Abstract
Background
An altered diurnal blood pressure (BP) pattern has been linked to risk of developing heart failure (HF). We tested whether an altered diurnal BP pattern is associated with adverse outcomes (hospitalization due to HF exacerbation or death) in HF patients.
Methods and Results
One hundred eighteen HF patients were enrolled from a tertiary care HF clinic and followed for death or heart failure hospitalization for up to 4 years. 24-hour ambulatory BP was monitored. Forty patients (34%) had normal BP dipping pattern (night-day ambulatory BP ratio < 0.9), 44 (37%) had a non-dipping pattern (0.9 ≤ night-day ambulatory BP ratio < 1.0) and 34 (29%) had a reverse dipping BP pattern (night-day ambulatory BP ratio ≥ 1.0). A total of 39 patients had an adverse outcome. Adverse outcome rates were the lowest in dippers and the highest in reverse dippers (Log rank p=0.052). Predictors of adverse outcomes, selected based on log likelihood contrast, were NYHA functional class (Hazard ratio (HR) 1.96, 95% confidence interval (CI) 1.11-3.44), anemia (HR 2.50, 95% CI 1.23-5.08) and dipping status (HR 1.65, 95% CI 1.08-2.50).
Conclusions
In addition to other traditional predictors, blood pressure dipping status may be an important prognostic factor in HF.
Introduction
Blood pressure (BP) varies minute to minute1 and BP variability may be an important prognostic factor in cardiovascular disease.2,3 Although conventional office-based BP monitoring is the basis of diagnosis and treatment of hypertension, it does not consider the variations of BP throughout the day. Alternatively, 24-hour ambulatory BP monitoring can capture this variability and provides additional information about the circadian pattern of BP.2 Numerous studies in hypertension have shown that 24-hour ambulatory BP data are better predictive of adverse cardiovascular outcomes than office-based BP.4-7 The diurnal BP pattern is also an important piece of data which 24-hour ambulatory BP monitoring can provide. While there are considerable data regarding diurnal BP patterns in patients with hypertension, there is much less information on the diurnal BP pattern in patients with heart failure (HF). Recently, the reverse nighttime BP dipping pattern, in which mean nighttime BP is higher than mean daytime BP, was associated with a 2.2-fold higher incidence of HF in 951 Swedish patients.8 Regarding HF prognosis, only one study has considered 24-hour ambulatory BP data.9 This study reported that mean 24-hour systolic BP <105 mmHg was significantly associated with increased risk of death in severe HF patients, however only 38 patients were studied and the circadian pattern of BP was not evaluated. Thus, the aim of our study was to examine 24-hour ambulatory BP data in a larger group of patients, and to determine whether diurnal BP patterns are associated with prognosis in patients with symptomatic HF.
Methods
Patients and protocol
The study was a part of a Care Coordination-Home Telehealth program, which started in 2001 at the Malcom Randall Veterans Affairs Medical Center, Gainesville, Florida. Only patients who were enrolled by September 20, 2005 were included for analysis, so that there was a minimum 1 year follow-up on all patients. The study design has been previously published.10 Briefly, adult veterans with chronic HF were enrolled in the study. Inclusion criteria were symptomatic (New York Heart Association (NYHA) functional class II-IV) HF with documented left ventricular ejection fraction <40%, age greater than 18 years old, active enrollment in the primary care clinic, and new onset (within 6 months) or difficult-to-manage symptoms of HF. Patients were excluded for a documented history of medication noncompliance and for active substance abuse. The protocol was reviewed and approved by the local Institutional Review Board, and all patients gave written informed consent prior to participating in the study.
At baseline, demographic characteristics, weight, height, HF etiology, left ventricular ejection fraction, serum sodium and creatinine levels, blood hemoglobin level, NYHA functional class, and medication profile were recorded. Patients with a documented history of myocardial infarction, percutaneous transluminal coronary angioplasty, coronary artery bypass graft or > 50% diameter stenosis of any of the three major epicardial coronary arteries were classified as having ischemic HF. Other patients were classified as having nonischemic HF. Left ventricular ejection fraction was determined by 2-dimensional echocardiography. Glomerular filtration rate was estimated by a Modification of Diet in Renal Disease equation.11 Anemia was defined as blood hemoglobin level <13 g/dL in men and <12 g/dL in women.12
Ambulatory BP and heart rate were recorded over a 24-hour period during the patient's normal daily activities. The monitors (A&D Medical, Milpitas, CA, model TM-2430EG), which have been validated,13 were programmed to obtain readings at intervals of not more than 30 minutes between 8 a.m. and 10 p.m. and at intervals of not more than 60 minutes between 10 p.m. and 8 a.m. Criteria for acceptable BP and heart rate recordings included: 1) a minimum of 75% of readings were available for interpretation, and 2) BP and heart rate readings were physiologically reasonable.
Patients were followed in clinic or by telephone for up to 4 years. The primary outcome was time to death or hospitalization due to HF exacerbation, whichever came first. There were two secondary outcomes: 1) time to death and 2) time to hospitalization due to HF. Death was confirmed via medical record and/or population death registry. Hospitalization was confirmed with medical record review or patient interview. Duration of follow-up was defined as the interval from the date of enrollment to the date of the first adverse outcome, the last contact or the study closure.
Statistical analysis
The Statistical Analysis System (SAS, Version 9.1) was used for data analyses. Data are expressed as mean ± standard deviation unless indicated otherwise. Daytime was defined from 9 a.m. to 9 p.m. and nighttime was defined from 1 a.m. to 6 a.m. based on a fixed time method.14 Mean daytime and nighttime BPs and heart rates were calculated from hourly averages of each parameter during the periods above. The fixed time method, where early morning and late evening periods are excluded for 24-hour ambulatory BP data analysis, is a commonly used analysis approach due to a number of potential advantages. Specifically, this approach results in less BP variation between the old and the young, and essentially eliminates the effects of sleeping patterns, including cultural differences in sleeping patterns, on the diurnal BP pattern.14 Patients were stratified by diurnal BP pattern: Dippers were defined as patients with night to day mean ambulatory systolic blood pressure (SBP) ratio <0.9, non-dippers were defined as patients with a ratio between 0.9 and 1.0, and reverse dippers were patients with a ratio of ≥ 1.0.
Continuous variables were compared among the strata by analysis of variance and pair-wise t-test using the Bonferroni correction method. Adverse outcome rates were estimated by Kaplan-Meier analysis and were compared among the strata by log rank test. Variables with a p-value <0.1 in the univariable proportional hazard analyses were entered into the multivariable proportional hazard models. Models were selected based on log likelihood contrast. Final models were checked by the Cox-Snell residuals method. As exploratory analyses, death and hospital admission due to HF exacerbation were analyzed as secondary outcomes by Kaplan-Meier and proportional hazard analyses.
The fixed time method may introduce biases, by excluding some BP data obtained over the 24-hour period. Thus, as a sensitivity analysis, all the data collected were also analyzed by defining daytime as an interval from 6 a.m. to 11 p.m. and nighttime as an interval from 11 p.m. to 6 a.m. A p-value < 0.05 was considered statistically significant.
Results
A total of 118 patients were included for analysis. When they were stratified by nighttime BP dipping pattern, 40 (34%) were dippers, 44 patients (37%) were non-dippers and 34 (29%) were reverse dippers. Table 1 shows baseline characteristics of the study cohort stratified by nighttime BP dipping pattern. Only estimated glomerular filtration rate, percent of patients with diabetes and percent of patients treated with antihyperlipidemics were different among the strata. However, only for the estimated glomerular filtration rate was there an ordered relationship between dipping status and the parameters of interest. Over 90% of the study cohort received pharmacotherapy recommended by the consensus HF management guidelines: 91% of patients received either an angiotensin-converting enzyme inhibitor (ACEI, mostly fosinopril (56%)), an angiotensin receptor blocker (ARB) or both, and 90% of patients received a β-blocker (mostly metoprolol (84%)) at baseline. Importantly, proportions of the patients who received these drug classes were not statistically different among the strata. Also, there were 37% of patients who were prescribed diuretics more often than once a day. However, the frequency of diuretic administration was not associated with dipping status (p=0.4446 by Chi-square test). This suggests that frequency of diuretic administration may not play a role in confounding our data.
Table 1.
Baseline characteristics by nighttime BP dipping status
| Variable | N=118 | Dipper
(N=40) |
Non-dipper
(N=44) |
Reverse dipper
(N=34) |
|---|---|---|---|---|
| Age (years) | 65 ± 12.2 | 63.5 ± 13.7 | 66.8 ± 11.1 | 67.0 ± 11.7 |
| Male | 117 (99%) | 40 (100%) | 43 (98%) | 34 (100%) |
| Caucasians | 104 (88%) | 36 (90%) | 37 (84%) | 31 (91%) |
| Ischemic etiology | 87 (74%) | 32 (80%) | 29 (66%) | 26 (76%) |
| Heart failure duration > 1 year | 79 (67%) | 25 (63%) | 33 (75%) | 21 (62%) |
| NYHA functional class | ||||
| II | 41 (36%) | 14 (37%) | 17 (39%) | 10 (32%) |
| III | 58 (51%) | 17 (45%) | 24 (54%) | 17 (55%) |
| IV | 14 (13%) | 7 (18%) | 3 (7%) | 4 (13%) |
| Sodium (mmol/L) | 139 ± 3.1 | 138.7 ± 3.1 | 139.6 ± 3.4 | 140.2 ± 2.6 |
| Hemoglobin (g/dL) | 13.5 ± 1.9 | 13.4 ± 2.0 | 13.7 ± 1.8 | 13.2 ± 2.0 |
| Anemia | 49 (42%) | 18 (45%) | 16 (36%) | 15 (44%) |
| Ejection fraction | 0.25 ± 0.09 | 0.24 ± 0.10 | 0.26 ± 0.08 | 0.26 ± 0.09 |
| Body mass index (kg/m2) | 29.5 ± 5.8 | 29.1 ± 5.4 | 29.9 ± 5.6 | 29.5 ± 6.7 |
| Obesity (%) | 46.6 | 37.5 | 47.7 | 55.9 |
| Estimated GFR (ml/min/1.73 m2)* | 65.5 ± 23.5 | 71.6 ± 25.7 | 66.2 ± 20.8 | 57.3 ± 22.6 |
| Past medical history | ||||
| Myocardial infarction | 70 (59%) | 24 (60%) | 24 (55%) | 22 (65%) |
| Coronary artery disease | 77 (65%) | 25 (63%) | 29 (66%) | 23 (68%) |
| Implantable cardioverter defibrillator | 31 (26%) | 7 (18%) | 14 (32%) | 10 (29%) |
| Hypertension | 72 (61%) | 22 (55%) | 25 (57%) | 25 (74%) |
| Diabetes mellitus** | 48 (41%) | 16 (40%) | 12 (27%) | 20 (59%) |
| Atrial fibrillation | 34 (29%) | 12 (30%) | 12 (27%) | 10 (29%) |
| Chronic obstructive pulmonary disease | 33 (28%) | 13 (33%) | 9 (20%) | 11 (32%) |
| Medications | ||||
| ACEI/ARB | 108 (91%) | 35 (88%) | 42 (95%) | 31 (91%) |
| β-blocker | 106 (90%) | 34 (85%) | 39 (89%) | 33 (97%) |
| Diuretics | 104 (88%) | 34 (85%) | 40 (91%) | 30 (88%) |
| Digoxin | 57 (48%) | 18 (45%) | 22 (50%) | 17 (50%) |
| Spironolactone | 28 (24%) | 9 (23%) | 11 (25%) | 8 (24%) |
| Antiplatelets | 77 (65%) | 26 (65%) | 30 (68%) | 21 (62%) |
| Warfarin | 50 (42%) | 19 (48%) | 18 (41%) | 13 (38%) |
| Calcium antagonist | 15 (13%) | 4 (10%) | 6 (14%) | 5 (15%) |
| Antihyperlipidemics*** | 92 (78%) | 36 (90%) | 28 (64%) | 28 (82%) |
p=0.032
p=0.019
p=0.011
NYHA: New York Heart Association, GFR: Glomerular filtration rate, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, ACEI: Angiotensin-converting enzyme inhibitor, ARB: Angiotensin receptor blocker
Dipper: Night to day mean ambulatory systolic blood pressure ratio <0.9
Non-reverse dipper: 0.9 ≤ Night to day mean ambulatory systolic blood pressure ratio <1.0
Reverse dipper: Night to day mean ambulatory systolic blood pressure ratio ≥1.0
There were no differences in clinic SBP and DBP among patients with different diurnal BP patterns (Table 2). However, for data collected by 24-hour ambulatory blood pressure monitoring, all BP categories except for mean daytime DBP were significantly different among the strata. As expected, BPs tended to be lowest in dippers although daytime BPs were similar between non-dippers and reverse dippers. Thus daytime ambulatory data would not allow us to separate these two groups. There were also no differences in distribution of hypertension as a co-morbidity or in heart rate (Table 1 and Table 2).
Table 2.
Blood pressure and heart rate data according to diurnal blood pressure pattern
| Variable | Dipper | Non-dipper | Reverse dipper | p-value |
|---|---|---|---|---|
| Clinic SBP at entry | 113.1 ± 18.6 | 124.3 ± 22.5 | 120.8 ± 26.3 | 0.088 |
| Clinic DBP at entry | 66.2 ± 8.8 | 69.1 ± 12.8 | 67.8 ± 13.8 | 0.53 |
| Clinic heart rate at entry | 78.3 ± 13.9 | 77.8 ± 15.0 | 75.4 ± 13.0 | 0.66 |
| Mean 24-hr SBP | 111.3 ± 10.2 | 125.4 ± 18.1* | 128.6 ± 19.3* | <0.0001 |
| Mean daytime SBP | 116.9 ± 11.0 | 127.2 ± 18.6** | 124.9 ± 19.0 | 0.015 |
| Mean night time SBP | 97.7 ± 10.5 | 120.7 ± 17.7* | 136.0 ± 20.7*, *** | <0.0001 |
| Mean 24-hr DBP | 64.5 ± 6.2 | 69.7 ± 8.8** | 70.9 ± 9.7* | 0.002 |
| Mean daytime DBP | 67.9 ± 6.7 | 70.1 ± 8.9 | 68.9 ± 9.5 | 0.51 |
| Mean night time DBP | 57.0 ± 5.8 | 67.9 ± 9.4* | 76.0 ± 14.0*, *** | <0.0001 |
| Mean 24-hr heart rate | 70.3 ± 7.3 | 71.1 ± 9.6 | 72.1 ± 12.1 | 0.74 |
| Mean daytime heart rate | 72.7 ± 7.7 | 73.5 ± 10.0 | 73.2 ± 12.7 | 0.93 |
| Mean night time heart rate | 66.6 ± 8.2 | 67.2 ± 10.3 | 69.7 ± 12.5 | 0.41 |
SBP: Systolic blood pressure, DBP: Diastolic blood pressure, Unit of BP: mmHg, Unit of heart rate: bpm
Dipper: Night to day mean ambulatory systolic blood pressure ratio <0.9
Non-reverse dipper: 0.9 ≤ Night to day mean ambulatory systolic blood pressure ratio <1.0
Reverse dipper: Night to day mean ambulatory systolic blood pressure ratio ≥1.0
vs. dipper: p<0.01
vs. dipper: p=0.015
vs. non-dipper: p<0.01
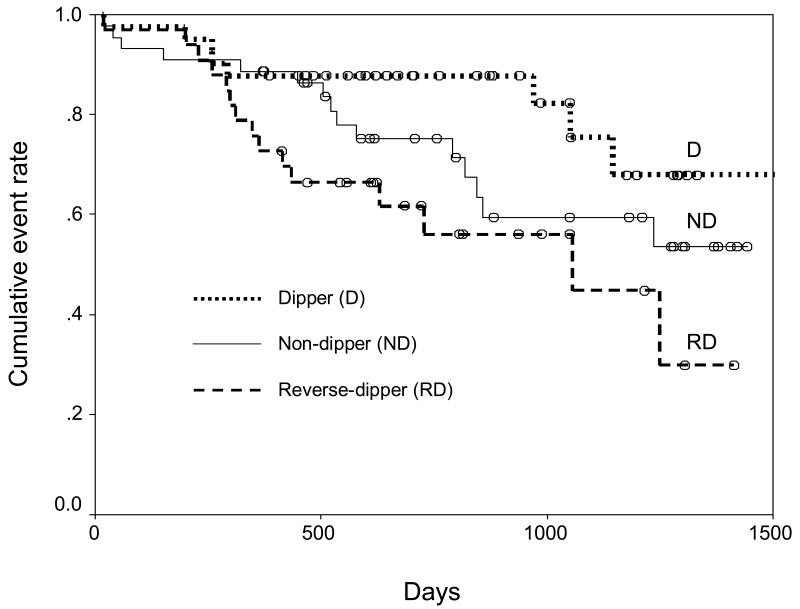
There were a total of 39 primary outcome events (deaths or hospitalizations, whichever came first) that occurred over a median 2-year follow-up (Table 3, Primary Outcomes). Kaplan-Meier analysis showed a trend suggesting that dippers had the lowest cumulative primary outcome rate and reverse dippers had the highest rate (Figure 1, log rank p=0.052). Seven variables including nighttime BP dipping status (dipper, non-dipper and reverse dipper) had a p-value <0.1 in univariable proportional hazard regression analyses (Table 4). When contrasted by -2log likelihood values among different multivariable models that included all permutations of the seven variables, a model with 3 variables was the most parsimonious one with all coefficients not statistically zero (Table 5). In this model, NYHA functional class and anemia were significantly associated with increased risk of death or hospitalization with hazard ratios (HRs) of 1.96 (95% confidence interval (CI) 1.11-3.44) and 2.50 (95% CI 1.23-5.08), respectively. In addition, HRs in non-dippers vs. dippers and reverse dippers vs. dippers were 1.65 (95% CI 1.08-2.50) and 2.72 (95% CI 2.29-3.13), respectively. This suggests that nighttime BP dipping status is a significant predictor of the primary outcome. The model appeared to fit the data well when it was verified by the Cox-Snell residuals method. Since BP readings in patients with atrial fibrillation may be inaccurate,15 and 29% of our patients had atrial fibrillation, a repeat analysis was conducted excluding the patients with atrial fibrillation. Our findings remained valid in this reanalysis, where we noted that NYHA functional class (HR 2.25, 95% CI 1.02-4.94) and dipping status (HR 2.14, 95% CI 1.21-3.77) remained significantly associated with the primary outcomes.
Table 3.
Outcomes and follow-up
| Outcome | Number | Median time to event (days) |
|---|---|---|
| Primary outcomes | 39 | 414 |
| Death | 15 | 630 |
| Hospitalization | 24 | 305 |
|
| ||
| Secondary outcomes | ||
| Death | 25 | 448 |
| Hospitalization | 24 | 305 |
|
| ||
| Overall follow up | 761 | |
Figure 1.
Cumulative adverse outcome rate among the strata (log rank p=0.052). Dipper = Night to day mean ambulatory systolic blood pressure ratio <0.9, Non-dipper = 0.9 ≤ Night to day mean ambulatory systolic blood pressure ratio <1.0, Reverse dipper = Night to day mean ambulatory systolic blood pressure ratio ≥1.0.
Table 4.
Variables with p-value <0.1 in univariable proportional hazard regression analyses
| Variables | HR | 95% CI | p-value |
|---|---|---|---|
| Anemia | 3.32 | 1.70 - 6.45 | 0.0004 |
| NYHA functional class | 2.36 | 1.42 - 3.92 | 0.0009 |
| Dipping status | 1.66 | 1.09 - 2.53 | 0.0173 |
| Age | 1.04 | 1.01 - 1.07 | 0.0205 |
| Estimated GFR | 0.98 | 0.97 - 0.99 | 0.0209 |
| HF duration | 2.13 | 0.94 - 4.84 | 0.0706 |
| Etiology | 2.14 | 0.89 - 5.13 | 0.0889 |
HR: Hazard ratio, CI: Confidence interval, NYHA: New York Heart Association, GFR: Glomerular filtration rate, HF: Heart failure
Anemia is defined as blood hemoglobin level <13 g/L in men and <12 g/L in women.
Codings were as follows:
Anemia: 0 (non-anemic) and 1 (anemic)
Dipping status: 0 (dipper), 1 (non-dipper) and 2 (reverse dipper)
HF duration: 0 (<1 year) and 1 (≥ 1 year).
Etiology: 0 (non-ischemic) and 1 (ischemic).
Table 5.
Multivariable models of proportional hazard regression
| Variable | HR | 95% CI | p-value |
|---|---|---|---|
| NYHA functional class | 1.96 | 1.11 - 3.44 | 0.020 |
| Anemia | 2.50 | 1.23 - 5.08 | 0.011 |
| Dipping status | 1.65 | 1.08 - 2.50 | 0.020 |
HR: Hazard ratio, CI: Confidence interval, NYHA: New York Heart Association
Codings were as follows:
Anemia: 0 (non-anemic) and 1 (anemic)
Dipping status: 0 (dipper), 1 (non-dipper) and 2 (reverse dipper)
In the secondary analysis, there were 25 deaths and 24 patients with hospitalizations. Ten patients experienced both events, with hospitalization as their first event, followed by death (Table 3; Secondary Outcomes). Secondary outcome analyses showed that the cumulative death rate differed significantly by strata (log rank p=0.04), with the lowest death rate in dippers and the highest in reverse dippers (12.5% in dippers vs. 20.5% in non-dippers vs. 32.3% in reverse dippers). In addition, dipping status was significantly associated with death rate (HR 1.90, 95% CI 1.13-3.20) after adjustment for NYHA functional class (HR 2.15, 95% CI 1.05-4.39) and anemia (HR 2.71, 95% CI 1.13-6.50). However, the cumulative hospitalization rate was not associated with nighttime BP dipping status. Sensitivity analysis showed that when all the ambulatory blood pressure data collected over 24 hours were utilized, dipping status was of borderline statistical significance in death or hospitalization (p=0.06). In this analysis, the HRs of each of the three variables (NYHA functional class, anemia and dipping status) were 2.02 (95% CI 1.16-3.54), 2.42 (95% CI 1.20-4.86) and 1.60 (95% CI 0.98-2.62), respectively, which was consistent with the results from the fixed time method.
Discussion
To our knowledge, this is the largest study to date of diurnal BP patterns in patients with established severe chronic heart failure. Additionally, ours is the first report to identify that the diurnal BP pattern, measured by 24-hour ambulatory BP monitoring, is an independent prognostic factor in symptomatic HF patients. Specifically, non-dippers and reverse dippers had 1.65-fold and 2.72-fold higher risk of death or hospitalization due to HF exacerbation than dippers, after adjustment for several known prognostic factors. The presence of NYHA functional class and anemia in our model as significant prognostic factors supports the validity of the model since NYHA functional class and anemia have been identified as prognostic factors for HF in other studies.16-18 In addition, since dipping status was not associated with NYHA functional class (Fisher's exact test p=0.5735), dipping status may be a prognostic factor independent of NYHA functional class. Our sensitivity analysis results also support the validity of the model.
Other studies addressing prognostic factors in heart failure have found additional clinical variables that are commonly associated with poor prognosis. However, these prognostic factors are not identical across studies, with left ventricular ejection fraction being an example of a variable that is positively associated with prognosis in some studies19,20 but not others.21,22 This is likely explained by a number of factors, including the population sample size, the severity of HF in the population, and the clinical variables that are considered, among others. Thus, the fact that our analysis did not reveal LVEF or renal function to be significantly associated with prognosis, does not invalidate our model, since significant prognostic factors are often different across studies.
We did not see an association between adverse outcomes and the use of β-blockers or ACE inhibitors in our study, which is likely due to the fact that over 90% of our study cohort received these drug classes at baseline and the drug therapy was maintained throughout the study. In addition, this suggests that diurnal BP pattern may be an important prognostic factor in HF patients even among those treated with appropriate pharmacotherapy as recommended by HF management guidelines.23 This also implies that HF patients without a nighttime BP dipping pattern may require more diligent monitoring and/or additional therapy.
Although the results of our secondary outcomes should be viewed as exploratory, it is interesting that we saw a significant association of nighttime BP dipping pattern with cumulative death rate while there was no association with cumulative hospitalization rate. This may be explained in part by the intensive monitoring and follow-up that patients receive through the Telehome care program, with benefits including increased patient adherence to treatment, thereby reducing the total number of inpatient hospital days.10 On the other hand, this intensive monitoring program would not be expected to be able to have the same influence on preventing death as preventing hospitalizations.24 The physiological basis of our findings is not currently clear. However, the non-dipping BP pattern during the night has been associated with an elevated level of sympathetic activity25 and this elevated sympathetic activity plays a key role in HF progression.26 In our study, heart rates were not different for any period (Table 2), nor was the median β-blocker dose at baseline different among the patients with different diurnal BP pattern (median: 50 mg/day of metoprolol equivalent dose in all strata, p=0.32 by the Kruskal-Wallis test). These data suggest that the patients in each stratum had a similar degree of β-blockade with similar β-blocker doses. Therefore, it seems that subtle alterations in the sympathetic nervous system in the non-dippers rather than generalized elevation in sympathetic activity per se may have contributed to the outcome differences in our study. In fact, there are data that non-dippers may have higher sensitivity of vascular α1-adrenergic receptors than dippers, even with similar degrees of β-adrenergic receptor responsiveness.25 Given that 84% of our population received metoprolol, which has no α1-adrenergic receptor blocking effects, the dipping status differences may have been larger than would be observed with carvedilol, which blocks α1-adrenergic receptors.
Abnormal activity of the parasympathetic nervous system has also been noted to be an important prognostic factor in heart failure, reflected by decreased heart rate variability.27,28 Since 24 hour ambulatory electrocardiographic data are needed for thorough assessment of heart rate variability, and such data are not available in this study, we cannot exclude that the findings regarding BP dipping status and outcomes are somehow related to heart rate variability.
Perhaps the most likely explanation for our findings is that the differences in nighttime BP are markers for obstructive sleep apnea,29 which consequently contributed to the adverse outcomes.30 In our study, 6 patients (5%) had a diagnosis of obstructive sleep apnea at baseline. Because our study population is almost exclusively male, among whom the prevalence of sleep apnea in HF (11 to 37%)31,32 is higher than our reported range, sleep apnea was probably under-diagnosed in our study population. Recently, it has been found that mortality is significantly higher in chronic HF patients with sleep apnea compared to patients without sleep apnea.33 Therefore, further research is needed to evaluate the link between BP dipping patterns in HF, sleep apnea and adverse outcomes.
Our study supports the importance of measuring 24-hour ambulatory BPs (Table 2). Clinic SBP and DBP were not statistically different among the strata. However, almost all BP data by 24-hour ambulatory BP monitoring were statistically different among the strata, and reverse dippers had significantly higher mean nighttime SBP and DBP than dippers and non-dippers. If BP had been taken only in the clinic, these differences could not have been appreciated. Therefore, our data provide evidence that BPs obtained by 24-hour ambulatory BP may be more predictive of adverse cardiovascular outcomes than office-based BP in HF, just as is the case in hypertension.6,7 In addition, it is important to note that the diurnal BP patterns noted in these HF patients were different from those previously reported in patients with hypertension. Specifically, evidence of a reverse dipper pattern is scant in the hypertension literature. Further, only 34% of HF patients in our study were classified as dippers while numerous studies suggest that 60-70% of hypertension patients are dippers.3,34,35 What is consistent between the hypertension literature and this study of HF patients is that those with the dipper phenotype have the best outcomes. Further studies are needed to elucidate why the rate of nondipping and reverse dipping is so much higher in HF than in hypertension.
A small sample size is one of the limitations of our study. Because of its nature as a non-experimental study, unmeasured biases might also have been introduced. The fact that our ambulatory BP monitor has not been validated in patients with atrial fibrillation is a limitation of the study. However, some data suggest that 24-hour ambulatory BP monitoring can be successfully used in patients with atrial fibrillation,36,37 and a reanalysis of the data with exclusion of atrial fibrillation patients supports our primary conclusions. In addition, the patients may have received greater than usual care because they were closely followed by cardiology nurses under the Care Coordination-Home Telehealth program, which might have differentially affected adverse outcomes among the strata. Since our study cohort is almost entirely comprised of males, our data may not be applicable to female HF patients given that women have better survival rates than males with heart failure.38 Finally, we do not have multiple determinations of the 24-hour ambulatory blood pressure in any of our patients, which may be a limitation of our study.
If our findings could be replicated in a larger cohort, this would suggest that 24-hour ambulatory BP monitoring should be routine in patients with symptomatic systolic HF, in order to identify those at high risk of adverse cardiovascular outcomes. Further, if replicated, our results would suggest that future studies may also need to focus on the high risk group of non-dippers and reverse dippers to identify therapies that might improve their outcomes.
Acknowledgments
We thank Vladimir Ortiz, RN and Eric Marshall for their assistance with patient recruitment.
This work was supported by NIH grant HL68834, Bethesda, MD, American Heart Association postdoctoral fellowship grant 0525474B, St. Petersburg, FL, and Department of Veterans Affairs/North Florida/South Georgia Veterans Health System University of Florida.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Staessen J, Fagard R, Lijnen P, Thijs L, van Hoof R, Amery A. Ambulatory blood pressure monitoring in clinical trials. J Hypertens Suppl. 1991;9:S13–9. [PubMed] [Google Scholar]
- 2.Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med. 2006;354:2368–74. doi: 10.1056/NEJMra060433. [DOI] [PubMed] [Google Scholar]
- 3.Khattar RS, Swales JD, Banfield A, Dore C, Senior R, Lahiri A. Prediction of coronary and cerebrovascular morbidity and mortality by direct continuous ambulatory blood pressure monitoring in essential hypertension. Circulation. 1999;100:1071–6. doi: 10.1161/01.cir.100.10.1071. [DOI] [PubMed] [Google Scholar]
- 4.Bjorklund K, Lind L, Zethelius B, Berglund L, Lithell H. Prognostic significance of 24-h ambulatory blood pressure characteristics for cardiovascular morbidity in a population of elderly men. J Hypertens. 2004;22:1691–7. doi: 10.1097/00004872-200409000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Clement DL, De Buyzere ML, De Bacquer DA, de Leeuw PW, Duprez DA, Fagard RH, et al. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348:2407–15. doi: 10.1056/NEJMoa022273. [DOI] [PubMed] [Google Scholar]
- 6.Staessen JA, Thijs L, Fagard R, O'Brien ET, Clement D, de Leeuw PW, et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators. JAMA. 1999;282:539–46. doi: 10.1001/jama.282.6.539. [DOI] [PubMed] [Google Scholar]
- 7.Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46:156–61. doi: 10.1161/01.HYP.0000170138.56903.7a. [DOI] [PubMed] [Google Scholar]
- 8.Ingelsson E, Bjorklund-Bodegard K, Lind L, Arnlov J, Sundstrom J. Diurnal blood pressure pattern and risk of congestive heart failure. JAMA. 2006;295:2859–66. doi: 10.1001/jama.295.24.2859. [DOI] [PubMed] [Google Scholar]
- 9.Canesin MF, Giorgi D, Oliveira MT, Jr, Wajngarten M, Mansur AJ, Ramires JA, et al. Ambulatory blood pressure monitoring of patients with heart failure. A new prognosis marker. Arq Bras Cardiol. 2002;78:83–9. doi: 10.1590/s0066-782x2002000100007. [DOI] [PubMed] [Google Scholar]
- 10.Schofield RS, Kline SE, Schmalfuss CM, Carver HM, Aranda JM, Jr, Pauly DF, et al. Early outcomes of a care coordination-enhanced telehome care program for elderly veterans with chronic heart failure. Telemed J E Health. 2005;11:20–7. doi: 10.1089/tmj.2005.11.20. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 12.Organisation WH. Nutritional anemia: report of a WHO scientific group. WHO Techinical Report Series. 1968 [Google Scholar]
- 13.Palatini P, Frigo G, Bertolo O, Roman E, Da Corta R, Winnicki M. Validation of the A&D TM-2430 device for ambulatory blood pressure monitoring and evaluation of performance according to subjects' characteristics. Blood Press Monit. 1998;3:255–60. [PubMed] [Google Scholar]
- 14.O'Brien E, Asmar R, Beilin L, Imai Y, Mallion JM, Mancia G, et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21:821–48. doi: 10.1097/00004872-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals, Part I: Blood pressure measurement in humans, a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 16.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–33. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 17.Anand I, McMurray JJ, Whitmore J, Warren M, Pham A, McCamish MA, et al. Anemia and its relationship to clinical outcome in heart failure. Circulation. 2004;110:149–54. doi: 10.1161/01.CIR.0000134279.79571.73. [DOI] [PubMed] [Google Scholar]
- 18.O'Meara E, Clayton T, McEntegart MB, McMurray JJ, Lang CC, Roger SD, et al. Clinical correlates and consequences of anemia in a broad spectrum of patients with heart failure: results of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Circulation. 2006;113:986–94. doi: 10.1161/CIRCULATIONAHA.105.582577. [DOI] [PubMed] [Google Scholar]
- 19.Berger R, Huelsman M, Strecker K, Bojic A, Moser P, Stanek B, et al. B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation. 2002;105:2392–7. doi: 10.1161/01.cir.0000016642.15031.34. [DOI] [PubMed] [Google Scholar]
- 20.Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35:681–9. doi: 10.1016/s0735-1097(99)00608-7. [DOI] [PubMed] [Google Scholar]
- 21.Bettencourt P, Ferreira A, Dias P, Pimenta J, Frioes F, Martins L, et al. Predictors of prognosis in patients with stable mild to moderate heart failure. J Card Fail. 2000;6:306–13. doi: 10.1054/jcaf.2000.20558. [DOI] [PubMed] [Google Scholar]
- 22.Maeda K, Tsutamoto T, Wada A, Mabuchi N, Hayashi M, Tsutsui T, et al. High levels of plasma brain natriuretic peptide and interleukin-6 after optimized treatment for heart failure are independent risk factors for morbidity and mortality in patients with congestive heart failure. J Am Coll Cardiol. 2000;36:1587–93. doi: 10.1016/s0735-1097(00)00912-8. [DOI] [PubMed] [Google Scholar]
- 23.Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2001;38:2101–13. doi: 10.1016/s0735-1097(01)01683-7. [DOI] [PubMed] [Google Scholar]
- 24.Lane RE, Cowie MR, Chow AW. Prediction and prevention of sudden cardiac death in heart failure. Heart. 2005;91:674–80. doi: 10.1136/hrt.2003.025254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherwood A, Steffen PR, Blumenthal JA, Kuhn C, Hinderliter AL. Nighttime blood pressure dipping: the role of the sympathetic nervous system. Am J Hypertens. 2002;15:111–8. doi: 10.1016/s0895-7061(01)02251-8. [DOI] [PubMed] [Google Scholar]
- 26.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–18. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 27.Nolan J, Flapan AD, Capewell S, MacDonald TM, Neilson JM, Ewing DJ. Decreased cardiac parasympathetic activity in chronic heart failure and its relation to left ventricular function. Br Heart J. 1992;67:482–85. doi: 10.1136/hrt.67.6.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giles Thomas D, Roffidal Louise, Quiroz Antonio, et al. Circadian Variation in Blood Pressure and Heart Rate in Nonhypertensive Congestive Heart Failure. J Cardiovascular Pharmacol. 1996;28:733–40. doi: 10.1097/00005344-199612000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki M, Guilleminault C, Otsuka K, Shiomi T. Blood pressure “dipping” and “non-dipping” in obstructive sleep apnea syndrome patients. Sleep. 1996;19:382–7. doi: 10.1093/sleep/19.5.382. [DOI] [PubMed] [Google Scholar]
- 30.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 31.Javaheri S, Parker TJ, Wexler L, Michaels SE, Stanberry E, Nishyama H, et al. Occult sleep-disordered breathing in stable congestive heart failure. Ann Intern Med. 1995;122:487–92. doi: 10.7326/0003-4819-122-7-199504010-00002. [DOI] [PubMed] [Google Scholar]
- 32.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–6. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Parker JD, Newton GE, Floras JS, Mak S, Chiu KL, Ruttanaumpawan P, Tomlinson G, Bradley TD. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49:1625–31. doi: 10.1016/j.jacc.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 34.Verdecchia P, Porcellati M, Schillaci G, Borgioni C, Ciucci A, Battistelli M, et al. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793–801. doi: 10.1161/01.hyp.24.6.793. [DOI] [PubMed] [Google Scholar]
- 35.Pierdomenico SD, Bucci A, Costantini F, Lapenna D, Cuccurullo F, Mezzetti A. Circadian Blood Pressure Changes and Myocardial Ischemia in Hypertensive Patients With Coronary Artery Disease. J Am Coll Cardiol. 1998;31:1627–34. doi: 10.1016/s0735-1097(98)00163-6. [DOI] [PubMed] [Google Scholar]
- 36.Olsen R, Amlie A, Omvik P. Twenty-four hour ambulatory blood pressure monitoring in atrial fibrillation. Blood Press Monit. 2002;7:149–56. doi: 10.1097/00126097-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Lip GY, Zarifis J, Beevers M, Beevers DG. Ambulatory blood pressure monitoring in atrial fibrillation. Am J Cardiol. 1996;78:350–3. doi: 10.1016/s0002-9149(96)00293-7. [DOI] [PubMed] [Google Scholar]
- 38.Mehta PA, Cowie MR. Gender and heart failure: a population perspective. Heart. 2006;92 3:iii14–8. doi: 10.1136/hrt.2005.070342. [DOI] [PMC free article] [PubMed] [Google Scholar]