Abstract
Natriuretic peptides are important in regulating salt and body-fluid balance. In cells, these peptides are made as precursor forms that are converted to active forms by proteolyic processing. Corin is a transmembrane serine protease identified in the heart. Corin converts pro-atrial natriuretic peptide (pro-ANP) to active ANP in a sequence-specific manner. In mice, lack of corin prevents the conversion of pro-ANP to ANP and causes salt-sensitive hypertension. The hypertensive phenotype is exacerbated when the mice become pregnant. In humans, single nucleotide polymorphisms (SNPs) in the corin gene have been identified in African-Americans with hypertension and cardiac hypertrophy. These data indicate that corin is important in maintaining normal blood pressure in vivo and that corin deficiency may contribute to hypertension and heart disease in patients.
Keywords: protease, hypertension, preeclampsia
THE NATRIURETIC PEPTIDE SYSTEM
The natriuretic peptide system, which was first discovered in the early 1980s by Adolfo de Bold,1 is an endocrine mechanism that regulates salt and body-fluid balance. The system is conserved in all vertebrates ranging from the most primitive species such as hagfish to human. For many teleost species, natriuretic peptides are critical for their adaptation to low and high salt environments. Salmon and some eels, for example, live in both fresh and salty water during different phases of their life cycles. Natriuretic peptides have been identified in these animals.2,3 In Japanese eel (Anguilla japonica), natriuretic peptides are shown to be important in controlling water drinking and regulating salt absorption and excretion in gills, guts, and kidneys when the animals switch between low-salt freshwater and high-salt seawater.3 Unlike the teleost fish, terrestrial mammals do not have to deal with constant surroundings of dangerously low or high salt. Nevertheless, natriuretic peptides are important in maintaining blood volume and electrolyte balance, as drinking water and food contents on earth may vary dramatically in different seasons and locations.
The mammalian natriuretic peptide family has three structurally related members, namely atrial natriuretic peptide or factor (ANP or ANF), brain or B-type natriuretic peptide (BNP), and C-type natriuretic peptide (CNP). ANP and BNP are made primarily in the heart whereas CNP is made in many organs including the brain, kidney, bone and vessels. The primary function of ANP and BNP is to regulate blood volume and pressure. Under high blood volume or pressure, ANP and BNP are released into the circulation. In target organs such as kidneys and peripheral blood vessels, the peptides activate their receptor, natriuretic peptide receptor-A (NPR-A), and increase intracellular cGMP production, leading to natriuresis, diuresis, and vasodilation, and thereby lowering blood volume and pressure. ANP and BNP also suppress renin and endothelin release, which is an additional mechanism to regulate vascular tone. In contrast, CNP binds to natriuretic peptide receptor-B (NPR-B), inhibits vascular smooth muscle cell proliferation, and prevents coronary artery restenosis in animal models. CNP and NPR-B also play a major role in chondrocyte differentiation and bone formation.4 There is a third natriuretic peptide receptor, NPR-C, which binds all three peptides and remove them from the circulation. Several recent articles have reviewed various aspects of the natriuretic peptide system in depth.5–7
BIOSYNTHESIS OF NATRIURETIC PEPTIDES
Like many peptide hormones, natriuretic peptides are synthesized as prepropeptides. After the signal peptide is removed, an additional proteolytic cleavage is required to convert the inactive propeptide to an active peptide (Fig. 1). This post-translational processing step represents an important mechanism in regulating the activity of the natriuretic peptides, but identifying the endogenous processing enzymes was challenging. Several laboratories, for example, showed that thrombin and kellikreins could process pro-ANP in vitro.8 However, it was difficult to prove that the observed activities were specific and to show that these enzymes function as the physiological pro-ANP convertase in vivo. Other studies also indicated that a cell membrane associated high-molecular weight trypsin-like enzyme in the heart may be the pro-ANP convertase but purification of this enzyme was unsuccessful.9 As a result, the enzymes responsible for processing natriuretic peptides remain undefined for many years.
Figure 1. Processing of natriuretic peptides.
Natriuretic peptides are synthesized as prepropeptides. The signal peptide (sp) is removed by signal peptidase. The propeptide is cleaved by propeptide convertase to produce an inactive N-terminal peptide and a C-terminal mature peptide that is biologically active.
THE CARDIAC SERINE PROTEASE CORIN
The discovery of corin was serendipitous. Our interests in serine proteases started with thrombin, a blood clotting enzyme whose activity is greatly enhanced in the presence of cell membranes. Later, we extended our work to include transmembrane serine proteases such as enteropeptidase and hepsin,10 which subsequently led us to search for other transmembrane serine proteases in the cardiovascular system. By searching databases for genes with trypsin-like sequences, we cloned a new membrane serine protease from the human heart and named it corin for its cardiac expression.11
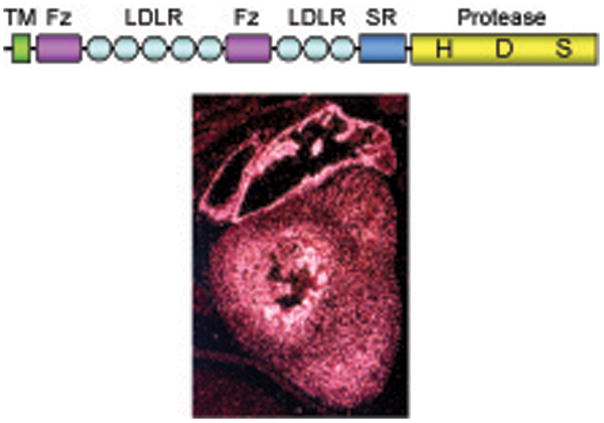
As shown in Fig. 2, corin is a mosaic protein consisting of a transmembrane domain near the N-terminus and two frizzled-like domains, eight LDL receptor repeats, a scavenger receptor –like domain, and a trypsin-like protease domain at the C-terminus. Such a protein domain arrangement is unique. To date, corin remains the only known trypsin-like protease that contains frizzled-like domains, which are mostly associated with Wnt signaling proteins. Moreover, human corin, as a serine protease, is exceptionally large with 1042 amino acids. There are 19 predicted N-linked glycosylation sites in its extracellular region.11 On SDS-PAGE and Western analysis, native and recombinant human, rat, and mouse corin proteins had apparent molecular masses of ~150–200 kDa.12–15 The human corin gene is on chromosome 4p12–13 with 22 exons and spans >200 kb,16 making it one of the biggest protease genes known to date.
Figure 2. Corin domain sturecture and mRNA expression in the heart.
Corin has a transmembrane domain (TM), two frizzled-like domains (Fz), eight LDL receptor repeats (LDLR), a scavenger receptor domain (SR), and a trypsin-like protease domain with three active site amino acids His (H), Asp (D), and Ser (S). Corin mRNA expression in the atrium and ventricle of a mouse heart at embryonic day 15.5 was detected by in situ hybridization.
Corin is made primarily in the heart. We and others detected corin mRNA and protein in fetal and adult cardiomyocytes.11,13 Corin mRNA expression appeared to be higher in the atrium than the ventricle (Fig. 2). Corin mRNA also was found in scar myofibroblasts in rat hearts.17 Lower levels of corin mRNA were detected in other tissues including developing kidneys and bones.11 Most recently, corin mRNA and protein were detected in mouse skin hair follicles.18 In contrast, corin mRNA was not found in other muscle-rich tissues such as stomach, small intestine, bladder, skeletal muscle, and non-pregnant uterus.11
CORIN IN PRO-ANP PROCESSING
We next set out to identify corin function. Because corin is a cardiac transmembrane protease with trypsin-like substrate specificity, it is likely that the corin substrate is a protein in the heart that is cleaved at a basic amino acid. Pro-ANP, which is made in the heart and proteolytically activated at Arg-98, could be a candidate substrate for corin. We did biochemical and cellular studies to test this hypothesis. In cell- and purified protein-based assays, corin converted pro-ANP to ANP in a sequence-specific manner.15,19,20 In cultured cardiomyocytes, overexpression of a negative mutant corin or transfection of small interfering RNA against the corin gene blocked pro-ANP processing,21 suggesting that corin is the pro-ANP convertase.
To define the functional importance of corin in vivo, we made corin null mice and showed that atrial tissues from these mice had pro-ANP but no ANP, indicating that pro-ANP processing was abolished in the knockout mice.22 Infusion of a soluble corin into these mice transiently restored pro-ANP processing, resulting in the release of active ANP into the circulation. These results have established corin as the physiological pro-ANP convertase and solved a long-standing puzzle in natriuretic peptide biology.23,24 In cell-based assays, corin also cleaved pro-BNP, although the reaction was less efficient than that for pro-ANP.15 Previous reports suggested that other enzymes may also process pro-BNP.25 Further studies are needed to define the pro-BNP convertase in vivo.
In contrast, corin did not cleave pro-CNP in similar experiments.26 In human kidney cells and chondrocytes, recombinant human pro-CNP was processed without corin. Corin overexpression did not enhance pro-CNP processing in these cells. The results are consistent with the fact that the pro-CNP cleavage sequence differs from that in pro-ANP and pro-BNP, and the fact that pro-CNP is processed intracellularly whereas corin acts extracellularly. We went on and tested furin, a trans-Golgi enzyme, as a candidate for pro-CNP convertase. Using furin-deficient LoVo cells and purified recombinant furin protein, we showed that pro-CNP is activated by furin.26 Thus, natriuretic peptides are processed by different enzymes, although they share significant sequence and structural similarities.
HYPERTENSION IN CORIN KNOCKOUT MICE
The ANP pathway is important in regulating blood pressure. Knockout mice lacking ANP or NPR-A are hypertensive.27,28 If corin is essential for pro-ANP activation, mice with corin deficiency are expected to have a similar hypertensive phenotype. Using radiotelemetry, we found that corin null mice, which are viable and fertile, indeed were hypertensive.22 Both male and female corin null mice had elevated systolic, diastolic, and mean arterial blood pressure compared to that in wild-type controls. Blood pressure increased further when the mice were fed a high-salt diet. Such a phenotype also was reported in ANP null mice.27 The data are consistent with corin being the pro-ANP convertase and show the importance of corin in maintaining normal blood pressure in vivo.
In Northern blotting, corin mRNA was found only in the heart. By in situ hybridization, however, abundant corin mRNA was detected in the decidual cells of the pregnant mouse uterus.11 Corin mRNA also was found in human endometrium and leiomyosarcoma cells.11 The finding of uterine corin expression was unexpected but may suggest a role of corin in pregnancy. As blood volume expands significantly during pregnancy, maintaining normal blood pressure becomes increasingly challenging. As a result, additional regulations may be needed to deal with this special condition. It is possible that the uterine corin expression is a previously unrecognized mechanism to prevent hypertension during pregnancy.
If this hypothesis is correct, we should expect further blood pressure increase when corin null mice become pregnant. By telemetric monitoring, we indeed found a significant increase of systolic blood pressure in pregnant corin null mice, which reached the highest level at mid-gestation but eventually returned to pre-pregnancy levels after delivery.22 The pregnant corin null mice also develop late-gestational proteinuria, reminiscent of preeclampsia in patients. Histological analysis indicated endotheliosis and ischemic changes in glomeruli in these mice. These results suggest that corin may be exploited during pregnancy to regulate hemodynamic changes and prevent hypertension. Corin null mice may be used as a new model of preeclampsia.
HUMAN CORIN VARIANTS AND HYPERTENSION
Single nucleotide polymorphisms (SNPs) in the ANP gene promoter or a deletion in the NPR-A gene promoter are associated with patients with hypertension and cardiac hypertrophy, suggesting that defects in the ANP pathway may contribute to hypertensive disease.29,30 Recently, two non-synonymous and non-conservative SNPs (T555I and Q568P) are found in the human corin gene.31 These two SNPs are in complete linkage disequilibrium in the population and, as a result, are co-localized in a minor allele (I555/P568). Epidemiological studies of large population-based cohorts, including the Dallas Heart Study, the Multi-Ethnic Study of Atherosclerosis (MESA), and the Chicago Genetics of Hypertension Study, have shown that the minor corin I555/P568 allele is more common in African-Americans than in Caucasians (~12% vs. <0.2% carrying one or more copies of the allele) and associated with an increased risk for hypertension.31
In addition, the I555/P568 corin minor allele was reported to be associated with an enhanced concentric cardiac hypertrophy in response to high systolic blood pressure in African-Americans from the Dallas Heart and MESA cohorts.32 Patients with this corin allele had a greater left ventricular mass compared to that of control patients with a wild-type allele but similar systolic blood pressure. The data linking the corin gene variant to myocardial pathology in patients is intriguing.33 Earlier animal studies have indicated that the ANP pathway has a local anti-hypertrophy function in the heart, which is independent of its systemic action on blood pressure.34 Consistently, we also showed that corin null mice developed cardiac hypertrophy.22 These data suggest that corin deficiency may contribute to hypertension and heart failure in African-Americans, a population known for its high prevalence of these cardiovascular diseases.
UNANSWERED QUESTIONS
For more than a quarter century, significant knowledge has been gained for the natriuretic peptides and their biological function and therapeutic potential35. And yet, much more remains to be learned. For example, both ANP and BNP are thought to bind NPR-A, but this concept may not be satisfactory. The affinity of ANP binding to the receptor was shown to be ~10 times higher than that for BNP binding.36 How, then, could BNP bind to the receptor in the presence of similar levels of ANP? More importantly, ANP- and BNP-knockout mice have very different phenotypes.27,37 If these peptides can substitute each other for the same receptor, why do ANP- and BNP-knockout mice have a phenotype at all? Some studies suggest that there may be another receptor for BNP but its identity remains unknown.38 As ANP and BNP are being developed to treat patients with heart failure,5 answers to these questions will be critical to understand the therapeutic efficacy and unwanted side-effects of these peptides.
The discovery of corin and characterization of the hypertensive phenotype in corin null mice highlight the importance of natriuretic peptide processing and point to a long-neglected area in this field. For years, radioimmunoassay and ELISA are used to measure ANP and BNP in a variety of clinical and experimental settings. The antibodies used in these assays may not distinguish processed from unprocessed natriuretic peptides. For example, antibodies bind to ANP may also recognize pro-ANP. As a result, it is not clear which molecular forms, i.e. ANP, pro-ANP, or both, are measured in these assays. Similar situation applies to BNP and pro-BNP assays. As plasma BNP is used as a diagnostic test for heart failure,39 this issue is of clinical importance.
Corin is a newly-discovered enzyme. We are only at the beginning toward understanding its biology.40 Many questions remain regarding corin function and regulation under physiological and pathological conditions. In animal models of heart failure, for example, corin mRNA expression was shown to be up-regulated in the ventricle41,42 but down-regulated in the atrium43. It appears that corin regulation is a part of the pathological process during heart failure but the results need to be verified in samples from patients with heart failure. Further studies of corin should provide new insights into the natriuretic peptide system and may help to design new strategies to diagnose and treat hypertension and heart disease.
Acknowledgments
We thank many former and current lab members who contributed corin studies, especially Dr. Wei Yan who prepared the picture in Fig. 2. We also thank Ms. Robin Lewis for helping with illustrations and Drs. Edward Plow and Martin Schreiber for their support. This work was supported by grants from the Bakken Heart-Brain Institute, the Cleveland Clinic, the National Institutes of Health, and the Ralph Wilson Medical Research Foundation.
Footnotes
Disclosure: None
References
- 1.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 2.Tervonen V, Arjamaa O, Kokkonen K, Ruskoaho H, Vuolteenaho O. A novel cardiac hormone related to A-, B- and C-type natriuretic peptides. Endocrinology. 1998;139:4021–4025. doi: 10.1210/endo.139.9.6292. [DOI] [PubMed] [Google Scholar]
- 3.Tsukada T, Takei Y. Integrative approach to osmoregulatory action of atrial natriuretic peptide in seawater eels. Gen Comp Endocrinol. 2006;147:31–38. doi: 10.1016/j.ygcen.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Chusho H, Tamura N, Ogawa Y, et al. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc Natl Acad Sci U S A. 2001;98:4016–4021. doi: 10.1073/pnas.071389098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CY, Burnett JC., Jr Natriuretic peptides and therapeutic applications. Heart Fail Rev. 2007;12:131–142. doi: 10.1007/s10741-007-9016-3. [DOI] [PubMed] [Google Scholar]
- 6.McGrath MF, de Bold ML, de Bold AJ. The endocrine function of the heart. Trends Endocrinol Metab. 2005;16:469–477. doi: 10.1016/j.tem.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 8.Seidah NG, Cromlish JA, Hamelin J, Thibault G, Chretien M. Homologous IRCM-serine protease 1 from pituitary, heart atrium and ventricle: a common pro-hormone maturation enzyme? Biosci Rep. 1986;6:835–844. doi: 10.1007/BF01117107. [DOI] [PubMed] [Google Scholar]
- 9.Imada T, Takayanagi R, Inagami T. Atrioactivase, a specific peptidase in bovine atria for the processing of pro-atrial natriuretic factor. Purification and characterization. J Biol Chem. 1988;263:9515–9519. [PubMed] [Google Scholar]
- 10.Wu Q. Type II transmembrane serine proteases. Curr Top Dev Biol. 2003;54:167–206. doi: 10.1016/s0070-2153(03)54009-1. [DOI] [PubMed] [Google Scholar]
- 11.Yan W, Sheng N, Seto M, Morser J, Wu Q. Corin, a mosaic transmembrane serine protease encoded by a novel cDNA from human heart. J Biol Chem. 1999;274:14926–14935. doi: 10.1074/jbc.274.21.14926. [DOI] [PubMed] [Google Scholar]
- 12.Gladysheva IP, Robinson BR, Houng AK, Kovats T, King SM. Corin is co-expressed with pro-ANP and localized on the cardiomyocyte surface in both zymogen and catalytically active forms. J Mol Cell Cardiol. 2008;44:131–142. doi: 10.1016/j.yjmcc.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Hooper JD, Scarman AL, Clarke BE, Normyle JF, Antalis TM. Localization of the mosaic transmembrane serine protease corin to heart myocytes. Eur J Biochem. 2000;267:6931–6937. doi: 10.1046/j.1432-1033.2000.01806.x. [DOI] [PubMed] [Google Scholar]
- 14.Liao X, Wang W, Chen S, Wu Q. Role of glycosylation in corin zymogen activation. J Biol Chem. 2007;282:27728–27735. doi: 10.1074/jbc.M703687200. [DOI] [PubMed] [Google Scholar]
- 15.Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci USA. 2000;97:8525–8529. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan J, Hinzmann B, Yan W, Wu F, Morser J, Wu Q. Genomic structures of the human and murine corin genes and functional GATA elements in their promoters. J Biol Chem. 2002;277:38390–38398. doi: 10.1074/jbc.M205686200. [DOI] [PubMed] [Google Scholar]
- 17.Calderone A, Bel-Hadj S, Drapeau J, et al. Scar myofibroblasts of the infarcted rat heart express natriuretic peptides. J Cell Physiol. 2006;207:165–173. doi: 10.1002/jcp.20548. [DOI] [PubMed] [Google Scholar]
- 18.Enshell-Seijffers D, Lindon C, Morgan BA. The serine protease Corin is a novel modifier of the Agouti pathway. Development. 2008;135:217–225. doi: 10.1242/dev.011031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knappe S, Wu F, Madlansacay MR, Wu Q. Identification of domain structures in the propeptide of corin essential for the processing of proatrial natriuretic peptide. J Biol Chem. 2004;279:34464–34471. doi: 10.1074/jbc.M405041200. [DOI] [PubMed] [Google Scholar]
- 20.Knappe S, Wu F, Masikat MR, Morser J, Wu Q. Functional analysis of the transmembrane domain and activation cleavage of human corin. J Biol Chem. 2003;278:52363–52370. doi: 10.1074/jbc.M309991200. [DOI] [PubMed] [Google Scholar]
- 21.Wu F, Yan W, Pan J, Morser J, Wu Q. Processing of pro-atrial natriuretic peptide by corin in cardiac myocytes. J Biol Chem. 2002;277:16900–16905. doi: 10.1074/jbc.M201503200. [DOI] [PubMed] [Google Scholar]
- 22.Chan JC, Knudson O, Wu F, Morser J, Dole WP, Wu Q. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc Natl Acad Sci U S A. 2005;102:785–790. doi: 10.1073/pnas.0407234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davenport RJ. Worth its salt. Elusive enzyme generates hormone that lowers blood pressure. Sci Aging Knowledge Environ. 2005;2005:nf5. doi: 10.1126/sageke.2005.2.nf5. [DOI] [PubMed] [Google Scholar]
- 24.Potter LR. “Corination” of the proANP converting enzyme. Cell Metab. 2005;1:88–90. doi: 10.1016/j.cmet.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Sawada Y, Suda M, Yokoyama H, et al. Stretch-induced hypertrophic growth of cardiocytes and processing of brain-type natriuretic peptide are controlled by proprotein-processing endoprotease furin. J Biol Chem. 1997;272:20545–20554. doi: 10.1074/jbc.272.33.20545. [DOI] [PubMed] [Google Scholar]
- 26.Wu C, Wu F, Pan J, Morser J, Wu Q. Furin-mediated processing of Pro-C-type natriuretic peptide. J Biol Chem. 2003;278:25847–25852. doi: 10.1074/jbc.M301223200. [DOI] [PubMed] [Google Scholar]
- 27.John SW, Krege JH, Oliver PM, et al. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science. 1995;267:679–681. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- 28.Lopez MJ, Wong SK, Kishimoto I, et al. Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide. Nature. 1995;378:65–68. doi: 10.1038/378065a0. [DOI] [PubMed] [Google Scholar]
- 29.Nakayama T, Soma M, Takahashi Y, Rehemudula D, Kanmatsuse K, Furuya K. Functional deletion mutation of the 5′-flanking region of type A human natriuretic peptide receptor gene and its association with essential hypertension and left ventricular hypertrophy in the Japanese. Circ Res. 2000;86:841–845. doi: 10.1161/01.res.86.8.841. [DOI] [PubMed] [Google Scholar]
- 30.Rubattu S, Bigatti G, Evangelista A, et al. Association of atrial natriuretic peptide and type a natriuretic peptide receptor gene polymorphisms with left ventricular mass in human essential hypertension. J Am Coll Cardiol. 2006;48:499–505. doi: 10.1016/j.jacc.2005.12.081. [DOI] [PubMed] [Google Scholar]
- 31.Dries DL, Victor RG, Rame JE, et al. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation. 2005;112:2403–2410. doi: 10.1161/CIRCULATIONAHA.105.568881. [DOI] [PubMed] [Google Scholar]
- 32.Rame JE, Drazner MH, Post W, et al. Corin I555(P568) allele is associated with enhanced cardiac hypertrophic response to increased systemic afterload. Hypertension. 2007;49:857–864. doi: 10.1161/01.HYP.0000258566.95867.9e. [DOI] [PubMed] [Google Scholar]
- 33.Burnett JC, Jr, Olson TM. Natriuretic peptides and myocardial structure: insights from population genetics. Hypertension. 2007;49:765–766. doi: 10.1161/01.HYP.0000258567.67263.96. [DOI] [PubMed] [Google Scholar]
- 34.Molkentin JD. A friend within the heart: natriuretic peptide receptor signaling. J Clin Invest. 2003;111:1275–1277. doi: 10.1172/JCI18389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burnett JC., Jr Novel therapeutic directions for the natriuretic peptides in cardiovascular diseases: what’s on the horizon. J Cardiol. 2006;48:235–241. [PMC free article] [PubMed] [Google Scholar]
- 36.Koller KJ, Goeddel DV. Molecular biology of the natriuretic peptides and their receptors. Circulation. 1992;86:1081–1088. doi: 10.1161/01.cir.86.4.1081. [DOI] [PubMed] [Google Scholar]
- 37.Tamura N, Ogawa Y, Chusho H, et al. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci U S A. 2000;97:4239–4244. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goy MF, Oliver PM, Purdy KE, et al. Evidence for a novel natriuretic peptide receptor that prefers brain natriuretic peptide over atrial natriuretic peptide. Biochem J. 2001;358:379–387. doi: 10.1042/0264-6021:3580379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruskoaho H. Cardiac hormones as diagnostic tools in heart failure. Endocr Rev. 2003;24:341–356. doi: 10.1210/er.2003-0006. [DOI] [PubMed] [Google Scholar]
- 40.Wu Q. The serine protease corin in cardiovascular biology and disease. Front Biosci. 2007;12:4179–4190. doi: 10.2741/2379. [DOI] [PubMed] [Google Scholar]
- 41.Jiang W, Cai DY, Pan CS, et al. Changes in production and metabolism of brain natriuretic peptide in rats with myocardial necrosis. Eur J Pharmacol. 2005;507:153–162. doi: 10.1016/j.ejphar.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 42.Tran KL, Lu X, Lei M, Feng Q, Wu Q. Upregulation of corin gene expression in hypertrophic cardiomyocytes and failing myocardium. Am J Physiol Heart Circ Physiol. 2004;287:H1625–1631. doi: 10.1152/ajpheart.00298.2004. [DOI] [PubMed] [Google Scholar]
- 43.Langenickel TH, Pagel I, Buttgereit J, et al. Rat corin gene: molecular cloning and reduced expression in experimental heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1516–1521. doi: 10.1152/ajpheart.00947.2003. [DOI] [PubMed] [Google Scholar]