Abstract
Insulin-like growth factor (IGF)-II is a hormone with mitogenic activity for many cell types and tissues. We demonstrate that its intracellular processing and secretion strictly depend on the endoplasmic reticulum chaperone glucose-regulated protein (GRP) 94. GRP94 interacts physically and transiently with pro-IGF-II intermediates, and its activity is essential for secretion of active IGF-II, thus establishing IGF-II as a client of GRP94. Embryonic stem (ES) cells that lack GRP94 are hypersensitive to stress conditions such as serum deprivation and die by apoptosis because they cannot respond to the stress by producing active IGF-II. This chaperone–client interaction may explain the previously documented antiapoptotic activity of GRP94 in a number of stress responses.
INTRODUCTION
Glucose-regulated protein 94 (GRP94) is a stress protein that is one of the most abundant constituents of the endoplasmic reticulum (ER; Argon and Simen, 1999). GRP94 expression is up-regulated when the cellular glucose tension drops (Lee et al., 1983) or in response to other metabolic stresses, such as hypoxia (Little et al., 1994; Paris et al., 2005) or excessive levels of reducing agents (Kim et al., 1987). GRP94 is also a major calcium-binding protein of the ER (Koch et al., 1986) and is involved in the response to perturbation of calcium homeostasis (Drummond et al., 1987; Biswas et al., 2007). Furthermore, GRP94 was recently linked to disposal of misfolded proteins by the ER-associated degradation pathway (Christianson et al., 2008).
As a stress protein, GRP94 has been repeatedly ascribed an antiapoptotic activity in a variety of cells. Its expression was correlated with resistance to thapsigargin-induced cell death (Liu et al., 1997; McCormick et al., 1997; Reddy et al., 1999) and with resistance to cytotoxic T-cell killing (Sugawara et al., 1993). GRP94 was shown to be cyto-protective when apoptosis of hematopoietic cells was induced by etoposide, and a fraction of the chaperone was reported to undergo Ca2+ regulated cleavage by calpain (Reddy et al., 1999).
Another study showed that GRP94 is important for protecting neurons against ischemic injury (Bando et al., 2003); overexpression of GRP94 protected neurons, whereas anti-sense RNA increased their susceptibility to stress (Bando et al., 2004). All these studies relate either the level of GRP94 expression or its activity status in the cell to cellular processes leading to apoptosis. However, a molecular mechanism that explains how GRP94 could prevent programmed cell death has not emerged. In particular, no previous study related the antiapoptotic property of GRP94 to its biochemical activity as a molecular chaperone.
Even though GRP94 is ubiquitously expressed and is a major constituent of the ER (Gilchrist et al., 2006), only very few proteins have so far been shown to be clients of GRP94 that require its activity for folding. This short list includes immunoglobulins (Melnick et al., 1994), some integrins (Randow and Seed, 2001), some Toll-like receptors (Yang et al., 2007), and plant CLAVATA proteins (Ishiguro et al., 2002). The cell-specific expression of these GRP94-dependent proteins does not account for the level of GRP94 expression or for its ubiquitousness. We previously showed that GRP94-deficient embryonic stem (ES) cells cannot differentiate into muscle cells and that this developmental block can be bypassed when the ES cells are supplied with exogenous insulin-like growth factor (IGF)-II (Wanderling et al., 2007). In the present study we demonstrate that IGF-II interacts with GRP94 directly during its biosynthesis and that the properties of the interaction satisfy the expectations for a chaperone–client pair. We further show that the interaction between GRP94 and IGF-II is necessary to protect cells from death induced by withdrawal of growth factors. Without the interaction, mature IGF-II is not secreted and the required IGF signaling does not occur. We propose that the antiapoptotic activity of GRP94 stems from its essential role in the biosynthesis of IGFs.
MATERIALS AND METHODS
Materials
Insulin, basic fibroblast growth factor, estradiol, suramin, and bis-benzimide (Hoechst No. 33258) were purchased from Sigma Chemicals (St. Louis, MO). FuGENE 6 transfection reagent was purchased from Roche (Nutley, NJ), Lipofectamine 2000 was from Invitrogen (Carlsbad, CA) and Amplify reagent from Amersham (Rockford, IL). Recombinant IGF-I was from R&D Systems (Minneapolis, MN), and recombinant IGF-II was purchased from GroPep (Adelaide, SA, Australia).
Antibodies
Anti-Flag M2 mAb (F-3165) was purchased from Sigma; biotinylated anti-mouse IGF-II (BAF792), monoclonal anti-IGF-II (MAB792), and polyclonal anti-mouse IGF-II (AF792) were from R&D Systems; and antibodies against cleaved caspase 3 (FITC-conjugated; no. 9667 and 9661) were from Cell Signaling (Beverly, MA). The anti-GRP94 mAb 9G10 (SPA-850) from Stressgen Biotechnologies (Victoria, BC, Canada), recognizes an epitope located in the charged linker of GRP94 and is a conformation-sensitive antibody (Vogen et al., 2002).
Plasmids
The murine GRP94 cDNA was PCR-amplified and cloned under the control of the EF1α promoter in the vector pBUDCE4 vector (Invitrogen). Pro-IGF-II-Flag expression plasmid (Duguay et al., 1998) was a generous gift of Dr. Duguay (University of Chicago).
Targeted Disruption of the Murine GRP94 Gene
The GRP94 gene was disrupted by homologous recombination of a targeting construct in which the sequence between exons 1 and 4 was replaced by the neomycin resistance gene. The targeting construct would allow expression of at most the first 63 amino acids of the protein, which do not contain any of its known active sites. In the resulting homozygous progeny, which are embryonic lethal, there is no detectable expression of GRP94 protein (Wanderling et al., 2007).
Cells
grp94−/− and +/+ cells and ES cells (clones 14.1 and 42.1, respectively) were established from the same litter of blastocysts, flushed from an F4 backcrossed female, as described in Wanderling et al. (2007). Cells that grew out of the blastocyst inner cell mass were cloned and genotyped by PCR, and stable clones were maintained by growth on a feeder layer of mouse embryonic fibroblasts. The two ES clones differ in their responses to a number, but not to all forms of stress. For example, the grp94−/− cells grow at the same rate as grp94+/− and +/+ cells in high-, medium-, and low-glucose media (Wanderling et al., 2007). However, the ES grp94−/− cells are hypersensitive to calcium perturbation (Biswas et al., 2007). As shown in Figure 5D, the absence of GRP94 is not accompanied by compensatory increases in other ER chaperones, such as BiP (binding immunoglobulin protein) and calreticulin. Heterozygous mice and cells are indistinguishable from wild-type (WT) cells in all the physiological and biochemical assays that we used (Wanderling et al., 2007).
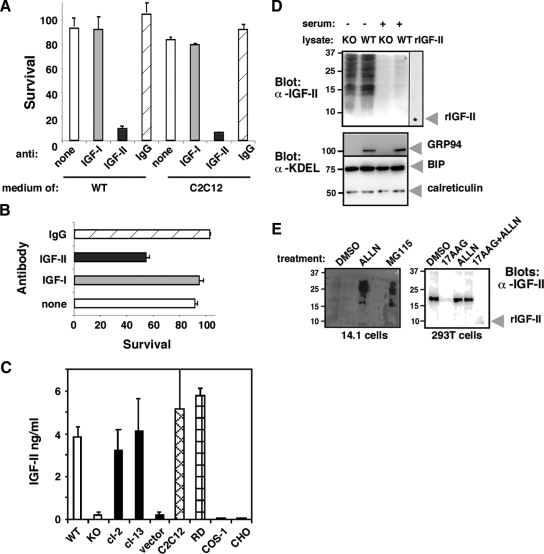
Figure 5.
The cytoprotective activity is due to IGF-II. (A) grp94−/− cells were grown for 24 h in conditioned media from either wild-type ES cells or from C2C12 cells that had previously been subjected to immunodepletion. The conditioned media were incubated with antibody (10 μg/ml) to either IGF-I, -II, or mouse IgG for 3 h and then added to the mutant cells. Viable cells were counted after 24-h incubation. The results represent the mean ± SD of three independent experiments. (B) Wild-type ES cells were grown in serum-free medium in the presence of either anti-IGF-I, -II, or anti-mouse IgG antisera (10 μg/ml each) for 24 h, and cell viability was determined. The results represent the mean ± SD of three independent experiments. (C) The concentration of IGF-II in the media of cells grown for 24 h without serum was determined by ELISA with anti-IGF-II antibodies. All cells shown were cultured at the same density. Data shown are averages and SEs, based on three independent determinations for WT cells, three for KO cells, two for clone 13 (transfected with exogenous GRP94), and one for clone 3 (transfected with empty vector). (D) Intracellular pro-IGF-II forms in ES cells. Wild-type (WT) or grp94−/− (KO) ES cells were grown in the presence of serum or shifted to serum-free medium for 15 h, a time point where 40% of the grp94−/− cells are alive (see Figure 1). NP-40 lysates of the cells were prepared, 100 μg total protein were resolved by SDS-PAGE, and blots were probed with polyclonal anti-IGF-II antiserum that reacts with multiple forms of the prohormone, as well as with mature IGF-II. The same samples were probed with anti-KDEL to normalize the total protein load. (E) Effect of proteasome inhibitors on intracellular pro-IGF-II. Left, grp94−/− (KO or 14.1) ES cells, grown as in D and incubated in the presence of 3–5 μM of either ALLN or MG115 for 4 h. DMSO treatment was used as a solvent control. Ten micrograms of total cell protein were resolved by SDS-PAGE and probed with anti-IGF-II antibody. Right, 293T cells transiently expressing FLAG-tagged pro-IGF-II were incubated for 4–6 h in the presence of 10 μM of 17AAG with or without ALLN or MG115 (5 or 10 μM, respectively). Immounoblots with anti-IGF-II antibody were performed as above.
Cell Culture
For the experiments described here, ES cells were cultured without a feeder layer on gelatin-coated tissue culture plates in HEPES-buffered DMEM, supplemented with 15% heat-inactivated fetal calf serum (Hyclone, Logan, UT), penicillin, streptomycin, and glutamine (Invitrogen), nonessential amino acids (Cellgro, Herndon, VA), 1000 IU/ml recombinant human leukemia inhibitory factor (LIF; ESGRO; Millipore, Billerica, MA), and 0.001%, β-mercaptoethanol (Sigma). The viability of ES cells in serum-free medium was assessed by Trypan Blue exclusion. Either grp94+/+ cells (hereafter referred to as WT), grp94−/− cells, or grp94−/− cells transfected with a functional GRP94 cDNA or with empty vector, were seeded on gelatin-coated 60-mm plates in growth medium at 50% density. The next day, the cells were washed in serum-free medium and incubated in the absence of serum, typically for 24 h. Viable cells that excluded trypan blue were counted at the indicated time points. Viability of ES cells in serum-free medium that also lacked LIF was poorer than in medium containing LIF (10–15% survival after 24 h compared with ∼20% in the presence of LIF). 293T cells were cultured in DMEM, supplemented with 10% heat-inactivated fetal calf serum (Gemini, Woodland, CA). They were transfected using the FuGENE 6 reagent (Roche Applied Science, Indianapolis, IN), according to the manufacturer's instructions. Transfected 293T cells were labeled in 100-mm plates with 600 μCi/ml 35S-labeled Met and Cys (Amersham, Arlington Heights, IL) for 20 min and chased in complete medium containing unlabeled methionine and cysteines. Detergent lysates were clarified by centrifugation and subjected to immunoprecipitation with the appropriate antibodies.
Immunoprecipitation
Cells were lysed with NP-40 as in Melnick et al., (1994). Detergent lysates representing known cell equivalents were incubated with primary antibodies to either anti-GRP94 (monoclonal 9G10), anti-IGF-II, anti-FLAG M2, or control anti-tubulin antibodies. The immune complexes were isolated using protein A-Sepharose for anti-IGF and anti-FLAG antibodies, or protein G-Sepharose for anti-GRP94. The beads were washed and eluted with 0.2 M glycine. Equal portions from each eluate were separated using 15% or gradient 6–20% SDS-PAGE. The gels were fixed, incubated in Amplify (Amersham) for 30 min, dried, and exposed to x-ray film.
Western Blot Analysis
Equal amounts of extracted proteins (10 μg) were loaded, separated by SDS-PAGE, and transferred to nitrocellulose membranes. The membranes were incubated in blocking buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% Tween 20, and 3% [wt/vol] nonfat dry milk) and then in 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, and 2% (wt/vol) nonfat dry milk with the first and secondary antibodies. Immunoblotting was performed with the following antibodies: anti-GRP94 (1:1000), anti-activated caspase 3 (1:500), anti-cleaved PARP (1:500), and anti-Raf (1:2000). Proteins were visualized using the enhanced chemiluminescence kit (ECL; Pierce, Rockford, IL).
Immunohistochemical Staining and Image Processing
Cells were fixed in formaldehyde and stained with Hoechst 33258 to visualize chromatin or with FITC-conjugated anti-caspase 3 (Cell Signaling). Cells were imaged on a Zeiss Axiovert 200M microscope (Thornwood, NY) equipped with a Roper Coolsnap FX-HQ camera (Tucson, AZ). No-neighbor deconvolution was performed using Slidebook v4.0 software (Intelligent Imaging Innovations, Denver, CO).
Quantitation of IGF-II
ELISA plates coated with anti-IGF-II (mAb 792, R&D Systems) were incubated with conditioned media of the indicated cells. The bound IGF-II was detected with a biotinylated anti-IGF-II antibody (BAF792, R&D Systems) and developed with streptavidin-HRP (R&D Systems) according to the manufacturer's recommended procedure. Optical density units were converted to concentrations of the hormone with a standard curve generated with recombinant IGF-II (792-MG; R&D Systems).
RESULTS
grp94−/− Cells Do Not Tolerate Serum Deprivation
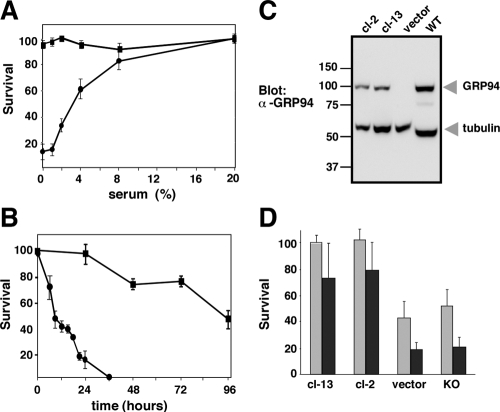
Previous work showed that grp94−/− cells are more susceptible to conditions known to cause ER stress, such as tunicamycin or Ca2+ ionophore treatments (Biswas et al., 2007; Wanderling et al., 2007). We therefore asked if these mutant ES cells are also more sensitive to serum withdrawal, a common mode of inducing metabolic stress, which is usually ascribed to depletion of growth factors. When grown in decreasing level of fetal bovine serum (FBS), there was a sharp decline in the survival of grp94−/− cells, whereas the genetically matched WT cells tolerated growth in low FBS serum much better (Figure 1A). Death of the mutant cells upon serum withdrawal was rapid: within 6 h, 25% detached from the plates and were dying, and only 20% survived after 24 h without serum. In contrast, no appreciable death of WT cells was observed even after 36 h (Figure 1B). To prove that the sensitivity to serum deprivation is due to the absence of GRP94 and not to a different adaptation of the mutant cells, we sought to complement the defect by reexpressing GRP94 in the mutant cells. Clones expressing various levels of GRP94 were selected (Figure 1C). Three clones (cl-2 and cl-13, as well as cl-17, not shown) were tested, and all were more resistant than the grp94−/− cells to serum withdrawal (Figure 1D). Clones transfected with an empty expression vector, which had been subjected to the same drug selection, were as sensitive as the nontransfected grp94−/− cells (Figure 1D).
Figure 1.
Sensitivity of GRP94-deficient cells to serum deprivation. (A) Dependence of grp94−/− ES cells on serum concentration. WT (■) and grp94−/− cells (•) were seeded in 60-mm plates in growth medium, as described in Materials and Methods. The next day, cells were washed in serum-free medium and incubated at the indicated concentration of serum. After 24 h, viable, attached cells were counted. The nonattached cells were dead, as judged by replating them in growth medium. The results represent the means ± SD of three independent experiments. (B) Time course of cell death. WT (■) and grp94−/− cells (•), grown as described above, were shifted to serum-free medium at time 0, and the percentage of live, plate-attached cells was determined at the indicated times thereafter. n = 3. (C) Levels of GRP94 expression. Total cell lysates (30 μg each) were derived from either WT cells, from two grp94−/− clones stably transfected with GRP94 (cl-2 and cl-13), or from grp94−/− transfected with empty vector. The lysates were separated by SDS-PAGE (all lanes are from the same gel, spliced together) and analyzed by Western blotting with the anti-GRP94 mAb 9G10. The level of tubulin served as a loading control, detected with anti-tubulin antibody. (D) Complementation of viability by reexpression of GRP94. Susceptibility of clone-2 and clone-13, shown in C, to serum deprivation was assessed after either 18 or 24 h in serum-free medium (gray and black bars, respectively), compared with cells transfected with empty vector.
The death of grp94−/− Cells Is Apoptotic
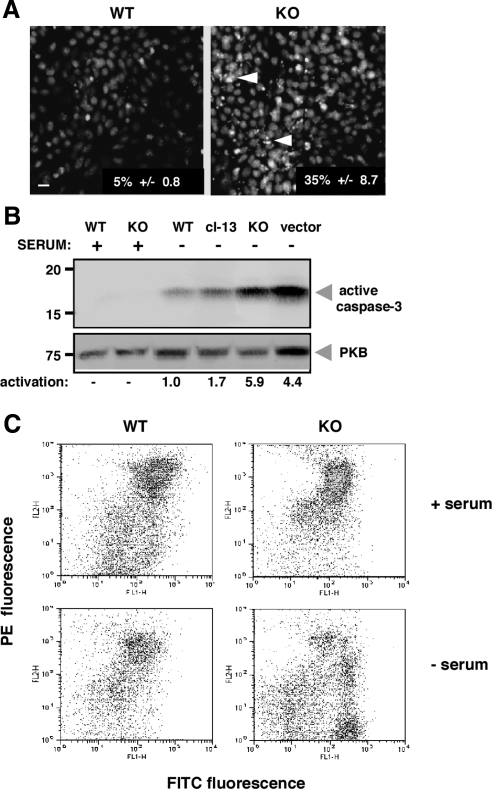
Three lines of evidence show that the mutant ES cells undergo programmed cell death. First, the nuclei of mutant cells become progressively condensed, as shown by Hoechst staining (Figure 2A). Second, serum-deprived mutant cells are positive by immunofluorescence with anti-activated caspase 3, whereas in WT cells, the percentage of activated caspase 3–positive cells was low (Table 1). Increased levels of the 17-kDa activated fragment of caspase 3 in serum-deprived mutant cells versus serum-deprived WT cells was also shown by immunoblots of whole cell lysates (Figure 2B). When lysates of mutant cells reexpressing GRP94 were examined by the same method, the activation level of caspase 3 mirrors that of WT cells, as did the fraction of dying cells. In contrast, vector-transfected mutant cells respond to the stress with the same high level of caspase 3 activation as do nontransfected mutant cells (Figure 2B). The third indication of apoptosis is the staining of cells with the mitochondrial voltage-sensitive dye JC-1. Serum-deprived mutant cells exhibit depolarization of their mitochondrial membrane potential, shown by the shift from orange to green fluorescence (Figure 2C).
Figure 2.
grp94−/− cells undergo apoptosis in the absence of serum. (A) WT and grp94−/− cells (KO) were incubated for 12 h in the absence of serum and then stained with Hoechst 33258 (bis-benzidine). The percentages of cells with condensed chromatin (arrowheads) were derived from counting at least 250 cells each, and the numbers listed in the figure represent the mean ± SD of three independent experiments. Scale bar, 20 μm. (B) To measure activation of caspase 3, cell extracts (40 μg) were derived from either WT, grp94−/− (KO) cells, or KO cells transfected with a grp94 expression vector (cl-13) or with an empty vector (cl-3). Lysates were separated by SDS-PAGE and immunoblotted with an antibody specific for cleaved caspase-3 (17-kDa band). The level of the cytosolic protein kinase B (PKB) served as a loading control. The results from densitometric analysis of the autoradiograms are shown below the gel, expressed in relative units of activated caspase 3. (C) FACS analysis of JC-1–stained cells. WT or grp94−/− cells were loaded with JC-1, a mitochondrial voltage-sensitive dye and analyzed by FACS after incubation in normal or serum-free media. Live cells are mostly orange (FL-2 axis), and the green shift (FL-1 axis) in serum-free condition reflects cells with depolarized mitochondria undergoing apoptosis.
Table 1.
Caspase 3 activation upon serum withdrawal
| Exp. | WT cells (%) | KO cells (%) |
|---|---|---|
| 1 | 5.2 ± 0.7a | 39.1 ± 4.3 |
| 2 | 3.8 ± 1.3 | 34 ± 2.8 |
| 3 | 7.4 ± 2.2 | 43 ± 3.7 |
a Percentage of cells positive for cleaved, activated caspase 3, as judged by immunofluorescence.
GRP94 Is Needed for a Secreted Growth Factor
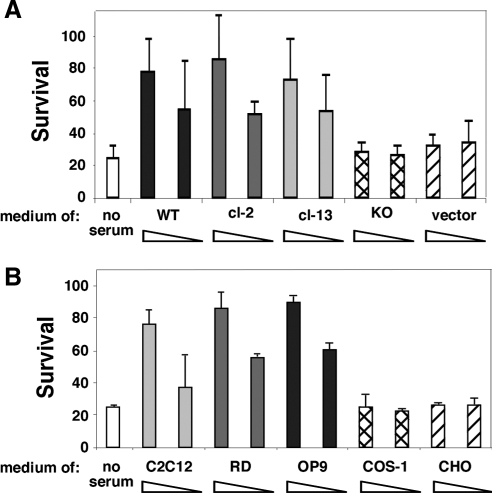
Because all the known GRP94 clients are either secreted proteins or membrane receptors, we asked whether survival of normal ES cells is due to a secreted growth factor. To this end, WT cells were grown in serum-free medium for 24 h, and their conditioned medium was then added to grp94−/− cells. The viability of mutant cells was markedly improved when cultured in such conditioned media and the level of rescue was proportional to the percentage of conditioned medium provided (Figure 3A). Media conditioned by two different GRP94-transfected grp94−/− ES cells (clones 2 and 13) were equally as effective as medium from the WT ES cells, whereas medium from cells expressing empty vector had no protective effect against serum deprivation (Figure 3A). Therefore, grp94−/− cells are incapable of secreting a factor(s) that wild-type cells secrete and that protects them in autocrine manner when serum is removed. Because apoptosis induced by growth factor withdrawal is a stress response common to many cells, we also tested the ability of serum-free medium conditioned by five other cell lines to rescue grp94−/− cells from apoptosis. Medium conditioned by either RD (human embryonal rhabdomyosarcoma), C2C12 (murine myoblast-like), or OP9 (murine stromal) cells was capable of rescuing the grp94−/− cells from apoptosis, whereas neither Chinese hamster ovary (CHO)-conditioned medium nor COS-1–conditioned medium had any detectable activity (Figure 3B).
Figure 3.
Conditioned media rescue grp94−/− cells from apoptosis. (A) Serum-free media conditioned by growth of the indicated cells for 24 h were collected as described in Materials and Methods. Then, grp94−/− cells were incubated either in the conditioned media of either WT ES cells (WT), mutant ES cells (KO), GRP94-transfected mutant cells (cl-2 and -13), or empty vector-transfected cells, and survival determined 24–48 h later. Each medium was tested both as 1/1 and 1/3 dilution in serum-free medium (triangles below each cell line). Data are means ± SD of at least five independent experiments. (B) A similar test to the one shown in A, using conditioned media from the cell lines C2C12, OP9, RD, COS, and CHO. All conditioned media were collected after 72–96 h of growth in serum-free cultures. Data are means ± SD of at least three independent experiments.
Identification of the Growth Factor
Several growth factors were likely candidates, given these experimental parameters. C2C12 and RD cells are known to produce IGF-II (Crouch and Helman, 1991; Rosen et al., 1993). Transferrin had no effect at concentrations up to 30 μg/ml, nor did estradiol (Figure 4A). Similarly, basic fibroblast growth factor (bFGF, at up to 3 μg/ml) could not protect grp94−/− cells from death, even when combined with heparin (1 mg/ml), a condition know to augment bFGF activity. Neither addition of recombinant tumor growth factor β nor bone morphogenic proteins 2 and 4 could not rescue grp94−/− from death (data not shown). Insulin, however, was capable of promoting survival, but only at 10 μg/ml or higher (Figure 4A), and this activity was reflected in lower levels of caspase 3 activation (Figure 4B).
Figure 4.
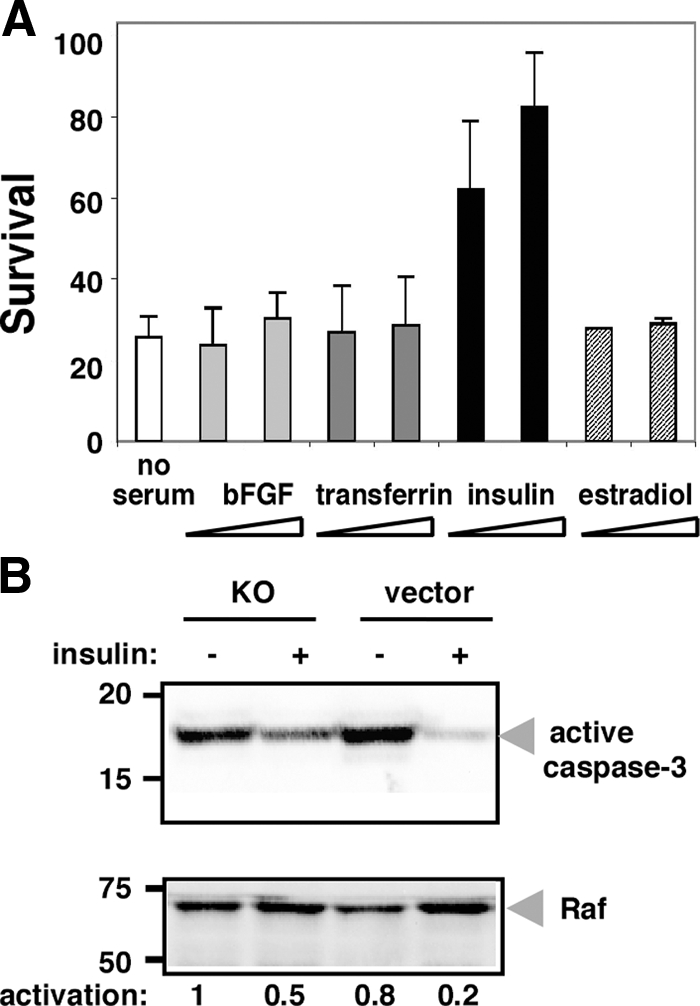
Insulin protects grp94−/− cells against apoptosis in the absence of serum. (A) grp94−/− cells were grown as described in Figure 1 and immediately after transfer to serum-free medium, supplemented with two different concentrations (3.3 and 10 μg/ml) of either bFGF, transferrin, insulin, or estradiol. Viable cells were counted after 24 h. The results are the means ± SD of three independent experiments. (B) Either grp94−/− cells or empty vector-transfected cells were grown for 12 h in serum-free medium in the presence or absence of insulin (10 μg/ml). Cell lysates (40 μg) were then separated by SDS-PAGE and analyzed by Western blotting with anti-activated caspase-3 antibody. The level of RAF served as a loading control. Normalized quantitation of the signals is shown below the blot. The results represent the mean ± SD of three independent experiments.
Because not only insulin, but also IGF-I and -II are known to act as survival factors for a number of cells (Baker et al., 1993; Grimberg and Cohen, 2000), conditioned media were treated with suramin, a known inhibitor of signaling of growth factors, including IGF-II (De Giovanni et al., 1995; Sullivan et al., 1997). At a dose of suramin that was not toxic to either ES cells or to C2C12 cells, this treatment partly inhibited the activity of the conditioned media (Table 2). To identify the factor(s) more directly, the conditioned media were then incubated with either anti-IGF-I or -II antibodies, and the effect on the activity tested. Anti-IGF-II, but not -I, neutralized the survival activity of medium conditioned by ES cells expressing GRP94 as well as C2C12-conditioned medium (Figure 5A). Moreover, addition of anti-IGF-II antiserum to cultures of WT ES cells, which can withstand the deprivation of serum, promoted extensive death among these cells as well (Figure 5B). We therefore measured the levels of IGF-II in the media directly, using ELISA. As shown in Figure 5C, conditioned media that were capable of rescuing the mutant cells (wild-type ES cells, two mutant ES clones that reexpress GRP94, C2C12, and RD) all contained IGF-II, whereas media that were incapable of rescue (mutant cells, mutant cells transfected with vector alone, COS1 and CHO cells) had undetectable levels of IGF-II. We determined that WT ES cells do not produce and secrete IGF-II constitutively, but rather begin producing IGF-II once shifted to serum-free medium (Figure 5D). After 15 h of growth without serum, several intracellular isoforms of IGF-II are readily detectable in lysates of either WT or grp94−/− ES cells (Figure 5D), but the grp94−/− ES cells fail to secrete the growth factor (Figure 5C). Treatment of grp94−/− ES cells with either MG115 or ALLN led to accumulation of intracellular pro-IGF intermediates (Figure 5E, left panel). Similarly, in 293T cells transfected with pro-IGF-II and treated with 17-allylamino-17-demethoxygeldanamycin (17-AAG) to inactivate GRP94, a further treatment with ALLN increased the level of intracellular pro-IGF-II (Figure 5E, right panel). The effects of the inhibitors suggest that when the cells are not able to process and secrete IGF-II, the intracellular intermediates are targeted to proteasomal degradation.
Table 2.
Inhibition of cytoprotective activity by suramin
| cl-2 medium | C2C12 medium | Serum-free medium | |
|---|---|---|---|
| grp94−/− cells; no addition | 84.5 ± 10a | 81.8 ± 8 | 27.5 ± 2 |
| grp94−/− cells; 500 μM suramin | 40.5 ± 4 | 39.1 ± 1 | 22.5 ± 4 |
| Wild-type cells; 500 μM suramin | ND | ND | 85 ± 7 |
| C2C12 cells; 500 μM suramin | ND | ND | 93 ± 7 |
a Percentage of live cells after 24 h.
To demonstrate that IGF-II is necessary and sufficient to explain the inability to respond to serum withdrawal, we complemented the serum-starved grp94−/− cells with exogenous IGF. Recombinant IGF, either IGF-I or -II, was capable of rescuing the cells' viability over the course of the experiment (Figure 6, A–C), almost to the same extent as conditioned media that contain IGF-II or -I, and likely other growth factors (Figure 6, D and E). The ability of either IGF to complement the deficiency is expected, because both IGF-I and -II signal primarily through the same IGF-I receptor (LeRoith and Roberts, 2003). We conclude that IGF-II is the growth factor that is normally essential for ES cell survival after withdrawal of serum and whose secretion is controlled by GRP94.
Figure 6.
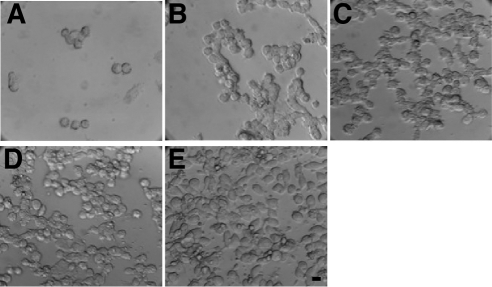
Rescue of grp94−/− cell survival by IGF. Representative fields of grp94−/− cells after 24 h of culture in serum-free medium under the following conditions: (A) in the absence of growth factors; (B) in the presence of 100 ng/ml recombinant IGF-I; (C) in the presence of 100 ng/ml recombinant IGF-II; (D) in the presence of medium collected from serum-starved p12 cells, which are fibroblasts that constitutively secreted IGF-I; and (E) medium collected from serum-starved C2C12, which produce IGF-II endogenously and secrete it when induced to differentiate. The images are representative of three independent experiments. Scale bar, 10 μm.
The GRP94–IGF-II Interaction Is Not Limited to ES Cells
We next asked if the control of IGF-II secretion by GRP94 is specific to ES cells or is a more general phenomenon. C2C12 cells were induced to differentiate, and at 24 h, before IGF-II secretion begins, they were treated with 17-AAG, to inhibit GRP94 (Schulte and Neckers, 1998). Twenty four hours later, when C2C12 differentiation should be peaking, at least 60% of the 17-AAG–treated cells were dead, and the myotubes that formed under these conditions were smaller than normal (data not shown). The culture medium contained only 0.06 ng/ml IGF-II, compared with 0.21 ng/ml in the medium of mock-treated cells (Figure 7A). Thus, IGF-II secretion is dependent on GRP94 not only in ES cells, but also in a myoblast cell line. Concomitantly, 17-AAG–treated C2C12 cells displayed activation of caspase-3 (Figure 7B), correlating the reduced secretion of IGF-II with increased death of C2C12 cells.
Figure 7.
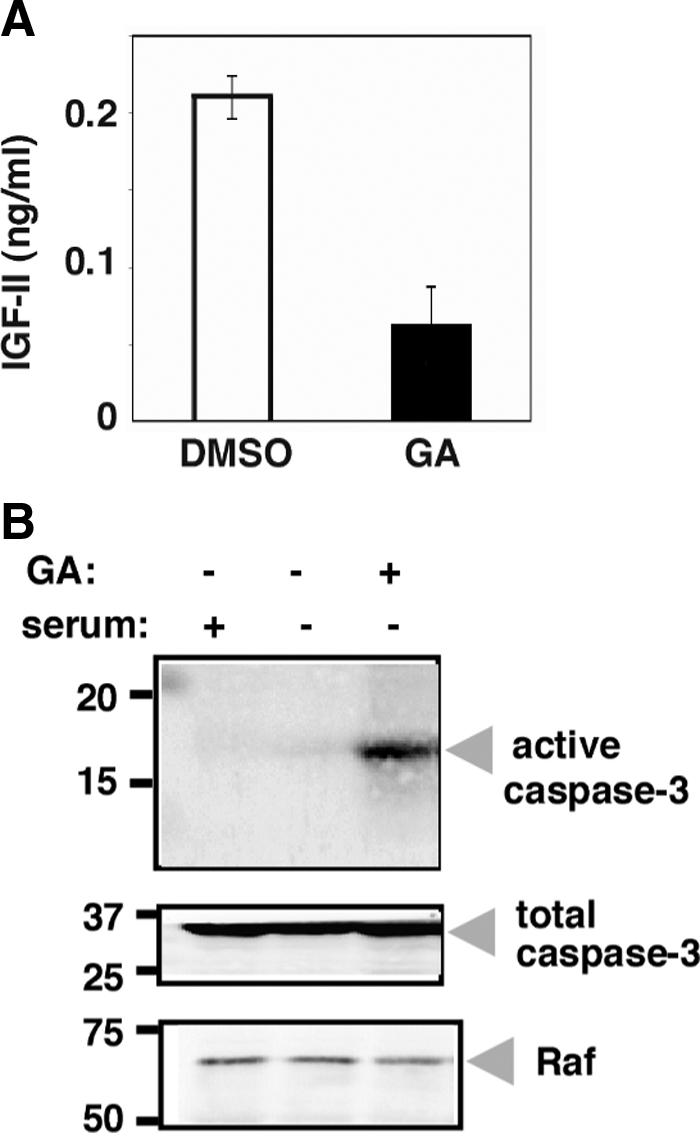
Similar action of GRP94 in C2C12 cells. (A) Cells were transferred to serum-free medium for 24 h and 17-AAG (GA) was added to a final concentration of 15 μM (or DMSO as control). After additional 24 h the medium was harvested, and the IGF-II levels were determined by ELISA. The results represent the mean ± SD of two independent experiments, each of which was measured in triplicates. (B) Detergent lysates of the same cultures as in A, as well as of C2C12 grown in normal medium, were resolved by SDS-PAGE and probed with antibodies specific for active caspase-3 (cas-3), total caspase-3, or RAF.
Direct Association of GRP94 with IGF-II
To determine whether there is physical association between IGF-II and GRP94, we used the C2C12 cell line, which expresses and secretes IGF-II when induced to differentiate into muscle (Rosen et al., 1993; also shown in Figure 3). C2C12 cells were allowed to differentiate for 72–96 h to achieve peak expression, and detergent (NP-40) lysates were immunoprecipitated with an mAb directed against GRP94 (9G10). The immunoprecipitates were then probed for IGF-II. Three isoforms of pro-IGF-II were detected in association with GRP94, with apparent molecular weights of 28, 23, and 17 kDa (Figure 8, A and B). The nature of these isoforms is not yet clear, but similar ones were reported in many cell types (Duguay et al., 1998) as well as human plasma (van Doorn et al., 2002). All three isoforms are too large to result from Golgi proteolytic processing of IGF-II and are attributable to disulfide bonds formation and O-glycosylation (Duguay et al., 1998). No mature IGF-II was coimmunoprecipitated with GRP94 (Figure 8B), consistent with ER localization of the interaction. Importantly, the ability to coimmunoprecipitate these pro-IGF-II isoforms with an anti-GRP94 antibody is dependent on the activity of GRP94. When the chaperone was inhibited by treatments with either 17-AAG or radicicol, under conditions known to inhibit most of the cellular pool of GRP94 without affecting cell viability (Vogen et al., 2002), coimmunoprecipitation of the IGF-II species was sharply reduced (Figure 8, B and D, bottom panel), because the mAb 9G10 failed to recognize the inhibited conformation of GRP94 (Figure 8, A and D, top panel; Vogen et al., 2002), even though the total level of GRP94 remained unchanged (Figure 8C).
Figure 8.
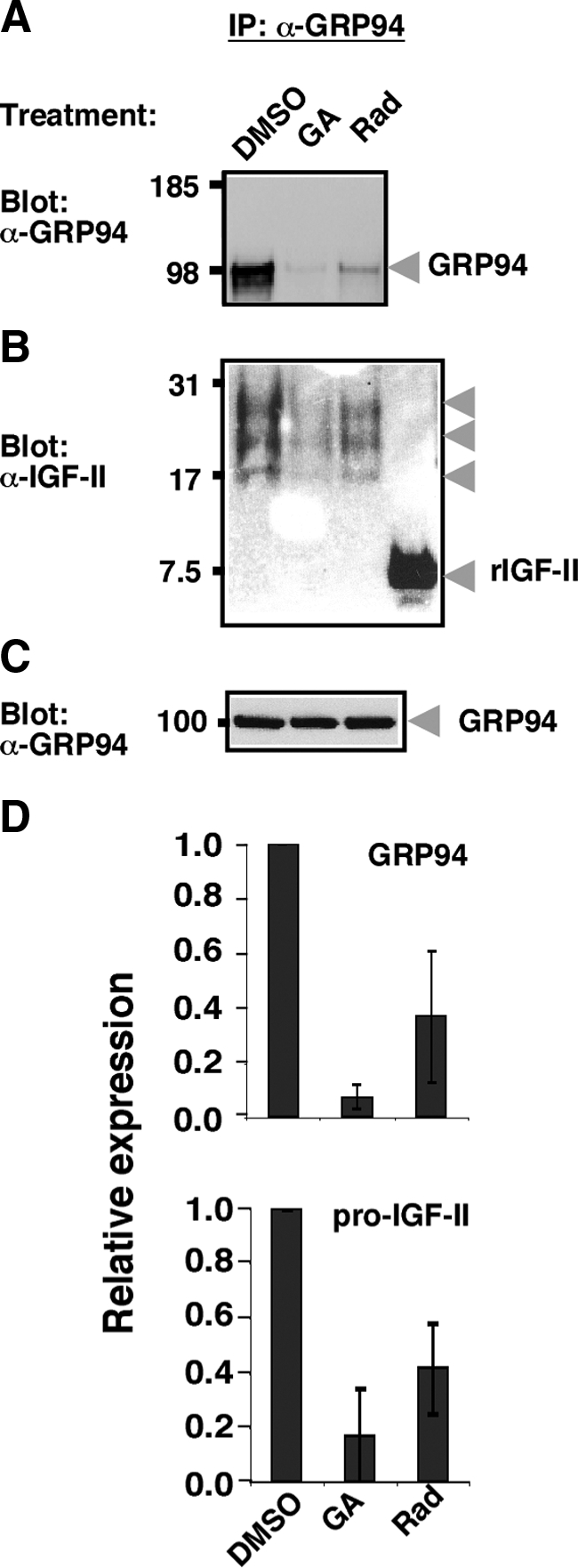
Coimmunoprecipitation of pro-IGF-II with anti-GRP94. (A) C2C12 cells were incubated in differentiation medium for 72 h, with the last 12 h being in the presence of 15 μM radicicol (Rad), 17AAG (GA), or DMSO control. The cells were lysed with detergent and immunoprecipitated with mAb 9G10, which is directed at the first charged domain of GRP94, is conformation-sensitive and only precipitates active GRP94 (Loo et al., 1998; Vogen et al., 2002). After SDS-PAGE, the top half of the gel was probed for GRP94 and developed by an ECL reaction. (B) The bottom half of the same gel as in A was probed for IGF-II. Arrowheads point to the three biosynthetic intermediates of pro-IGF-II that are coimmunoprecipitated with GRP94. Mature, recombinant murine IGF-II (rIGF-II) served as a positive control for antibody reactivity. (C) GRP94 immunoblot of total cell extracts of the samples in A and B before immunoprecipitation as a loading control, showing the total level of GRP94. (D) Top, quantitation of the GRP94 bands averaged from three experiments like the one shown in A. Bottom, quantitation of the pro-IGF-II bands averaged from two experiments like the one shown in B.
The association of pro-IGF-II with GRP94 was also demonstrated by the reciprocal immunoprecipitation: when endogenous pro-IGF-II was immunoprecipitated from C2C12 cells (using two different antibodies), GRP94 was detected in the immunoprecipitated material, but not in immunoprecipitates of the cytosolic protein RAF (Figure 9A). In cells that were treated with 17-AAG to inactivate GRP94, the association with pro-IGF-II was inhibited (Figure 9, A and B), whereas the total level of GRP94 in the lysates was not significantly changed (Figure 9C).
Figure 9.
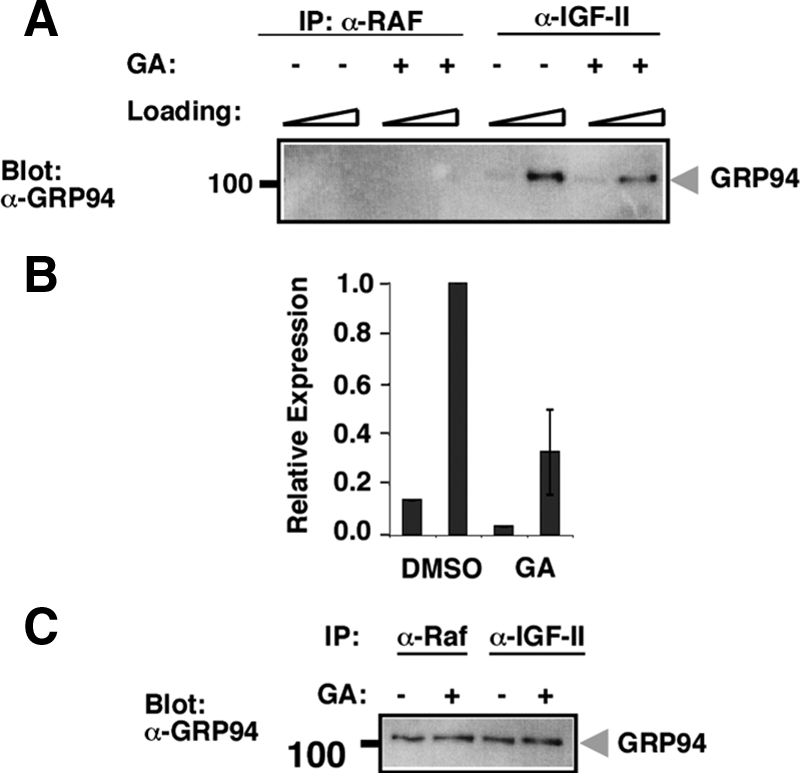
Reciprocal immunoprecipitation of GRP94 with anti-IGF-II. (A) The reciprocal experiment to the one shown in Figure 8 was performed on C2C12 cell lysates in the presence or absence of 17-AAG (GA). Samples were immunoprecipitated with mouse monoclonal anti-IGF-II (S1F2) or with an irrelevant monoclonal anti-Raf and then probed with anti-GRP94. One quarter and three quarters of each sample were loaded on adjacent lanes. Similar results were obtained by immunoprecipitation with rabbit anti-IGF-II (data not shown). (B) Quantitation of the GRP94 bands shown in A. The results represent the mean ± SD of two independent experiments, one of which is shown in A. (C) GRP94 immunoblots of total cell extracts of the samples described in A before immunoprecipitation, shown as a loading control.
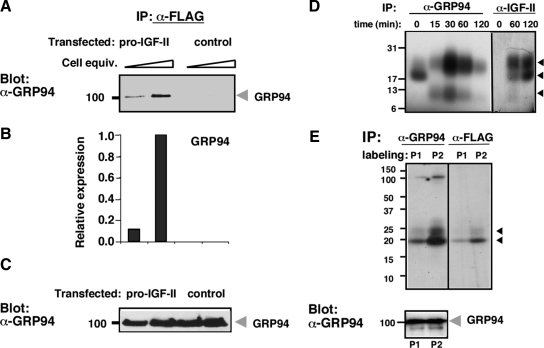
Association of IGF-II and GRP94 was also demonstrated in 293T cells expressing a FLAG-tagged version of pro-IGF-II. GRP94 was coimmunoprecipitated with anti-FLAG only from cells expressing pro-IGF-II and not from vector-transfected cells (Figure 10, A–C). The sizes of the pro-IGF intermediates in 293T cells were similar to those in C2C12 cells. The GRP94–pro-IGF-II association is time-dependent and is a property of some biosynthetic intermediates, as shown by pulse-chase analysis (Figure 10D). Immediately after a short pulse, a 17-kDa species is coimmunoprecipitated with GRP94. During the chase, one of the two major biosynthetic intermediates of IGF, a 23-kDa species, is coimmunoprecipitated, with peak association at about 30–60 min of chase (Figure 10D). In addition, an 11-kDa minor IGF species is associated with GRP94 with kinetics similar to the 23-kDa species. The 17- and 23-kDa species are similar to ones detected by coimmunoprecipitation in C2C12 cells, but the largest species (ca. 28 kDa, Figure 8B) is not detected in the pulse-chase analysis of 293T cells (Figure 10D). The identity of the labeled bands as biosynthetic intermediates of pro-IGF-II is further confirmed by sequential immunoprecipitation: the 19- and 23-kDa GRP94-associated bands are recognized by an anti-FLAG antibody to the N-terminally tagged pro-IGF-II construct (Figure 10E, top panel). It is notable that a 100-kDa band is clearly observed only when a longer pulse is used (Figure 10E), and immunoblotting of the same samples with anti-GRP94 antibody confirms the identity of this band as GRP94 (Figure 10E, bottom panel). This is consistent with the known long half-life of GRP94. Furthermore, no band at the expected size of BiP/GRP78 (∼78 kDa) is detectable in the anti-GRP94 immunoprecipitates. Taken together, we conclude that GRP94 associates physically with pro-IGF-II intermediates in transient and selective manner during the biosynthesis of the hormone, independently of BiP, as expected from an active chaperone.
Figure 10.
Association of GRP94 and pro-IGF-II in transfected 293T cells. (A) 293T cells transiently expressing FLAG-tagged pro-IGF-II were immunoprecipitated with M2 anti-FLAG antibody and the immunoprecipitates were probed with anti-GRP94 antibody. Vector-transfected 293T cells were used as controls. Two loads of each sample were used in adjacent lanes. (B) Quantitation of the GRP94 bands. The mean ± SD of band intensity from two independent experiments (one of them shown in A) are presented. (C) GRP94 immunoblots of total cell extracts of the samples described in A before immunoprecipitation, shown as a loading control. (D) GRP94 associates with pro-IGF-II intermediates transiently. Pulse-chase analysis of 35S-labeled 293T cells expressing FLAG-tagged pro-IGF-II. Cells were pulse-labeled with 35S-labeled Met and Cys for 15 min and then chased for the indicated times. Cell lysates from each time point were immunoprecipitated with anti-GRP94 antibody and protein G-Sepharose, and the unbound material from each time point was reimmunoprecipitated with monoclonal no. 166 anti-IGF-II. The migrations of the pro-IGF-II biosynthetic intermediates are indicated by arrows. (E) Sequential immunoprecipitation of GRP94 and IGF-II. 293T cells expressing FLAG-tagged pro-IGF-II were labeled with 35S-Met and Cys for 35–40 min (P1) or 60 min (P2). Top, cell lysates from each time point were immunoprecipitated with anti-GRP94 antibody, and 80% of the eluted material from each time point was sequentially immunoprecipitated with anti-FLAG antibody. The migrations of the pro-IGF-II biosynthetic intermediates are indicated by arrows. Bottom, 0.5% of immunoprecipitates with anti-GRP94 antibody (E) were resolved by SDS-PAGE, and blots were probed with anti-GRP94 antibody.
DISCUSSION
Despite sequence homology to the hsp90 chaperones and expression patterns consistent with activity in stress responses, no mechanism has yet been shown to explain the known antiapoptotic activity of GRP94. The novel insight that this study provides is that GRP94 regulates the secretion of IGF-II, a known prosurvival peptide hormone. IGF-I and -II are pleiotropic growth factors, well known to be involved in survival and programmed cell death of normal and transformed cells and in differentiation decisions during both embryogenesis and postnatal life. The expression and secretion of these insulin-like factors are often induced in response to changes in the external environment and are required for executing the cellular response programs. We show here that upon withdrawal of serum, ES cells respond by secreting IGF-II (but not IGF-I), which apparently acts in autocrine manner, binding to and signaling growth through the IGF receptor 1 (LeRoith and Roberts, 2003). GRP94-deficient ES cells cannot secrete IGF-II when serum is removed and consequently die by apoptosis. This deficiency is complemented when GRP94 is reexpressed in the mutant cells, showing that it is a direct effect of the presence of the chaperone. The effect on IGF-II secretion is likely to be due to the absence of GRP94 interaction rather than to altered balance of other chaperones, because we find that there is little if any up-regulation of BiP or calreticulin in grp94−/− cells (Figure 5D). Furthermore, this work shows that the association GRP94 and IGF-II is not only functional, but also physical: GRP94 associates with pro-IGF-II intermediates in cells that express the hormone endogenously, as well as in transfected cells. Therefore, we conclude that IGF-II is a client of GRP94, whose activity as a chaperone is necessary for IGF-II secretion. In the absence of GRP94 activity, IGF-II intermediates undergo proteasome-dependent degradation.
The mitogenic activity of IGF-II is important for embryonic development (Lee et al., 1990), for muscle differentiation (Prelle et al., 2000) and maintenance (Smith et al., 2000), and for tumor progression (LeRoith and Roberts, 2003). In each of these cases, signaling via the IGF pathway promotes cell survival, among other effects. Thus, it is likely that the control of IGF-II production and secretion by GRP94 operates in various cells and tissues, in normal physiology as well as pathological situations. The control of IGF production would also explain the numerous observations ascribing antiapoptotic activity to GRP94, through up-regulation of its expression (e.g., Bando et al., 2004; Reddy et al., 1999). The antiapoptotic activity of GRP94 may also be related to its recently described role in clearance of misfolded proteins from ER, through its association with the protein OS-9 (Christianson et al., 2008).
This interpretation does not exclude the possibility that other factors needed for cell growth and survival are also inactive in the absence of GRP94 function. In fact, it is likely that some integrins are not properly targeted to the cell surface without GRP94's activity (Randow and Seed, 2001). The absence of such clients may account for the incomplete rescue by recombinant IGF.
Despite the importance of IGF-II, -I, and insulin for growth and development of many tissues, there is almost no work on the regulation of their biosynthesis and secretion by chaperones. Binding sites for BiP/GRP78 were identified in misfolded insulin mutants (Schmitz et al., 1995) and persistent association of wild-type proinsulin with BiP has been demonstrated under ER stress conditions (Schmitz et al., 1995). It would be informative to determine which proinsulin folding intermediates are normally associated with BiP. High-resolution microscopy work colocalized the Cpn60 chaperonin to all compartments of the secretory route of insulin, including the secretory granules that also contain the PC1 convertase, suggesting by association that Cpn60 plays a role in either processing or packaging of proinsulin (Arias et al., 2000). However, Cpn60 was not shown to associate with insulin or affect it biosynthesis. To our knowledge, the present study is the first to show both functional and physical association of IGF with any chaperone. Preliminary results suggest that in other cell types the secretion of IGF-I is also dependent on the activity of GRP94 (Ostrovsky, Baserga, and Argon, unpublished data). Thus, it is possible that all insulin-family peptides require chaperone interactions for their maturation.
IGF-II biogenesis is a complex, multistep process. IGF-II is encoded by a single gene, but multiple promoters and alternative splicing yield multiple protein isoforms (Engstrom et al., 1998). The primary translation product is not N-glycosylated, but addition of N-acetylgalactosamine at four sites occurs upon transport to the Golgi complex, followed by addition of sialic acid (Duguay et al., 1998). In the Golgi complex, pro-IGF-II is cleaved at 3 Lys or Arg residues by subtilisin-like proprotein convertases, releasing the O-glycosylated E peptide from the mature peptide hormone (Duguay et al., 1998). An early folding step in the ER is formation and isomerization of the three disulfide bonds that characterize the entire insulin family of proteins. The complexity of IGF biosynthesis is reflected even in vitro, where it was shown that under conditions that mimic the redox state of the ER, both IGF-I and -II are unable to form their native disulfide bonds (Hober et al., 1999). Such an inherent folding problem would lead to dependence on molecular chaperones for in vivo folding. The topology of the three disulfides in IGF-II is a “cysteine knot,” which necessitates cysteine oxidation in a prescribed order, and governing this process maybe the role of GRP94, in much the same way that it is needed for the completion of oxidative folding of immunoglobulin light chains (Gidalevitz, Ostrovsky, Simen, Dul, and Argon).
Even without knowing precisely how GRP94 activity is needed for IGF-II processing and secretion, this work suggests a mechanism that explains the cyto-protective activity of GRP94 and is a natural extension of GRP94's known role in the folding of selected secretory and membrane proteins.
ACKNOWLEDGMENTS
We thank Cat Makarewich for invaluable help throughout this work, Lucille Guevarra and Julian Ostrovsky for tissue culture help, Drs. Don Steiner and Steve Duguay (the University of Chicago) for the IGF-II expression vectors, Dr. Shara Kabak (the University of Pennsylvania) for help with the C2C12 line, Ms. Rennell Dupree for help with microscopy, and Drs. Craig Bassing, Tali Gidalevitz, and Adda Grimberg for comments on the manuscript. This work was supported by grants from the National Institutes of Health (AG-18001 and NS-059367) and the W. W. Smith foundation to Y.A. O.O. was supported by postdoctoral fellowships from the Juvenile Diabetes Research Foundation and the Arthritis Foundation.
Glossary
Abbreviations used:
- ES
embryonic stem
- GRP
glucose-regulated protein
- IGF
insulin-like growth factor
- 17-AAG
17-allylamino-17-demethoxygeldanamycin.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-04-0346) on January 21, 2009.
REFERENCES
- Argon Y., Simen B. B. GRP94, an ER chaperone with protein and peptide binding properties. Semin. Cell Dev. Biol. 1999;10:495–505. doi: 10.1006/scdb.1999.0320. [DOI] [PubMed] [Google Scholar]
- Arias A. E., Velez-Granell C. S., Mayer G., Bendayan M. Colocalization of chaperone Cpn60, proinsulin and convertase PC1 within immature secretory granules of insulin-secreting cells suggests a role for Cpn60 in insulin processing. J. Cell Sci. 2000;113(Pt 11):2075–2083. doi: 10.1242/jcs.113.11.2075. [DOI] [PubMed] [Google Scholar]
- Baker J., Liu J. P., Robertson E. J., Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- Bando Y., Katayama T., Aleshin A. N., Manabe T., Tohyama M. GRP94 reduces cell death in SH-SY5Y cells perturbated calcium homeostasis. Apoptosis. 2004;9:501–508. doi: 10.1023/B:APPT.0000031446.95532.ad. [DOI] [PubMed] [Google Scholar]
- Bando Y., Katayama T., Kasai K., Taniguchi M., Tamatani M., Tohyama M. GRP94 (94 kDa glucose-regulated protein) suppresses ischemic neuronal cell death against ischemia/reperfusion injury. Eur. J. Neurosci. 2003;18:829–840. doi: 10.1046/j.1460-9568.2003.02818.x. [DOI] [PubMed] [Google Scholar]
- Biswas C., Ostrovsky O., Makarewich C. A., Wanderling S., Gidalevitz T., Argon Y. The peptide binding activity of GRP94 is regulated by calcium. Biochem. J. 2007;405:233–241. doi: 10.1042/BJ20061867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson J. C., Shaler T. A., Tyler R. E., Kopito R. R. OS-9 and GRP94 deliver mutant alpha1-antitrypsin to the Hrd1-SEL1L ubiquitin ligase complex for ERAD. Nat. Cell Biol. 2008;10:272–282. doi: 10.1038/ncb1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch G. D., Helman L. J. All-trans-retinoic acid inhibits the growth of human rhabdomyosarcoma cell lines. Cancer Res. 1991;51:4882–4887. [PubMed] [Google Scholar]
- De Giovanni C., Melani C., Nanni P., Landuzzi L., Nicoletti G., Frabetti F., Griffoni C., Colombo M. P., Lollini P. L. Redundancy of autocrine loops in human rhabdomyosarcoma cells: induction of differentiation by suramin. Br. J. Cancer. 1995;72:1224–1229. doi: 10.1038/bjc.1995.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond I. A., Lee A. S., Resendez E., Jr, Steinhardt R. A. Depletion of intracellular calcium stores by calcium ionophore A23187 induces the genes for glucose-regulated proteins in hamster fibroblasts. J. Biol. Chem. 1987;262:12801–12805. [PubMed] [Google Scholar]
- Duguay S. J., Jin Y., Stein J., Duguay A. N., Gardner P., Steiner D. F. Post-translational processing of the insulin-like growth factor-2 precursor. Analysis of O-glycosylation and endoproteolysis. J. Biol. Chem. 1998;273:18443–18451. doi: 10.1074/jbc.273.29.18443. [DOI] [PubMed] [Google Scholar]
- Engstrom W., Shokrai A., Otte K., Granerus M., Gessbo A., Bierke P., Madej A., Sjolund M., Ward A. Transcriptional regulation and biological significance of the insulin like growth factor II gene. Cell Prolif. 1998;31:173–189. doi: 10.1111/j.1365-2184.1998.tb01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist A., et al. Quantitative proteomics analysis of the secretory pathway. Cell. 2006;127:1265–1281. doi: 10.1016/j.cell.2006.10.036. [DOI] [PubMed] [Google Scholar]
- Grimberg A., Cohen P. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J. Cell Physiol. 2000;183:1–9. doi: 10.1002/(SICI)1097-4652(200004)183:1<1::AID-JCP1>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hober S., Lundstrom Ljung J., Uhlen M., Nilsson B. Insulin-like growth factors I and II are unable to form and maintain their native disulfides under in vivo redox conditions. FEBS Lett. 1999;443:271–276. doi: 10.1016/s0014-5793(98)01737-2. [DOI] [PubMed] [Google Scholar]
- Ishiguro S., Watanabe Y., Ito N., Nonaka H., Takeda N., Sakai T., Kanaya H., Okada K. SHEPHERD is the Arabidopsis GRP94 responsible for the formation of functional CLAVATA proteins. EMBO J. 2002;21:898–908. doi: 10.1093/emboj/21.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. K., Kim K. S., Lee A. S. Regulation of the glucose-regulated protein genes by β-mercaptoethanol requires de novo protein synthesis and correlates with inhibition of protein glycosylation. J. Cell Physiol. 1987;133:553–559. doi: 10.1002/jcp.1041330317. [DOI] [PubMed] [Google Scholar]
- Koch G., Smith M., Macer D., Webster P., Mortara R. Endoplasmic reticulum contains a common, abundant calcium-binding glycoprotein, endoplasmin. J. Cell Sci. 1986;86:217–232. doi: 10.1242/jcs.86.1.217. [DOI] [PubMed] [Google Scholar]
- Lee A. S., Delegeane A. M., Baker V., Chow P. C. Transcriptional regulation of two genes specifically induced by glucose starvation in a hamster mutant fibroblast cell line. J. Biol. Chem. 1983;258:597–603. [PubMed] [Google Scholar]
- Lee J. E., Pintar J., Efstratiadis A. Pattern of the insulin-like growth factor II gene expression during early mouse embryogenesis. Development. 1990;110:151–159. doi: 10.1242/dev.110.1.151. [DOI] [PubMed] [Google Scholar]
- LeRoith D., Roberts C. T., Jr The insulin-like growth factor system and cancer. Cancer Lett. 2003;195:127–137. doi: 10.1016/s0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- Little E., Ramakrishnan M., Roy B., Gazit G., Lee A. S. The glucose-regulated proteins (GRP78 and GRP94): functions, gene regulation, and applications. Crit. Rev. Eukaryot. Gene Expr. 1994;4:1–18. doi: 10.1615/critreveukargeneexpr.v4.i1.10. [DOI] [PubMed] [Google Scholar]
- Liu H., Bowes R.C., 3rd, van de Water B., Sillence C., Nagelkerke J. F., Stevens J. L. Endoplasmic reticulum chaperones GRP78 and calreticulin prevent oxidative stress, Ca2+ disturbances, and cell death in renal epithelial cells. J. Biol. Chem. 1997;272:21751–21759. doi: 10.1074/jbc.272.35.21751. [DOI] [PubMed] [Google Scholar]
- Loo M. A., Jensen T. J., Cui L., Hou Y., Chang X. B., Riordan J. R. Perturbation of Hsp90 interaction with nascent CFTR prevents its maturation and accelerates its degradation by the proteasome. EMBO J. 1998;17:6879–6887. doi: 10.1093/emboj/17.23.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick T. S., McColl K. S., Distelhorst C. W. Mouse lymphoma cells destined to undergo apoptosis in response to thapsigargin treatment fail to generate a calcium-mediated grp78/grp94 stress response. J. Biol. Chem. 1997;272:6087–6092. doi: 10.1074/jbc.272.9.6087. [DOI] [PubMed] [Google Scholar]
- Melnick J., Dul J. L., Argon Y. Sequential interaction of the chaperones BiP and GRP94 with immunoglobulin chains in the endoplasmic reticulum. Nature. 1994;370:373–375. doi: 10.1038/370373a0. [DOI] [PubMed] [Google Scholar]
- Paris S., Denis H., Delaive E., Dieu M., Dumont V., Ninane N., Raes M., Michiels C. Up-regulation of 94-kDa glucose-regulated protein by hypoxia-inducible factor-1 in human endothelial cells in response to hypoxia. FEBS Lett. 2005;579:105–114. doi: 10.1016/j.febslet.2004.11.055. [DOI] [PubMed] [Google Scholar]
- Prelle K., Wobus A. M., Krebs O., Blum W. F., Wolf E. Overexpression of insulin-like growth factor-II in mouse embryonic stem cells promotes myogenic differentiation. Biochem. Biophys. Res. Commun. 2000;277:631–638. doi: 10.1006/bbrc.2000.3737. [DOI] [PubMed] [Google Scholar]
- Randow F., Seed B. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat. Cell Biol. 2001;3:891–896. doi: 10.1038/ncb1001-891. [DOI] [PubMed] [Google Scholar]
- Reddy R. K., Lu J., Lee A. S. The endoplasmic reticulum chaperone glycoprotein GRP94 with Ca(2+)-binding and antiapoptotic properties is a novel proteolytic target of calpain during etoposide-induced apoptosis. J. Biol. Chem. 1999;274:28476–28483. doi: 10.1074/jbc.274.40.28476. [DOI] [PubMed] [Google Scholar]
- Rosen K. M., Wentworth B. M., Rosenthal N., Villa-Komaroff L. Specific, temporally regulated expression of the insulin-like growth factor II gene during muscle cell differentiation. Endocrinology. 1993;133:474–481. doi: 10.1210/endo.133.2.8393762. [DOI] [PubMed] [Google Scholar]
- Schmitz A., Maintz M., Kehle T., Herzog V. In vivo iodination of a misfolded proinsulin reveals co-localized signals for Bip binding and for degradation in the ER. EMBO J. 1995;14:1091–1098. doi: 10.1002/j.1460-2075.1995.tb07092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T. W., Neckers L. M. The benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds to HSP90 and shares important biologic activities with geldanamycin. Cancer Chemother. Pharmacol. 1998;42:273–279. doi: 10.1007/s002800050817. [DOI] [PubMed] [Google Scholar]
- Smith J., Goldsmith C., Ward A., LeDieu R. IGF-II ameliorates the dystrophic phenotype and coordinately down-regulates programmed cell death. Cell Death Differ. 2000;7:1109–1118. doi: 10.1038/sj.cdd.4400738. [DOI] [PubMed] [Google Scholar]
- Sugawara S., Takeda K., Lee A., Dennert G. Suppression of stress protein GRP78 induction in tumor B/C10ME eliminates resistance to cell mediated cytotoxicity. Cancer Res. 1993;53:6001–6005. [PubMed] [Google Scholar]
- Sullivan K. A., Kim B., Buzdon M., Feldman E. L. Suramin disrupts insulin-like growth factor-II (IGF-II) mediated autocrine growth in human SH-SY5Y neuroblastoma cells. Brain Res. 1997;744:199–206. doi: 10.1016/S0006-8993(96)01078-5. [DOI] [PubMed] [Google Scholar]
- van Doorn J., Hoogerbrugge C. M., Koster J. G., Bloemen R. J., Hoekman K., Mudde A. H., van Buul-Offers S. C. Antibodies directed against the E region of pro-insulin-like growth factor-II used to evaluate non-islet cell tumor-induced hypoglycemia. Clin. Chem. 2002;48:1739–1750. [PubMed] [Google Scholar]
- Vogen S. M., Gidalevitz T., Biswas C., Simen B. S., Stein E., Gulmen F., Argon Y. Radicicol-sensitive peptide binding to the N-terminal portion of GRP94. J. Biol. Chem. 2002;277:40742–40750. doi: 10.1074/jbc.M205323200. [DOI] [PubMed] [Google Scholar]
- Wanderling S., Simen B. B., Ostrovsky O., Ahmed N. T., Vogen S., Gidalevitz T., Argon Y. GRP94 is essential for mesoderm induction and muscle development because it regulates IGF secretion. Mol. Biol. Cell. 2007;18:3764–3775. doi: 10.1091/mbc.E07-03-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Liu B., Dai J., Srivastava P. K., Zammit D. J., Lefrancois L., Li Z. Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity. 2007;26:215–226. doi: 10.1016/j.immuni.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]