effects of AD treatment (F2,100=4.233, p=0.0172), genotype (F2,100=20.03, p<0.0001) and the interaction of both (F2,100=5.085, p=0.0009).
(C–D) Running for 6 weeks failed to increase the number of Ki67 (C) or Doublecortin (D) positive cells in the DG of TrkBhGFAP mice. N=6–13 for each. ANOVA (GLM) revealed significant effects of running (F1,36=13.64, p=0.0007 for Ki67; F1,36=16.01, p=0.0003 for Doublecortin), genotype (F1,36=141.1, p<0.0001 for Ki67; F1,36=58.46, p<0.0001 for Doublecortin) and the interaction of the two (F1,36=12.66, p=0.0010 for Ki67; F1,36=14.79, p=0.0005 for Doublecortin).
(E–F) TrkBhGFAP mice did not display decrease in latency to feed (E, NSFT) or duration of immobility (F, TST) after 6 weeks of running, compared to sedentary controls. Data were shown as percentage of sedentary control. N=8–16 for each. NSFT (F1,41=15.09, p=0.0004 for genotype), TST (F1,41=9.082, p=0.0044 for genotype; F1,41=8.273, p=0.0064 for exercise).
*p<0.05; **p<0.01; ***p<0.001.
TrkB is required for behavioral improvement induced by ADs and exercise
To determine whether the lack of neurogenic response in the TrkBhGFAP mice was coupled with general insensitivity to chronic ADs and exercise, we compared the depression and anxiety-like behaviors in control and TrkBhGFAP mice. First, mice treated with fluoxetine, imipramine, or saline for 21 days were examined in the novelty-suppressed feeding test (NSFT), a conflict paradigm in which the latency to feed in a novel environment is used as an indicator of anxiety level (Santarelli et al., 2003). In agreement with the general capacity of chronic ADs to ease anxiety, 24 hours after the last dose, control mice receiving fluoxetine or imipramine displayed significantly shorter latency to feed compared to saline-treated control mice (Figure 6A; F2,100=8.022, p=0.0006 for treatment; p<0.001 for post hoc test both AD-to-saline comparisons). The TrkBhGFAP mice, on the contrary, were insensitive to the effects of either AD (Figure 6A; F2,100=10.49, p<0.0001 for genotype). Similarly, TrkBhGFAP mice exposed to 6 weeks of running showed no improvement in the NSFT, whereas the control runners displayed clear decrease in latency compared to sedentary animals (Figure 6E; F1,41=15.09, p=0.0004 for genotype; p<0.05 for post hoc test of running effect in control mice). No difference in home cage consumption, or body weight loss was observed across genotypes (not shown).
Next we examined depression-like behavior in the control and TrkBhGFAP mice by using the tail-suspension test (TST), a paradigm of inescapable stress (Porsolt et al., 1987). All mice were tested 48 hours after the last dose of AD or saline to exclude the acute effects on behaviors that do not correlate with prior duration of drug treatment, or clinical responses. Control runners and AD treated control mice showed decreased immobility (a state of “behavioral despair”), compared to sedentary or saline treated control mice, respectively (Figure 6F: F1,41=9.082, p=0.0044 for exercise, post hoc p<0.01; Figure 6B: F2,100=20.03, p<0.0001 for AD, post hoc p<0.01 for fluoxetine, p<0.001 for imipramine). The TrkBhGFAP mice again failed to display any appreciable response to either treatment (Figure 6B: F2,100=4.233, p=0.0172 for genotype; Figure 6F: F1,41=9.082, p=0.0044 for genotype).
Despite the lack of responses to ADs and exercise in the behavioral paradigms of NSFT and TST, the saline-treated TrkBhGFAP mice performed similarly compared to the control mice, suggesting relatively normal depression and anxiety-like behaviors at the basal level. To further investigate this finding, we examined a cohort of control and TrkBhGFAP mice in a series of behavioral measures (Supplemental Figure 5; n=17–21 for each). We observed no significant differences within these two groups in the dark-light test in both the length of time spent and activity in the light compartment. In the open field test, the TrkBhGFAP mice were equivalent to controls in the time spent in the center. In the elevated-plus maze test, the TrkBhGFAP mice spent more time in the open arm compared to littermate controls. Together these results suggest normal (and in some case reduced) baseline anxiety-like behaviors in the TrkBhGFAP mice. Similarly, in the forced swim test, the TrkBhGFAP mice displayed normal latency to immobility, and a non-significant trend toward decreased total length of immobility, thereby supporting earlier observations of normal baseline depression-like behavior. Based on the above observations, we conclude that the lack of responses to AD and exercise in the TrkBhGFAP mice cannot be explained by alterations in baseline behaviors, but rather is a result of insensitivity to the molecular and cellular changes induced by chronic AD and exercise.
Normal sensitivity to chronic ADs in mice lacking TrkB in differentiated neurons
To explore whether the deficit in increased neurogenesis contributed to the abolished behavior sensitivity to chronic AD, we tested the TrkBSyn mice in the TST and the NSFT. Similar to control mice, upon chronic treatment with fluoxetine and imipramine, the TrkBSyn mice showed significant decreases in anxiety (Figure 6A; post hoc p<0.05 for both ADs) and depression-like behaviors (Figure 6B; post hoc p<0.05 for both ADs). This result, in conjunction with the observation that chronic AD treatment increased DG proliferation and neurogenesis in these mice (Figure 5), indicates that despite the lack of TrkB in differentiated neurons, the unaffected TrkB signaling in the NPCs was sufficient for the TrkBSyn mice to display a behavioral response to chronic AD.
Specific ablation of TrkB in adult NPCs is sufficient to block sensitivity to AD
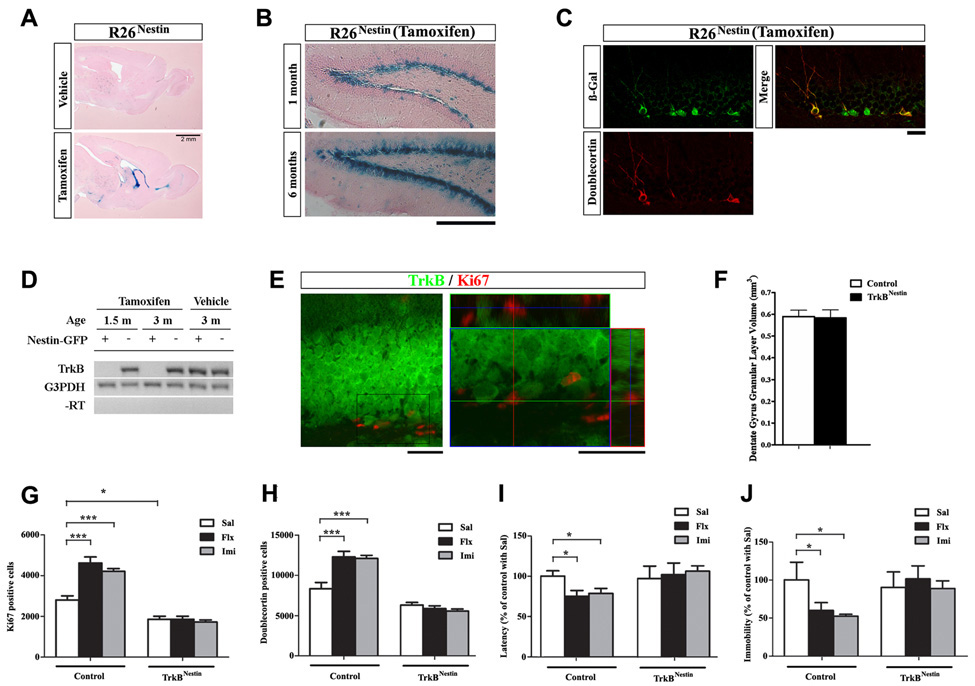
To further delineate whether TrkB function in adult NPCs alone was required for AD induced neurogenic and behavioral responses, we utilized a tamoxifen-inducible form of Cre recombinase, CreERT2, expressed under the regulatory element of the Nestin gene in which TrkB ablation could be confined to the adult neurogenic niches. When interbred with the R26 reporter mice (R26Nestin), Cre activity in the Nestin-CreER mice could be visualized either in embryonic or in adult CNS, in a tamoxifen-dependent manner. Specifically, when 1-month old R26Nestin mice were injected with vehicle or tamoxifen and analyzed 1 month afterwards, spontaneous recombination (vehicle treated) was minimal, while tamoxifen-induced recombination was restricted to the DG, sub-ventricular zone (SVZ), rostral migratory stream, olfactory bulb and cerebellum (Figure 7A and not shown). In the DG, recombination occurred specifically in the inner granular layer and SGZ. To evaluate the efficiency of the Nestin-CreER to target NPCs, we induced R26Nestin mice at 1 month and analyzed them at 2 months and 7 months of age. The number of X-gal stained cells was dramatically increased in the 6 months post-injection group, indicating effective recombination in the NPCs that were capable of proliferation and self-renewal (Figure 7B). Additional confirmation was obtained by double-immunostaining for β-gal and doublecortin at 4 months of age, where the majority of doublecortin positive cells also co-express β-gal (Figure 7C).
Figure 7. Specific ablation of TrkB from adult NPCs was sufficient to block AD sensitivity.
(A) X-gal staining on sagittal brain sections of R26Nestin mice, treated with vehicle or tamoxifen at 1 month and analyzed at 2 months of age.
(B) X-gal staining on DG sections of R26Nestin mice, treated with tamoxifen at 1 month and analyzed 1 month or 6 months afterwards. Note the increase in X-gal stained cells 6 months after tamoxifen injection. Scale bar, 200um.
(C) Co-staining for β-gal (green) and Doublecortin (red) on DG sections from R26Nestin mice, treated with tamoxifen at 1 month and analyzed at 4 months of age. Scale bar, 10um.
(D) RT-PCR detection of TrkB and G3PDH transcripts in FACS sorted Nestin-GFP positive and negative cells from the DG of TrkBflox/flox; Nestin-CreER mice treated with tamoxifen (TrkBNestin) or vehicle (control) at 1 month old.
(E) Co-staining for TrkB (green) and Ki67 (red) on DG sections from TrkBNestin mice at the age of 3 months (2 months post-tamoxifen injection), note the lack of co-localization of TrkB and Ki67 in the SGZ. Scale bars, 10um and 5um.
(F) TrkBNestin mice at the age of 3 months had normal DG volume.
(G–H) Quantitative analysis of Ki67 (G) and Doublecortin (H) positive cells demonstrated lack of increases in the TrkBNestin mice after fluoxetine or imipramine treatments. N=8–10 for each. ANOVA (GLM) found significant effects of AD treatment (F2,50=12.45, p<0.0001 for Ki67; F2,50=7.014, p=0.0021 for Doublecortin), genotype (F1,50=176.7, p<0.0001 for Ki67; F1,50=141.9, p<0.0001 for Doublecortin) and an interaction of the two (F2,50=13.57, p<0.0001 for Ki67; F2,50=12.56, p<0.0001 for Doublecortin).
(I–J) TrkBNestin mice did not display decrease in latency to feed (I, NSFT) or duration of immobility (J, TST) after chronic exposure to fluoxetine or imipramine, compared to control mice. Data were shown as percentage of control treated with saline. N=8–10 for each. NSFT: F1,50=13.68, p=0.0005 for genotype, F2,50=4.206, p=0.0205 for the interaction of genotype and AD treatment. TST: F1,50=18.15, p<0.0001 for genotype, F2,50=6.848, p=0.0024 for AD and F2,50=9.488, p=0.0003 for an interaction of the two.
*p<0.05. ***p<0.001.
As described above, hGFAP-Cre mediated ablation of TrkB resulted in a smaller DG that we attribute to the lack of TrkB signaling in early postnatal NPCs to sustain rapid proliferation required for normal structural development. To bypass this essential phase of DG postnatal morphogenesis, we subjected TrkBflox/flox; Nestin-CreER mice to tamoxifen (TrkBNestin) at 1 month of age. Littermate TrkBflox/flox; Nestin-CreER mice injected with vehicle, and TrkBflox/flox mice injected with tamoxifen were analyzed, and collectively presented as the control group. At the age of 6 weeks and 3 months, TrkB mRNA was virtually undetectable by RT-PCR in the DG NPCs of TrkBNestin mice, using FACS sorted Nestin-GFP positive cells (Figure 7D). TrkB protein was also absent from the proliferating cells of the SGZ, while its level in the majority of granular neurons was unaffected (Figure 7E). As expected, ablation of TrkB at 1 month did not lead to reduction in DG granular layer volume, when examined at 3 months of age (Figure 7F; n=6 for each; p>0.2). Basal proliferation in the SGZ at this age (Ki67 positive) was modestly decreased in the TrkBNestin mice, whereas no aberrant cell death was observed (Supplemental Figure 6). Upon exposure to fluoxetine (10ug/g) or imipramine (20ug/g) for 21 days (n=8–10 for each), the TrkBNestin mice did not display increased proliferation as measured by the numbers of Ki67 (Figure 7G; F1,50=176.7, p<0.0001 for genotype; F2,50=12.45, p<0.0001 for treatment; F2,50=13.57, p<0.0001 for interaction) and phosphorylated histone-H3 positive cells (Supplemental Figure 6; F1,50=131.9, p<0.0001 for genotype; F2,50=17.46, p<0.0001 for treatment; F2,50=17.34, p<0.0001 for interaction). The number of newly generated neurons as labeled by Doublecortin also did not change in the TrkBNestin mice (Figure 7H; F1,50=141.9, p<0.0001 for genotype; F2,50=7.014, p=0.0021 for treatment; F2,50=12.56, p<0.0001 for interaction). The absence of neurogenic response in the TrkBNestin mice coincided with a lack of behavioral improvements both in the NSFT (Figure 7I; F1,50=13.68, p=0.0005 for genotype) and the TST (Figure 7J; F1,50=18.15, p<0.0001 for genotype). These observations refine and confirm the preceding studies, indicating that ablation of TrkB from adult NPCs alone is sufficient to block sensitivity to chronic ADs.
Discussion
Regulation of postnatal and adult neurogenesis in the dentate gyrus
The identification of NPCs in the largely post-mitotic adult brain has transformed our perspective on the development, physiology and pathogenesis of this organ. Anatomically, NPCs in the postnatal and adult brain reside in the SVZ of the lateral ventricle and the SGZ of the DG, regions considered to be the residues of the embryonic germinal niche (Alvarez-Buylla and Lim, 2004). However, adult NPCs, especially those in the DG, deviate from the “embryonic properties” and have been noted to possess limited self-renewal, proliferation and migration capacities (Bull and Bartlett, 2005; Seaberg and van der Kooy, 2002). Paradoxically, DG NPCs gain a remarkable capacity to respond to various extrinsic and intrinsic stimuli (Lledo et al., 2006; Ming and Song, 2005). The signaling mechanisms that specify these unique properties of DG NPCs, both on the basal level and the induced state, remain unidentified.
Our study shows that TrkB-expressing NPCs in the postnatal and adult DG respond to BDNF. As demonstrated in the TrkBhGFAP mice, ablation of TrkB from the DG NPCs blocked BDNF-induced neurosphere growth in vitro, and impaired proliferation and neurogenesis in vivo. Although the Cre-mediated deletion of TrkB in the TrkBhGFAP mice was not restricted to DG NPCs, the requirement for TrkB in proliferation was cell-autonomous as evidenced by: (1) neurospheres generated from the DG of the TrkBhGFAP mice had impaired proliferation in vitro; (2) In vivo, the dividing cells in the DG of the TrkBhGFAP mice were more prone to prematurely exit the cell cycle; (3) Proliferation in the DG was unaffected in the TrkBSyn mice where Cre-mediated recombination occurred exclusively in differentiated neurons; (4) Temporal and spatial specific ablation of TrkB from young adult NPCs (TrkBNestin) also resulted in impaired proliferation and neurogenesis.
Neurotrophic factors, particularly BDNF, have been shown to promote proliferation of NPCs in vitro (Barnabe-Heider and Miller, 2003). Whether this effect resonates with a similar physiological requirement in vivo is less apparent. BDNF germline heterozygous mice have been reported to have decreased (Lee et al., 2000) or increased proliferation (Sairanen et al., 2005); as well as decreased (Sairanen et al., 2005) or normal long-term neurogenesis (Rossi et al., 2006). By directly ablating the BDNF receptor, TrkB, in NPCs, our data ascertain an unambiguous cell autonomous requirement for TrkB in maintaining proliferation and neurogenesis in the DG. It is worth noting that as TrkB is the high-affinity receptor for both BDNF and neurotrophin 4 (NT4), the phenotype observed in the TrkBhGFAP and TrkBNestin mice could be a combinatory outcome of lacking signaling response to both ligands, although the expression level of NT4 in the postnatal brain is lower than BDNF. We have examined BDNFhGFAP conditional mutant mice and observed a similar degree of reduction in DG granular layer volume to that of the TrkBhGFAP mice. NT4 null mice are viable (Liebl et al., 2000) and appear to have normal DG volume (not shown).
The specific impairment of postnatal DG morphogenesis observed in the TrkBhGFAP mice appears to be a unique phenomenon. Although NPCs in the embryonic CNS express TrkB and have been shown to benefit from exogenous BDNF in vitro (Barnabe-Heider and Miller, 2003), ablation of TrkB or BDNF in vivo, as demonstrated in TrkB and BDNF germline knockouts as well as the TrkBhGFAP mice, do not result in aberrant neurogenesis of the prenatal CNS. The TrkBhGFAP mice were born with, and continued to have normal brain size throughout adulthood. The reduction of the DG granular layer volume was absent at birth, and only becomes appreciable after P10. Absence of TrkB did not affect proliferation in the primary dentate neuroepithelium at E16.5, nor the migration and resettling of the secondary dentate matrix at P0 (not shown). Therefore, we reveal here a regulatory mechanism that uncouples postnatal DG neurogenesis from embryonic development. Interestingly, while early ablation of TrkB (TrkBhGFAP) dramatically reduced proliferation in adult DG, we observed a more modest impairment in basal proliferation when TrkB is removed at the young adult age (TrkBNestin). This likely reflects the combined outcome of TrkB ablation in the early postnatal period on affecting individual cell division by promoting cell cycle exiting, and diminishing the overall pool of progenitor cells available for proliferation. In contrast, late deletion of TrkB cannot significantly disturb the progenitor pool size and thus the phenotype in the TrkBNestin mice likely represents the role of TrkB in regulating the behaviors of individual adult NPCs.
Our studies demonstrate that deletion of TrkB from DG NPCs (TrkBhGFAP and TrkBNestin) abolishes the proliferative and neurogenic effects of chronic AD treatments and voluntary exercise. This is consistent with earlier observations that both AD and running induce significant increases in BDNF level (Neeper et al., 1996; Nibuya et al., 1995). The inability of TrkB null NPCs to respond to AD and running was most likely due to the lack of sensitivity to BDNF, but not from developmental deficits that compromise cell division, as evidenced by their capacity to react to other exogenous factors, including EGF, bFGF and oxygen conditions in vitro (not shown). Conversely, when TrkB was ablated only from differentiated neurons (TrkBSyn), both the proliferation and neurogenesis responses to chronic AD were maintained. It is intriguing that we observed a slightly more robust response to ADs in the TrkBSyn mice, suggesting that when surrounded by TrkB null neurons, NPCs and immature neurons with unaffected TrkB signaling may have a selective advantage in growth and/or survival.
TrkB and the behavioral efficacy of antidepressants
There has been considerable evidence that genetic ablation of BDNF or TrkB may interfere with the normal function of the adult brain, depending on the regions affected. Conditional knockout animals lacking TrkB in forebrain neurons (with broader recombination than TrkBhGFAP and TrkBSyn) have impaired spatial learning behavior, but display normal anxiety level in the open field test (Minichiello et al., 1999). The latter is consistent with our observation that the TrkBhGFAP mice exhibited normal basal anxiety-like behavior in the NSFT, the open field test, and the dark-light preference test. Likewise, the TrkBhGFAP mice seemed to have normal depression-like behavior at the basal level, as measured by the TST and the forced-swim test. The TrkBNestin mice only lack TrkB in a highly specific population of cells, and were phenotypically indistinguishable from control mice at baseline. Previous studies with BDNF mutants regarding these anxiety and depression-like behaviors have yielded mixed results: some were normal (Gorski et al., 2003; Monteggia et al., 2004; Saarelainen et al., 2003); while others were not (Chen et al., 2006; Monteggia et al., 2007; Rios et al., 2001). This discrepancy may be explained by the difference in the brain regions affected by the genetic ablation. BDNF is a secreted protein that can be transported and deposited across long distances both in retrograde and anterograde directions. Therefore local inactivation of the BDNF gene may well result in global disturbance of BDNF protein level, thus confounding the interpretation of results. Recent discovery that BDNF in different regions of the brain may have opposing function in modulating stress response supports this notion (Berton et al., 2006).
Unlike control mice, the TrkBhGFAP (and TrkBNestin) mice were insensitive to chronic AD and exercise-induced improvement in depression and anxiety-like behaviors. This finding provides compelling evidence that TrkB is required for a shared molecular mechanism through which AD and exercise act. This process is likely to be BDNF-dependent, echoing previous studies showing elevated levels of BDNF with chronic AD (Nibuya et al., 1995) as well as blunted behavioral response to AD in BDNF mutant animals (Chen et al., 2006; Monteggia et al., 2004; Saarelainen et al., 2003). Interestingly, TrkBSyn mice show normal behavioral responses to chronic AD treatment, indicating that neuronal expression of TrkB is not required for the behavioral efficacy of AD. Though the mechanism via which ADs elicit increase in hippocampal BDNF level remains undefined, this process only occurs after chronic exposure to ADs (Nibuya et al., 1995), implicating BDNF elevation and the subsequent TrkB activation as an indirect downstream event from the acute accumulation of monoamine, such as serotonin and noradrenaline. It has been suggested that chronic ADs promote the cAMP pathways and increase CREB activity (Nibuya et al., 1996) – a transcriptional factor that activates the BDNF gene, among a multitude of other targets. While these observations provide important insight into the molecular underpinning of AD response, future work is needed to validate and further delineate the involvement of this pathway in regulating neurogenesis and mood (Conti et al., 2002; Nakagawa et al., 2002).
Functional link between neurogenesis and sensitivity to antidepressants
It has been debated whether neurogenesis in the adult hippocampus bears functional significance in the action of chronic AD, or the physiology of the hippocampus in general (Leuner et al., 2006; Scharfman and Hen, 2007; Zhao et al., 2008). In the current study, we selectively abolished the neurogenic sensitivity and a corresponding blockade of behavioral responses to chronic AD by ablating TrkB in adult NPCs (TrkBNestin), NPCs and neurons (TrkBhGFAP), but not neurons alone (TrkBSyn). Therefore we conclude that TrkB-dependent increase in neurogenesis is required for the effects of chronic ADs in control and TrkBSyn mice in behavioral paradigms of NFST and TST. The hGFAP-Cre and Nestin-CreER transgenes elicit recombination in the neurogenic niches of SVZ as well as hippocampus. However, prior studies have excluded a possible link for SVZ neurogenesis and the behavioral effects of AD in rodents (Malberg et al., 2000; Santarelli et al., 2003). Thus our data provide genetic support and mechanistic insight to previous reports that irradiation-mediated ablation of dividing cells from the hippocampal region of rodents abolished their behavioral responses to ADs (Airan et al., 2007; Santarelli et al., 2003; Wang et al., 2008a). A recent study reports differential response to chronic AD in inbred strains of mice suggesting differing molecular and cellular mechanisms (Holick et al. 2008). Thus in contrast to the present findings and previous reports (Encinas et al., 2006; Santarelli et al., 2003; Wang et al., 2008a), chronic fluoxetine produces antidepressive behavioral effects in BALB/cJ mice by mechanisms independent of serotonin 1A receptor and DG neurogenesis. This marked strain difference highlights the existence of multiple mechanisms by which chronic AD changes anxiety and depression-like behaviors, and raises the intriguing possibility that genetic variations may be involved in determining the path of AD efficacy.
Our results also suggest that reduced levels of basal proliferation in the DG of TrkBhGFAP and TrkBNestin mice do not directly lead to obvious affective impairment, an observation consistent with recent findings using irradiation-mediated NPC ablation method (Airan et al., 2007; Santarelli et al., 2003; Wang et al., 2008a). Rather, these animal studies suggest ADs and exercise-stimulated increase in DG neurogenesis may translate into enhanced synaptic plasticity in the pertinent neural circuits (Wang et al., 2008a), which subsequently manifests behavioral responsiveness to such treatments. One possible explanation for this dissociation between basal and induced neurogenesis may reside in functional differences between neurons generated at the constant state and stimulated state (Jakubs et al., 2006). The exact nature of these differences and the molecular mechanism that encodes them remain to be determined. Nonetheless, our data suggests that TrkB regulates DG NPCs at both states.
Experimental Procedures
A detailed description of all procedures is included in the Supplemental Data.
Animals
The floxed alleles of TrkB and BDNF were generated previously (Luikart et al., 2005; Monteggia et al., 2007). All mouse protocols were approved by the Institutional Animal Care and Research Advisory Committee at University of Texas Southwestern Medical Center.
Histology and Quantitative Analysis
Mice were intra-cardially perfused with PBS followed by 4% (w/v) paraformaldehyde (PFA) in PBS, and the dissected brains postfixed in 4% PFA at 4 degree. In situ hybridization, TUNEL assay, X-gal, Nissl, and immuno-staining were performed as previously described (Lei et al., 2005; Luikart et al., 2005). Detailed methods for these assays and the stereological quantifications were described in Supplemental Experimental Procedures.
Protein Analysis
Total protein was extracted and measured for western blotting and BDNF ELISA assay. Total protein from the supernatant was used for western blotting as described (Lush et al., 2008). BDNF level was measured using a BDNF ELISA kit (Promega) as per manufacturer’s instructions.
Neurosphere cultures
Neurosphere culture was established and maintained as described (Bull and Bartlett, 2005) with some modifications. Briefly, DG of adult mice were dissected (Seaberg and van der Kooy, 2002) and digested in 0.1% trypsin-EDTA (Sigma) followed by mechanical trituration until smooth. Cells were plated at a density of 20 cells/ul in complete growth medium, consisting of mouse NeuroCult NSC basal medium (StemCell Technologies), mouse NeuroCult NSC proliferation supplements (StemCell Technologies), 2 ug/ml heparin (Sigma), EGF (20 ng/ml, Gibco) and bFGF (10 ng/ml, Sigma). Treatment with BDNF (50 ng/ml, R&D Systems) started from the first day, or 30min before harvest in the case of phospho-Trk490 western blotting.
FACS
For FACS isolation of GFP positive or negative cells, primary cells were prepared from the DG of Nestin-GFP transgenic mice at the age of postnatal day 2, 15 and 2 months following the procedure for establishing neurosphere culture. GFP positive and negative fractions of the live cells (PI negative) were analyzed and sorted on FACSAria.
Tamoxifen treatment
Tamoxifen (Sigma) was dissolved in a sunflower oil/ethanol mixture (9:1) at 6.7 mg/ml. Vehicle (9:1 sunflower oil/ethanol) or tamoxifen was injected intraperitoneally into 1-month-old mice at 250 µl/20 g (83.8 mg/kg) twice a day for 5 consecutive days. All injected mice were observed daily for neurological-related abnormality. TrkBflox/flox;Nestin-CreERT2 mice injected with vehicle and TrkBflox/flox mice injected with tamoxifen were indistinguishable and therefore pooled as the control group.
Antidepressant treatment, voluntary exercise and behavioral tests
All animals used for AD treatment, running and subsequent behavioral tests were littermates. Detailed information on the cohorts of animals used and the procedures of behavioral tests were described in Supplemental Data. Briefly, immediately after the last dose of AD or saline, or last day of running, mice were deprived of food for 24 hours and then subjected to the NSFT. 24 hours afterwards, the same groups of mice were subjected to the TST. Latency to feed in the NSFT and length of immobility in the TST were rated as previously described (Santarelli et al., 2003; Svenningsson et al., 2006), by investigators blind to genotypes. Mice were sacrificed 24 hours after the TST for tissue collection and subsequent analyses.
Supplementary Material
Acknowledgements
The authors would like to thank Shawna Kennedy, Dustin Corgan, Linda McClellan, Steven McKinnon for technical assistance, Drs. Jingsheng Yan and Joshua Koch for advice on statistical analyses, and Dr. Louis Reichardt for providing the TrkB antibody. We are indebted to the members of the Parada lab, in particular Drs. Lei Lei and Mark Lush for sharing reagents and helpful suggestions. We wish to thank Dr. Eric J. Nestler for insightful discussions and Dr. Amelia Eisch for critical reading of this manuscript. This work was supported by the NINDS grant R37NS033199, NIMH Conte Center grant P50MH66172 and the ACS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990;301:365–381. doi: 10.1002/cne.903010304. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A. Neurogenesis and plasticity in the CNS of adult birds. Exp Neurol. 1992;115:110–114. doi: 10.1016/0014-4886(92)90232-f. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Barnabe-Heider F, Miller FD. Endogenously produced neurotrophins regulate survival and differentiation of cortical progenitors via distinct signaling pathways. J Neurosci. 2003;23:5149–5160. doi: 10.1523/JNEUROSCI.23-12-05149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Bull ND, Bartlett PF. The adult mouse hippocampal progenitor is neurogenic but not a stem cell. J Neurosci. 2005;25:10815–10821. doi: 10.1523/JNEUROSCI.3249-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi MJ, Kang RH, Lim SW, Oh KS, Lee MS. Brain-derived neurotrophic factor gene polymorphism (Val66Met) and citalopram response in major depressive disorder. Brain Res. 2006;1118:176–182. doi: 10.1016/j.brainres.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Coe CL, Kramer M, Czeh B, Gould E, Reeves AJ, Kirschbaum C, Fuchs E. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry. 2003;54:1025–1034. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- Conti AC, Cryan JF, Dalvi A, Lucki I, Blendy JA. cAMP response element-binding protein is essential for the upregulation of brain-derived neurotrophic factor transcription, but not the behavioral or endocrine responses to antidepressant drugs. J Neurosci. 2002;22:3262–3268. doi: 10.1523/JNEUROSCI.22-08-03262.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci U S A. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini A, Kupfer DJ. Is treatment-resistant depression a unique subtype of depression? Biol Psychiatry. 2003;53:640–648. doi: 10.1016/s0006-3223(02)01670-0. [DOI] [PubMed] [Google Scholar]
- Frazer A. Pharmacology of antidepressants. J Clin Psychopharmacol. 1997;17 Suppl 1:2S–18S. doi: 10.1097/00004714-199704001-00002. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski JA, Balogh SA, Wehner JM, Jones KR. Learning deficits in forebrain-restricted brain-derived neurotrophic factor mutant mice. Neuroscience. 2003;121:341–354. doi: 10.1016/s0306-4522(03)00426-3. [DOI] [PubMed] [Google Scholar]
- He XP, Kotloski R, Nef S, Luikart BW, Parada LF, McNamara JO. Conditional deletion of TrkB but not BDNF prevents epileptogenesis in the kindling model. Neuron. 2004;43:31–42. doi: 10.1016/j.neuron.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33:406–417. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- Jakubs K, Nanobashvili A, Bonde S, Ekdahl CT, Kokaia Z, Kokaia M, Lindvall O. Environment matters: synaptic properties of neurons born in the epileptic adult brain develop to reduce excitability. Neuron. 2006;52:1047–1059. doi: 10.1016/j.neuron.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Jiang X, Xu K, Hoberman J, Tian F, Marko AJ, Waheed JF, Harris CR, Marini AM, Enoch MA, Lipsky RH. BDNF variation and mood disorders: a novel functional promoter polymorphism and Val66Met are associated with anxiety but have opposing effects. Neuropsychopharmacology. 2005;30:1353–1361. doi: 10.1038/sj.npp.1300703. [DOI] [PubMed] [Google Scholar]
- Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30:1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Klein R, Martin-Zanca D, Barbacid M, Parada LF. Expression of the tyrosine kinase receptor gene trkB is confined to the murine embryonic and adult nervous system. Development. 1990;109:845–850. doi: 10.1242/dev.109.4.845. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Long JM, Ingram DK, Mattson MP. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J Mol Neurosci. 2000;15:99–108. doi: 10.1385/JMN:15:2:99. [DOI] [PubMed] [Google Scholar]
- Lei L, Laub F, Lush M, Romero M, Zhou J, Luikart B, Klesse L, Ramirez F, Parada LF. The zinc finger transcription factor Klf7 is required for TrkA gene expression and development of nociceptive sensory neurons. Genes Dev. 2005;19:1354–1364. doi: 10.1101/gad.1227705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Liebl DJ, Klesse LJ, Tessarollo L, Wohlman T, Parada LF. Loss of brain-derived neurotrophic factor-dependent neural crest-derived sensory neurons in neurotrophin-4 mutant mice. Proc Natl Acad Sci U S A. 2000;97:2297–2302. doi: 10.1073/pnas.040562597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Luikart BW, Nef S, Virmani T, Lush ME, Liu Y, Kavalali ET, Parada LF. TrkB has a cell-autonomous role in the establishment of hippocampal Schaffer collateral synapses. J Neurosci. 2005;25:3774–3786. doi: 10.1523/JNEUROSCI.0041-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lush ME, Li Y, Kwon CH, Chen J, Parada LF. Neurofibromin is required for barrel formation in the mouse somatosensory cortex. J Neurosci. 2008;28:1580–1587. doi: 10.1523/JNEUROSCI.5236-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahmias C, Young LT. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Minichiello L, Korte M, Wolfer D, Kuhn R, Unsicker K, Cestari V, Rossi-Arnaud C, Lipp HP, Bonhoeffer T, Klein R. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron. 1999;24:401–414. doi: 10.1016/s0896-6273(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, Meuth S, Nagy A, Greene RW, Nestler EJ. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci U S A. 2004;101:10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parada LF, Nestler EJ. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry. 2007;61:187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Frazer A. Antidepressants and brain monoaminergic systems: a dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int J Neuropsychopharmacol. 2004;7:193–218. doi: 10.1017/S1461145704004080. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, Zhang YJ, Nestler EJ, Duman RS. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci. 2002;22:3673–3682. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera TD, Coplan JD, Lisanby SHC, Lipira CM, Arif M, Carpio C, Spitzer G, Santarelli L, Scharf B, Hen R, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 2007;27:4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Egan MF, Meyer-Lindenberg A, Weinberger DR. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Chermat R, Lenegre A, Avril I, Janvier S, Steru L. Use of the automated tail suspension test for the primary screening of psychotropic agents. Arch Int Pharmacodyn Ther. 1987;288:11–30. [PubMed] [Google Scholar]
- Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, Lechan RM, Jaenisch R. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F, Fabbri ME, Tessarollo L, Maffei L, Berardi N, Caleo M. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci. 2006;24:1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, Agerman K, Haapasalo A, Nawa H, Aloyz R, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23:349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairanen M, Lucas G, Ernfors P, Castren M, Castren E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clin Psychol Rev. 2001;21:33–61. doi: 10.1016/s0272-7358(99)00032-x. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Hen R. Neuroscience. Is more neurogenesis always better? Science. 2007;315:336–338. doi: 10.1126/science.1138711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schule C, Zill P, Baghai TC, Eser D, Zwanzger P, Wenig N, Rupprecht R, Bondy B. Brain-derived neurotrophic factor Val66Met polymorphism and dexamethasone/CRH test results in depressed patients. Psychoneuroendocrinology. 2006;31:1019–1025. doi: 10.1016/j.psyneuen.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci. 2002;22:1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME. Bilateral hippocampal volume reduction in adults with posttraumatic stress disorder: a meta-analysis of structural MRI studies. Hippocampus. 2005;15:798–807. doi: 10.1002/hipo.20102. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, Vaugeois JM, Nomikos GG, Greengard P. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science. 2006;311:77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- Tardito D, Perez J, Tiraboschi E, Musazzi L, Racagni G, Popoli M. Signaling pathways regulating gene expression, neuroplasticity, and neurotrophic mechanisms in the action of antidepressants: a critical overview. Pharmacol Rev. 2006;58:115–134. doi: 10.1124/pr.58.1.7. [DOI] [PubMed] [Google Scholar]
- Thase ME, Rush AJ. Treatment-resistant depression. In: B FE, K DJ, editors. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press; 1995. pp. 1081–1098. [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008a;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, Dranovsky A, Hen R. The when and where of BDNF and the antidepressant response. Biol Psychiatry. 2008b;63:640–641. doi: 10.1016/j.biopsych.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Yu TS, Dandekar M, Monteggia LM, Parada LF, Kernie SG. Temporally regulated expression of Cre recombinase in neural stem cells. Genesis. 2005;41:147–153. doi: 10.1002/gene.20110. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhou XF, Parada LF, Soppet D, Rush RA. Distribution of trkB tyrosine kinase immunoreactivity in the rat central nervous system. Brain Res. 1993;622:63–70. doi: 10.1016/0006-8993(93)90802-t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.