Abstract
Purpose
Osteoprotegerin (OPG) inhibits osteoclast activation and reduces osteolysis in bone tumors. We hypothesized that tumor-tropic neural progenitor cells (NPCs) engineered to express OPG would reduce neuroblastoma disease burden in the bone.
Methods
Stable expression of green fluorescent protein (NPC-GFP) and OPG (NPC-OPG) was established in human NPCs by lentivirus-mediated transduction. Bone disease was established by intrafemoral injection of luciferase-expressing human neuroblastoma (CHLA-255) cells into 20 SCID mice. Three weeks later, mice began receiving IV injection of 2×106 NPC-OPG or NPC-GFP (control) every 10 days × 3 doses. Disease was monitored with quantitative bioluminescent imaging (BLI) and X-ray images, which were evaluated on a scale of 0 to 4. These studies were IACUC approved.
Results
OPG treatment in vitro produced no direct toxicity to tumor cells. Co-culture of tumor cells with bone marrow significantly increased activation of bone-marrow derived osteoclasts as assessed by TRAP staining (156±10.8osteoclasts/well) compared to bone marrow culture alone (91.67±4.7, p=0.005). This increase was abrogated by adding OPG-containing media (68.3±2.8, p=0.001). NPC-OPG slowed tumor progression (108-fold increase from pre-treatment) compared to mice treated with NPC-GFP (538-fold), as judged by BLI. X-rays subjectively demonstrated less bone disease in NPC-OPG-treated mice (2.27±0.25) compared to NPC-GFP-treated mice (3.25±0.22, p=0.04).
Conclusions
NPC-mediated delivery of OPG slowed disease progression in a pre-clinical model of neuroblastoma bone metastasis. The decrease in bone disease was not from direct tumor cell toxicity but likely occurred indirectly through inhibition of osteoclast-directed bone resorption. Thus, targeted delivery of OPG by NPCs may be effective in the treatment of neuroblastoma bone metastasis.
Keywords: Neuroblastoma, Osteoprotegerin, Neural progenitor cells, Osteoclast, Bone metastasis
Introduction
Neuroblastoma is the most common malignancy of infancy and the most common extracranial solid tumor of childhood. It comprises approximately 7% of childhood malignancies, while accounting for 15% of pediatric oncology deaths.1 The course of the disease can be extremely variable, but disseminated neuroblastoma in older children continues to have a poor prognosis despite intensive multimodality therapy. Unfortunately, approximately 60% of patients older than one year of age present with metastatic disease at the time of diagnosis.2 Bone and bone marrow are frequent sites of metastasis and can result in significant morbidity, including pain, fracture, hypercalcemia, nerve compression, thrombocytopenia, and anemia.2,3
Neuroblastoma tends to form osteolytic lesions when it metastasizes to bone; these osteolytic lesions form through the recruitment and activation of osteoclasts which break down the bone.4 Growth factors released from bone degradation stimulate tumor growth and in a positive feedback loop, further stimulate osteoclasts.3,5 The osteoclast is a critical component of this “vicious cycle” of bone destruction leading to further tumor growth which results in the release of cytokines stimulating further osteoclast activity.6 Several factors contribute to the activation and recruitment of osteoclasts in bone metastasis, including interleukin-1, interleukin-6, interleukin-11, parathyroid hormone related peptide, matrix metalloproteinase-9, and receptor activator of nuclear factor κB ligand (RANKL).3,7 RANKL binds the RANK receptor on osteoclasts and is an important factor in osteoclast activation in neuroblastoma bone metastasis.7 RANKL can be produced by mesenchymal cells in response to tumor cells, tumor cells alone, and osteoblasts in response to growth factors released from bone degradation.
Osteoprotegerin (OPG) is a soluble protein in the TNF receptor family that can hinder RANKL activation of the RANK receptor protein by binding RANKL and acting as a decoy receptor for RANKL.8 Studies in other tumors metastatic to bone have shown that OPG can abrogate the effect of RANKL, slowing the progression of bone lesions.9,10 In vitro application of OPG to bone marrow co-culture with neuroblastoma cells significantly reduces osteoclast formation and activity through the RANKL/RANK system.7,11
Neural progenitor cells (NPCs) may provide a unique means of delivering OPG directly to the tumor in bone. NPCs have been shown to have a remarkable capacity to migrate to tumors after introduction at local sites of disease or following intravascular injection.12,13 Consequently, NPCs have potential as a vehicle for delivering gene-mediated therapy directly to the tumor.15-17 We hypothesized that tumor-tropic NPCs engineered to express OPG would slow progression of osteolytic lesions and reduce neuroblastoma disease burden in the bone.
Materials and Methods
Cell Lines
The F3.C1 NPC line was derived by Dr. Seung Kim with permission from the Clinical Screening Committee of the University of British Columbia, and the F3.C1 clonal cell line was immortalized by retroviral transduction with v-myc, as described previously.18 The human neuroblastoma cell line, CHLA-255, was provided by C. Patrick Reynolds (Los Angeles, CA). These cells were engineered to constitutively express firefly luciferase and maintained in culture, as previously described.19
Osteoprotegerin transduction
To engineer stable OPG- and green fluorescent protein (GFP)- expressing NPCs, their cDNAs were cloned into lentiviral vector plasmids from which lentiviral supernatants were produced in the St. Jude Vector Lab using calcium phosphate transfection of 293T cells. The lentiviral expression vector vCL10-MSCV-OPG was provided by Kwee Yong, London, UK. NPCs were then transduced with these supernatants in polybrene for 6 hours.
Effects of osteoprotegerin in vitro
CHLA-255 cells were plated at a density of 2 × 105 cells per well in 24-well tissue culture plates (Costar, Corning, NY). The following day, 50μl of supernatant from NPCs transduced with the OPG gene and verified by ELISA to contain OPG protein was added to the culture medium of one third of the plates. Another third received supernatant from NPC-GFP cell culture, and the remaining third were untreated controls. Twenty-four hours later, Annexin V fluorescence-activated cell sorting and cell cycle analysis were performed on the tumor cells. Additional cells were incubated for 48 hours with conditioned media, after which cells were counted on a hemocytometer to evaluate any change in cell count with OPG treatment.
The effect of OPG on osteoclasts was analyzed in vitro with the use of cocultures of human neuroblastoma cells with murine bone marrow cells. The bone marrow of CB-17 mice (Charles River Laboratories, Wilmington, MA) was harvested and plated in α-MEM (Mediatech), as previously described.20 Supernatant from GFP-expressing NPCs (150 μl) was added to a subset of wells. Additional wells were treated with supernatant from OPG-expressing NPCs (150 μl at approximately 1ng/ml of OPG). Cultures were maintained for 6 days, and subsequently fixed and stained for tartrate–resistant acid phosphatase (TRAP) activity (Sigma-Aldrich, St. Louis, MO). Osteoclasts were counted as TRAP-positive multinucleated cells. Each experiment was run in triplicate.
Animal model
All murine experiments were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee of St. Jude Children's Research Hospital. A model of neuroblastoma bone metastasis was established by injection of 2 × 105 luciferase-expressing CHLA-255 human neuroblastoma cells into the medullary cavity of the right femur of 4- to 6-week-old male CB-17 SCID mice (Charles River Laboratories), as previously described.20 After three weeks of tumor growth, mice were divided into two groups of equivalent tumor burden based on bioluminescence imaging (BLI), and treatment was initiated. Two million NPCs were given intravenously by tail-vein injection every ten days, for a total of three doses. Control mice received GFP-expressing NPCs, and treated mice received OPG-expressing NPCs. After four weeks of treatment (seven weeks of tumor growth), mice were euthanized, and tissue was harvested.
Levels of human OPG were measured in plasma and tumor tissue using a commercially available immunoassay (ELISA, R&D Systems, Minneapolis, MN). Serum calcium was measured spectrophotometrically on a Vitros DT 60 Chemistry Analyzer (Ortho-Clinical Diagnostic, Rochester, New York) by the St. Jude Animal Diagnostic Lab.
Animal Imaging
For real-time in vivo assessment of intrafemoral tumor burden, animals were imaged with the use of an IVIS Imaging System 100 Series (Xenogen Corporation, Alameda, Calif), as previously described.20 Using this BLI, mice were divided into two groups of equivalent tumor burden (n=10mice/group). BLI signal was measured again after three weeks of treatment and was divided by baseline BLI for each mouse to determine the relative tumor burden. Radiographs were obtained weekly with the use of a MX20 Faxitron X-ray machine (Faxtiron X-Ray Corp, Wheeling, Ill) at 22 kV with a 10-second exposure time. Radiographs of the femora were interpreted and scored according to grade of osteolytic lesions (0 -- none, 1 -- minimal, 2 -- moderate, 3 -- severe, 4 -- pathologic fracture) by a surgeon and a radiologist blinded to the treatment groups.
Tumor tissue analysis
Tumor tissue and bone were harvested after mouse euthanasia. Formalin-fixed, paraffin-embedded 5μm tissue sections were stained with hematoxylin and eosin or analyzed by immunohistochemistry. Immunohistochemistry was performed with rabbit anti-GFP antibody (Molecular Probes, Eugene, OR) and an anti-rabbit polymer conjugated to horse-radish peroxidase from DAKO (Rabbit EnVision, Carpinteria, CA). Additional tumor tissue was snap-frozen in liquid nitrogen. Protein lysates were made by homogenizing tissue in 1ml of lysis buffer, as previously described.22 The resulting homogenized protein was then analyzed in the same manner as cell culture for levels of OPG using an ELISA immunoassay (R&D Systems).
NPC localization
A separate cohort of mice was used to confirm NPC localization to sites of tumor growth. The cells were labeled with the lipophilic tracer CellTracker CM-DiI (C-7000; Invitrogen Molecular Probes, Eugene, Ore) immediately before injection, as previously described.19 Four days later, mice were sacrificed and tumors and tumor-naïve organs were harvested for histology. DAPI and hematoxylin and eosin–stained sections were viewed and digitally photographed using an Olympus U-SPT microscope (Olympus, Center Valley, PA) as previously described.19
Statistical Analyses
Results were reported as mean ± SE. Femoral radiograph scores were compared between treated and control mice with SAS software (Version 9.1, SAS Institute, Cary, NC) using a Wilcoxon rank sum, and congruency between scorers was analyzed with the same program using a Kappa weighted statistic. The Sigmaplot program (SPSS, Inc, Chicago, IL) was used to graphically present all other data and analyze statistical differences in the results using an unpaired Student's t-test.
Results
Transduction of NPCs
Successful transduction of the NPCs was confirmed by demonstrating GFP expression on fluorescent microscopy. Stable expression of OPG was confirmed by collection of the supernatant from NPCs in growth phase. ELISA for OPG in the supernatant from the NPC-OPG cultures demonstrated the presence of OPG in concentrations of 993.8pg/ml (from 5 × 106 NPC-OPG cells), while supernatant from NPC-GFP cell cultures had no detectable OPG. Smaller amounts of OPG were also detectable in the supernatant of CHLA-255 neuroblastoma cell cultures (80pg/ml) indicating that the tumor cells also produced a small amount of OPG.
Effects of OPG on NB tumor cells in vitro
Supernatant from NPC-OPG or NPC-GFP cell cultures was then added to the media of CHLA-255 human neuroblastoma cells in culture. There was no direct toxicity to the tumor cells from OPG. Cell counts after 48 hours of treatment with OPG-containing supernatant (7.64 × 104 ± 2 × 104cells/ml) were not significantly different from CHLA-255 cells treated with conditioned medium from NPC-GFP cultures (8.46 × 104 ± 3.88 × 103cells/ml, p=0.773) or control cell cultures receiving unconditioned medium (7.31 × 104 ± 4.38 × 103cells/ml, p=0.907) (Figure 1). Furthermore, treatment with OPG produced no change in cell cycle, as analyzed by FACS, or apoptosis, as measured by Annexin V staining when compared to in vitro treatment with control medium (data not shown). However, OPG did appear to have an effect on osteoclast development in co-cultures of CHLA-255 tumor cells and bone marrow. The addition of CHLA-255 cells to bone marrow cultures significantly increased the number of osteoclasts on TRAP staining (156 ± 10.8osteoclasts/well) compared to bone marrow without the addition of tumor cells (91.67 ± 4.7osteoclasts/well, p=0.005). However, the addition of OPG-containing medium to a co-culture of bone marrow and tumor cells significantly decreased the number of osteoclasts (68.3 ± 2.8osteoclasts/well, p=0.001) compared to untreated bone marrow and tumor cells or bone marrow and tumor cells treated with NPC-GFP conditioned media (159 ± 14.9osteoclasts/well, p=0.003) (Figure 2).
Figure 1. OPG exhibits no direct toxicity on neuroblastoma cells.
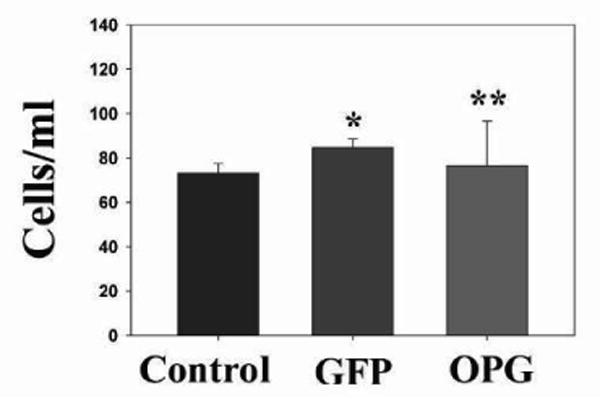
Bar graph showing cell counts in CHLA-255 neuroblastoma cell cultures after 48 hours of NPC-OPG or NPC-GFP supernatant exposure compared to untreated controls. (*p value =0.773 and **p value=0.907)
Figure 2. In vitro effects of OPG on osteoclast formation in bone marrow/neuroblastoma cell co-culture.
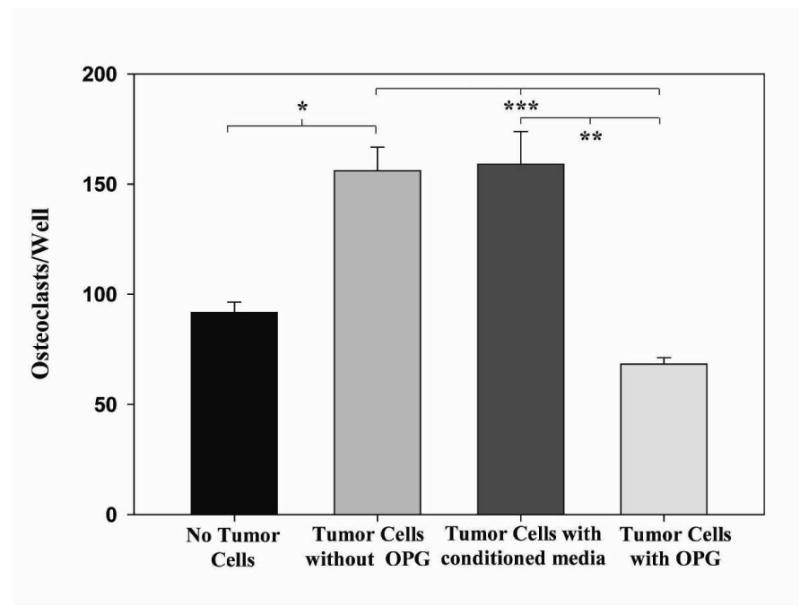
Bar graph comparing numbers of osteoclasts present in bone marrow culture alone, bone marrow and neuroblastoma co-culture, bone marrow/neuroblastoma co-culture treated with conditioned NPC-GFP medium, and bone marrow/neuroblastoma co-culture treated with NPC-OPG medium. (*p value=0.005, **p value=0.001, ***p value=0.003)
Establishment of neuroblastoma bone tumors
To test the effect of NPC-OPG on neuroblastoma bone metastasis in vivo, tumors were established in the femurs of SCID mice by direct injection of luciferase-expressing neuroblastoma cells. Three weeks later, the presence of tumors was confirmed with BLI. Ninety percent of the injected mice developed BLI evident tumors.
NPC localization to tumor
The migration of NPCs to tumors was demonstrated by two means. Tissue harvested from mice at the completion of the study was stained for GFP which is expressed by all of the injected NPCs. Scattered GFP-stained cells could be seen within the tumor, but no GFP-stained cells were seen in tissue from non-tumor bearing organs (data not shown). We sought to further confirm the localization of NPCs to tumor tissue using a lipohilic tracer. NPCs labeled with CM-DiI were injected into a separate cohort of mice with established femoral tumors. Four days later tumor tissue was harvested from the mice, and labeled NPCs were evident in the tumor (Figure 3A and B) confirming their migration to tumor-bearing tissue. Tumor from mice not injected with labeled-NPCs showed no background red fluorescence in the tumor tissue (Figure 3C). Likewise, disease-free organs from tumor-bearing mice also showed no evidence of labeled-NPCs (Figure 3D-F).
Figure 3. Localization of NPCs to tumor tissue but not tumor-free tissue.
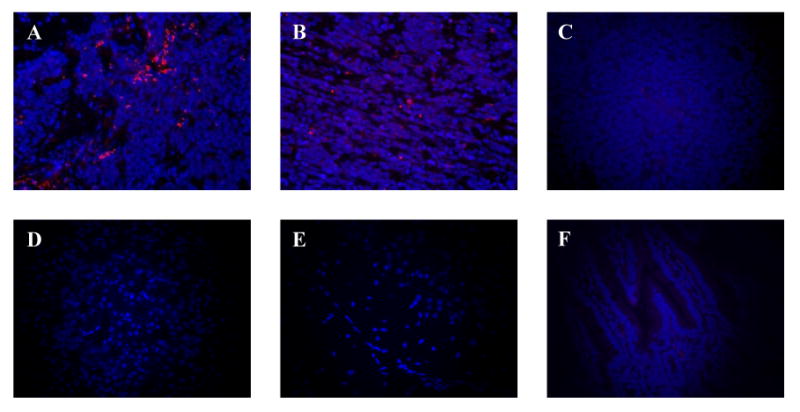
CM-diI-labeled NPCs (red) against a background of DAPI-stained (blue) tumor cells in bone (A) and bone marrow (B). No red background is evident in DAPI-stained tumor not receiving labeled-NPCs (C) or in tumor-free kidney, brain, and intestine, after injection with labeled NPCs (D-F, respectively). Images were taken at 400×.
Effects of NPC-delivered osteoprotegerin on neuroblastoma bone lesions
We have previously shown that BLI signal from luciferase-expressing neuroblastoma cells correlates with tumor burden in mouse xenograft models.19 Using BLI, tumor burden within the right femur of mice was measured one week after the last treatment. There was less tumor progression as measured by BLI (Figure 4) in mice treated with NPC-OPG; the mean relative tumor burden in NPC-OPG treated mice was twenty percent (108 ± 31.2 relative tumor burden) of the relative tumor burden of mice treated with control NPC-GFP (538 ± 366 relative tumor burden, p=0.232).
Figure 4. Restricted tumor growth with NPC-OPG.
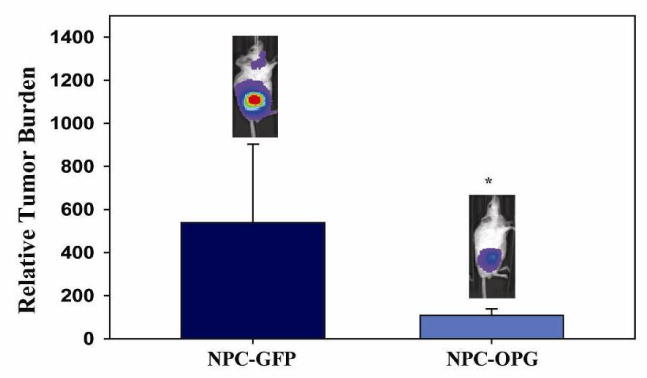
Bar graph showing the relative increase in tumor burden from baseline in mice treated with NPC-GFP or NPC-OPG as measured by bioluminescence signal. (*p value=0.232)
Two blinded investigators independently reviewed X-rays of the osteolytic lesions to assess the severity of bone disease in tumor-bearing mice. Their independent scoring of the lesions showed good correlation with a weighted Kappa statistic of 0.6673 (0.4627, 0.8647). X-rays of the mouse femurs demonstrated less bone disease progression in NPC-OPG-treated mice (median score 2 for both investigators) compared to NPC-GFP-treated mice (median score 4, p=0.037 and p=0.107 for respective investigators) (Figure 5). Serum calcium levels were also lower in mice treated with NPC-OPG (6.92mg/dl) compared to mice treated with NPC-GFP (8.81mg/dl, p=0.11) likely due to less bone degradation.
Figure 5. Analysis of radiographs of oseolytic lesions in the femora of tumor-bearing SCID mice.
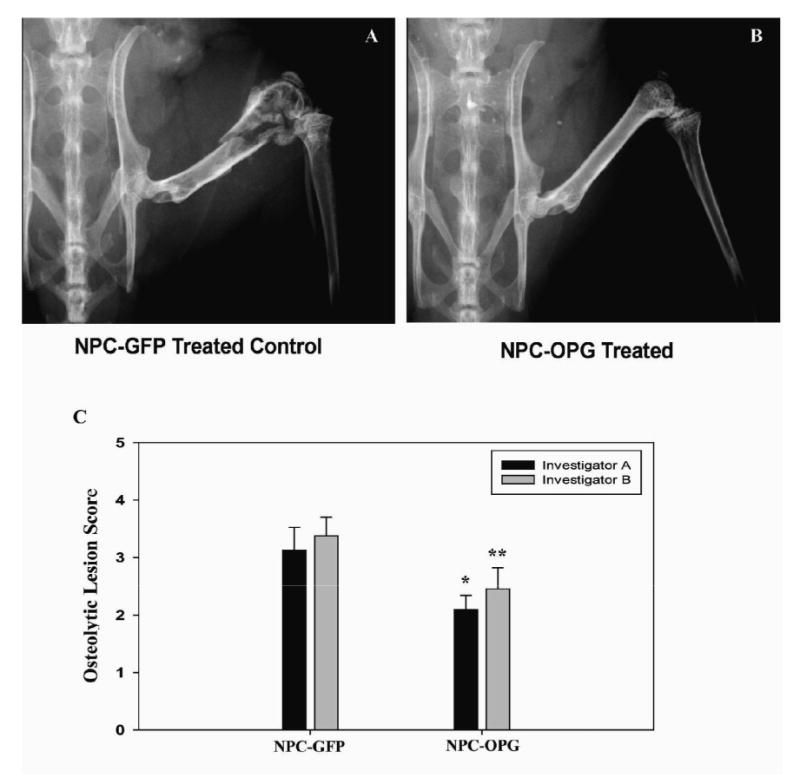
Representative radiographs of the right hind limb of a mouse treated with NPC-GFP (A) or NPC-OPG (B). Bar graph (C) showing the mean and standard error for blinded evaluation of x-rays from mice treated with NPC-GFP or NPC-OPG after introduction of CHLA-255 neuroblastoma cells into the right femur. (*p value vs. NPC-GFP=0.037 and **p value vs. NPC-GFP=0.107)
Osteoprotegerin Levels
The presence of human OPG was analyzed in the plasma and tumor tissue of tumor-bearing mice. No OPG was detectable by ELISA in the plasma of any of the mice. Human OPG was present in the tumor tissue, however, at the time of harvest. Mice treated with NPC-OPG had almost three times as much OPG present in the tumor tissue (90.17 ± 24.06pg/ml) as NPC-GFP-treated mice (34.8 ± 8.56pg/ml, p=0.061) (Figure 6). While CHLA-255 tumors may have been producing low levels of OPG, delivery of OPG to tumors using NPCs increased OPG expression within the tumor considerably. This increase in OPG expression appears to have been localized just to the tumor, as systemic levels of OPG were undetectable.
Figure 6. Osteoprotogerin levels in tumor tissue.
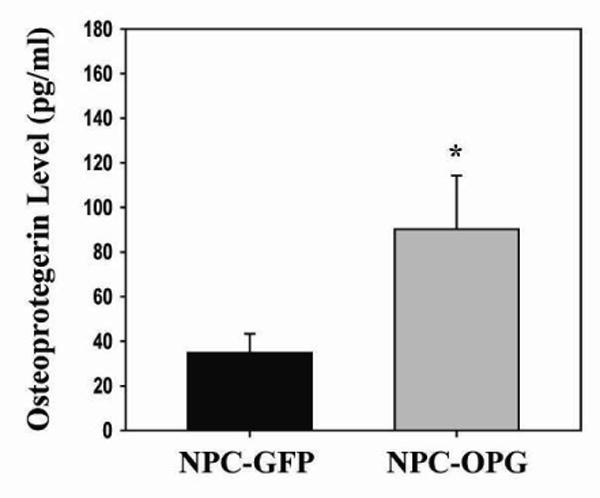
Bar graph comparing ELISA measured levels of tumor OPG in mice treated with either NPC-GFP or NPC-OPG. (*p value=0.061)
Discussion
Osteoprotegerin has been shown to inhibit RANK activation, development, and upregulation of osteoclasts by competitively binding RANKL. The interaction of OPG, RANK, and RANKL plays an important role in bone metabolism; RANK knockout mice develop osteopetrosis,23,24 and unhindered RANK activation in OPG knockout mice results in severe osteoporosis.25 Utilization of this system has recently been applied in the treatment of cancer bone metastasis. Pre-clinical use of OPG to treat bone lesions in breast cancer and prostate cancer has slowed progression of osteolytic lesions.10,26 In this study, we showed that NPC-mediated delivery of OPG also slowed progression of osteolytic lesions in a pre-clinical model of neuroblastoma bone metastasis. While OPG did not have a direct cytotoxic effect on tumor cells, treatment with OPG in this manner did correspond to decreased osteoclast activation in vitro. Osteoclasts are responsible for bone degradation, thereby facilitating further tumor growth. As evidence of decreased bone breakdown, systemic calcium levels were lower in tumor-bearing mice treated with NPC-OPG compared to control mice. Growth factors are also released from bone during osteoclast activity and may stimulate tumor growth within the bone.6 This may explain the decreased tumor burden in NPC-OPG treated tumors compared to control-treated tumors.
This study has shown that OPG localized to the tumor can slow progression of bone disease and tumor burden in the absence of detectable systemic levels of OPG. This localized OPG therapy was achieved by targeted delivery of the protein using NPCs. NPCs provide a unique vehicle for targeting therapy directly to sites of pathology. NPC-delivered OPG appears to produce a local therapeutic effect without generating high systemic levels of the therapeutic agent. In fact, OPG was not detectable in mouse plasma after NPC-OPG administration. This unique delivery system even allows treatment within the bone marrow and bone lesions of metastatic neuroblastoma, a location of neuroblastoma disseminated disease that is felt to be more difficult to treat. Approval has recently been granted for clinical trials utilizing NPCs, and studies are underway to further clarify how NPCs target and migrate toward tumors. Further studies are also needed to optimize NPC-OPG dosing and to clarify the long-term effects of NPC-mediated delivery.
In conclusion, NPC-mediated delivery of OPG slowed disease progression in a pre-clinical model of neuroblastoma bone metastasis. The decrease in bone disease was not from direct tumor cell toxicity but likely occurred indirectly through inhibition of osteoclast-directed bone resorption. Thus, NPC-mediated OPG warrants further investigation for the treatment of neuroblastoma since OPG may be effective in the treatment of neuroblastoma metastatic to bone using this targeted method of delivery.
Acknowledgments
This work was supported by the Assisi Foundation of Memphis, the US Public Health Service Childhood Solid Tumor Program Project Grant No. CA23099, the Cancer Center Support Grant No. 21766 from the National Cancer Institute, and by the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller RW, Young JL, Jr, Novakovic B. Childhood cancer. Cancer. 1995;75(1 Suppl):395–405. doi: 10.1002/1097-0142(19950101)75:1+<395::aid-cncr2820751321>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 2.Black CT. Neuroblastoma. In: Andrassy RJ, editor. Pediatric Surgical Oncology. Philadelphia, PA: W.B. Saunders; 1998. pp. 175–211. [Google Scholar]
- 3.Roodman GD. Biology of osteoclast activation in cancer. J Clin Oncol. 2001;19:3562–3571. doi: 10.1200/JCO.2001.19.15.3562. [DOI] [PubMed] [Google Scholar]
- 4.Sohara Y, Shimada H, DeClerck YA. Mechanisms of bone invasion and metastasis in human neuroblastoma. Cancer Lett. 2005;228:203–209. doi: 10.1016/j.canlet.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 5.Guise TA, Mundy GR. Cancer and bone. Endocr Rev. 1998;19:18–54. doi: 10.1210/edrv.19.1.0323. [DOI] [PubMed] [Google Scholar]
- 6.Wittrant Y, Theoleyre S, Chipoy C, et al. RANKL/RANK/OPG: new therapeutic targets in bone tumours and associated osteolysis. Biochim Biophys Acta. 2004;1704:49–57. doi: 10.1016/j.bbcan.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Michigami T, Ihara-Watanabe M, Yamazaki M, et al. Receptor activator of nuclear factor kappaB ligand (RANKL) is a key molecule of osteoclast formation for bone metastasis in a newly developed model of human neuroblastoma. Cancer Res. 2001;61:1637–1644. [PubMed] [Google Scholar]
- 8.Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 9.Body JJ, Greipp P, Coleman RE, et al. A phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer. 2003;97(3 Suppl):887–892. doi: 10.1002/cncr.11138. [DOI] [PubMed] [Google Scholar]
- 10.Keller ET, Dai J, Escara-Wilke J, et al. New trends in the treatment of bone metastasis. J Cell Biochem. 2007;102:1095–1102. doi: 10.1002/jcb.21540. [DOI] [PubMed] [Google Scholar]
- 11.Granchi D, Amato I, Battistelli L, et al. In vitro blockade of receptor activator of nuclear factor-kappaB ligand prevents osteoclastogenesis induced by neuroblastoma cells. Int J Cancer. 2004;111:829–838. doi: 10.1002/ijc.20308. [DOI] [PubMed] [Google Scholar]
- 12.Aboody KS, Najbauer J, Schmidt NO, et al. Targeting of melanoma brain metastases using engineered neural stem/progenitor cells. Neuro Oncol. 2006;8:119–126. doi: 10.1215/15228517-2005-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bendetti S, Pirola B, Pollo B, et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–450. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- 14.Brown A, Yang W, Schmidt N, et al. Intravascular delivery of neural stem cell lines to target intracranial and extracranial tumors of neural and non-neural origin. Hum Gene Therapy. 2003;14:1777–1785. doi: 10.1089/104303403322611782. [DOI] [PubMed] [Google Scholar]
- 15.Aboody K, Brown A, Rainov N, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danks MK, Yoon KJ, Bush R, et al. Tumor-targeeted enzyme/prodrug therapy mediates long-term disease-free survival of mice bearing disseminated neuroblastoma. Cancer Res. 2007;67:22–25. doi: 10.1158/0008-5472.CAN-06-3607. [DOI] [PubMed] [Google Scholar]
- 17.Dickson PV, Hamner JB, Burger R, et al. Intravascular administration of tumor tropic neural progenitor cells permits targeted delivery of interferon-β and restricts tumor growth in a murine model of disseminated neuroblastoma. J Pediatr Surg. 2007;42:48–53. doi: 10.1016/j.jpedsurg.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 18.Kim S, Nakagawa E, Hatori K, et al. Production of immortalized human neural crest stem cells. Methods Mol Biol. 2002;198:55–65. doi: 10.1385/1-59259-186-8:055. [DOI] [PubMed] [Google Scholar]
- 19.Dickson PV, Hamner B, Ng CY, et al. In vivo bioluminescence imaging for early detection and monitoring of disease progression in a murine model of neuroblastoma. J Pediatr Surg. 2007;42:1172–1179. doi: 10.1016/j.jpedsurg.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 20.Dickson PV, Hamner JB, Cauthen LA, et al. Efficacy of zoledronate against neuroblastoma. Surgery. 2006;140:227–235. doi: 10.1016/j.surg.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Dickson PV, Hamner JB, Sims TL, et al. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res. 2007;13:3942–3950. doi: 10.1158/1078-0432.CCR-07-0278. [DOI] [PubMed] [Google Scholar]
- 22.Davidoff AM, Ng CY, Zhang Y, et al. Careful decoy receptor titering is required to inhibit tumor angiogenesis while avoiding adversely altering VEGF bioavailability. Mol Ther. 2005;11:300–310. doi: 10.1016/j.ymthe.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Dougall WC, Glaccum M, Charrier K, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong YY, Boyle WJ, Penninger JM. Osteoprotegerin ligand: a common link between osteoclastogenesis, lymph node formation and lymphocyte development. Immunol Cell Biol. 1999;77:188–193. doi: 10.1046/j.1440-1711.1999.00815.x. [DOI] [PubMed] [Google Scholar]
- 25.Mizuno A, Amizuka N, Irie K, et al. Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun. 1998;247:610–615. doi: 10.1006/bbrc.1998.8697. [DOI] [PubMed] [Google Scholar]
- 26.Canon JR, Roudier M, Bryant R, et al. Inhibition of RANKL blocks skeletal tumor progression and improves survival in a mouse model of breast cancer bone metastasis. Clin Exp Metastasis. 2008;25:119–129. doi: 10.1007/s10585-007-9127-1. [DOI] [PubMed] [Google Scholar]


