Abstract
Muscle development involves the specification and morphogenesis of muscle fibers that attach to tendons. After attachment, muscles and tendons then function as an integrated unit to transduce force to the skeletal system and stabilize joints. The attachment site is the myotendinous junction, or MTJ, and is the primary site of force transmission. We find that attachment of fast-twitch myofibers to the MTJ correlates with the formation of novel microenvironments within the MTJ. The expression or activation of two proteins involved in anchoring the intracellular cytoskeleton to the extracellular matrix, Focal adhesion kinase (Fak) and β-dystroglycan is up-regulated. Conversely, the extracellular matrix protein Fibronectin (Fn) is down-regulated. This degradation of Fn as fast-twitch fibers attach to the MTJ results in Fn protein defining a novel microenvironment within the MTJ adjacent to slow-twitch, but not fast-twitch, muscle. Interestingly, however, Fak, laminin, Fn and β-dystroglycan concentrate at the MTJ in mutants that do not have slow-twitch fibers. Taken together, these data elucidate novel and dynamic microenvironments within the MTJ and indicate that MTJ morphogenesis is spatially and temporally complex.
Keywords: myofiber, morphogenesis, fibronectin, somite, myotendinous junction, MTJ, muscle, Hedgehog, zebrafish, tendon, laminin, Fak
Muscle specification and morphogenesis during early development are critical for normal muscle physiology throughout adult life. In vertebrates, the main source of muscle is the embryonic myotome. The myotome is comprised of skeletal muscle fibers derived from somites. Somites are segmentally reiterated structures that are delineated by the formation of somite boundaries. As development proceeds, a portion of the somite gives rise to skeletal muscle fibers that comprise the myotome. Because they are derived from somites, myotomes are also segmentally reiterated and separated by boundaries. The boundaries that separate myotomes have been called many different terms including myotendinous junction (MTJ), myoseptum, myoseptal tendon, axial tendon and myomyomal junction (Bassett and Currie, 2003; Gemballa and Vogel, 2002; Long et al., 2002; Summers and Koob, 2002). To explicitly distinguish the myotome boundary from the initial epithelial somite boundary, and because the myotome boundary gives rise to the MTJ, we will refer to the myotome boundary as the MTJ. In fishes, the MTJ is the major site of force transmission during swimming behavior (Gemballa and Vogel, 2002). As in humans, the MTJ can also react to compressive loads (Summers and Koob, 2002), thus the zebrafish tendon is functionally homologous to the mammalian tendon.
The formation of myotomes correlates with a wave of slow-twitch muscle migration during development. In zebrafish, slow- and fast-twitch muscle fibers are spatially segregated. In the adult, slow-twitch fibers are the most lateral (superficial) layer of muscle. In the early embryo, however, slow-twitch muscle cells are initially the most medial cells in a somite. Shortly after somite formation, slow-twitch muscle cells migrate laterally through the fast-twitch muscle domain to generate the most lateral layer of muscle (Devoto et al., 1996). This dynamic medial to lateral displacement of slow-twitch muscle cells is accompanied by a medial to lateral wave of fast-twitch muscle cell elongation (Cortes et al., 2003). In fact, slow-twitch muscle migration is both necessary and sufficient for timely muscle cell elongation (Henry and Amacher, 2004). Once muscle cells elongate, they attach strongly to the nascent MTJ. The molecular mechanisms that underlie both the initial attachment and homeostatic maintenance of attachment in vivo are unknown.
Complex and dynamic interactions between fiber types and various extracellular matrix (ECM) components are vital to the functional physiology of the adult fish. Contractile force generated at sarcomeres within muscle fibers is transduced outside the cell through the basement membrane, to the collagen rich connective tissue, to the tendons, and finally to the skeletal system to move the bones. Disruption of many components of this force relay results in severe muscle diseases such as the muscular dystrophies. Muscle and tendons function to mobilize the skeletal system as well as to stabilize joints. Despite the importance of muscle fiber formation and attachment to the nascent tendon, neither myofiber nor tendon morphogenesis in vertebrates are well understood.
1. Results and discussion
1.1. Fn is degraded medial to migrating slow-twitch muscle fibers, but laminin levels remain constant
Elongated muscle fibers attach to the MTJ that is derived from the initial epithelial somite boundary. We hypothesized that fiber attachment to the nascent MTJ might result in changes in the MTJ itself. We analyzed MTJ morphogenesis through time in order to test this hypothesis. We first analyzed composition of the initial epithelial somite boundary prior to slow-twitch muscle migration. Cells can adhere to the extracellular matrix (ECM) through either integrin-based focal adhesions or the dystroglycan complex (DGC), which does not contain integrins (Durbeej and Campbell, 2002; Mitra and Schlaepfer, 2006; Sgambato and Brancaccio, 2005). Two major ligands for Integrin receptors, the ECM proteins Fibronectin (Fn) and laminin, are homogenously distributed throughout the medial to lateral extent of the initial epithelial somite boundary (Fig. 1A–B). The focal adhesion proteins Paxillin (Crawford et al., 2003) and phosphorylated Fak (PY 397-Fak, Fig. 1C) also accumulate at initial epithelial somite boundaries. However, although DGC proteins are strongly concentrated at MTJs (Bassett et al., 2003; Parsons et al., 2002a), β-dystroglycan (n = 6) does not concentrate at initial somite boundaries (data not shown). These data indicate that the initial epithelial somite boundary contains focal adhesion proteins, but not DGC proteins primarily involved in muscle function.
Figure 1. The ECM proteins laminin-1, Fn and Fak concentrate at the initial epithelial somite boundary.
All panels are Leica confocal micrographs. All panels side view, anterior left, dorsal top, of 18- somite wild-type embryos. Panels A–C are superficial views and panels numbered 1 are medial views of the same Z stack. β-catenin which outlines all cells is in red, and laminin, Fn and Fak are each in green. Laminin-1 (A, A1, white arrows), Fn (B, B1, white arrows), and PY397-Fak (C, C1, white arrows) are all concentrated at the somite boundaries throughout the medial-lateral axis of the embryo.
Although Fn inhibits myoblast fusion in vitro (Ohtake et al., 2006; Podleski et al., 1979; Puri et al., 1979), roles for Fn during muscle development in vivo have not yet been elucidated. During zebrafish development, Fn is highly concentrated at somite boundaries (Crawford et al., 2003) and is critical for somite boundary formation (Julich et al., 2005; Koshida et al., 2005). After the initial epithelial somite boundary has formed, slow-twitch muscle fibers translocate laterally (Devoto et al., 1996) and trigger fast-twitch myofiber elongation (Cortes et al., 2003; Henry and Amacher, 2004). Therefore, the position of slow-twitch fibers in the medial-lateral axis can serve as a marker for fast-twitch muscle cell elongation. We find that Fn is down-regulated at the boundary medial to migrating slow-twitch muscle fibers (Fig. 2B–D, note that in the transverse view in C1 Fn (green) is highly expressed lateral to migrating slow-twitch muscle (white), but is not concentrated medial [M, to the left] to slow-twitch muscle, n = 10, see supplemental movie 1). This down-regulation of Fn medial to migrating slow-twitch fibers proceeds throughout slow-twitch muscle migration and results in the specific concentration of Fn adjacent to slow-twitch fibers at the MTJ (Fig. 2, note that Fn (green) is expressed adjacent to lateral slow-twitch fibers (white) in panels B, C and D, also see projections in panels B1 and C1 and note that Fn does not extend away from slow-twitch muscle fibers). Fn is downregulated medial to migrating slow-twitch fibers in both anterior and posterior somites (data not shown). An alternative explanation for the decrease in Fn immunoreactivity is that Fn undergoes a conformational change in which epitopes for the Fn stain are altered. However, we find this explanation unlikely because the Fn antibody used is polyclonal.
Figure 2.
The ECM composition of the myoseptal tendon changes during early muscle development. See also supplemental movies 1 and 2. Laminin persists, whereas Fn is downregulated, resulting in Fn only concentrating adjacent to slow fibers. All panels were obtained on a Zeiss ApoTome. All Z-series are taken from the transition region of the embryo in which slow-twitch muscle is proceeding laterally. F59, which denotes slow-twitch muscle, is in white.
A: Cartoon summarizing methods. Embryos were stained for slow-twitch muscle (red in cartoons, white in data panels B–G) and Fn (green) or laminin (yellow). Z-series were taken and 3-dimensionaly reconstructed. Transverse views were obtained by rotating the 3-dimensional projection 90 degrees.
B–G: Projections of 20 somite-stage embryos (somite number indicated by S#) stained with F59 to visualize slow muscle (white), Fn (green, the Fn antibody recognizes Fn1 and Fn3 in zebrafish), or laminin (yellow). Lettered panels are side views, anterior left, dorsal top, red arrows denote staining at the myoseptal tendon. Panels numbered 1 are transverse views of the corresponding lettered panel. D, G: Both Fn and laminin are observed in newly formed somites prior to slow muscle migration. Transverse reconstructions show that Fn and laminin are concentrated both medially (D1, G1, white stars, medial (M) is to the left) and laterally (red stars, lateral is to the right). C, F: In slightly older somites, Fn is downregulated medial to migrating slow muscle (C1, medial left, white star medial to the slow fibers shows very little Fn medially). Laminin distribution, however, does not change (F1, white star, laminin is robustly concentrated medially). B, E: In myotomes, where slow muscle migration is complete, Fn is concentrated adjacent to slow fibers but not fast fibers (B1, white star). Laminin, however, is observed throughout the medial-lateral extent of the myoseptal tendon (E1, white star).
H: Cartoon summarizing dynamic changes in the myoseptal tendon. Slow-twitch fibers (red) are medial in younger somites (posterior, to the right), and laminin (yellow) and Fn (green) are located throughout the medial-lateral extent of the nascent myoseptal tendon. As slow-twitch muscle migrates laterally (in older anterior somites, to the left) Fn is downregulated at the nascent myoseptal tendon medial to migrating slow-twitch fibers. Scale bar: 50μm.
The dynamic down-regulation of Fn is striking when compared to laminin-1 localization: laminin-1 concentration at the boundary does not change during fast-twitch muscle elongation (Fig. 2E–G, compare Fig. 2 E1 to B1 and note that laminin (yellow) extends medial to slow-twitch muscle fibers, see supplemental movie 2). The differential localization of Fn therefore defines two novel microenvironments within the MTJ: the lateral MTJ adjacent to mononucleate slow-twitch muscle that contains Fn, and the medial MTJ adjacent to multinucleate fast-twitch muscle that does not contain Fn. This data is the first indication of molecular complexity within the MTJ correlating with fiber type. Furthermore, degradation of Fn adjacent to fast-twitch muscle fibers that will be multinucleate suggests the hypothesis that Fn degradation may be permissive for myoblast fusion in vivo as well as in vitro.
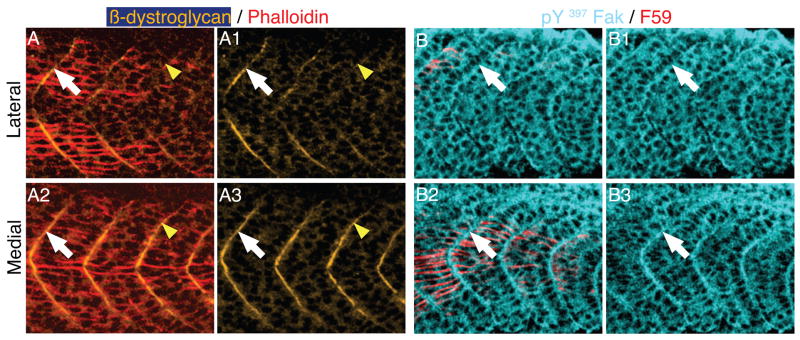
1.2 The laminin receptor β-dystroglycan as well as the integrin focal adhesion protein Fak are up-regulated medial to migrating slow-twitch fibers
We find that, in contrast to Fn, other proteins involved in adhesion to the ECM are strikingly up-regulated medial to slow-twitch muscle fibers. β-dystroglycan is a component of the Dystroglycan complex that is a receptor for laminin. β-dystroglycan concentration at the MTJ is up-regulated coincident with slow-twitch muscle migration and fast-twitch fiber elongation (Fig. 3A-A3, n = 6). Concentration of another component of the dystroglycan complex at the MTJ, dystrophin, increases shortly after fast-twitch fibers elongate (data not shown). An additional complex involved in adhesion to the ECM is the integrin-based focal adhesion complex. Activation of a cytoplasmic component of focal adhesions, focal adhesion kinase (Fak) by phosphorylation at tyrosine 397, also increases in concentration at the nascent myoseptal tendon compared to within the myotome when fast-twitch fibers elongate (Fig. 3B-B3, n = 12). Similarly, the concentration of paxillin, an additional cytoplasmic component of focal adhesions, increases shortly after fast-twitch fibers elongate (data not shown).
Figure 3.
β-Dystroglycan and pY397FAK concentrate at the myoseptal tendon medial to migrating slow muscle fibers. All panels are ApoTome images, side views, anterior left, dorsal top, of the transition region, where slow muscle is migrating, in 19–21 somite embryos. Phalloidin, denoting actin, is red in panel A and F59, denotes slow-twitch muscle in red for panel B. Panels numbered 1 and 3 show only β-Dystroglycan and pY397FAK, respectively.
A: Lateral to migrating slow fibers, β-Dystroglycan (green) does not concentrate at the boundary (A, A1, yellow arrowheads) but does concentrate slightly anteriorly where slow-twitch muscle has migrated (white arrows, A, A1). β-Dystroglycan concentrates at the boundary medial to migrating slow fibers (A2, A3, note robust concentration adjacent to yellow arrowheads where β-Dystroglycan was not concentrated laterally). B: Similarly, pY397FAK concentration at the boundary is increased medial and adjacent to slow muscle fibers (B2, B3, white arrowheads).
The dynamic changes in molecular composition of the nascent MTJ during zebrafish muscle morphogenesis are similar to dynamic changes in integrin receptor expression during mouse myogenesis (Cachaco et al., 2005). During mouse muscle development, Integrin receptors for Fn are expressed in the initial muscle masses, but Integrin receptors for laminin are expressed as myotubes form (Cachaco et al., 2005). These data suggest that molecular mechanisms of muscle morphogenesis may be conserved between teleosts and mammals. Thus, zebrafish and mouse muscle and tendon morphogenesis may be molecularly as well as functionally homologous.
1.3 Inhibition of Hedgehog signaling frequently disrupts expression of MTJ proteins
We have shown that changes in expression/activation of three MTJ proteins (Fak, β-dystroglycan and Fn) correlate with the medial-lateral displacement of slow-twitch muscle fibers. In zebrafish, slow-twitch muscle fibers are specified by Hedgehog signaling (Barresi et al., 2000; Coutelle et al., 2001; Currie and Ingham, 1996; Du et al., 1997; Roy et al., 2001; Wolff et al., 2003). We asked if Hedgehog signaling was necessary for the expression of MTJ components by analyzing their expression in slow muscle omitted (smu) mutant embryos. Smu encodes zebrafish Smoothened (Varga et al., 2001), a transmembrane receptor required for Hedgehog signaling (Ingham and McMahon, 2001). We used smu/smo mutant embryos (Chen et al., 2002; Chen et al., 2001; Varga et al., 2001) or cyclopamine treatment to test if Hedgehog signaling is required for protein localization at the MTJ. Cyclopamine is a steroidal alkaloid that binds to Smoothened and blocks transmission of the Hedgehog signal (Chen et al., 2002; Varga et al., 2001).
We have previously shown that there are sometimes large gaps between myotomes in smu/smo mutant embryos (Henry and Amacher, 2004). Perhaps not surprisingly, the expression of some MTJ components is sometimes reduced or absent at these gaps (Fig. 4, yellow arrowheads). When there are not gaps, laminin, PY-397 Fak and Fn localize to the MTJ (Fig. 4 white arrows). Localization of Fak (data not shown) and Fn (Fig. 4 C1, green arrowhead) to the MTJ in Hh signaling deficient embryos is sometimes reduced (5/17 embryos had reduced/absent Fn localization at the MTJ, 2/14 embryos had reduced/absent Fak localization at the MTJ). Although we are unable to explain the variability in the MTJ phenotype in smu- mutant embryos, these data indicate that Hedgehog signaling is not necessary for concentration of MTJ components at the MTJ. Interestingly, Fn and PY-397 Fak concentration at the MTJ changes in a medial-lateral sequence as in wild-type embryos (data not shown). Therefore, slow-twitch muscle migration is not required for changes in the protein composition of the MTJ that normally occur coincident with slow-twitch muscle migration.
Figure 4.
Slow-twitch muscle specification and subsequent migration is not required for localization of laminin, Fak and Fn at the MTJ during muscle morphogenesis, however expression is sometimes disrupted. All panels were obtained using a Zeiss ApoTome. Side views, anterior left, dorsal top of 24 hpf embryos.
A: Laminin (yellow) is highly concentrated at the MTJ of wild-type embryos (A, white arrow). In smu embryos, there are frequently gaps in the myotome boundary, and laminin often congregates at these gaps (A1, arrowheads) in addition to the MTJ (A1, arrow).
B: pY397FAK (blue) is concentrated at the MTJ of wild-type embryos (B, white arrow). In smu embryos, pY397FAK concentrates at the MTJ (B1, white arrow) but not at gaps (B1, yellow arrowheads).
C: Fn (green) is concentrated at the lateral MTJ of wild-type embryos (C, white arrow). In smu embryos, Fn concentrates at the MTJ (C1, white arrow) but is sometimes reduced/absent (C1, green arrowhead).
1.4 Conclusion
In summary, we have investigated morphogenesis of the nascent MTJ in zebrafish embryos. The MTJ is derived from the initial epithelial somite boundary and is analogous to the mammalian tendon in that it is the primary site of force transduction from muscle to the skeletal system. We show that there is dynamic modulation of microenvironments within the nascent MTJ. During zebrafish muscle development, slow-twitch fibers translocate laterally from their initial medial location (Devoto et al., 1996). Fn protein localization at the MTJ is dramatically down-regulated medial to migrating slow-twitch muscle fibers (Fig. 2B–D). In contrast, laminin levels remain constant and the concentration of two intracellular proteins involved in adhesion to the ECM is up-regulated. These data indicate that there are at least three classes of MTJ protein components during early muscle development when fast-twitch fibers attach to the MTJ: (1) proteins that are degraded (2) proteins that do not change in concentration and (3) proteins that are up-regulated at the MTJ. Taken together, our data suggest that multiple dynamic interactions between muscle fibers and the MTJ mediate normal musculoskeletal development.
Experiments performed more than 2 decades ago suggest that Fn can inhibit myotube formation in cell culture. Addition of exogenous Fn reduces fusion whereas mild trypsinization, which removes surface Fn, increases fusion (Podleski et al., 1979). Interestingly, Fn is not required for myoblast fusion in suspension culture (Puri et al., 1979). It has recently been shown that Membrane-type 1 matrix metalloproteinase (MT1-MMP) degrades Fn and MT1-MMP activity is required for myoblast fusion in C2C12 cells (Ohtake et al., 2006). Taken together, these data suggest that Fn degradation may be an important regulator of myoblast fusion. The spatial and temporal regulation of Fn protein at the nascent MTJ during muscle development in zebrafish supports this hypothesis. Fn is degraded at the MTJ adjacent to fast muscle cells that are multinucleate, but Fn is not degraded at the MTJ adjacent to mononucleate slow muscle cells (Fig. 2). This result suggests that degradation of Fn in vivo may be permissive for cell fusion.
2. Experimental Procedures
2.1. Zebrafish husbandry
Zebrafish embryos were obtained from natural spawning of adult fish kept at 28.5°C on a 16 h light/8 h dark cycle and were staged according to (Kimmel et al., 1995).
2.2. Immunocytochemistry
F59 was utilized to visualize slow-twitch muscle myosin as previously described (Crow and Stockdale, 1986; Devoto et al., 1996). Alexa Fluor 488 and 546 phalloidin were obtained from Molecular Probes. β-catenin, fibronectin, laminin, Fak, paxillin, β-dystroglycan, phalloidin and dystrophin staining were also performed as previously described (Bassett et al., 2003; Crawford et al., 2003; Henry and Amacher, 2004; Henry et al., 2001; Parsons et al., 2002b; Topczewska et al., 2001). For antibody staining, embryos were fixed in 4% paraformaldehyde (PFA) for 4 hours at room temperature and incubated in block for 1 hour. Staining was conducted in PBDT (1% BSA, 1% DMSO, 1% Triton X-100 in PBS). For visualization of actin using phalloidin staining, embryos were fixed as above, followed by permeabilized in 2% Triton X-100/PBS for 1.5 hours and incubated in 1:20 Alexa-Fluor 488 or 543 conjugated phalloidin (Invitrogen) for 1 hour. Embryos were then rinsed overnight prior to proceeding with antibody staining.
Antibodies used were: mouse monoclonal anti-slow-twitch myosin (F59) (Devoto, et al. 1996, generous gift of Frank Stockdale) 1:10, rabbit polyclonal anti-fibronectin 1+3 (LabVision) 1:100, rabbit polyclonal anti-laminin 1 (LabVision) 1:100, rabbit polyclonal anti-pY397FAK (Biosource) 1:50, mouse monoclonal anti-dystrophin clone MANDRA-1 (Sigma) 1:100, mouse monoclonal anti-β-dystroglycan (Novocastra) 1:50, mouse monoclonal anti-β-catenin (Sigma) 1:500, rabbit polyclonal anti-β-catenin (abcam) 1:500, mouse monoclonal anti-paxillin (BD Transduction Labs) 1:100, Alexa-Fluor 488, 546 and 633 conjugated goat anti-mouse and goat anti-rabbit secondary antibodies (Invitrogen) 1:200.
2.3. Imaging
Images were acquired using a Leica SP2 confocal microscope and a Zeiss ApoTome running on a Zeiss Axio Imager Z1. For confocal images, embryos were mounted in 100% glycerol and visualized using a 40x oil immersion lens. Image quality was optimized using 2–16 line averaging depending on staining quality. For ApoTome images, embryos were mounted in 80% glycerol/20% PBS with 1.5mm cover slips and visualized using a 20x objective. Image quality was optimized by averaging 4–5 frames. Staining longevity for ApoTome images was improved using SlowFade reagent (Invitrogen). Images were processed in Adobe Photoshop and collated in Adobe Illustrator.
2.4. Projection, reconstruction, and movie construction
Zeiss 4D imaging software was used to project and rotate Z-series. Images were then exported as Tiff files and processed in Adobe Photoshop. When appropriate for the supplemental movies, lettering detailing staining, etc. was added in Adobe Photoshop. The files were flattened and saved as Tiffs. Next, Image J was used to compile the individual files into a Z-stack that was then exported as a Quick-time movie.
Supplementary Material
Acknowledgments
The authors would like to thank Stephen Devoto, Robert Jones, Emma Oster, and Dorothy Croall for helpful discussions as well as Ian McNulty who did some initial pilot experiments. The authors also thank Mark Nilan and Mary Simon for fish care. This research was supported by the Muscular Dystrophy Association and also in part by NIH grant RO1 HD052934-01A1 as well as NIH grant P20 RR-016463 from the INBRE program of the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barresi MJ, et al. The zebrafish slow-muscle-omitted gene product is required for Hedgehog signal transduction and the development of slow muscle identity. Development. 2000;127:2189–99. doi: 10.1242/dev.127.10.2189. [DOI] [PubMed] [Google Scholar]
- Bassett DI, et al. Dystrophin is required for the formation of stable muscle attachments in the zebrafish embryo. Development. 2003;130:5851–60. doi: 10.1242/dev.00799. [DOI] [PubMed] [Google Scholar]
- Bassett DI, Currie PD. The zebrafish as a model for muscular dystrophy and congenital myopathy. Hum Mol Genet. 2003;12:R265–70. doi: 10.1093/hmg/ddg279. Spec No 2. [DOI] [PubMed] [Google Scholar]
- Cachaco AS, et al. Integrin repertoire on myogenic cells changes during the course of primary myogenesis in the mouse. Dev Dyn. 2005;232:1069–78. doi: 10.1002/dvdy.20280. [DOI] [PubMed] [Google Scholar]
- Chen JK, et al. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16:2743–8. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, et al. Analysis of the zebrafish smoothened mutant reveals conserved and divergent functions of hedgehog activity. Development. 2001;128:2385–96. doi: 10.1242/dev.128.12.2385. [DOI] [PubMed] [Google Scholar]
- Cortes F, et al. Cadherin-mediated differential cell adhesion controls slow muscle cell migration in the developing zebrafish myotome. Dev Cell. 2003;5:865–76. doi: 10.1016/s1534-5807(03)00362-9. [DOI] [PubMed] [Google Scholar]
- Coutelle O, et al. Hedgehog signaling is required for maintenance of myf5 and myoD expression and timely terminal differentiation in zebrafish adaxial myogenesis. Dev Biol. 2001;236:136–50. doi: 10.1006/dbio.2001.0193. [DOI] [PubMed] [Google Scholar]
- Crawford BD, et al. Activity and Distribution of Paxillin, Focal Adhesion Kinase, and Cadherin Indicate Cooperative Roles during Zebrafish Morphogenesis. Mol Biol Cell. 2003;14:3065–81. doi: 10.1091/mbc.E02-08-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow MT, Stockdale FE. Myosin expression and specialization among the earliest muscle fibers of the developing avian limb. Dev Biol. 1986;113:238–54. doi: 10.1016/0012-1606(86)90126-0. [DOI] [PubMed] [Google Scholar]
- Currie PD, Ingham PW. Induction of a specific muscle cell type by a hedgehog-like protein in zebrafish. Nature. 1996;382:452–5. doi: 10.1038/382452a0. [DOI] [PubMed] [Google Scholar]
- Devoto SH, et al. Identification of separate slow and fast muscle precursor cells in vivo, prior to somite formation. Development. 1996;122:3371–80. doi: 10.1242/dev.122.11.3371. [DOI] [PubMed] [Google Scholar]
- Du SJ, et al. Positive and negative regulation of muscle cell identity by members of the hedgehog and TGF-beta gene families. J Cell Biol. 1997;139:145–56. doi: 10.1083/jcb.139.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbeej M, Campbell KP. Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models. Curr Opin Genet Dev. 2002;12:349–61. doi: 10.1016/s0959-437x(02)00309-x. [DOI] [PubMed] [Google Scholar]
- Gemballa S, Vogel F. Spatial arrangement of white muscle fibers and myoseptal tendons in fishes. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:1013–37. doi: 10.1016/s1095-6433(02)00186-1. [DOI] [PubMed] [Google Scholar]
- Henry CA, Amacher SL. Zebrafish slow muscle cell migration induces a wave of fast muscle morphogenesis. Dev Cell. 2004;7:917–23. doi: 10.1016/j.devcel.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Henry CA, et al. Roles for zebrafish focal adhesion kinase in notochord and somite morphogenesis. Dev Biol. 2001;240:474–87. doi: 10.1006/dbio.2001.0467. [DOI] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–87. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Julich D, et al. Integrinalpha5 and delta/notch signaling have complementary spatiotemporal requirements during zebrafish somitogenesis. Dev Cell. 2005;8:575–86. doi: 10.1016/j.devcel.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, et al. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Koshida S, et al. Integrinalpha5-dependent fibronectin accumulation for maintenance of somite boundaries in zebrafish embryos. Dev Cell. 2005;8:587–98. doi: 10.1016/j.devcel.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Long JH, et al. Force transmission via axial tendons in undulating fish: a dynamic analysis. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:911–29. doi: 10.1016/s1095-6433(02)00211-8. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006 doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Ohtake Y, et al. Multifunctional roles of MT1-MMP in myofiber formation and morphostatic maintenance of skeletal muscle. J Cell Sci. 2006;119:3822–32. doi: 10.1242/jcs.03158. [DOI] [PubMed] [Google Scholar]
- Parsons MJ, et al. Removal of dystroglycan causes severe muscular dystrophy in zebrafish embryos. Development. 2002a;129:3505–12. doi: 10.1242/dev.129.14.3505. [DOI] [PubMed] [Google Scholar]
- Parsons MJ, et al. Zebrafish mutants identify an essential role for laminins in notochord formation. Development. 2002b;129:3137–46. doi: 10.1242/dev.129.13.3137. [DOI] [PubMed] [Google Scholar]
- Podleski TR, et al. Fibronectin delays the fusion of L6 myoblasts. Exp Cell Res. 1979;122:317–26. doi: 10.1016/0014-4827(79)90308-2. [DOI] [PubMed] [Google Scholar]
- Puri EC, et al. Fibronectin-independent myoblast fusion in suspension cultures. Biochem Biophys Res Commun. 1979;90:883–9. doi: 10.1016/0006-291x(79)91910-7. [DOI] [PubMed] [Google Scholar]
- Roy S, et al. The u-boot mutation identifies a Hedgehog-regulated myogenic switch for fiber-type diversification in the zebrafish embryo. Genes Dev. 2001;15:1563–76. doi: 10.1101/gad.195801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgambato A, Brancaccio A. The dystroglycan complex: from biology to cancer. J Cell Physiol. 2005;205:163–9. doi: 10.1002/jcp.20411. [DOI] [PubMed] [Google Scholar]
- Summers AP, Koob TJ. The evolution of tendon--morphology and material properties. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:1159–70. doi: 10.1016/s1095-6433(02)00241-6. [DOI] [PubMed] [Google Scholar]
- Topczewska JM, et al. The winged helix transcription factor Foxc1a is essential for somitogenesis in zebrafish. Genes Dev. 2001;15:2483–93. doi: 10.1101/gad.907401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga ZM, et al. Zebrafish smoothened functions in ventral neural tube specification and axon tract formation. Development. 2001;128:3497–509. doi: 10.1242/dev.128.18.3497. [DOI] [PubMed] [Google Scholar]
- Wolff C, et al. Multiple muscle cell identities induced by distinct levels and timing of hedgehog activity in the zebrafish embryo. Curr Biol. 2003;13:1169–81. doi: 10.1016/s0960-9822(03)00461-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.