Abstract
Background
A blunted decline in waking to sleep blood pressure (BP) is more common in African–American (AA) than European–American (EA) women. The causes of reduced BP ‘dipping’ in AA women are not known, although several factors including ethnic differences in catecholamine sensitivity have been suggested. The purpose of this study was to investigate the possible contribution of catecholamine influences on BP to ethnic differences in BP dipping in a sample of working women.
Participants and methods
Healthy female participants wore ambulatory BP monitors over the course of 1 work day and night. Urine samples for assay of epinephrine and norepinephrine were collected at work (approximately 11.00–15.00 h), home (approximately 06.00–22.00 h) and during sleep (approximately 22.00–06.00 h). Analysis of covariance was used to assess the relationships between changes in BP and the catecholamines by ethnicity.
Results
AA women (n= 51; age = 38.9 ± 8.5 years) had smaller proportional BP changes from work to sleep and home to sleep than EA women (n =110; age = 37.1 ±9.2 years). Overall, the work to sleep change in epinephrine excretion was positively associated with changes in both SBP (P <0.003) and DBP (P < 0.001); however, there was an ethnic difference in the epinephrine–BP relationship. For AA women, these associations were highly positive and significant, but for EA women, there was little correlation. Nonetheless, the analysis also revealed that overall, the work to sleep BP changes were not directly related to ethnic differences in catecholamine variation.
Conclusion
The AA–EA difference in waking–sleep BP changes (dipping) is not directly related to ethnic differences in catecholamine variation; however, AA seem to have a greater BP sensitivity to epinephrine.
Keywords: ambulatory blood pressure, blood pressure change, epinephrine
Introduction
In healthy individuals, blood pressure (BP) usually drops or ‘dips’ 10% or more from waking to sleep [1]. A decline of less than 10% (nondipping) has been associated with an increased risk of cardiovascular morbidity, particularly in women [2–4]. A growing body of evidence also indicates that African–Americans are more likely to be nondippers than European–Americans [4–9]. Why African–Americans are more likely to have smaller BP declines with sleep is not well understood, although renal sodium handling [7], psychophysiological responses to stress, in general [10], and the actions of catecholamines in particular [11] have all been suggested as mechanisms contributing to the differences. The idea that catecholamines might play a role in the ethnic differences in BP variation is supported by data suggesting that there may be ethnic differences in β-adrenergic receptor sensitivity, and particularly by data that show that African–Americans have greater receptor sensitivity than European–Americans [12–15].
Several observational studies show that under real life conditions, the level of stress-related changes in catecholamines during the day is correlated with the magnitude of BP decline with sleep [16–18]. These associations have often been related to the experience of stress during the day, particularly work (employment)-related stress [16,18]. These studies suggest that, in general, there is a relationship between diurnal catecholamine and BP variation that is possibly related to work stress. However, there are very few studies examining ethnic differences in this catecholamine–BP relationship, particularly in women. Sherwood et al. [19] recently reported that African–American men and women were not only more likely to be nondippers, but that they also had attenuated day–night catecholamine changes. The associations between the variables were not directly tested in that study, however, nor were the results evaluated independently for men and women. The purpose of this study was to investigate whether changes in catecholamines between daily waking environments to sleep contribute to ethnic differences in waking–sleep BP between African–American and European–American women.
Methods
Design and participants
To assess whether the relationship of diurnal catecholamine and BP changes differed by ethnic group, a natural experimental approach was used in which urinary catecholamines and BPs were collected and compared across three distinct daily microenvironments (work, home, and sleep) following James et al. [16]. The 161 women (110 European–American and 51 African–American) evaluated in this study were part of a larger study that was designed to examine the psychobiological effects associated with different levels of familial risk for breast cancer [20,21]. The participants ranged in age from 28 to 48 years, and were recruited through advertisements posted in the staff areas of three major medical centers in New York City. Fewer than 10% of the women, who responded to the advertisements and were found eligible, refused to participate. Women were excluded from the study if they: (i) did not speak English, (ii) had a history of HIV or cancer, (iii) were on medications other than birth control pills, and/or (iv) were participating in any other research trial that could affect the study variables. All participants signed written informed consents. Ethnicity was based on self-report. All participants worked day shifts (e.g. 09.00–17.00 h), and were studied between March 1998 and December 2000 on a typical mid-week workday (e.g. Tuesday–Thursday). Table 1 shows relevant biological and demographic characteristics of the study sample. Overall, the European–American and African–American women were quite similar in lifestyle characteristics.
Table 1.
Characteristics of the European–American and African–American women in the study samplea
| Total |
|||
|---|---|---|---|
| Characteristic | EA (n = 110) | AA (n = 51) | Significance |
| Age (years) | 37.5 ± 9.3 | 38.7 ± 7.4 | 0.416 |
| Height (m) | 1.59 ± 0.66 | 1.59 ± 0.64 | 0.686 |
| Weight (kg) | 58.6 ± 10.3 | 68.3 ± 18.2 | 0.010 |
| BMI (kg/m2) | 24.3 ± 4.7 | 28.7 ± 7.2 | 0.001 |
| Family history of breast cancer | 42% | 26% | 0.057 |
| Employment (full-time) | 85% | 90% | 0.265 |
| Occupation | |||
| Professional | 62% | 60% | 0.422 |
| Clerical | 38% | 40% | |
| Currently married | 40% | 37% | 0.265 |
| Premenopausal | 78% | 77% | 0.484 |
| Smoker | 13% | 8% | 0.239 |
| Drank caffeine on assessment day | 82% | 75% | 0.202 |
| Drank alcohol on assessment day | 12% | 6% | 0.166 |
AA, African–American; EA, European–American.
Mean ± standard deviation or percent within ethnic groups. Fisher’s exact significance and P values for t-tests.
Over half of the women in both groups reported being unmarried, having one or more children living at home, having a college education, and working in professional jobs. In addition, over 70% of the participants were premenopausal and were in the follicular phase of their cycle at the time of the study. The women who did report being menopausal were not on hormone replacement, nor were there significant differences (P<0.484) found between the two groups. Although the numbers were small, European–American women tended to smoke more and indulge in caffeine and alcohol slightly more than the African–American women on the day they were studied. Preliminary analyses, however, found no significant (P<0.05) relationship between these variables and the ethnic effects on the BP variables. It should be noted that the women in this study were recruited for a familial breast cancer risk study (see Table 1 – 42% of the European–Americans and 26% of the African–Americans had a family history of breast cancer). Preliminary analyses were therefore conducted to evaluate whether having a family history of breast cancer affected the ethnic comparisons of the BP variables. As with smoking, caffeine, and alcohol, the analysis revealed there was no effect (at P<0.05).
Procedures
At the beginning of their workday (e.g. between 08.00 and 09.00 h), participants came to the study room, completed informed consent and baseline questionnaires. The ambulatory BP monitor was then placed and calibrated and urine collection procedures explained. The specific urine collection procedures were based on those described by James et al. [16]. In brief, approximately 1–2 h after the collection of the baseline data (around 11.00 h), the women were contacted at their place of work and asked to go to the bathroom and empty their bladder. This urine specimen was not collected, but the time was noted and represented the beginning of the work urine collection period for the study. The participants were given a 3-l polyethylene bottle and a collection ‘hat’ for the toilet and were asked to collect all their urine for the next 4 h. At approximately 15.00 h the participants were again contacted at their workplace and asked to empty their bladder and dispense the urine into the polyethylene bottle. The time was noted for this final collection of the work period.
After the end of the work period, participants were given two additional 3-l polyethylene bottles and hats for urine collections at home in the evening, and for the following morning (overnight). They were instructed upon arriving home (approximately 18.00 h) to void (uncollected) and note the time. This was the start of their home period. They were instructed to collect all urine samples until bedtime (approximately 22.00 h), following the identical procedure as that at work. Finally, the participants emptied their bladder upon awakening (approximately 06.00 h) into the remaining polyethylene bottle, noting the time which marked the end of the sleep period.
The participants were instructed to return the samples the following morning to the respective research centers. The three contrasting daily environments studied were thus defined as work (approximately 11.00–15.00 h), home (approximately 18.00–22.00 h), and sleep (approximately 22.00–06.00 h). The timed urine samples were collected in 0.5 g of sodium metabisulphite (a preservative for the catecholamines). The total volume of each sample and the length of time of the collection (to the nearest minute) were recorded for each urine collection. A 5-ml aliquot was taken from each sample and frozen at −60°C before batch blind assay.
Concentrations of epinephrine and norepinephrine were determined using high-pressure liquid chromatography with electrochemical detection following the procedures noted by Brown and James [22]. For the purpose of analysis, the catecholamines were expressed as rate of excretion (ng/min) over each sampling period. These rates were calculated by multiplying the urine production rate (ml/min) by the measured concentration (ng/ml). All catecholamine assays were performed by the GCRC Core Laboratory at the Weil College of Medicine of Cornell University.
Ambulatory BP data were collected across the day using the Spacelabs model 90207 (Redmond, Washington, USA) ambulatory BP monitor [16,23,24]. Monitors were calibrated to a mercury column as they were fitted to each individual at the beginning of the study day, after baseline data were collected. The monitors were set to take pressures every 15 min from 08.00 to 22.00 h and every 30 min from 22.00 to 08.00 h the following morning using manufacturer provided software [23]. A uniform data-editing algorithm [25] was used to confirm acceptability of the record and to exclude extreme outliers. Minimal editing was required, however, with less than 0.1% of the readings excluded. BP averages at work, home, and overnight were calculated from measurements taken over the urine collection time periods, so that BPs and urinary excretion rates would correspond.
Psychosocial stress measures
The participants were instructed to keep a simple diary; recording mood (happy, sad, anxious, anger, or neutral), position (standing, sitting, and reclining), activity and location (work, home, elsewhere) to coincide with ambulatory BP readings. For this study, an index of negative moods was calculated based on the number of times anxiety, anger, or sadness were reported at each ambulatory BP measurement during the urine collection period (% of BPs with associated negative moods/total BPs taken over each microenvironmental study period= negative mood experience) following the procedure of Broege et al. [26]. This index was included as a covariate in the analysis to assess the role of mood in the BP change, as several earlier studies suggest that negative moods can increase BP[27–29].
Statistical analyses
The proportional changes from work or home to sleep in BP and catecholamines were calculated following James et al. [16]. One-way analysis of variance and independent sample t-tests was used to compare the unadjusted BPs and catecholamine excretion rates between the European –American and African–American women. Analysis of covariance models were used to evaluate the associations between diurnal BP changes and changes in catecholamines by ethnic group. In each model, ethnicity was a fixed factor, and negative mood experience and percent change in catecholamine levels were included as random covariates to evaluate their influence on the changes in average systolic and diastolic BPs (the dependent variables). In addition, because studies have shown that adipose tissue (body fat or body mass) is positively associated with catecholamine excretion [30,31] and because there was an ethnic difference in BMI (see Table 1), BMI was also used as a covariate in the models. Only main effects and two-way interactions were examined.
Results
Table 2 shows the comparisons of the unadjusted systolic and diastolic BP and the catecholamine excretion rates at home, work, and during sleep between the ethnic groups. As indicated, there were no significant differences in catecholamine excretion rates in any of the microenvironments or BPs measured at home. However, the African–American women did have significantly higher systolic BPs at work (P<0.033) and during sleep (P<0.001) than the European–American women.
Table 2.
Comparison of ambulatory blood pressures and urinary norepinephrine and epinephrine excretion measured at work, home, and during sleep between European–American (n = 110) and African–American (n = 51) women
| EA | AA | Significance | |
|---|---|---|---|
| Home | |||
| Systolic blood pressure (mmHg) | 118.7 ± 10.4 | 121.6 ± 12.3 | 0.123 |
| Diastolic blood pressure (mmHg) | 78.6 ± 9.9 | 78.5 ± 10.9 | 0.979 |
| Norepinephrine rate (ng/min) | 14.3 ± 16.4 | 15.4 ± 17.4 | 0.692 |
| Epinephrine rate (ng/min) | 3.2 ± 5.1 | 3.3 ± 4.9 | 0.844 |
| Work | |||
| Systolic blood pressure (mmHg) | 120.3 ± 10.1 | 124.2 ± 11.5 | 0.033 |
| Diastolic blood pressure (mmHg) | 81.5 ± 10.1 | 81.3 ± 29.2 | 0.935 |
| Norepinephrine rate (ng/min) | 13.7 ± 11.9 | 17.1 ± 26.4 | 0.304 |
| Epinehrine rate (ng/min) | 3.4 ± 2.0 | 4.4 ± 7.2 | 0.218 |
| Sleep | |||
| Systolic blood pressure (mmHg) | 104.6 ± 9.2 | 110.9 ± 12.9 | 0.001 |
| Diastolic blood pressure (mmHg) | 65.0 ± 9.7 | 66.8 ± 10.2 | 0.297 |
| Norepinephrine rate (ng/min) | 5.6 ± 3.7 | 6.1 ± 3.5 | 0.429 |
| Epinephrine rate (ng/min) | 0.81 ± 0.72 | 0.83 ± 0.59 | 0.858 |
AA, African–American; EA, European–American.
Comparisons of the proportional changes in BP and the catecholamines from work or home to sleep between the European–American and African–American women are presented in Table 3.
Table 3.
Comparison of the mean proportional changes (%) in blood pressure and catecholamines in European–American and African–American women from sleep to work (a) and sleep to home (b)
| EA | AA | Significance | |
|---|---|---|---|
| Sleep to work (a) | |||
| Systolic blood pressure | 12.9 ± 5.7 | 10.5 ± 8.0 | 0.035 |
| Diastolic blood pressure | 20.1 ± 7.0 | 17.7 ± 9.6 | 0.064 |
| Norepinephrine | 198 ± 271 | 214 ± 337 | 0.826 |
| Epinephrine | 532 ± 564 | 780 ± 1398 | 0.285 |
| Sleep to home (b) | |||
| Systolic blood pressure | 11.7 ± 5.6 | 8.7 ± 6.3 | 0.003 |
| Diastolic blood pressure | 17.0 ± 7.7 | 14.7 ± 8.8 | 0.070 |
| Norepinephrine | 227 ± 437 | 241 ± 482 | 0.430 |
| Epinephrine | 521 ± 1504 | 431 ± 612 | 0.793 |
AA, African–American; EA, European–American.
As indicated, African–American women had significantly lower proportional changes in systolic BPs from work to sleep (P<0.035) and from home to sleep (P<0.003). Although the ethnic differences in work to sleep and home to sleep changes in diastolic pressure were not quite statistically significant (P<0.05), they were close (P<0.064 and <0.07, respectively). No statistically significant differences or trends for differences were found for the proportional changes in epinephrine or norepinephrine between the ethnic groups.
Table 4 shows the analysis of covariance results for the work to sleep change in systolic and diastolic BP. As indicated, the change in epinephrine excretion was significantly related to the work to sleep change in both systolic (P<0.002) and diastolic (P<0.001) pressure, independent of ethnicity. However, there were significant interactions between epinephrine and ethnicity as predictors of both systolic (P<0.009) and diastolic BP (P<0.001). In addition, there was a significant independent effect of negative mood on systolic BP, such that a greater number of negative moods reported at work was associated with smaller work–sleep changes in systolic pressure (P<0.018; β= − 11.488).
Table 4.
ANCOVA results examining factors predicting proportional changes in ambulatory blood pressures from work to sleep
| Source of variation | Degrees of freedom | Mean squared error | F | Significance |
|---|---|---|---|---|
| Systolic blood pressure | ||||
| Ethnicity | 1 | 737.390 | 16.667 | 0.001 |
| BMI | 1 | 130.296 | 2.945 | 0.088 |
| Epinephrinea | 1 | 452.290 | 10.223 | 0.002 |
| Negative mood | 1 | 446.578 | 10.094 | 0.002 |
| Ethnicity × epinephrine | 1 | 310.389 | 7.016 | 0.009 |
| Ethnicity × negative mood | 1 | 253.449 | 5.729 | 0.018 |
| BMI × epinephrine | 1 | 436.280 | 9.861 | 0.002 |
| Error | 6768.982 | |||
| R | 0.158 | |||
| Diastolic blood pressure | ||||
| Ethnicity | 1 | 864.808 | 14.941 | 0.010 |
| BMI | 1 | 369.627 | 6.386 | 0.013 |
| Epinephrinea | 1 | 640.297 | 11.062 | 0.001 |
| Negative mood | 1 | 0.006 | 0.000 | 0.992 |
| Ethnicity × epinephrine | 1 | 775.654 | 13.384 | 0.010 |
| Ethnicity × negative mood | 1 | 24.397 | 0.422 | 0.517 |
| BMI × epinephrine | 1 | 648.072 | 11.197 | 0.001 |
| Error | 8855.731 | |||
| R | 0.121 | |||
ANCOVA, analysis of covariance.
Proportional change in urinary epinephrine excretion from work to sleep.
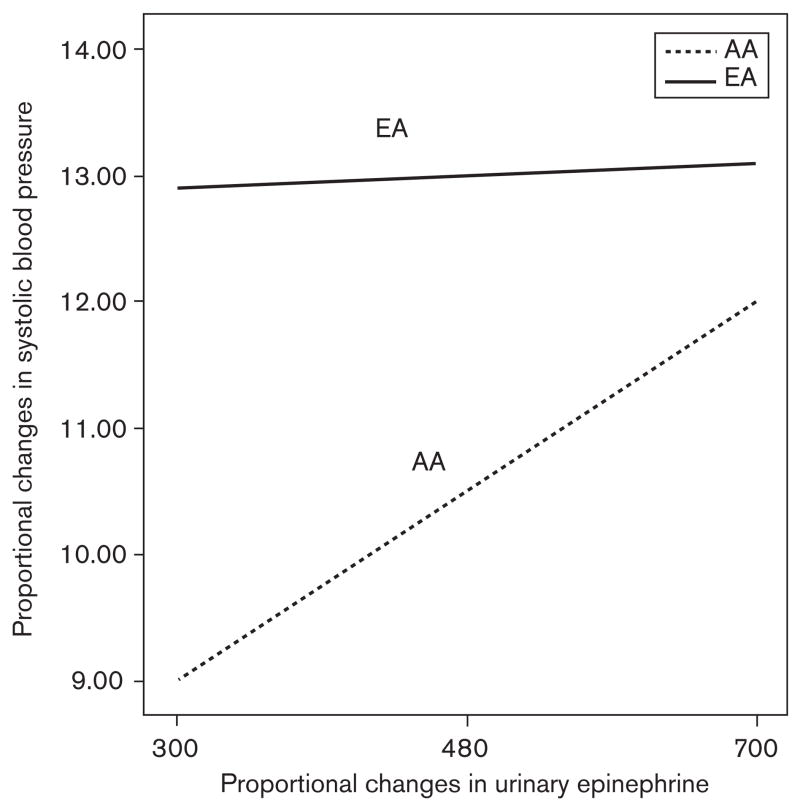
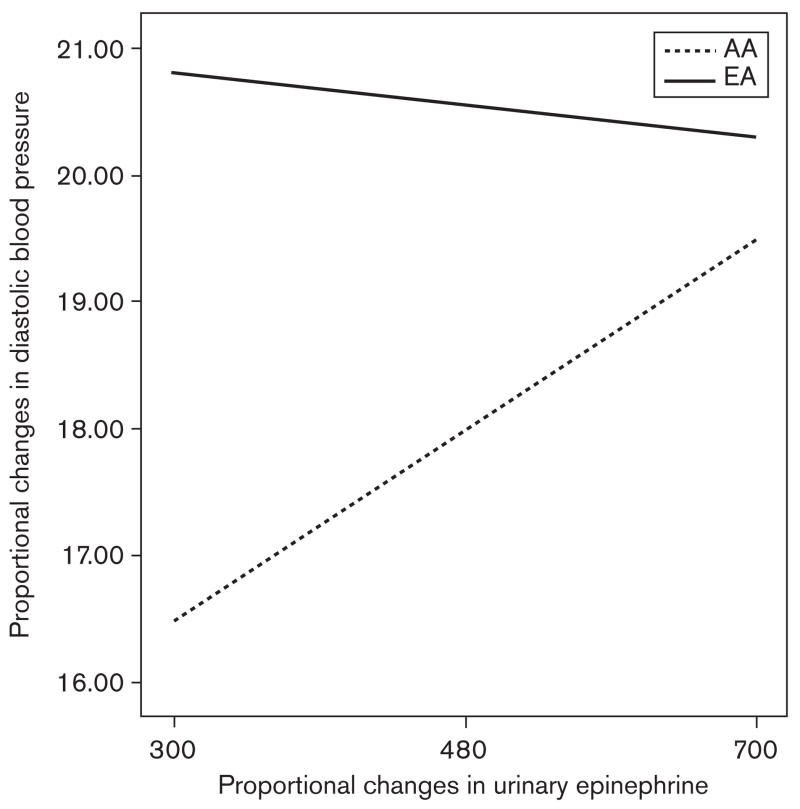
To further explore the interactive effects of epinephrine change and ethnicity on BP changes, a parameter estimates model was used to create comparative graphs (see Figs. 1 and 2). As can be seen in the figures, the work to sleep systolic BP change among European–American women varied very little with the change in urinary epinephrine excretion (had a relatively flat slope). A similar pattern with diastolic BP was seen. However, among the African–American women, there was a strong positive relationship between the proportional work– sleep changes in both systolic and diastolic BP and the proportional work–sleep change in epinephrine (i.e. had substantial positive slopes). Finally, for both the systolic and diastolic work–sleep BP changes, the levels of the European–American women are consistently greater than those of the African–American women, regardless of the proportional change in epinephrine, although when epinephrine change is very high, the ethnic difference in the proportional change in BP is less.
Fig. 1.
Ethnic differences in the relationship between the work–sleep proportional changes in urinary epinephrine excretion and systolic blood pressure in European–American (EA) and African–American (AA) women (mean centered ± 2SD).
Fig. 2.
Ethnic differences in the relationship between the work–sleep proportional changes in urinary epinephrine excretion and diastolic blood pressure in European–American (EA) and African–American (AA) women (mean centered ± 2SD).
Analyses of the effects of epinephrine change and ethnicity as predictors of the proportional home to sleep BP changes revealed no significant effects (not shown). In addition, no significant relationships were found between the proportional changes in norepinephrine excretion and the proportional BP changes for either work to sleep or home to sleep.
Discussion
The results show that the African–American women had significantly smaller proportional changes in both systolic and diastolic BP than did the European–American women from either work or home to sleep. These BP results are consistent with that of many other studies which show that African–Americans, both hypertensive and normotensive adults [4–7] as well as adolescents [6], exhibit a smaller decline in BP from waking to sleep than do individuals of European descent.
Although there were no ethnic differences in the level or changes in catecholamines, there was a strong association in the entire sample between the work–sleep changes in both systolic and diastolic pressure and changes in epinephrine excretion, consistent with previous research that shows that changes in catecholamines during the day are correlated with the magnitude of BP decline with sleep [16–18]. The results, however, also show that the ethnic difference in diurnal BP change is probably not a function of epinephrine levels, as the BP changes among the European–Americans are consistently greater than that of the African–Americans regardless of the change in epinephrine excretion (see Figs. 1 and 2). These figures, however, also indicate that the BPs of the African– Americans are likely more sensitive to the changes in epinephrine than that of the European–Americans, as the slopes of the African–American BP–epinephrine relationships are much steeper. This last finding is consistent with the literature supporting an ethnic difference in β-adrenergic receptor sensitivity and density [12–14].
Epinephrine is the most potent endogenous agonist of the β-adrenergic receptors, which are involved in modulation of cardiovascular activity [32]. Repeated elicited β-adrenergic responses, (e.g. during behavioral challenges that invoke active coping), can lead to sustained high BP[33]. Several studies have suggested that ethnic differences in the efficacy of β-blocking antihypertensive medications could be due to differences in end-organ β-adrenergic receptor sensitivity [12–14], but there are studies which do not show the effect [34,35]. Mills et al. [15] found that African–American men with a history of hypertension had more sensitive and a higher density of β-receptors than European– American men. The results of this study may also suggest a functional component for the role of epinephrine in the stimulation of β-adrenergic receptors and BP responses, particularly among the African–Americans.
Finally, the results also showed that women who experienced more frequent negative moods during work had a smaller decline in systolic pressure from work to sleep independent of the effects of changes in catecholamines. It may be speculated that greater dissatisfaction in the form of negative moods may influence the quality or quantity of sleep. In other words, if you are having a bad day at work, you may not sleep as well, and as a consequence, there is a lesser decline in sleep BP. Earlier studies suggest that poor sleep quality (leading to an increased number of awake pressures during the sleep period) can affect the proportional awake–sleep BP change [36].
Limitations of this study mandate caution in extrapolating the results to the general population. The European– American and African–American women in this study are not representative of the general population as they are selected from a limited number of occupational settings. It should also be noted that the data for this study were not collected specifically to evaluate ethnic differences in cardiovascular parameters. In addition, the analyses did not evaluate other potential causes of the ethnic difference in dipping, such as those related to ethnic differences in sodium handling [7]. Nonetheless, the findings of this study do suggest that (i) the BPs of the African–American women dip less than that of the European–American women, consistent with previous research (ii) this ethnic difference is not directly related to an ethnic difference in the diurnal change in catecholamines, although work–sleep changes in epinephrine are associated with work–sleep BP changes overall, and (iii) women of African–American descent do appear to have significantly stronger systolic and diastolic responses to epinephrine than European–American women. Further research is needed to verify these findings.
Acknowledgments
Preparation of this manuscript was partially supported by National Institutes of Health, Bethesda, MD, Grant CA 72357. Portions of the data in this study were presented at the 31st Annual Meeting of the Human Biology Association, 8–9 March 2006.
Footnotes
Potential conflicts of interest: none.
References
- 1.Dimsdale JE, von Kanel R, Profant J, Nelesen R, Ancoli-Israel S, Ziegler M. Reliability of nocturnal blood pressure dipping. Blood Press Monit. 2000;5:217–221. doi: 10.1097/00126097-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Verdecchia P, Clement D, Fagard R, Palatini P, Parati G. Blood pressure monitoring task force III: target-organ damage, morbidity and mortality. Blood Press Monit. 1999;4:303–317. doi: 10.1097/00126097-199912000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Verdecchia P, Schillaci G, Reboldi G, Franklin SS, Porcellati C. Ambulatory monitoring for prediction of cardiac and cerebral events. Blood Press Monit. 2001;6:211–215. doi: 10.1097/00126097-200108000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Wilson DK, Kliewer W, Teasley N, Plybon L, Sica DA. Violence exposure, catecholamine excretion, and blood pressure nondipping status in African American male versus female adolescents. Psychosom Med. 2002;64:906–915. doi: 10.1097/01.psy.0000024234.11538.d3. [DOI] [PubMed] [Google Scholar]
- 5.Hyman DJ, Ogbonnaya K, Taylor AA, Ho K, Pavlik VN. Ethnic differences in nocturnal blood pressure decline in treated hypertensives. Am J Hypertens. 2000;13:884–891. doi: 10.1016/s0895-7061(00)00279-x. [DOI] [PubMed] [Google Scholar]
- 6.Harshfield GA, Alpert BS, Willey ES, Somes GW, Murphy JK, Dupaul LM. Race and gender influence ambulatory blood pressure patterns of adolescents. Hypertension. 1989;14:598–603. doi: 10.1161/01.hyp.14.6.598. [DOI] [PubMed] [Google Scholar]
- 7.Harshfield GA, Hwang C, Grim CE. Circadian variation of blood pressure in blacks: influence of age, gender and activity. J Hum Hypertens. 1990;4:43–47. [PubMed] [Google Scholar]
- 8.Staessen JA, Bieniaszewski L, O’Brien E, Gosse P, Hayashi H, Imai Y, et al. Nocturnal blood pressure fall on ambulatory monitoring in a large international database. The ‘ad hoc’ working group. Hypertension. 1997;29(1 Pt 1):30–39. doi: 10.1161/01.hyp.29.1.30. [DOI] [PubMed] [Google Scholar]
- 9.James GD. Race and perceived stress independently affect the diurnal variation of blood pressure in women. Am J Hypertens. 1991;4(4 Pt 1):382–384. doi: 10.1093/ajh/4.4.382. [DOI] [PubMed] [Google Scholar]
- 10.Harshfield GA, Wilson ME, Treiber FA, Alpert BS. A comparison of ambulatory blood pressure patterns across populations. Blood Press Monit. 2002;7:265–269. doi: 10.1097/00126097-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Brown DE, James GD, Aki SL, Mills PS, Etrata MB. A comparison of awake– sleep blood pressure variation between normotensive Japanese–American and Caucasian women in Hawaii. J Hypertens. 2003;21:2045–2051. doi: 10.1097/00004872-200311000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Sharoky M, Perkal M, Turner R, Lesko LJ. Steady state relative bioavailability and pharmacokinetics of oral propranolol in black and white North Americans. Biopharm Drug Dispos. 1988;9:447–456. doi: 10.1002/bod.2510090503. [DOI] [PubMed] [Google Scholar]
- 13.Zhou HH, Silberstein DJ, Koshakji RP, Wood AJ. Interindividual differences in beta-receptor density contribute to variability in response to beta-adrenoceptor antagonists. Clin Pharmacol Ther. 1989;45:587–592. doi: 10.1038/clpt.1989.78. [DOI] [PubMed] [Google Scholar]
- 14.Rutledge DR. Are there beta-adrenergic receptor response differences between racial groups? Ann Pharmacother. 1991;25:824–834. doi: 10.1177/106002809102500719. [DOI] [PubMed] [Google Scholar]
- 15.Mills PJ, Dimsdale JE, Ziegler MG, Nelesen RA. Racial differences in epinephrine and beta 2-adrenergic receptors. Hypertension. 1995;25:88–91. doi: 10.1161/01.hyp.25.1.88. [DOI] [PubMed] [Google Scholar]
- 16.James GD, Schlussel YR, Pickering TG. The association between daily blood pressure and catecholamine variability in normotensive working women. Psychosom Med. 1993;55:55–60. doi: 10.1097/00006842-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Arita M, Minami E, Nakamura C, Ueno Y, Nishio I, Masuyama Y. Role of the sympathetic nervous system in the nocturnal fall in blood pressure. Hypertens Res. 1996;19:195–200. doi: 10.1291/hypres.19.195. [DOI] [PubMed] [Google Scholar]
- 18.James GD, Brown DE. The biological stress response and lifestyle: catecholamines and blood pressure. Annu Rev Anthropol. 1997;26:313–335. [Google Scholar]
- 19.Sherwood A, Steffen PR, Blumenthal JA, Kuhn C, Hinderliter AL. Nighttime blood pressure dipping: the role of the sympathetic nervous system. Am J Hypertens. 2002;15(2 Pt 1):111–118. doi: 10.1016/s0895-7061(01)02251-8. [DOI] [PubMed] [Google Scholar]
- 20.James GD, van Berge-Landry Hv, Valdimarsdottir H, Montgomery HB, Bovbjerg DH. Urinary catecholamine levels in daily life are elevated in women at familial risk of breast cancer. Psychoneuroendocrinology. 2004;29:831–838. doi: 10.1016/S0306-4530(03)00150-1. [DOI] [PubMed] [Google Scholar]
- 21.Dettenborn L, James GD, van Berge-Landry H, Valdimarsdottir HB, Montgomery GH, Bovbjerg DH. Heightened cortisol responses to daily stress in working women at familial risk for breast cancer. Biol Psychol. 2005;69:167–179. doi: 10.1016/j.biopsycho.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Brown DE, James GD. Physiological stress responses in Filipino American immigrant nurses: the effects of residence time, life-style, and job strain. Psychosom Med. 2000;62:394–400. doi: 10.1097/00006842-200005000-00013. [DOI] [PubMed] [Google Scholar]
- 23.James GD, Bovbjerg DH. Age and perceived stress independently influence daily blood pressure levels and variation among women employed in wage jobs. Am J Hum Biol. 2001;13:268–274. doi: 10.1002/1520-6300(200102/03)13:2<268::AID-AJHB1038>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 24.Santucci S, Cates EM, James GD, Schussel YR, Steiner D, Pickering TG. A comparison of two ambulatory blood pressure monitors, the Del Mar Avionics Pressurometer IV and the Spacelabs 90202. Am J Hypertens. 1989;2:797–799. doi: 10.1093/ajh/2.10.797. [DOI] [PubMed] [Google Scholar]
- 25.Pickering TG, Harshfield GA, Kleinert HD, Blank S, Laragh JH. Blood pressure during normal daily activities, sleep, and exercise. Comparison of values in normal and hypertensive subjects. JAMA. 1982;247:992–996. [PubMed] [Google Scholar]
- 26.Broege PA, James GD, Peters M. Anxiety coping style and daily blood pressure variation of female nurses. Blood Press Monit. 1997;2:155–159. [PubMed] [Google Scholar]
- 27.Meininger JC, Liehr P, Chan W, Smith G, Mueller WH. Developmental, gender, and ethnic group differences in moods and ambulatory blood pressure in adolescents. Ann Behav Med. 2004;28:10–19. doi: 10.1207/s15324796abm2801_3. [DOI] [PubMed] [Google Scholar]
- 28.Brown DE, James GD, Nordloh L. Comparison of factors affecting daily variation of blood pressure in Filipino-American and Caucasian nurses in Hawaii. Am J Phys Anthropol. 1998;106:373–383. doi: 10.1002/(SICI)1096-8644(199807)106:3<373::AID-AJPA9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro JP, Welker CJ, Pierce JL. An evaluation of residential treatment for sexually aggressive youth. J Child Sex Abus. 2001;10:1–21. doi: 10.1300/j070v10n01_01. [DOI] [PubMed] [Google Scholar]
- 30.James GD, Crews DE, Pearson JD. Catecholamines and stress. In: Little MA, Haas JD, editors. Human population biology: a transdiciplinary science. London: Oxford University Press; 1989. [Google Scholar]
- 31.Leonetti DL, Bergstrom RW, Shuman WP, Wahl PW, Jenner DA, Harrison GA, et al. Urinary catecholamines, plasma insulin and environmental factors in relation to body fat distribution. Int J Obes. 1991;15:345–357. [PubMed] [Google Scholar]
- 32.Fraser CM, Venter JC, Kaliner M. Autonomic abnormalities and autoantibodies to beta-adrenergic receptors. N Engl J Med. 1981;305:1165–1170. doi: 10.1056/NEJM198111123052001. [DOI] [PubMed] [Google Scholar]
- 33.Obrist PA. Cardiovascular psychophysiology: a perspective. New York: Plenum Press; 1981. [Google Scholar]
- 34.Stein CM, Lang CC, Singh I, He HB, Wood AJ. Increased vascular adrenergic vasoconstriction and decreased vasodilation in blacks. Additive mechanisms leading to enhanced vascular reactivity. Hypertension. 2000;36:945–951. doi: 10.1161/01.hyp.36.6.945. [DOI] [PubMed] [Google Scholar]
- 35.Venter JC, Fraser CM. Beta-adrenergic receptor structure, synthesis, antibodies and human disease. Bull Eur Physiopathol Respir. 1985;21:13s–18s. [PubMed] [Google Scholar]
- 36.James GD, Toledano T, Datz G, Pickering TG. Factors influencing the awake–sleep difference in ambulatory blood pressure: main effects and sex differences. J Hum Hypertens. 1995;9:821–826. [PubMed] [Google Scholar]