Abstract
Intermittent administration of parathyroid hormone (PTH) stimulates bone formation on the surface of cancellous and periosteal bone by increasing the number of osteoblasts. Previous studies of ours in mice demonstrated that intermittent PTH increases cancellous osteoblast number at least in part by attenuating osteoblast apoptosis, but the mechanism responsible for the anabolic effect of the hormone on periosteal bone is unknown. We report that daily injections of 100 ng/g of PTH(1–34) to 4–6 month old mice increased the number of osteoblasts on the periosteum of lumbar vertebrae by 2–3 fold as early as after 2 days. However, the prevalence of apoptotic periosteal osteoblasts was only 0.2% in vehicle treated animals, which is ~20-fold lower than is the case for cancellous osteoblasts. Moreover, PTH did not have a discernable effect on periosteal osteoblast apoptosis. Administration of BrdU for 4 days failed to label periosteal osteoblasts under either basal conditions or following administration of PTH. Cancellous osteoblasts, on the other hand, were labeled under basal conditions, but PTH did not increase the percentage of BrdU-positive cells. Thus, intermittent PTH does not increase cancellous or periosteal osteoblast number by stimulating the proliferation of osteoblast progenitors. Consistent with high turnover of cancellous osteoblasts as compared to that of periosteal osteoblasts, ganciclovir-induced ablation of replicating osteoblast progenitors in mice expressing thymidine kinase under the control of the 3.6kb rat Col1A1 promoter resulted in disappearance of osteoblasts from cancellous bone over a 7–14 day period, whereas periosteal osteoblasts were unaffected. However, 14 days of pre-treatment with ganciclovir prevented PTH anabolism on periosteal bone. We conclude that in cancellous bone, attenuation of osteoblast apoptosis by PTH increases osteoblast number because their rate of apoptosis is high, making this effect of the hormone profound. However, in periosteal bone where the rate of osteoblast apoptosis is low, PTH must exert pro-differentiating and/or pro-survival effects on post-mitotic pre-osteoblasts. Targeting the latter cells is an effective mechanism for increasing osteoblast number in periosteal bone where the production of osteoblasts from replicating progenitors is slow.
Keywords: bone formation, PTH, osteoblasts, apoptosis, periosteal bone, cancellous bone
Introduction
Daily injections of parathyroid hormone (PTH) are the only FDA-approved therapy that stimulates bone formation in osteoporotic individuals [1–3]. Studies in animals and humans have shown that intermittent administration of PTH, or PTH-related protein, increases the connectivity and thickness of trabeculae [1, 4–6]. In addition, PTH increases cortical bone mass by stimulating bone formation on the endosteal as well as the periosteal surface [4, 7–9]. The stimulation of periosteal bone formation leads to an increase in cortical diameter and thereby the cross-sectional moment of inertia, which is an important determinant of resistance to fracture.
The increase in bone formation caused by intermittent PTH is due mainly to an increase in osteoblast number rather than vigor. Thus PTH must influence the production and/or survival of osteoblasts. Osteoblasts develop from mesenchymal stem cells via a series of proliferative and differentiation steps that are governed by locally produced cytokines and growth factors [10]. The replication of osteoblast progenitors gradually declines as differentiation proceeds, leading to a population of preosteoblasts that are dividing slowly or not at all in adults [11, 12]. Some preosteoblasts also undergo apoptosis [13, 14]. The fully differentiated osteoblasts elaborate and calcify a collagenous bone matrix. In cancellous bone, some osteoblasts become incorporated into this matrix as osteocytes and some become flat lining cells that cover the quiescent bone surface, but the majority die by apoptosis [15–17]. Osteoblasts presumably have the same fates in periosteal tissue, but this presumption has not been tested, and the prevalence of periosteal osteoblast apoptosis has not been quantified.
Extensive in vitro evidence has shown that PTH has pleiotropic effects on osteoblasts and osteoblast progenitors including inhibition of apoptosis, stimulation or inhibition of mitosis depending on the cell model and culture conditions, and promotion of differentiation [18]. Consistent with the in vitro evidence, mice in which PTHrP production was specifically ablated in cells of the osteoblast lineage exhibit reduced bone mass associated with decreased osteoblastogenesis, increased osteoblast apoptosis, and decreased osteoblast number [19]. However, the only cellular effect of daily PTH injection that has been associated with the rapid increase in osteoblast number in murine cancellous bone is attenuation of apoptosis [5, 20]. The importance of osteoblast apoptosis as a determinant of osteoblast number and the rate of bone formation is supported by evidence from genetically manipulated mice, and from several murine models of osteoporosis [21].
Heretofore, the cellular mechanism(s) responsible for PTH anabolism in periosteal bone have remained unknown. Nevertheless, several lines of evidence indicate that it might differ from the mechanism that operates in cancellous bone. First, the rate of osteoblast differentiation from replicating progenitors in periosteal bone is much slower than in cancellous bone [22]. Second, osteoblasts and osteoblast progenitors of periosteal and cancellous bone reside in different environments, and often respond differently to mechanical, hormonal, and pharmacologic stimuli [23].
In experiments reported here, we sought to determine whether the mechanisms that underlie the anabolic effect of intermittent PTH in cancellous and periosteal bone are the same or different. We report that, unlike the situation in cancellous bone, attenuation of osteoblast apoptosis does not contribute to the increase in periosteal osteoblast number caused by intermittent PTH in the lumbar vertebrae of adult mice. Moreover, the PTH-induced increase in osteoblast number in either cancellous or periosteal bone tissue cannot be accounted for by stimulation of osteoblast progenitor replication. However, conditional ablation of replicating osteoblast progenitors in a transgenic mouse model prevents the PTH-induced increase in periosteal osteoblasts. Taken together, these observations indicate that PTH anabolism at the periosteum results from actions of PTH on post-mitotic preosteoblasts.
Materials and Methods
Generation of transgenic mice
Constitutive and osteoblastic lineage-specific expression of thymidine kinase (tk) was achieved by placing the tk cDNA downstream of the 3.6-kb rat collagen type I promoter [24], which was provided to us by Alex Lichtler (University of Connecticut, Storrs Connecticut). To supply a heterologous intron and a polyadenylation site, a DNA fragment derived from pIRESpuro2 (Clontech, Palo Alto, CA) containing an artificial intron, internal ribosome entry site, puromycin resistance gene, and a poly adenylation site, was inserted downstream from the tk cDNA. Purified DNA was microinjected into fertilized FVB/N eggs and tk transcripts in transgenic founders were identified by PCR using the following primer sequences: for (5′-TGGACTCCTTTCCCTTCCTT-3′) and -rev (5′-GTAAGTCATCGGCTCGGGTA-3′). Transgenic mice were maintained in the FVB genetic background, and bred using transgenic females and wild type males in view of previous evidence for tk transgene-induced sterility in males [25]. Both male and female 3.6Col1A1-tk mice were used for experiments as there is no evidence of sex specific response of the animals to ganciclovir or PTH. All protocols for the generation and maintenance of these mice, as well as for experiments using these mice and their wild-type littermates, were approved by the Institutional Animal Care and Use Committees of the University of Arkansas for Medical Sciences and the Central Arkansas Veterans Healthcare System.
Quantitative PCR (pPCR)
Total RNA from tibia, calvaria, vertebra L6, liver, and spleen of wild-type and 3.6Col1A1-tk transgenic mice was extracted using Ultraspec reagent (Biotecx Laboratories, Inc., Houston, TX) following instructions of the manufacturer. Equal amounts of RNA (2 μg) from each sample were reverse-transcribed using a High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Transcripts were subsequently amplified from the first strand cDNA by real-time PCR using Assay on Demand or Assay by Design primer probe pair sets and TaqMan Universal PCR Master Mix (Applied Biosystems). Amplification and detection were carried out on an ABI Prism 7700 Sequence Detection System (Applied Biosystems) as follows: 5-min denaturation at 95 C for 10 min, 40 cycles of amplification including denaturation at 94C for 15 sec and annealing/extension at 60C for 1 min. Gene expression was quantified using the comparative threshold cycle (Ct) method by subtracting the GAPDH Ct value from the Ct value of the gene of interest. Amplification efficiencies of each transcript measured were equivalent.
Animal studies
The data shown in Figure 1 were obtained using vertebral bone sections prepared from 4–6 month old female Swiss Webster left untreated, or treated with 100 ng PTH(1–34)/g/d or vehicle control, for up to 28 days in experiments that we have reported previously [20]. 3.6Col1A1 tk mice, or littermate controls, were given bovine PTH(1–34) (Bachem California, Inc., Torrance, CA) or buffer (0.9% saline, 10 μM β-mercaptoethanol, 0.01% acetic acid) by daily injection at 100 ng/g for up to 28 days as previously described [20]. Ganciclovir (Cytovene-IV, Roche Laboratories Inc., Nutley, NJ) was given at 8 μg/g i.p. twice daily in saline. Tetracycline (30 mg/kg) was injected (i.p.) at 8 and 4 days before the end of the experiment. BrdU was administered using slow release pellets delivering approximately 1 mg/day (Innovative Research of America, Sarasota, FL). BMD was measured by dual-energy x-ray absorption using a QDR 2000 Plus densitometer (Hologic Inc., Bedford, MA) adapted for measurement of murine skeletal subregions with the aid of customized software developed by Hologic for use with mice. Resolution was enhanced by increasing the line spacing and using a smaller collimator. Sensitivity was increased by reducing the scan speed [26, 27]. Serum was obtained by retroorbital bleeding or by cheek bleeding for measurement of osteocalcin (Biomedical Technologies, Soughton, MA). Colony forming osteoblast progenitors of the femur were determined in ex-vivo cultures as described [28].
Figure 1. The stimulatory effect of intermittent PTH on periosteal bone formation is not associated with attenuation of osteoblast apoptosis.
(A–C) Female Swiss-Webster mice were injected daily with 100 ng/g PTH, or vehicle (Veh), for 28 days (N=5 per group). Nondecalcified sections of lumbar vertebrae were used to determine (A) the number of periosteal osteoblasts (Ob) visualized by staining with toluidene blue (arrows), (B) tetracycline-based bone formation rate, and (C) the number of apoptotic periosteal osteoblasts visualized with ISEL-staining. Arrow head, ISEL-labeled periosteal osteoblast; black arrows, viable periosteal osteoblasts; red arrows viable endosteal and cancellous osteoblasts. (A–C) b, cortical bone; original magnification = 400X. (D) Female Swiss-Webster mice were injected daily with 100 ng/g PTH for the indicated number of days, or left untreated (N=5 per group), and periosteal osteoblast number in lumbar vertebrae determined. *P<0.05 vs. vehicle-treated (A,B) or untreated (D) animals. (C,D) There was no statistically significant effect of PTH on the prevalence of periosteal osteoblast apoptosis as determined by Poisson test of homogeneity.
Bone histomorphometry
After fixation in Millonigs, lumbar vertebrae (L1–L3 or L1–L4) were embedded undecalcified in methyl methacrylate, and stained with toluidene blue, as previously described [20, 26, 27]. Histomorphometric measurements were done using a computer and digitizer tablet (Osteomeasure Version 3.00, Osteometrics Inc., Atlanta, Georgia, USA) interfaced to a Zeiss Axioscope (Carl Zeiss Inc., Thornwood, New York, USA) with a drawing tube attachment. The identity of the sample was unknown to the reader. Measurements were made with a Zeiss Plan-Neofluar 40X (0.75 numerical aperture) objective. Cancellous measurements were made in the entire secondary spongiosa of 3–4 vertebrae. Periosteal measurements in were restricted to the cortex adjacent to the secondary spongiosa. Cortex adjacent to pedicles was omitted. Variables were measured and reported as previously described [20] using terminology recommended by the Histomorphometry Nomenclature Committee of American Society for Bone and Mineral Research [29].
Detection of apoptotic osteoblasts by in situ nick-end labeling (ISEL) was performed on nondecalcified sections as previously described [20]. A positive control with a known number of ISEL-labeled osteoblasts was included in each staining run to assure reproducibility. Determination of the percentage of periosteal and cancellous osteoblasts exhibiting ISEL-labeling was done at the same time except for the data shown in Figure 1, in which ISEL-labeled cancellous osteoblasts had been determined in a previous study [20]. Reanalysis of ISEL-labeled cancellous osteoblasts was performed in selected sections of this experiment and the results were similar to that obtained earlier.
Immunocytochemistry
Lumbar vertebrae (L1–L3 or L1–L4) were fixed in 2% paraformaldehyde containing 0.075 M lysine and 0.01 M periodate overnight at 4 C, and then decalcified with 5% EDTA solution (pH=7.0) for 5–7 days at 4 C. Bones were processed, embedded in paraffin and sectioned using established procedures [30]. After sections were deparaffinized, endogenous peroxidase activity was quenched with 3% H2O2 treatment for 10 minutes. Immunostaining was carried out at room temperature. To detect tk, sections were blocked with 10% rabbit serum for one hour in PBS. Serum was removed and sections were incubated for 3 h with rabbit polyclonal anti-thymidine kinase antibody (W. Summers, Bethesda, MD) diluted 1:100 in 2% goat serum. Normal rabbit serum was used as control. After rinsing with PBS, sections were incubated for 1 hour with goat anti-rabbit -HRP conjugated secondary antibody (Santa Cruz Biotechnologies, Santa Cruz, CA) diluted 1:300 in 2% goat serum. After rinsing and development with DAB substrate-chromogen system (Dako Corp., Carpinteria, CA) for 1 to 5 minutes, sections were counterstained with methyl green. A BrdU staining kit (# 93–3944, invitrogen, Carlsbad, CA) was used to detect incorporated BrdU.
Statistics
Data were analyzed using SigmaStat (SPSS Science, Chicago, IL) or SAS software (SAS Institute Inc., Cary, NC). Values are reported as the mean ± s.d. Treatment effects were evaluated using Student’s t-test or ANOVA. P values were Bonferroni-adjusted for the relevant number of post-hoc comparisons to control the overall error rate. Because ISEL-stained periosteal osteoblasts were rare, estimates of the prevalence of apoptotic periosteal osteoblasts were determined by pooling data from all of the animals in each treatment group. To detect statistically significant differences in the prevalence of rare apoptotic periosteal osteoblasts among treatment groups, the Poisson test of homogeneity was used [31], as implemented in the StatXact8 software package (Cytel Inc., Cambridge, MA; 2007). The exact procedure was used to calculate p-values. P<0.05 was considered significant.
Results
The stimulatory effect of intermittent PTH on periosteal bone formation does not result from attenuation of osteoblast apoptosis
We first examined the relationship between osteoblast apoptosis and PTH anabolism at the periosteum using bone specimens from a previous study of ours in which we had focused on the anabolic response in cancellous tissue of 4–6 month old Swiss-Webster mice [20]. As was the case in cancellous bone in our earlier report, administration of 100 ng PTH(1–34)/g/d for 28 days increased osteoblast number and bone formation rate on the periosteal bone surface of lumbar vertebrae (Figure 1A,B). This effect was shown earlier in C57BL/6 mice by others [4]. Periosteal osteoblasts were identified as toluidene blue-stained cells adjacent to the periosteal bone perimeter; and they were sometimes arranged in palisades in mice receiving PTH (Figure 1A). Few periosteal osteoclasts were seen in either vehicle or PTH treated mice (not shown).
Periosteal osteoblasts exhibiting in situ end labeling (ISEL), indicative of apoptosis, were so rarely observed that ISEL-labeled osteoblast counts from all of the animals in each treatment group were pooled in order to obtain an estimate of the prevalence of apoptotic periosteal osteoblasts (Figure 1C). Using the Poisson test of homogeneity for detecting a statistically significant difference in the frequency of rare events [31], we determined that there was no effect of PTH on the prevalence of apoptotic periosteal osteoblasts in this experiment. In contrast, using the same bone sections, we have shown earlier that 13.0% ± 2.2% of cancellous osteoblasts were apoptotic in control animals, and that intermittent PTH caused a statistically significant decline to a prevalence of 5.6% ± 0.8% [20].
As in the case of cancellous osteoblasts [20], the PTH-stimulated increase in periosteal osteoblasts was evident as early as 2 days after beginning hormone treatment, and progressively increased thereafter (Figure 1D). ISEL-labeled periosteal osteoblasts were also rare in untreated animals of this experiment, and there was no detectable effect of PTH (Figure 1E). In our earlier study of apoptotic osteoblasts in cancellous bone of the same sections, 11.0% ± 1.3% of osteoblasts exhibited ISEL-labeling in untreated mice, and PTH caused a progressive decrease in the prevalence of the phenomenon reaching 5.5% ± 1.2% after 6 days of treatment [20].
The PTH-stimulated increase in osteoblast number on cancellous and periosteal bone does not result from increased osteoblast progenitor replication
To determine whether the rapid PTH-induced increase in cancellous and periosteal osteoblast number is due to increased replication of osteoblast progenitors, groups of mice were implanted with slow release BrdU pellets at the same time as the initiation of daily injections of PTH (or vehicle), or at 24, 48 or 72 hours later simultaneously with subsequent hormone injections, as illustrated in Figure 2A and previously described [32]. This BrdU labeling protocol permits calculation of the percentage of osteoblasts that become labeled during each 24 h period of the experiment as depicted in Figure 2A and described in the Figure legend.
Figure 2. The PTH-stimulated increase in osteoblast number on cancellous and periosteal bone is not due to increased osteoblast progenitor replication.
(A) Left panel. BrdU labeling protocol used to obtained data shown in panels B and C. Groups of 5 month old female Swiss-Webster mice (indicated by bars, N=5–6 per group) were implanted with BrdU pellets at the indicated times during treatment with vehicle (Veh) or PTH (100 ng/g/d) by daily injection. Right panel. BrdU-labeled cancellous osteoblasts (arrows). b, cancellous bone; original magnification = 400X. (B) The total number of osteoblasts, and the number that were BrdU-labeled (BrdU+), on cancellous bone (left panel) or periosteal bone (right panel) were determined in decalcified sections of lumbar vertebrae. The number of BrdU-labeled periosteal osteoblasts in PTH-treated animals are too low to be depicted in the Figure (0.14 per mm the 48–96 h group, and the 0.03/mm in the 72–96 h group). (C) The percentage of cancellous osteoblasts labeled during each of the labeling intervals depicted in panel A was determined as follows. The percentage of BrdU-positive osteoblasts labeled during the 24–72 h period was subtracted from that labeled during the 0–96 h period to yield the percentage of osteoblasts that developed from progenitors that had divided during the first 24 h of the experiment, as indicated by the bracket in panel A. Similar calculations yield labeling indices for the 24–48 h and 48–72 h intervals. The 72–96 h labeling index was determined directly. * P<0.05 vs. respective vehicle control.
PTH increased both cancellous and periosteal osteoblast number by 2–3 fold (Figure 2B,C) and doubled the level of circulating osteocalcin (Vehicle: 50.5±14.1 ng/ml; PTH: 108.3±40.6, n=7–8/group, P<0.05). There was an increase in BrdU-positive cancellous osteoblasts with progressively longer labeling periods, and PTH increased both BrdU-negative and BrdU-positive osteoblast number (Figure 2B). For the most part, the percentage of cancellous osteoblasts labeled during each 24 h period of the experiment was the same in both vehicle and PTH-treated mice, indicating that PTH had no effect on the replication of progenitors that differentiated into osteoblasts (Figure 2C). Consistent with in vitro studies [33], PTH attenuated replication in the 0–24 h labeling interval. However, it is unclear why this was not seen in subsequent labeling intervals.
In contrast to cancellous osteoblasts, no BrdU-positive periosteal osteoblasts were observed in animals receiving vehicle (Figure 2B). In PTH-treated animals, there were only 0.14 BrdU-positive osteoblasts/mm in the 48–96 h labeling group and 0.03/mm in the 72–96 h labeling group. Thus, less than 1% of periosteal osteoblasts were labeled in these samples. No BrdU-labeled osteoblasts were observed in the other labeling groups of animals receiving PTH. These findings demonstrate that stimulation of osteoblast progenitor replication does not account for the rapid PTH-induced increase in osteoblasts in either cancellous or periosteal bone.
Conditional ablation of osteoblast progenitors causes rapid loss of osteoblasts in cancellous but not periosteal bone
Additional studies of the turnover of cancellous and periosteal osteoblasts, as well as the role of osteoblast progenitors in the anabolic response to intermittent PTH in these two compartments, were performed using transgenic mice in which replicating osteoblast progenitors can be conditionally ablated. Specifically, we generated mice expressing the herpes simplex thymidine kinase (tk) gene under the control of the 3.6 kb rat Col1A1 promoter, and designated as 3.6Col1A1-tk mice (Figure 3A). This promoter has been shown previously to be active in early osteoblast progenitors and their osteoblast and osteocyte progeny [24]. Upon exposure to the nucleoside analog ganciclovir, replicating osteoblast progenitors expressing tk will convert ganciclovir into a toxic nucleotide that incorporates into DNA during cell division, leading to DNA double-strand breaks and subsequent apoptosis [34]. We expected that replicating osteoblast progenitors expressing tk, but not their post-mitotic progeny, would be killed by administration of the nucleoside analog ganciclovir, thereby halting the flow of dividing osteoblast progenitors into the population of post-mitotic preosteoblasts and matrix synthesizing osteoblasts. As a result, the population of post-mitotic preosteoblasts would eventually decline as they differentiate into osteoblasts. And, osteoblasts would disappear as they died naturally by apoptosis, or become osteocytes and lining cells.
Figure 3. Characteristics of 3.6Col1A1-tk mice.
(A) The 3.6Col-tk transgene construct contains 3.6 kb of the rat collagen 1A1 5′-flanking region upstream from the herpes virus thymidine kinase (HSV tk) cDNA. To provide a heterologous intron and polyadenylation site, a fragment containing an artificial intron (int) followed by an internal ribosome entry site-puromycin-resistance cassette (IRES-puro) and polyadenylation site (PA) was inserted downstream from the tk cDNA. (B) Expression of tk mRNA in bone and other tissues from 3.6Col1A1-tk mice, determined by qPCR. Data shown represent the mean of samples from 3 mice relative to GAPDH (ND = not detected). (C) Immunostaining for tk in osteoblasts (arrows), and osteocytes (arrow heads) in cancellous and periosteal bone of lumbar vertebrae. b, bone; bm, bone marrow, original magnification = 400X.
At 4 months of age, the weight and BMD of 3.6Col1A1-tk mice were indistinguishable from that of wild type littermates (data not shown). Quantitative PCR (qPCR) analysis of tissues from 3.6Col1A1-tk mice demonstrated high levels of tk expression in calvarial, tibial, and vertebral bone, but minimal or undetectable levels in spleen, liver, lung, and kidney (Figure 3B). Specific immunostaining for tk was observed in osteoblasts and osteocytes of cancellous and periosteal bone (Figure 3C). The pattern of tk expression was practically identical to that seen in mice expressing green fluorescent protein under the control of the same 3.6 kb rat collagen promoter [24].
Twice daily injections of ganciclovir to 3.6Col1A1-tk mice decreased the level of circulating osteocalcin (Figure 4A). The magnitude of this effect increased with time and reached 10% of untreated control values by 28 days. Ganciclovir administration had no effect on osteocalcin levels in wild type mice (not shown). After 28 days of ganciclovir, transcript levels of osteocalcin and Col1A1 were reduced by 70–90% in vertebral bone (Figure 4B). As expected, transcript levels of the osteocyte-specific gene Sost were not affected by ganciclovir because the drug only kills replicating cells expressing tk. Transcript levels of tk were also reduced, but the reduction was not as extensive as for osteocalcin or Col1A1, likely reflecting the lack of effect on tk-expressing osteocytes. Histomorphometric measurements of 3.6Col1A1-tk mice revealed that osteoblast number, osteoid perimeter, and bone formation rate were dramatically reduced in cancellous bone, compared to vehicle controls, after 14 days of ganciclovir administration (Figure 4C).
Figure 4. Effect of ganciclovir on 3.6Col1A1-tk mice.
(A,B) 3.6Col1A1-tk mice (2 months old, N=5–7 per group) were given twice daily injections of vehicle (veh) or ganciclovir for 28 days. Serum osteocalcin level (A) was determined at the indicated times, and transcript levels of osteocalcin, Col1A1, Sost, and tk were determined in vertebral bone by qPCR (B) at the end of the experiment. (C) 3.6Col1A1-tk mice (4–5 months old, N=6 per group) were given twice daily injections of vehicle or ganciclovir for 14 days. Osteoblast (Ob) number and bone formation rate in cancellous bone of lumbar vertebrae were determined by histomorphometry. (D) 3.6Col1A1-tk mice (2 months old, N=5–7 per group) were given twice daily injections of vehicle or ganciclovir for 6 days. Osteoblasts (arrows) adjacent to osteoid in a nondecalcified section of vertebral cancellous bone after 6 days of ganciclovir administration. toluidene blue staining; original magnification 400X. b, bone; Apoptotic osteoblasts were quantified by ISEL staining in wild type and 3.6Col1A1-tk mice after 6 days of ganciclovir administration. (E) Femoral colony forming osteoblast progenitors were determined in ex-vivo cultures in 6-well plates after 14 days of vehicle or ganciclovir administration in the experiment shown in panel C. Mineralized matrix was visualized with Von Kossa staining. (F) Osteoblast number and indices of bone formation in periosteal bone of lumbar vertebrae determined by histomorphometry in the experiment shown in panel C. * P<0.05 vs. vehicle.
Although osteoblasts of 3.6Col1A1-tk mice express tk (Figure 3C), ganciclovir did not have nonspecific cytotoxic effects on these cells. Thus, plump cuboidal osteoblasts overlying osteoid were still observed in vertebral cancellous bone sections from animals given ganciclovir for 6 days (Figure 4D). And in the same experiment, ganciclovir had no effect on the prevalence of osteoblast or osteocyte apoptosis in either wild type or 3.6Col1A1-tk mice.
We also searched for so-called “bystander” effects of ganciclovir. This effect is thought to be due to release of toxic phosphorylated ganciclovir from tk-expressing cells to neighboring non-tk expressing cells [35]. We found that ganciclovir did not affect uncommitted mesenchymal stem cells of the bone marrow as evidenced by the retention of colony forming osteoblast progenitors in the femurs of ganciclovir-treated 3.6Col1A1-tk mice (Figure 4E). Consistent with this, cessation of ganciclovir administration resulted in an increase in circulating osteocalcin within 14 days, indicative of resumption of osteoblast production from early progenitors (not shown). Ganciclovir also had little or no effect on the number of femoral marrow cells in 4–5 month old 3.6Col1A1-tk mice (not shown). On the other hand, other experiments with 1–2 month old animals confirmed ganciclovir-induced depletion of hematopoietic cells as previously reported for mice expressing tk under the control of a 2.3-kb fragment of the Type I collagen promoter [36].
In view of the above evidence, the ganciclovir-induced decline in cancellous osteoblasts is best explained by the killing of tk-expressing replicating osteoblast progenitors, thereby halting the production of the new osteoblasts that are needed to replace those that die by apoptosis or become osteocytes or lining cells. Moreover, the finding that approximately 90% of osteoblasts had disappeared after 14 days of ganciclovir administration is consistent with previous evidence that the life span of murine cancellous osteoblasts is 7–10 days [26].
Unlike the situation in cancellous bone, 14 days of ganciclovir had no effect on periosteal osteoblast number or on tetracycline-based indices of the rate of bone formation, i.e. single labeled perimeter, double labeled perimeter, and mineral apposition rate (Figure 4F). The lack of an effect of 14 days of ganciclovir on periosteal osteoblast number indicates that osteoblast progenitors divide infrequently and enter into the pool of osteoblasts slowly, consistent with the slow rate at which periosteal osteoblasts become labeled with marked nucleosides, as noted in the present study employing BrdU and earlier studies using 3H-thymidine [22].
The PTH-stimulated increase in cancellous and periosteal osteoblasts is preserved when co-administered with ganciclovir for 5 days
The presence of BrdU-labeled osteoblasts in cancellous bone after 1–4 days of BrdU administration (Figure 2B), indicated that the differentiation of replicating osteoblast progenitors into matrix-synthesizing osteoblasts takes place within this period of time. We therefore used 3.6Col1A1-tk mice to examine whether the actions of PTH on post-mitotic preosteoblasts and/or osteoblasts could account for the ability of PTH to rapidly increase osteoblast number. To do this, mice were given ganciclovir 1 day prior to beginning daily injections of PTH for 5 days, while continuing to give ganciclovir, to minimize any contribution of effects of PTH on replicating progenitors to the increase in osteoblasts.
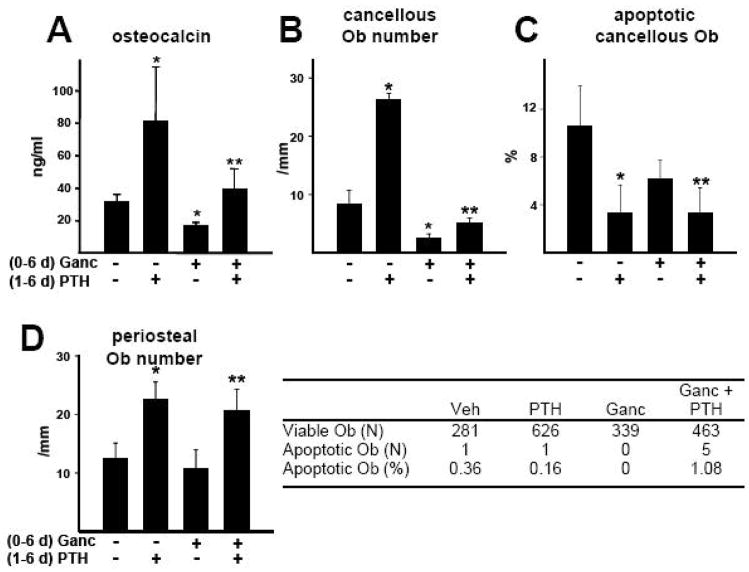
Consistent with the data of Figure 4, administration of ganciclovir to 3.6Col1A1-tk mice for 6 days reduced the level of circulating osteocalcin, as well as the number of cancellous osteoblasts in lumbar vertebrae by 50%; but the drug did not affect the prevalence of osteoblast apoptosis (Figure 5A–C). Co-administration of PTH for 5 days beginning 1 day after initiation of ganciclovir treatment ameliorated the ganciclovir-induced decrease in circulating osteocalcin and cancellous osteoblast number; and these responses were associated with a reduction in apoptotic cancellous osteoblasts. However, the magnitude of the increase in osteoblast number caused by administration of PTH alone was reduced by ganciclovir from 3.1±0.8 fold to 1.7±0.9 (P<0.05 by t-test).
Figure 5. The PTH-stimulated increase in cancellous and periosteal osteoblasts is preserved when co-administered with ganciclovir for 6 days.
Col1A1-tk mice (4 months old, N=6 per group) were injected twice daily with PBS or ganciclovir (Ganc) as in Figure 4 for 1 day, followed by 6 days of 100 ng/g PTH or vehicle injections while maintaining the ganciclovir administration protocol. (A) Circulating osteocalcin was determined at the end of the experiment. (B–D) Osteoblast (Ob) number and the prevalence of osteoblast apoptosis was detemined in cancellous and periosteal bone of lumbar vertebrae. * P<0.05 vs. mice receiving neither PTH nor ganciclovir; ** P<0.05 vs. mice receiving ganciclovir alone. There was no statistically significant difference in the prevalence of periosteal osteoblast apoptosis among the treatment groups as determined by Poisson test of homogeneity.
As in the experiment of Figure 4F, 6 days of ganciclovir had no effect on the number of periosteal osteoblasts (Figure 5D). More important, the PTH-stimulated increase in osteoblast number was not affected by co-administration of ganciclovir; and there was no detectable effect of either ganciclovir or PTH on the number of apoptotic periosteal osteoblasts. Combined with the observation that 4 days of intermittent PTH administration did not stimulate the replication of osteoblast progenitors, these findings indicate that the new periosteal osteoblasts must have arisen from the actions of PTH on post-mitotic preosteoblasts.
Figure 6. The increase in periosteal osteoblasts caused by 5 daily PTH injections is lost following pretreatment with ganciclovir for 14 days.
Col1A1-tk mice (4 months old, N=5–7 per group) were injected twice daily for 14 days with PBS or ganciclovir (Ganc), followed by 5 days of 100 ng/g PTH, or vehicle, injections while maintaining the ganciclovir administration protocol. Periosteal osteoblast (Ob) number and the prevalence of periosteal osteoblast apoptosis was determined in lumbar vertebrae. * P<0.05 vs. mice receiving neither ganciclovir nor PTH. There was no statistically significant difference in the prevalence of periosteal osteoblast apoptosis among the treatment groups as determined by Poisson test of homogeneity.
Fourteen days of pre-treatment with ganciclovir prevents the anabolic effect intermittent PTH on periosteal bone
In agreement with earlier evidence in the literature [22], preliminary studies of ours revealed the presence of BrdU-labeled osteoblasts on the periosteal bone perimeter of murine lumbar vertebrae 16 days after administration of BrdU (not shown). Therefore, we extended the ganciclovir pre-treatment period to 14 days in an attempt to deplete the periosteal tissue of both replicating progenitors and their post-mitotic progeny, and thereby test whether the latter are targets of the anabolic actions of intermittent PTH. Consistent with this contention, pretreatment of 3.6Col1A1-tk mice with ganciclovir for 14 days prevented the increase in periosteal osteoblasts caused by 5 days of PTH administration (Figure 6). As in the studies presented above, there was no effect of either PTH or ganciclovir on periosteal osteoblast apoptosis, and ganciclovir alone did not affect osteoblast number.
To confirm the ability of 14 days of ganciclovir treatment to block PTH-stimulated periosteal bone formation, we extended the duration of PTH administration to 14 days in order to accommodate tetracycline-based measurements of bone formation rate. 28 days of cumulative administration of ganciclovir alone did not reduce overall osteoblast number or increase osteoblast apoptosis (Figure 7A), but did cause a significant reduction in bone formation rate due to reduced double-labeled perimeter and mineral apposition rate (Figure 7B–E). More important, ganciclovir prevented the PTH-stimulated increase in periosteal osteoblast number, double and single labeled perimeter, and bone formation rate without affecting osteoblast apoptosis.
Figure 7. The increase in periosteal osteoblasts and bone formation rate caused by 14 daily PTH injections is lost following pretreatment with ganciclovir for 14 days.
Col1A1-tk mice (4 months old, N=5–7 per group) were injected twice daily for 14 days with PBS or ganciclovir (Ganc), followed by 14 days of 100 ng/g PTH, or vehicle, injections while maintaining the ganciclovir administration protocol. Periosteal osteoblast (Ob) number and the prevalence of periosteal osteoblast apoptosis (A), as well as indices of bone formation (B–E) were determined in lumbar vertebrae. * P<0.05 vs. mice receiving neither ganciclovir nor PTH. There was no statistically significant difference in the prevalence of periosteal osteoblast apoptosis among the treatment groups as determined by Poisson test of homogeneity.
Discussion
The present studies show that, unlike the situation in cancellous bone, the anabolic effect of intermittent PTH on periosteal bone cannot be accounted for by suppression of osteoblast apoptosis. We also elucidated that PTH does not increase cancellous or periosteal osteoblast number by stimulating the replication of osteoblast progenitors. Long term (14 days) pre-treatment of 3.6Col1A1-tk mice with ganciclovir to kill replicating osteoblast progenitors, however, blocked the anabolic effect of intermittent PTH on periosteal bone. Based on these observations, we conclude that PTH increases osteoblast number in periosteal bone via actions on post-mitotic pre-osteoblasts either by stimulating their differentiation, attenuating their apoptosis, or both.
So few periosteal osteoblasts die in periosteal bone that suppression of this process by PTH would have little impact on osteoblast number. Parfitt came to a similar conclusion based on the low rate of bone formation at this site [37]. Arguably, the small number of osteoblasts available for inspection on the periosteal surface of murine lumbar vertebrae, combined with the small percentage that exhibit ISEL-staining, makes it difficult to accurately determine whether PTH suppresses the apoptosis of periosteal osteoblasts. However, combining all of the observations from the studies presented in this paper, we detected 3 of 1380 (0.22%) periosteal osteoblasts with ISEL-labeling in vertebral sections from animals receiving vehicle, as compared to 19 of 4968 osteoblasts (0.38%) in the case of PTH treated animals. Using the Poisson test of homogeneity to detect significant differences in rare events [31], we determined that PTH had no effect on the prevalence of apoptotic osteoblasts at this site. Thus, the prosurvival effect of intermittent PTH, which is so prominent among the population of cancellous osteoblasts, does not contribute to the rapid increase in periosteal osteoblasts and bone formation caused by intermittent PTH.
Our studies also indicate that osteoblast turnover, which is a function of their birth from replicating progenitors and death by apoptosis, is markedly lower in periosteal as compared to cancellous bone of murine lumbar vertebrae. The fact that cancellous, but not periosteal, osteoblasts were labeled with BrdU following 4 days of administration is consistent with previous observations in the rat femur that the development of periosteal osteoblasts from replicating progenitors is slower than is the case for endosteal osteoblasts [22]. The low prevalence of osteoblast apoptosis in periosteal bone, as compared to cancellous bone, together with the low rate of replication of periosteal osteoblast progenitors explains why 4 weeks of ganciclovir administration did not significantly reduce periosteal osteoblast number. On the other hand, the rapid decline in cancellous osteoblasts following administration of ganciclovir to 3.6Col1A1 mice is explained by the high rate of replication of cancellous osteoblast progenitors combined with the fact that most cancellous osteoblasts die by apoptosis [16, 17].
Consistent with earlier studies in cancellous bone of the rat tibia [38], we observed that PTH did not increase the replication of osteoblast progenitors in cancellous or periosteal tissue of vertebral bone of adult mice. Nevertheless, intermittent PTH may stimulate the replication of osteoblast progenitors during experimental fracture healing in the rat [39], and during the bone formation that occurs following subcutaneous implantation of osteoblast progenitors into immunocompromised mice [40]. These observations suggest that the mechanisms by which PTH stimulates bone formation in these particular conditions are distinct from those that contribute to the increase in periosteal and cancellous osteoblasts in adult mice.
Our findings in the 3.6Col1A1-tk model raise the possibility that, in addition to attenuation of osteoblast apoptosis in cancellous bone, PTH also exerts pro-differentiating and/or anti-apoptotic actions on post-mitotic preosteoblasts at this site. In the experiment of Figure 4, the decline in osteoblast number caused by 6 days of ganciclovir was prevented by co-administration of PTH. Because ganciclovir blocks osteoblast production, this effect of PTH must be due to actions on remaining post-mitotic preosteoblasts, osteoblasts, or both. Ganciclovir reduced the magnitude of the PTH-stimulated increase in cancellous osteoblasts by ~50% in this experiment. This is probably because ganciclovir reduced the number of both of these potential target cells during the course of the experiment. Specifically, no new post-mitotic preosteoblasts could be made because their replicating progenitors were killed. And, the number of osteoblasts declined as they become osteocytes or still underwent apoptosis to some extent even with intermittent PTH administration.
PTH may act directly on post-mitotic periosteal preosteoblasts to increase differentiation as suggested by evidence that PTH increased the expression of Runx2, and stimulated Runx2 transcriptional activity, in cultured rat osteoblastic cells [41]. The stimulatory effect of PTH on periosteal bone formation may also be secondary to the well known ability PTH to modulate the synthesis of autocrine/paracrine factors that regulate the production of osteoblasts, such as IGF-I and sclerostin, the latter an antagonist of osteogenic Wnt signaling [18]. Indeed, PTH failed to stimulate periosteal bone formation in mice lacking IGF-I [42]. Moreover, transient downregulation of the synthesis of osteocyte-specific sclerostin [43, 44] following injection of PTH could be involved in the increased periosteal bone formation as administration of an anti-sclerostin antibody to ovariectomized rats increased the rate of bone formation on both endocortical and periosteal surfaces of the femur [45].
The disparate mechanisms by which intermittent PTH administration stimulates bone formation in cancellous vs. periosteal bone appear to reflect the pleiotropic effects of the hormone on osteoblasts and osteoblast progenitors. As illustrated in Figure 8, we propose that in cancellous bone, the anti-apoptotic effect of PTH on osteoblasts is the predominant mechanism for increasing osteoblast number because the rate at which they die is high, making the anti-apoptotic response effective. However, attenuation of periosteal osteoblast apoptosis, if it occurs at all, does not contribute to anabolism at this site because the rate of osteoblast apoptosis is already very low. Instead, pro-differentiating and/or pro-survival effects of PTH on post-mitotic preosteoblasts represent potent mechanisms for increasing osteoblast number in periosteal bone where the production of osteoblasts from replicating progenitors is slow. It is also possible that periosteal and cancellous osteoblast progenitors respond differently to PTH. Future progress in understanding how daily injections of PTH increase osteoblast number and stimulate bone formation in both cancellous and periosteal tissue will require in vivo studies that focus on actions of the hormone on the behavior of osteoblast progenitors.
Figure 8. Intermittent PTH stimulates bone formation in periosteal and cancellous bone by different mechanisms.
Mitotic osteoblast progenitors give rise to post-mitotic preosteoblasts and eventually mature osteoblasts, which then die by apoptosis or become osteocytes or lining cells. Intermittent PTH exerts anti-apoptotic and/or pro-differentiating effects on post-mitotic preosteoblasts in periosteal bone, and anti-apoptotic effects on mature osteoblasts in cancellous bone. The result in both sites is an increase in the number of osteoblasts and thereby an increase in bone formation rate. See text for further details.
Acknowledgments
The authors thank S. Berryhill, T. Chambers, J. Crawford, A. DeLoose, J. Doss, L. Hemmings, W. Webb, C. Wiggins III, R. Wynne, for their contributions to this work. This work was supported by the National Institutes of Health (R01 AR046823 to R.L.J., R01 AR049794 to C.A.O., R01 AR046191 to R.S.W., and P01 AG013918 to S.C.M.), the Department of Veterans Affairs (Merit Reviews to R.L.J., R.S.W. and S.C.M., REAP to S.C.M.), and the Arkansas Biosciences Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Compston JE. Skeletal actions of intermittent parathyroid hormone: effects on bone remodelling and structure. Bone. 2007;40:1447–52. doi: 10.1016/j.bone.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Hodsman AB, Bauer DC, Dempster D, Dian L, Hanley DA, Harris ST, Kendler D, McClung MR, Miller PD, Olszynski WP, Orwoll E, Yuen CK. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005;26:688–703. doi: 10.1210/er.2004-0006. [DOI] [PubMed] [Google Scholar]
- 3.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 4.Iida-Klein A, Lu SS, Cosman F, Lindsay R, Dempster DW. Effects of cyclic vs. daily treatment with human parathyroid hormone (1–34) on murine bone structure and cellular activity. Bone. 2007;40:391–8. doi: 10.1016/j.bone.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104:439–46. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L, Shane E, Plavetic K, Muller R, Bilezikian J, Lindsay R. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res. 2001;16:1846–53. doi: 10.1359/jbmr.2001.16.10.1846. [DOI] [PubMed] [Google Scholar]
- 7.Hirano T, Burr DB, Turner CH, Sato M, Cain RL, Hock JM. Anabolic effects of human biosynthetic parathyroid hormone fragment (1–34), LY333334, on remodeling and mechanical properties of cortical bone in rabbits. J Bone Miner Res. 1999;14:536–45. doi: 10.1359/jbmr.1999.14.4.536. [DOI] [PubMed] [Google Scholar]
- 8.Lindsay R, Zhou H, Cosman F, Nieves J, Dempster DW, Hodsman AB. Effects of a one-month treatment with parathyroid hormone (1–34) on bone formation on cancellous, endocortical and periosteal surfaces of the human ilium. J Bone Miner Res. 2007;22:495–502. doi: 10.1359/jbmr.070104. [DOI] [PubMed] [Google Scholar]
- 9.Rehman Q, Lang TF, Arnaud CD, Modin GW, Lane NE. Daily treatment with parathyroid hormone is associated with an increase in vertebral cross-sectional area in postmenopausal women with glucocorticoid-induced osteoporosis. Osteoporos Int. 2003;14:77–81. doi: 10.1007/s00198-002-1312-0. [DOI] [PubMed] [Google Scholar]
- 10.Aubin JE, Triffitt JT. Mesenchymal stem cells and osteoblast differentiation. In: Bilezikian JP, Raisz LG, Rodan G, editors. Principles of Bone Biology. 2. San Diego: Academic Press; 2002. pp. 59–81. [Google Scholar]
- 11.Owen M. Cellular dynamics of bone. In: Bourne GH, editor. Development and Growth. III. New York, NY: Academic Press; 1971. pp. 271–98. [Google Scholar]
- 12.Kember NF. Cell population kinetics of bone growth: the first ten years of autoradiographic studies with tritiated thymidine. Clin Orthop. 1971;76:213–30. doi: 10.1097/00003086-197105000-00029. [DOI] [PubMed] [Google Scholar]
- 13.Rice DP, Kim HJ, Thesleff I. Apoptosis in murine calvarial bone and suture development. Eur J Oral Sci. 1999;107:265–75. doi: 10.1046/j.0909-8836.1999.eos107406.x. [DOI] [PubMed] [Google Scholar]
- 14.Li G, Dickson GR, Marsh DR, Simpson H. Rapid new bone tissue remodeling during distraction osteogenesis is associated with apoptosis. J Orthop Res. 2003;21:28–35. doi: 10.1016/S0736-0266(02)00097-9. [DOI] [PubMed] [Google Scholar]
- 15.Parfitt AM. Bone-forming cells in clinical conditions. In: Hall BK, editor. Bone. Vol. 1. Boca Raton, FL: Telford Press and CRC Press; 1990. pp. 351–429. The Osteoblast and Osteocyte. [Google Scholar]
- 16.Jilka RL, Weinstein RS, Bellido T, Parfitt AM, Manolagas SC. Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res. 1998;13:793–802. doi: 10.1359/jbmr.1998.13.5.793. [DOI] [PubMed] [Google Scholar]
- 17.Boyce BF, Xing L, Jilka RL, Bellido T, Weinstein RS, Parfitt AM, Manolagas SC. Apoptosis in Bone Cells. In: Bilezikian JP, Raisz LG, Rodan G, editors. Principles of Bone Biology. 2. San Diego: Academic Press; 2002. pp. 151–68. [Google Scholar]
- 18.Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–46. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miao D, He B, Jiang Y, Kobayashi T, Soroceanu MA, Zhao J, Su H, Tong X, Amizuka N, Gupta A, Genant HK, Kronenberg HM, Goltzman D, Karaplis AC. Osteoblast-derived PTHrP is a potent endogenous bone anabolic agent that modifies the therapeutic efficacy of administered PTH 1–34. J Clin Invest. 2005;115:2402–11. doi: 10.1172/JCI24918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellido T, Ali AA, Plotkin LI, Fu Q, Gubrij I, Roberson PK, Weinstein RS, O’Brien CA, Manolagas SC, Jilka RL. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts: a putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem. 2003;278:50259–72. doi: 10.1074/jbc.M307444200. [DOI] [PubMed] [Google Scholar]
- 21.Jilka RL, Weinstein RS, Parfitt AM, Manolagas SC. Quantifying osteoblast and osteocyte apoptosis: challenges and rewards. J Bone Miner Res. 2007;22:1492–501. doi: 10.1359/jbmr.070518. [DOI] [PubMed] [Google Scholar]
- 22.Tonna EA, Cronkite EP. Skeletal cell labeling following continuous infusion with tritiated thymidine. Lab Invest. 1968;19:510–5. [PubMed] [Google Scholar]
- 23.Allen MR, Hock JM, Burr DB. Periosteum: biology, regulation, and response to osteoporosis therapies. Bone. 2004;35:1003–12. doi: 10.1016/j.bone.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Kalajzic I, Kalajzic Z, Kaliterna M, Gronowicz G, Clark SH, Lichtler AC, Rowe D. Use of type I collagen green fluorescent protein transgenes to identify subpopulations of cells at different stages of the osteoblast lineage. J Bone Miner Res. 2002;17:15–25. doi: 10.1359/jbmr.2002.17.1.15. [DOI] [PubMed] [Google Scholar]
- 25.al Shawi R, Burke J, Jones CT, Simons JP, Bishop JO. A Mup promoter-thymidine kinase reporter gene shows relaxed tissue-specific expression and confers male sterility upon transgenic mice. Mol Cell Biol. 1988;8:4821–8. doi: 10.1128/mcb.8.11.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids: potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–82. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jilka RL, Weinstein RS, Takahashi K, Parfitt AM, Manolagas SC. Linkage of decreased bone mass with impaired osteoblastogenesis in a murine model of accelerated senescence. J Clin Invest. 1996;97:1732–40. doi: 10.1172/JCI118600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Gregorio GB, Yamamoto M, Ali AA, Abe E, Roberson P, Manolagas SC, Jilka RL. Attenuation of the self-renewal of transit-amplifying osteoblast progenitors in the murine bone marrow by 17β-estradiol. J Clin Invest. 2001;107:803–12. doi: 10.1172/JCI11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 30.Skinner RA. Decalcification and paraffin processing techniques. In: An YH, Martin KL, editors. Handbook of Histology Methods for Bone and Cartilage. Totowa, NJ: Humana Press; 2003. pp. 167–84. [Google Scholar]
- 31.Fisher LD, van Belle G. Biostatistics: A methodology for the health sciences. New York: John Wiley and Sons, Inc; 1993. Counting Data; pp. 176–245. [Google Scholar]
- 32.Turner CH, Owan I, Alvey T, Hulman J, Hock JM. Recruitment and proliferative responses of osteoblasts after mechanical loading in vivo determined using sustained-release bromodeoxyuridine. Bone. 1998;22:463–9. doi: 10.1016/s8756-3282(98)00041-6. [DOI] [PubMed] [Google Scholar]
- 33.Qin L, Li X, Ko JK, Partridge NC. Parathyroid hormone uses multiple mechanisms to arrest the cell cycle progression of osteoblastic cells from G1 to S phase. J Biol Chem. 2005;280:3104–11. doi: 10.1074/jbc.M409846200. [DOI] [PubMed] [Google Scholar]
- 34.Borrelli E, Heyman R, Hsi M, Evans RM. Targeting of an inducible toxic phenotype in animal cells. Proc Natl Acad Sci U S A. 1988;85:7572–6. doi: 10.1073/pnas.85.20.7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mesnil M, Piccoli C, Tiraby G, Willecke K, Yamasaki H. Bystander killing of cancer cells by herpes simplex virus thymidine kinase gene is mediated by connexins. PNAS. 1996;93:1831–5. doi: 10.1073/pnas.93.5.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Visnjic D, Kalajzic I, Gronowicz G, Aguila HL, Clark SH, Lichtler AC, Rowe DW. Conditional ablation of the osteoblast lineage in Col2.3Δtk transgenic mice. J Bone Miner Res. 2001;16:2222–31. doi: 10.1359/jbmr.2001.16.12.2222. [DOI] [PubMed] [Google Scholar]
- 37.Parfitt AM. Parathyroid hormone and periosteal bone expansion. J Bone Miner Res. 2002;17:1741–3. doi: 10.1359/jbmr.2002.17.10.1741. [DOI] [PubMed] [Google Scholar]
- 38.Dobnig H, Turner RT. Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology. 1995;136:3632–8. doi: 10.1210/endo.136.8.7628403. [DOI] [PubMed] [Google Scholar]
- 39.Nakajima A, Shimoji N, Shiomi K, Shimizu S, Moriya H, Einhorn TA, Yamazaki M. Mechanisms for the enhancement of fracture healing in rats treated with intermittent low-dose human parathyroid hormone (1–34) J Bone Miner Res. 2002;17:2038–47. doi: 10.1359/jbmr.2002.17.11.2038. [DOI] [PubMed] [Google Scholar]
- 40.Pettway GJ, Meganck JA, Koh AJ, Keller ET, Goldstein SA, McCauley LK. Parathyroid hormone mediates bone growth through the regulation of osteoblast proliferation and differentiation. Bone. 2008;42:806–18. doi: 10.1016/j.bone.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krishnan V, Moore TL, Ma YL, Helvering LM, Frolik CA, Valasek KM, Ducy P, Geiser AG. PTH bone anabolic action requires Cbfa1/Runx2-dependent signaling. Mol Endocrinol. 2003;17:423–35. doi: 10.1210/me.2002-0225. [DOI] [PubMed] [Google Scholar]
- 42.Bikle DD, Sakata T, Leary C, Elalieh H, Ginzinger D, Rosen CJ, Beamer W, Majumdar S, Halloran BP. Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. J Bone Miner Res. 2002;17:1570–8. doi: 10.1359/jbmr.2002.17.9.1570. [DOI] [PubMed] [Google Scholar]
- 43.Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O’Brien CA, Manolagas SC, Jilka RL. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146:4577–83. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- 44.Keller H, Kneissel M. SOST is a target gene for PTH in bone. Bone. 2005;37:148–58. doi: 10.1016/j.bone.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 45.Niu Q, Warmington KS, Grisanti M, Tan H, Ominsky MS, Simonet WS, Robinson M, Kostenuik PJ, Ke HZ, Paszty C, Li X. Sclerostin inhibition leads to increased periosteal and endocortical bone formation as well as decreased cortical porosity in aged ovariectomized rats. Journal of Bone and Mineral Research. 2007;22:S65. [Google Scholar]