Abstract
The transplantation of multiple abdominal viscera, including liver-duodenum-pancreas, liver-stomach-duodenum-pancreas and liver-intestine, is being performed with increasing frequency and success. These procedures and other variations are derived from a seldom used multivisceral operation in which all of the foregoing organs are transplanted en bloc. It is described herein how the full multivisceral transplantation and its less extensive derivatives are based on the same principles of procurement, preservation and postoperative management. With all of these multiple organ permutations and with intestinal transplantation alone, management is complicated by inclusion in the grafts of a large lymphoreticular component that is capable of causing graft versus host disease (GVHD). Because of a systematic error in therapeutic philosophy, past efforts have been directed at altering or damaging the lymphoreticular cells by pretreatment of the donor or of the organs with drugs, irradiation or other means. From recent observations, the alternative approach is suggested of keeping these lymphoid depots intact, which then become the site of two way cell traffic after transplantation. With the use of powerful immunosuppression, such as that provided with FK 506, the donor lymphoreticular cells can circulate in the recipient without causing clinical GVHD, and the lymphoreticular cells in the graft become those of the recipient (local chimerism) without causing rejection. Even with avoidance of rejection and GVHD, metabolic interrelations between the grafted organs, and also between the graft organs and retained recipient viscera can affect the fate of the individual transplanted organs or retained recipient organs. The best delineated of these metabolic influences are mediated by the endogenous splanchnic hepatotrophic factors, of which insulin has been the most completely studied. An understanding of these various immunologic and nonimmunologic factors combined with more potent immunosuppression that is now available is sure to stimulate efforts at transplantation of abdominal organs and particularly of the hollow viscera that have resisted such clinical efforts.
In the course of early research on transplantation of the liver, multivisceral transplantation was described in dogs 30 years ago (1, 2). The multivisceral procedure had no apparent clinical application, but as it has turned out, this operation has been the conceptual centerpiece for any intra-abdominal grafting procedure that involves more than one organ. We will describe herein the interrelationship of the various operations in both the donor and recipient. These technical permutations are certain to be more frequently used in the future (3) now that improved immunosuppression has made it possible to reliably control rejection of the hollow splanchnic viscera (4–9) and to prevent or reverse graft versus host disease (GVHD) caused by these lymphoid rich organs (4–9) or by bone marrow (10, 11).
PRINCIPLE INVOLVED
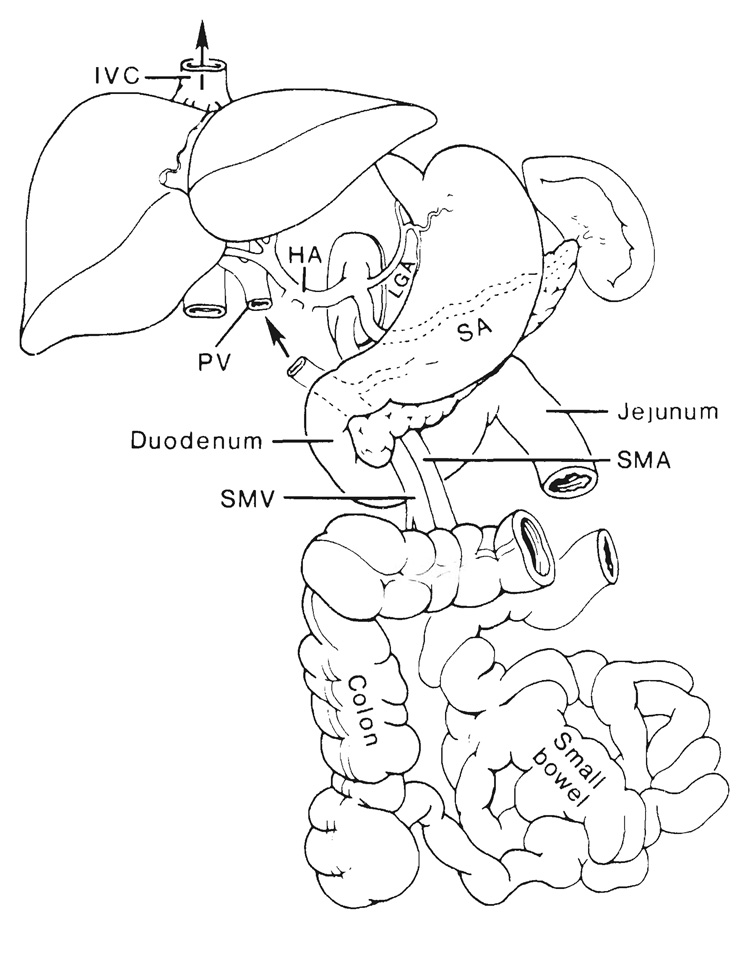
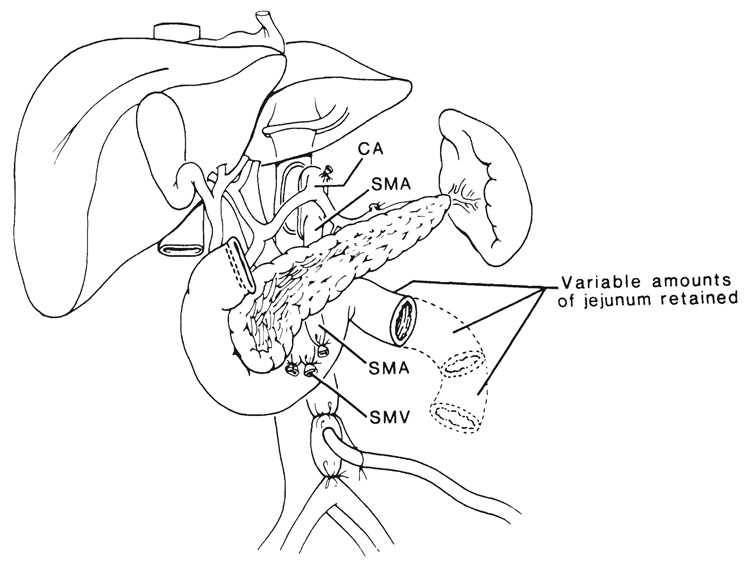
The complete multivisceral specimen is envisioned as a grape cluster with a double central stem consisting of the celiac axis and superior mesenteric artery (Fig. 1). The grapes, or individual organs, can be removed or retained according to the surgical objectives, but both arterial stem structures are preserved except when the intestine only is to be transplanted. The venous outflow from the grape cluster is entirely hepatofugal and is also kept intact up to or beyond the liver (Fig. 1).
FIG. 1.
The arterial pedicles and venous outflow of multivisceral allografts. IVC, Inferior vena cava; HA, hepatic artery; PV, portal vein; SA, splenic artery, SMA, superior mesenteric artery, and SMV, superior mesenteric vein.
VASCULAR RECONSTRUCTION
The anastomoses needed to revascularize any of the multivisceral grafts are fewer than for an isolated transplantation of the liver. However, there is a frequent need for vascular conduits. To meet all exigencies, the donor team must bring back segments of thoracic aorta, pulmonary artery and vena cava in addition to free grafts of iliac vein and artery (12–14).
Venous outflow
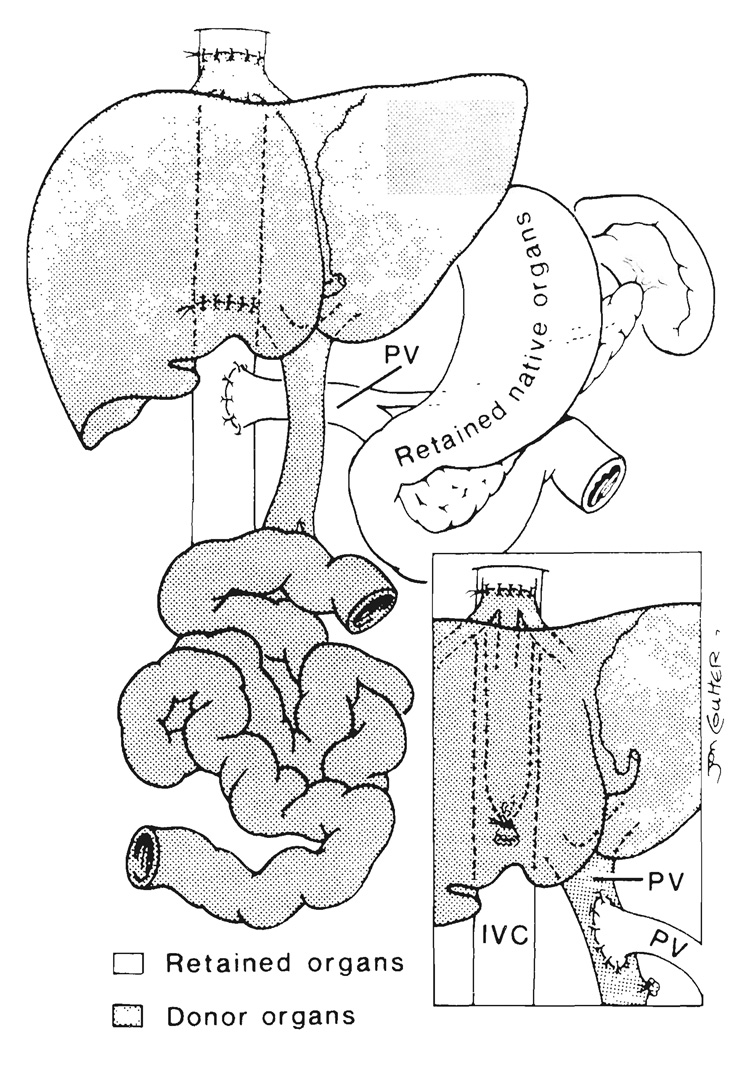
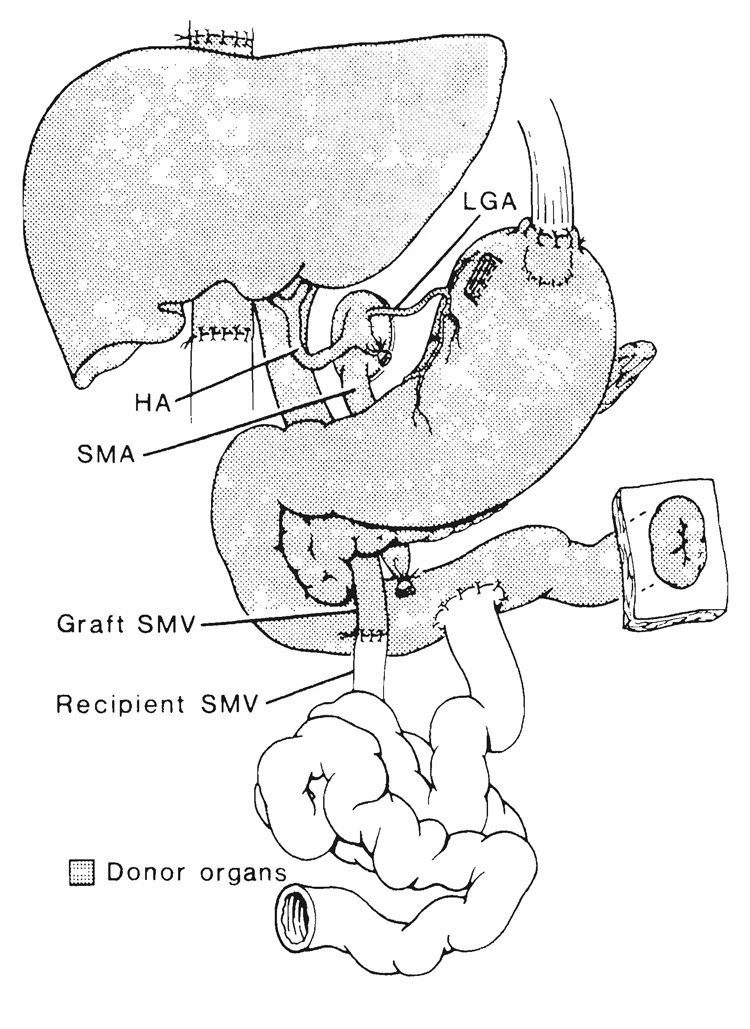
From grafts that include liver.—The venous outflow of composite transplants, which include the liver, is into a short length of retrohepatic inferior vena cava that is used to replace the recipient vena cava if the companion recipient segment is resected (Fig. 2). When the recipient vena cava is preserved, the upper end of the graft vena caval segment is anastomosed “piggyback” (15) to the anterior wall of the recipient vessel at an ostium made at the site of previous entry into it of the main hepatic veins of the excised native liver (Fig. 2, inset). In this event, the lower end of the graft vena caval segment is tied.
FIG. 2.
Liver-small intestinal transplantation in which a segment of donor retrohepatic vena cava is used to replace the excised recipient. Note that the venous outflow of the retained recipient viscera is directed into the recipient inferior vena cava (IVC) by portacaval shunt. Inset, “Piggyback” method of transplant venous drainage with anastomosis of the graft inferior vena cava to the anterior wall of the retained recipient inferior vena cava. Note the additional option of anastomosing the recipient portal vein (PV) to the graft portal vein, a maneuver designed to expose the hepatic allograft to hepatotrophic constituents from the retained viscera.
If the residual splanchnic viscera are not excised as part of the recipient operation, an additional requirement is provision for their venous drainage. Otherwise, venous hypertension in the retained host organs will not be relieved or will be made worse by removal of their natural drainage route through the native liver. We, as well as others (16), have resolved this problem by performing an end to side portacaval shunt in recipients of multiorgan grafts whose native stomach, pancreas and duodenum were left in place (Fig. 2). Alternatively, the native portal vein can be anastomosed end to side to the portal or superior mesenteric vein of the graft (Fig. 2, inset).
From grafts not containing liver.—If the liver is not part of the graft (as with an isolated intestinal graft), the transplant effluent is drained by anastomosing its portal or superior mesenteric vein end to end or end to side to the recipient portal vein in the hepatic hilum if the host vessel can be found. If not, the anastomosis is to the anterior wall of the recipient vena cava.
Arterialization
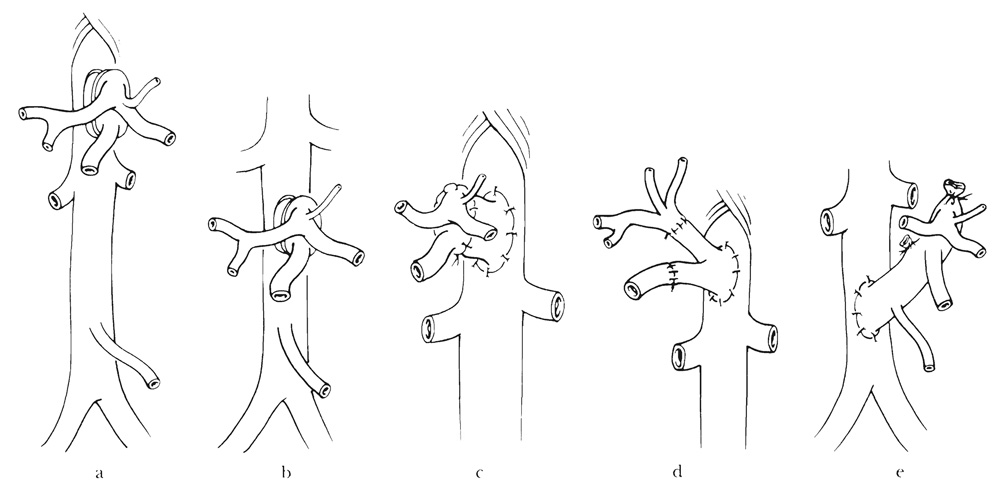
Both the celiac axis and superior mesenteric artery are revascularized in all of the multiple organ visceral graft combinations. A Carrel patch with the origins of these vessels can be anastomosed directly to the recipient aorta above or below the level of the renal arteries (Fig. 3a and b) or through an interposition graft of donor thoracic or abdominal aorta (Fig. 3c). Alternatively, the limbs of an aortoiliac bifurcation graft sewed to recipient aorta can be anastomosed individually to the celiac axis and superior mesenteric artery (Fig. 3d). If the donor aorta is kept in continuity with its celiac axis and superior mesenteric artery for aortoaortic anastomosis (Fig. 3e), it is necessary to sacrifice the origins of the donor renal arteries on both sides. This was the technique used for the first multivisceral procedures in dogs (1, 2) and clinically (17); but it is not acceptable because it causes wastage of donor kidneys that are needed for other patients.
FIG. 3.
Anastomosis of Carrel patch of organ graft above (a and c) or below (b) the recipient renal arteries. An interposition vascular graft may (c and d) or may not (a and b) be needed. This is a less satisfactory technique because it spoils the arterial supply of potential renal graft in transplantation of the donor aorta in continuity with its celiac axis and superior mesenteric artery (e). e, The procedure was used in the original multivisceral operation in dogs and humans.
PROCUREMENT AND PRESERVATION OF VISCERAL GRAFTS
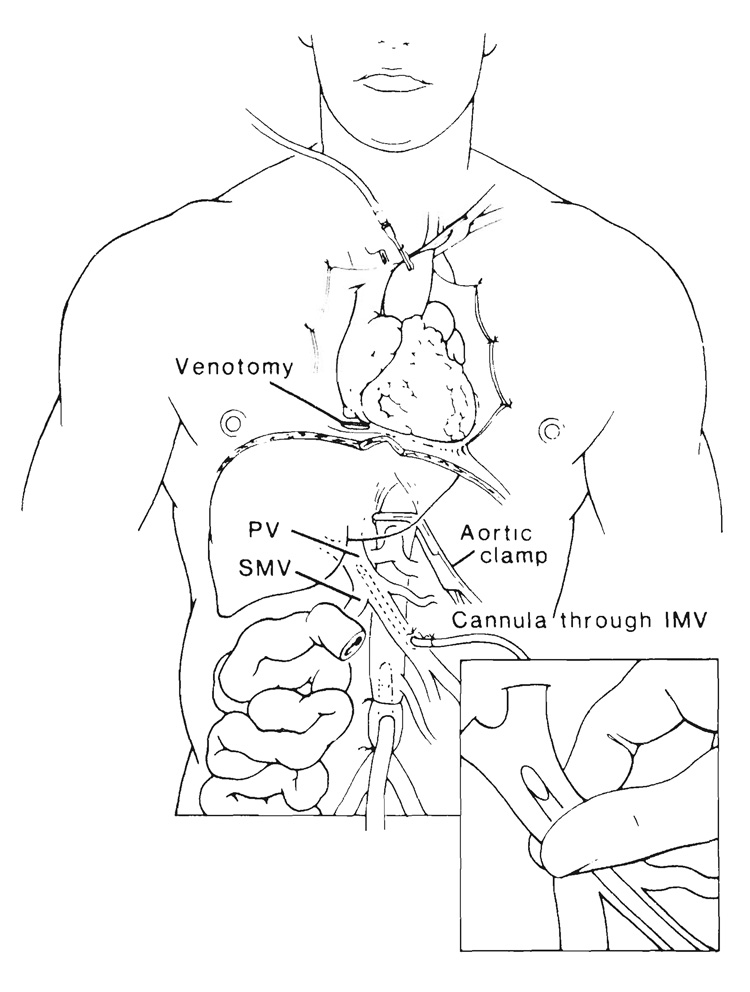
The key steps are generic but the details are tailored after discussion between the donor and recipient surgeons. If there is time, the intestinal tract of the heart beating cadaveric donor can be sterilized with a nonabsorbable antibiotic instilled in the stomach after suspension in Golytely® (a hyperosmolar cathartic solution). Removal of the graft is with modifications (17–19) of the so-called “flexible” procurement technique (13, 14). The objective is to cool those organs that are to be transplanted by infusion of chilled infusates into the arterial supply. The aorta is encircled proximally near the diaphragm for later cross clamping when circulation is discontinued. The distal part of the aorta is cleaned and encircled at or below the origin of the inferior mesenteric artery for insertion of the cannula used for entry of the cold preservation fluid (Fig. 4), which in our present practice is the University of Wisconsin (UW) solution (20, 21). As soon as the proximal aorta is cross clamped, in situ perfusion is begun, and the venous beds are decompressed by a venotomy of the suprahepatic vena cava (Fig. 4). The amount of infusate is variable, and guided by blanching of the organs and cooling, which is judged by touch. At least 2 liters usually are needed for adults. Frequently, the intestines and pancreas become chilled and bloodless while the liver remains discolored and feels warm. Then, the liver can be secondarily perfused through a cannula that is inserted through a tributary, such as the inferior mesenteric vein and advanced into the portal vein (Fig. 4). By finger compression below the catheter tip to prevent retrograde leakage, all of the infusate can be directed into the liver as illustrated in the inset in Figure 4.
FIG. 4.
Procurement of composite abdominal organ grafts. The technique is similar for the different kinds of specimens with perfusion of a cold solution into an isolated segment of abdominal aorta. If desired, portal perfusion of the liver can be secondarily done through another cannula placed through a side branch, such as the inferior mesenteric vein (IMV) with the cannula tip compressed to prevent leakage (inset). Heart procurement can proceed simultaneously. PV, Portal vein, and SMV, superior mesenteric vein.
The composite graft must be removed without contaminating the wound or ruining other organs, such as the kidneys, which are not part of the multivisceral specimen. Whether or not intestinal sterilization has been possible, the hollow viscera of the planned graft are sealed off by stapling and transecting them at their planned superior and inferior margins. The enteric contents are left undisturbed through the preservation period in preference to the traumatic and messy alternative of washing them during the procurement operation or on the back table.
Because the kidneys invariably are needed for other recipients, the plane separating the intra-abdominal organs and extraperitoneal kidneys must be developed with care. This can be done while the donor has an intact circulation or in situ after the organs have been made bloodless by perfusion as in the conventional procurement operation (13, 14). Alternatively, the separation can be done on the back table. As with standard combined liver-kidney procurement (13, 14), all of the inferior vena cava is left in continuity with the kidneys and renal veins except for that segment (Fig. 2) that is part of the hepatic graft. Protection of the right renal artery, which originates close to the aortic mouth of the superior mesenteric artery, is the foremost consideration. The risk of injury to the renal artery is minimized by preliminary dissection of the proximal celiac axis and the superior mesenteric artery before excising the Carrel patch that includes their origin.
An alternative to the separate removal of the multivisceral graft and kidneys during the actual procurement is to perform the separation of specimens on the back table. If this is planned, the kidneys are removed en bloc with the multivisceral specimen at the deeper plane used by pathologists at autopsy with the so-called Rokitansky techniques (22). This principle has been used to remove renal and pancreatic grafts after circulatory arrest (23).
THE FULL MULTIVISCERAL OPERATION
Complete multivisceral transplantation (Fig. 5) has been performed upon a handful of patients (16, 17, 24–26). The patient who survived the longest (six months) was a three and one-half year old child who died of Epstein-Barr virus associated lymphoma (17); the B lymphocytes in the tumor were of recipient phenotype. In another study, one patient died of recurrent carcinoma of the pancreas at eight and two-third months (26). Both of these patients had function of all of the organs in the complex grafts. More clinical attempts undoubtedly will be made with this operation. The routine chronic survival of healthy and normally developing rat recipients treated with FK 506 has demonstrated its feasibility (4, 7).
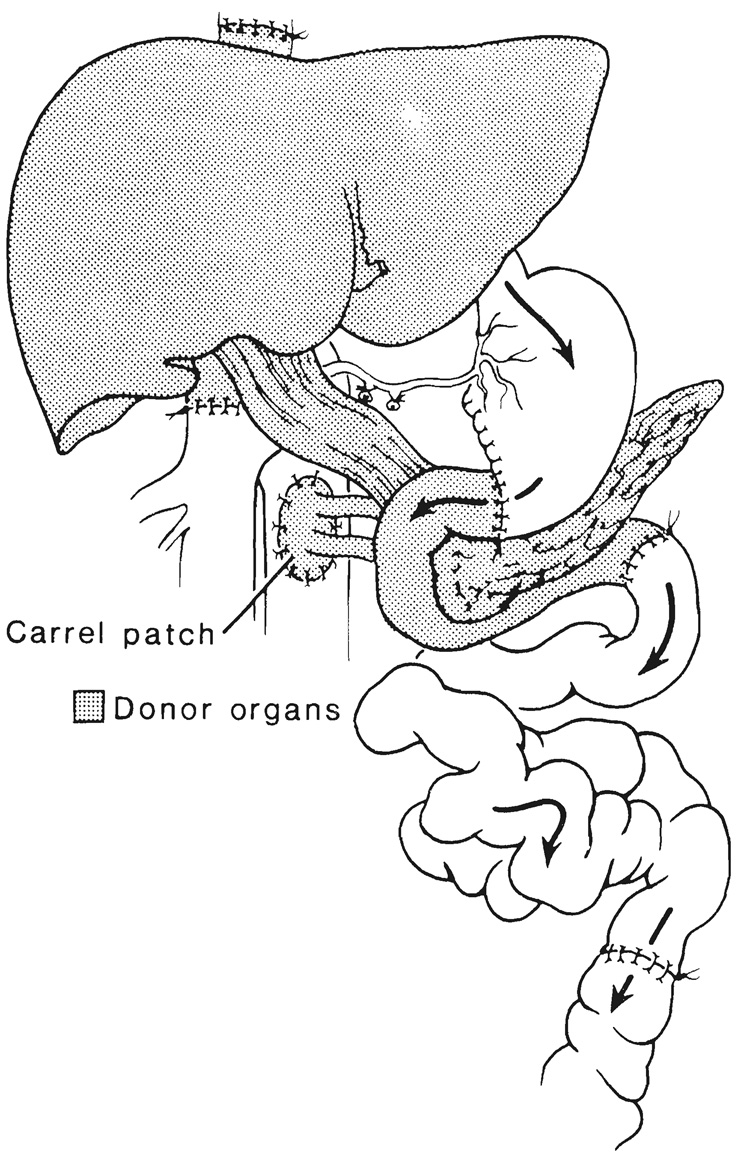
FIG. 5.
Complete multivisceral transplantation. HA, Hepatic artery; GDA, gastroduodenal artery; SMA, superior mesenteric artery, and LGA, left gastric artery of graft. Note that the host left gastric artery was retained to nourish a small recipient gastric remnant. Without this expedient, all of the recipient stomach must be removed as in Figure 7.
UPPER ABDOMINAL GRAFTS (CLUSTER)
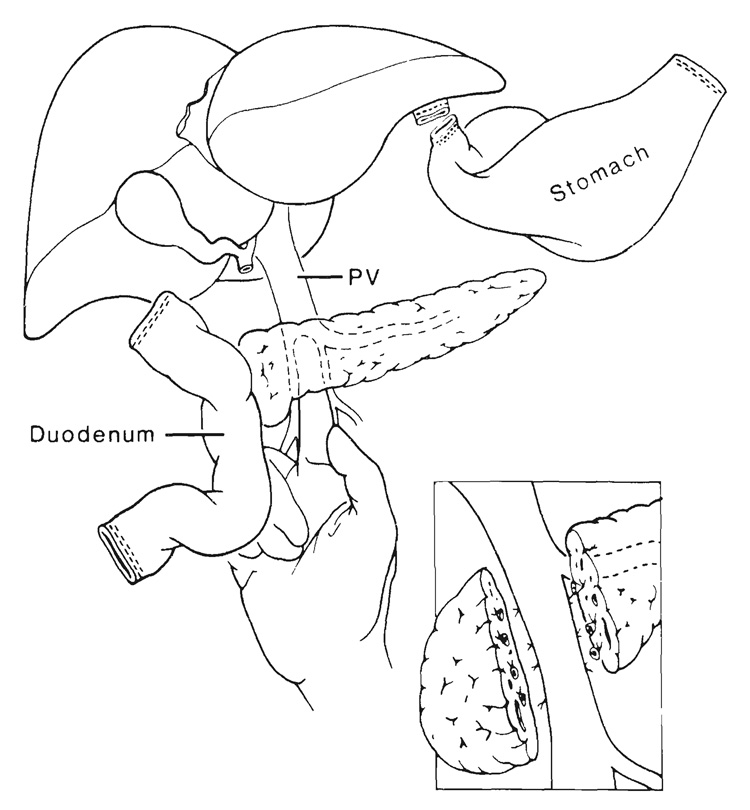
Cluster transplants have been used after removal of the recipient liver, pancreas, stomach, spleen, duodenum, proximal part of the jejunum and part of the colon for malignant lesions involving the liver and pancreas (18). The replacement graft contains liver, pancreas and variable amounts of duodenum and jejunum (Fig. 6). Because patients undergoing such operations have serious nutritional problems (18, 27, 28), the possibility of retaining the stomach has been entertained (Fig. 7). One patient who had this procedure died 14 days postoperatively of complications of a segmental venous infarction of the recipient jejunum. At autopsy, the transplanted stomach and other organs in the graft were normal.
FIG. 6.
Cluster graft used to replace resected viscera after exenteration of the upper part of the abdomen. CA, celiac axis; SMA, superior mesenteric artery, and SMV, superior mesenteric vein.
FIG. 7.
Cluster graft including stomach. This operation has been performed in a human (see text). Note jejunal chimney from graft for postoperative decompression. LGA, left gastric artery; HA, hepatic artery; SMA, superior mesenteric artery, and SMV, superior mesenteric vein.
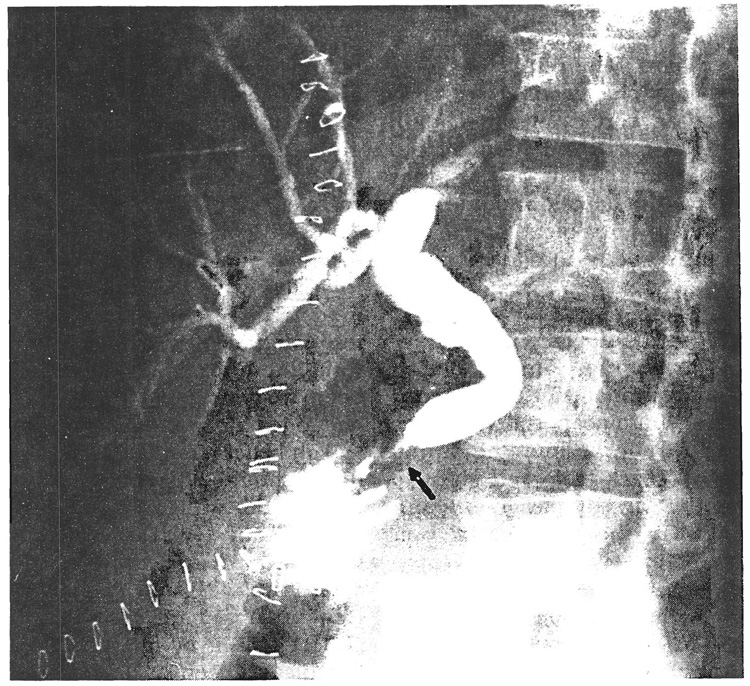
Cluster operations have been performed 21 times in Pittsburgh more than a year ago, and several other times elsewhere. Our perioperative mortality rate (first three months) was 24 per cent. Pancreatitis was the most important cause of lethal or serious technical problems. With follow-up periods of 12 to 29 months, the two year actuarial survival rate is 35.5 per cent. Of special interest were two patients whose grafted duodenums always were part of the mainstream continuity of the gastrointestinal tract (Fig. 8). The original diagnosis in the first patient was sarcoma of the duodenum with massive hepatic metastases. After transplantation. endoscopic biopsies of the duodenal homograft showed rejection at three weeks, widespread replacement of the duodenal mucosa with granulation at two months and normal histopathologic structure at one and two years. This patient is clinically well after 26 months. The second patient with carcinoma of the cecum and widespread hepatic metastases had ampullary dysfunction of the grafted common duct (Fig. 9), necessitating common duct anastomosis to a Roux-limb of recipient jejunum at a second operation. This patient died of recurrent carcinoma after eight and one-half months.
FIG. 8.
Cluster graft in which a segment of the duodenum becomes part of the gastrointestinal main stream, and thus, a functioning segmental enteric graft as soon as the patient begins to eat. The longest follow-up period for this type of patient is 26 months after exenteration of the upper part of the abdomin for a massive duodenal sarcoma with hepatic metastases.
FIG. 9.
Ampullary dysfunction (arrow) in a cluster graft causing biliary obstruction and jaundice of the recipient after the operation depicted in Figure 8. The dilated distal common duct was detached from the allograft and anastomosed to a Roux-limb constructed from the jejunum of the recipient.
HEPATIC-INTESTINAL GRAFTS
With these operations, the small intestine and liver are retained from the basic multivisceral graft (Fig. 2). Removal of the other organs from the stem vascular structures can be done during procurement, on the back table after removal of the perfused complete specimen or (as we recommend) by a combination of these approaches. With the combination method, a decision is made early during procurement about the proximal and distal limits of the intestine to be grafted. The intestine is stapled and transected at these points, after making sure that the blood supply is intact to the proximal and distal ends. Removal of the organs to be discarded is facilitated by their piecemeal mobilization after stapling and transection (Fig. 10) at the esophogogastric junction, the proximal jejunum and the distal ileum near the ileocolic valve. The added exposure and mobility gained are important also for protection of the structures to be saved. The most inaccessible vessel, the superior mesenteric vein, can be approached by inserting a finger along its avascular anterior surface and transecting of the neck of the pancreas (Fig. 10, inset). This allows the numerous medial and lateral tributaries to be ligated under direct vision. Further liberation of the skeletonized superior mesenteric vein from the uncinate process and duodenum is best done on the back table.
FIG. 10.
Removal of pancreas and other organs during trimming of viscera for liver-small intestinal transplantation. PV, Portal vein.
Successful transplantation of the liver and small intestine in continuity was first accomplished by Grant and others (29). At the time of reporting one year later, a patient in their study had normal hepatic and intestinal function. We have used this operation to treat two children and one adult with good results after follow-up periods of two, six and six and one-half months. In the two patients with the longest follow-up period, the venous drainage of the pancreas and other residual viscera of the upper part of the abdomen was decompressed with a portacaval shunt (Fig. 2). depriving the liver permanently of endogenous hepatotrophic substances that emanate mainly from the pancreas (30). Even after resumption of diet, moderate hypoalbuminemia was seen (2.8 grams per cent). The results of biopsy of the liver showed fatty changes that are characteristic of those after portacaval shunt (30). Consequently, a similar portacaval shunt placed intraoperatively in a third patient in our study was detached as soon as the hepatic and intestinal graft was in place and the recipient portal vein was anastomosed to the side of the donor portal vein (Fig. 2, inset).
ISOLATED INTESTINAL TRANSPLANTATION
If the intestine is to be transplanted alone, it is useful to retain long segments of the superior mesenteric artery and draining portal vein. The liver can be separated from the intestine during procurement or on the back table and given to another recipient along with the celiac axis and upper portal vein, while the distal portal vein and complete superior mesenteric artery are retained with the intestine. One of the patients in our study, now eight and one-half months after operation, is the longest known survivor after a complete transplantation of the small intestine (9). However, other nearly complete grafts of the small intestine are known to be functioning after 23 months (31–34).
RESTORATION OF CONTINUITY OF THE GASTROINTESTINAL TRACT
These techniques are dependent on the nature of the allograft. If the intestine is part of the graft, an exteriorizing ostomy is advisable at its upper and lower end because prolonged enteric decompression usually is required postoperatively. After the graft has settled in and is free of rejection or other complications, upper or lower enteric anastomoses can be performed as described elsewhere (17, 18, 31). Examples are shown in Figure 5, Figure 7 and Figure 8.
DISCUSSION
The intestinal component has been the Achilles heel of abdominal multiorgan grafts and especially with the full multivisceral operation, in part because it appears to be more vulnerable to rejection than the liver and other abdominal organs (35). When rejection occurs, bacterial leakage through the disrupted mucosal barrier can be expected with overwhelming systemic sepsis. In spite of these difficulties, there has been a slowly growing number of chronically functioning intestinal segments (25, 31, 32) duodenal C-loops in two of the cluster recipients in our study (maximum 26 months), a complete cadaveric graft of the small intestine in our series after (eight and one-half months) (9), complete transplants of the small intestine in combination with hepatic grafts in Grant’s experience (29) and ours (9), and a complete intestine in two full multivisceral recipients (17, 26). Clinical successes may be easier to achieve with the new immunosuppressive drug, FK 506, which has provided results superior to cyclosporine in several rat intestinal transplant models (4–7). All four of the human small intestinal transplant recipients in our study, one with intestine alone and three with liver, have been treated with FK 506 for the two to eight and one-half months of survival.
With better immunosuppressive therapy, the possibility should be reduced that lymphoid tissues in the graft will damage the recipient (GVHD). Evidence of GVHD was seen 30 years ago in the first untreated canine recipients of multivisceral grafts (2), and studied precisely by others (36) in inbred rat recipients of intestines. The GVHD caused by intestinal and multivisceral allografts has been surprisingly easy to control with FK 506 in animals and humans (4–7, 9). The same ability to prevent or reverse GVHD with FK 506 has been demonstrated after bone marrow transplantation (10, 11, 37). These observations have changed our therapeutic strategy for control of GVHD. In our first experience with multivisceral instances, in which immunosuppression was with cyclosporine (17, 24), an attempt was made to deplete the graft lymphoid population with OKT3 treatment of the donor or by irradiation of the intestine after it had been implanted. These steps were omitted for the last four intestinal or liver-intestinal grafts whose recipients had FK 506.
In the latter four patients, circulating donor lymphoid cells were found during the first postoperative month (9) as had been previously described under cyclosporine by Grant (29), but in contrast with that experience, there was no comtemporaneous evidence of clinical GVHD. Concomitantly in the patients in our study, the lymphoreticular population of the intestinal lamina propria was replaced by cells of the recipient. The same kind of repopulation previously had been demonstrated in rat intestinal or multivisceral grafts, which also had a complete cell transformation in the Peyer’s patches and mesenteric nodes (35). The consistent development of this special kind of chimera is of considerable basic and practical interest. It remains to be seen if the cell traffic to and from the graft had any relation to the Epstein-Barr virus associated B cell lymphomas that developed in the first three multivisceral recipients one month or more after multivisceral transplantation using cyclosporine (16, 17, 24). The three patients were small children with grafts that were treated with OKT3 or irradiation. No further examples of this complication were seen in subsequent adult or pediatric recipients in our study of cluster, intestinal, liver-intestinal or multivisceral transplants using supression with either cyclosporine or FK 506 with grafts that were not altered by donor pretreatment.
With complete multivisceral grafts, the venous effluent from all of the nonhepatic splanchnic organs contributes to the portal blood supply of the graft liver, assuring first pass delivery to this new liver of intestinal nutrients and of the so-called portal hepatotrophic substances that are important for normal hepatocyte structure, function and capacity for regeneration (30, 38–43). The hepatotrophic factors of which endogenous insulin is the single most important are multiple and apparently cumulative. When partial multivisceral grafts are used, such as the liver-intestine, it is preferable to direct the gastroduodenal and pancreatic effluent from the retained recipient organs into the portal circulation of the new liver (Fig. 2, inset) instead of using the more expedient option of decompressing this venous bed by a portacaval shunt. Otherwise, subtle injury of the liver can be expected (19, 40), as was first shown in auxiliary hepatic graft experiments performed in another study (44).
Additional information is needed about the vigor of rejection of the visceral organs when these are part of an organ composite versus fate when they are transplanted alone. Although this question prompted the original experiments with the multivisceral operation (1, 2), it has not been answered completely, as discussed elsewhere (7, 35). There is evidence that intestines are less vigorously rejected when accompanied by a liver than when transplanted alone (7, 29, 35, 45), but this is far from conclusive. The related question of whether or not the liver mitigates the rejection of intestines whose venous drainage is transportal, as opposed to systemic, also remains controversial (46–48).
SUMMARY
Multivisceral allografts and lesser variations of it have in common the liver as a constituent organ, the use of the same blood vessels and vascular surgical techniques for arterialization and provision of venous outflow and the variable inclusion of hollow viscera, such as intestine, which have resisted previous allempts at clinical transplantation. The metabolic relationship among the transplanted organs and between them and the retained recipient viscera must be considered in planning any of these operations. An additional complexity is the risk of GVHD, which probably is proportional to the amount of lymphoid rich intestine that is included in the graft. The lymphoid and dendritic tissues in the multivisceral grafts are rapidly repopulated by recipient lymphoreticular cells postoperatively at the same time as the donor lymphoreticular cells leave the graft for a new home in the recipient tissues. This two way traffic was seen under immunosuppression with FK 506 without either clinical rejection or GVHD. There has been extended survival after complete multivisceral transplantation and all of its principal variants. Understanding the principles involved should facilitate patient care and progress in this field, including transplantation of the intestine alone.
Acknowledgments
Supported by Research Grants from the Veterans Administration and Project Grant No. DK 29961 from the National Institutes of Health, Bethesda, Maryland.
REFERENCES
- 1.Starzl TE, Kaupp HA., Jr Mass homotransplantation of abdominal organs in dogs. Surg. Forum. 1960;11:28–30. [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Kaupp HA, Jr, Brock DR, et al. Homotransplantation of multiple visceral organs. Am. J. Surg. 1962;103:219–229. doi: 10.1016/0002-9610(62)90491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaffe BM. Visceral interchange. Am. J. Surg. 1989;157:2–5. doi: 10.1016/0002-9610(89)90411-x. [DOI] [PubMed] [Google Scholar]
- 4.Murase N, Kim D, Todo S, et al. Induction of liver, heart, and multivisceral graft acceptance with a short course of FK 506. Transplant Proc. 1990;22:74–75. [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffman AL, Makowka L, Banner B, et al. The use of FK 506 for small intestine allotransplantation: Inhibition of acute rejection and prevention of fatal graft-versus-host disease. Transplantation. 1990;49:483–490. doi: 10.1097/00007890-199003000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee K, Stangl MJ, Todo S, et al. Successful orthotopic small bowel transplantation with short term FK 506 immunosuppressive therapy. Transplant Proc. 1990;22:78–79. [PMC free article] [PubMed] [Google Scholar]
- 7.Murase N, Demetris AJ, Matsuzaki T, et al. Long survival in rats after multivisceral versus isolated small bowel allotransplantation under FK 506. Surgery. in press. [PMC free article] [PubMed] [Google Scholar]
- 8.Starzl TE, Abu-Elmagd K, Tzakis A, et al. Selected topics on FK 506: With special references to rescue of extrahepatic grafts, transplantation of “for-bidden organs”, side effects, mechanisms, and practical pharmacokinetics. Transplant Proc. in press. [PubMed] [Google Scholar]
- 9.Iwaki Y, Starzl TE, Yagihasi A, et al. Replacement of donor lymphoid tissue in human small bowel transplants under FK 506 immunosuppression. Lancet. doi: 10.1016/0140-6736(91)92517-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markus PM, Cai X, Ming W, et al. Prevention of graft-versus-host disease following allogeneic bone marrow transplantation in rats using FK 506. Transplantation. doi: 10.1097/00007890-199110000-00002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Markus PM, Cai X, Ming W, et al. FK 506 reverses acute graft-versus-host disease following allogeneic bone marrow transplantation in rats. Surgery. in press. [PMC free article] [PubMed] [Google Scholar]
- 12.Starzl TE, Halgrimson CG, Koep LJ, et al. Vascular homografts from cadaveric organ donors. Surg. Gynecol. Obstet. 1979;149:76–77. [PMC free article] [PubMed] [Google Scholar]
- 13.Starzl TE, Hakala TR, Shaw BW, Jr, et al. A flexible procedure for multiple cadaveric organ procurement. Surg. Gynecol. Obstet. 1984;158:223–230. [PMC free article] [PubMed] [Google Scholar]
- 14.Starzl TE, Miller C, Broznick B, Makowka L. An improved technique for multiple organ harvesting. Surg. Gynecol. Obstet. 1987;165:343–348. [PMC free article] [PubMed] [Google Scholar]
- 15.Tzakis A, Todo S, Starzl TE. Piggyback orthotopic liver transplantation with preservation of the interior vena cava. Ann. Surg. 1989;210:649–652. doi: 10.1097/00000658-198911000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams JW, Sankary HN, Foster PF, et al. Splanchnic transplantation an approach to the infant dependent on parenteral nutrition who develops irreversible liver disease. J. A. M. A. 1989;261:1458–1462. doi: 10.1001/jama.261.10.1458. [DOI] [PubMed] [Google Scholar]
- 17.Starzl TE, Rowe M, Todo S, et al. Transplantation of multiple abdominal viscera. J. A. M. A. 1989;261:1449–1457. [PMC free article] [PubMed] [Google Scholar]
- 18.Starzl TE, Todo S, Tzakis A, et al. Abdominal organ cluster transplantation for the treatment of upper abdominal malignancies. Ann. Surg. 1989;210:374–386. doi: 10.1097/00000658-198909000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Starzl TE, Tzakis AG. Pancreatico-duodenal transplantation with enteric exocrine drainage. In: Groth CG, editor. Pancreatic Transplantation. Philadelphia: W. B. Saunders Co.; 1988. pp. 113–129. [Google Scholar]
- 20.Kalayoglu M, Sollinger WH, Stratta RJ, et al. Extended preservation of the liver for clinical transplantation. Lancet. 1988;1:617–619. doi: 10.1016/s0140-6736(88)91416-x. [DOI] [PubMed] [Google Scholar]
- 21.Todo S, Nery J, Yanaga K, et al. Extended preservation of human liver grafts with UW solution. J. A. M. A. 1989;261:711–714. [PMC free article] [PubMed] [Google Scholar]
- 22.Mallory FB. Pathological Technique: A practical manual for workers in pathological histology, including directions for the performance of autopsies and microphotography. Philadelphia: W. B. Saunders Co.; 1938. [Google Scholar]
- 23.Bjorken C, Lundgren G, Ringden O, et al. A technique for rapid harvesting of cadaveric renal and pancreatic grafts after circulatory arrest. Br. J. Surg. 1976;63:517–519. doi: 10.1002/bjs.1800630705. [DOI] [PubMed] [Google Scholar]
- 24.Jaffe R, Trager JDK, Zeevi A, et al. Multivisceral intestinal transplantation: Surgical pathology. Pediatr. Pathol. 1989;9:633–654. doi: 10.3109/15513818909022372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroeder P, Goulet O, Lear PA. Small bowel transplantation: European experience. Letter. Lancet. 1990;336:110–111. doi: 10.1016/0140-6736(90)91621-g. [DOI] [PubMed] [Google Scholar]
- 26.1990. Dec, Follow-up with R. Margreiter, personal communication.
- 27.Mieles L, Todo S, Tzakis A, Starzl TE. The treatment of upper abdominal malignancies with organ cluster procedures. Clin. Transplant. 1990;4:63–67. [PMC free article] [PubMed] [Google Scholar]
- 28.Tzakis AG, Todo S, Madariaga J, et al. Upper abdominal exenteration in transplantation for extensive malignancies of the upper abdomen: An update. Transplantation. doi: 10.1097/00007890-199103000-00035. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grant D, Wall W, Mimeault R, et al. Successful small-bowel/liver transplantation. Lancet. 1990;335:181–184. doi: 10.1016/0140-6736(90)90275-a. [DOI] [PubMed] [Google Scholar]
- 30.Starzl TE, Porter KA, Francavilla A. The Eck fistula in animals and humans. Curr. Probl. Surg. 1983;20:687–752. doi: 10.1016/s0011-3840(83)80010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goulet O, Revillon Y, Jan D, et al. Small-bowel transplantation in children. Transplant. Proc. 1990;22:2499–2500. [PubMed] [Google Scholar]
- 32.1991. Feb, Follow-up of reference 31, Goulet, personal communication.
- 33.Deltz E, Schroeder P, Gebhardt H, et al. Successful clinical small bowel transplantation: Report of a case. Clin. Transplant. 1989;3:89–91. [Google Scholar]
- 34.1991. Feb, Follow-up of reference 33 by Deltz, personal communication.
- 35.Murase N, Demetris AJ, Kim DG, et al. Rejection of the multivisceral allografts in rats: A sequential analysis with comparison to isolated orthotopic small bowel and liver grafts. Surgery. 1990;108:880–889. [PMC free article] [PubMed] [Google Scholar]
- 36.Monchik GJ, Russell PS. Transplantation of the small bowel in the rat: technical and immunologic considerations. Surgery. 1971;70:693–702. [PubMed] [Google Scholar]
- 37.Tzakis AG, Fung JJ, Todo S, et al. Use of FK 506 in pediatric patients. Transplant. Proc. in press. [PMC free article] [PubMed] [Google Scholar]
- 38.Starzl TE, Francavilla A, Halgrimson CG, et al. The origin, hormonal nature, and action of hepatotrophic substances in portal venous blood. Surg. Gynecol. Obstet. 1973;137:179–199. [PMC free article] [PubMed] [Google Scholar]
- 39.Starzl TE, Porter KA, Kashiwagi N, et al. The effect of diabetes mellitus on portal blood hepatotrophic factors in dogs. Surg. Gynecol. Obstet. 1975;140:549–562. [PMC free article] [PubMed] [Google Scholar]
- 40.Starzl TE, Lee IY, Porter KA, Putnam CW. The influence of portal blood upon lipid metabolism in normal and diabetic dogs and baboons. Surg. Gynecol. Obstet. 1975;140:381–396. [PMC free article] [PubMed] [Google Scholar]
- 41.Starzl TE, Porter KA, Kashiwagi N, Putnam CW. Portal hepatotrophic factors, diabetes mellitus and acute liver atrophy, hypertrophy and regeneration. Surg. Gynecol. Obstet. 1975;141:843–858. [PMC free article] [PubMed] [Google Scholar]
- 42.Starzl TE, Francavilla A, Porter KA, et al. The effect of splanchnic viscera removal upon canine liver regeneration. Surg. Gynecol. Obstet. 1978;147:193–207. [PMC free article] [PubMed] [Google Scholar]
- 43.Starzl TE, Watanabe K, Porter KA, Putnam CW. Effects of insulin, glucagon, and insulin/glucagon infusions on liver morphology and cell division after complete portacaval shunt in dogs. Lancet. 1976;1:821–825. doi: 10.1016/s0140-6736(76)90477-3. [DOI] [PubMed] [Google Scholar]
- 44.Marchioro TL, Porter KA, Dickinson TC, et al. Physioloigic requirements for auxiliary liver homotransplnatation. Surg. Gynecol. Obstet. 1965;121:17–31. [PMC free article] [PubMed] [Google Scholar]
- 45.Zhong R, He G, Sakai Y, et al. Combined small bowel and liver transplantation in the rat: possible role of the liver in preventing intestinal allograft rejection. Transplantation. doi: 10.1097/00007890-199109000-00033. in press. [DOI] [PubMed] [Google Scholar]
- 46.Schraut WH, Abraham VS, Lee KW. Portal versus caval venous drainage of small bowel allografts: Technical and metabolic consequences. Surgery. 1986;99:193–198. [PubMed] [Google Scholar]
- 47.Koltun WA, Kirkman RL. Nutritional and metabolic aspects of total small bowel transplantation in inbred rats. Transplant. Proc. 1987;19:1120–1122. [PubMed] [Google Scholar]
- 48.Shaffer D, Diflo T, Love W, et al. Immunologic and metabolic effects of caval versus portal venous drainage in small-bowel transplantation. Surgery. 1988;104:518–524. [PubMed] [Google Scholar]