Abstract
The Aurora kinase complex, also called the chromosomal passenger complex (CPC), is essential for faithful chromosome segregation and completion of cell division. In Fungi and Animalia, this complex consists of the kinase Aurora B/AIR-2/Ipl1p, INCENP/ICP-1/Sli15p, and Survivin/BIR-1/Bir1p. A fourth subunit, Borealin/Dasra/CSC-1, is required for CPC targeting to centromeres and central spindles and has only been found in Animalia. Here we identified a new core component of the CPC in budding yeast, Nbl1p. NBL1 is essential for viability and nbl1 mutations cause chromosome missegregation and lagging chromosomes. Nbl1p colocalizes and copurifies with the CPC, and it is essential for CPC localization, stability, integrity, and function. Nbl1p is related to the N-terminus of Borealin/Dasra/CSC-1 and is similarly involved in connecting the other CPC subunits. Distant homology searching identified nearly 200, mostly unannotated, Borealin/Dasra/CSC-1–related proteins from nearly 150 species within Fungi and Animalia. Analysis of the sequence of these proteins, combined with comparative protein structure modeling of Bir1p-Nbl1p-Sli15p using the crystal structure of the human Survivin–Borealin–INCENP complex, revealed a striking structural conservation across a broad range of species. Our biological and computational analyses therefore establish that the fundamental design of the CPC is conserved from Fungi to Animalia.
INTRODUCTION
Accurate segregation of genetic material into daughter cells requires the coordination of complex cellular events. The chromosomal passenger complex (CPC) is conserved from yeast to humans and plays a crucial role in many of these processes. It regulates chromosome condensation, chromosome biorientation, signaling to the spindle checkpoint machinery, spindle assembly, and cytokinesis (reviewed in Ruchaud et al., 2007). CPC functions are associated with dynamic changes in localization throughout the cell cycle. In animals, the CPC is diffusely associated with chromosomes in interphase, focused at inner centromeres in metaphase, localized at the central spindle in anaphase, and concentrated at the spindle midbody in telophase (reviewed in Ruchaud et al., 2007). In the budding yeast Saccharomyces cerevisiae, CPC localization is reported to be similar with the exception that it follows the plus ends of depolymerizing spindle microtubules (MTs) rather than remaining at the midzone in late anaphase (Buvelot et al., 2003).
In humans, the CPC consists of a serine-threonine kinase, Aurora B, and three other subunits (INCENP, Survivin, and Borealin/Dasra) that regulate kinase activity and localization. INCENP stimulates Aurora B kinase activity and, with Survivin and Borealin/Dasra, targets the kinase to the central spindle and midbody (Jeyaprakash et al., 2007). Borealin/Dasra (Gassmann et al., 2004; Sampath et al., 2004) also targets the CPC to the centromere via its C-terminus (Jeyaprakash et al., 2007).
The initial discovery of Aurora kinase (Ipl1p) was made in budding yeast (Chan and Botstein, 1993). This is the only Aurora kinase in yeast, whereas animals have Aurora A and B. Yeast homologues of only two of the other three CPC components (INCENP/Sli15p and Survivin/Bir1p) have been found (Kim et al., 1999; Cheeseman et al., 2002). In humans, three α-helices, one each from INCENP, Survivin, and Borealin/Dasra, interact to form a three-helix bundle (THB; Jeyaprakash et al., 2007). Because the resulting complex is essential for proper CPC targeting, whether a comparable mechanism exists in yeast is an important and unanswered question.
Here we report the identification of a new component of the yeast CPC that fills the role of Borealin/Dasra/CSC-1. Because homologues of Borealin/Dasra/CSC-1 have not been detected outside of Animalia, we performed Hidden Markov Model (HMM)–based searches for distant homologues and identified nearly 200 Borealin/Dasra/CSC-1–related proteins from nearly 150 species including 54 Fungi and one species outside the Fungi/Animalia group. More than 80% of these proteins have yet to be meaningfully annotated. Protein sequence alignment of Borealin/Dasra/CSC-1–, Survivin/Bir1p-, and INCENP/Sli15p-related proteins identified widely conserved residues that are essential for the integrity of the CPC. These results establish that the fundamental design of the CPC is conserved from fungi to animals.
MATERIALS AND METHODS
Strains and Growth Conditions
C-terminal 3GFP (Sun et al., 2007), mCherry (Wong et al., 2007), and S-TEV-ZZ (Cheeseman et al., 2002) tags were integrated into the endogenous loci of genes as previously described (Longtine et al., 1998). The nbl1Δ strain was constructed by replacing its open reading frame (ORF) with the NATR gene. Temperature-sensitive alleles of NBL1 were generated and integrated using a previously described method (Cheeseman et al., 2001), and the resulting 12,000 transformants were tested for temperature sensitivity. Yeast were grown on 2% glucose with yeast extract/bacto-peptone or synthetic minimal medium supplemented as needed.
CPC Purifications
In vivo cross-linking of the CPC for mass spectrometry analysis was done as previously described (Sanchatjate and Schekman, 2006) with a few modifications. Dithiobis[succinimidylpropionate] (Thermo Fisher Scientific, Rockford, IL) was added to a final concentration of 1.3 mM, and all steps were conducted at 37°C. Purification of the complex using Sli15-TAP (tandem affinity purification) was done as previously described (Cheeseman et al., 2002) except 300 mM KCl was used in purification buffers.
Protein Identification
The protein digest was pressure-loaded onto a fused silica capillary column containing 18 cm of 5-μm Polaris C18-A material (Metachem, Ventura, CA) packed into a 100-μm id capillary with a 5-μm pulled tip. The column with loaded peptides was washed with buffer containing 95% water, 5% acetonitrile, and 0.1% formic acid for desalting. It was then placed inline with an Agilent 1100 quaternary HPLC (Palo Alto, CA) and analyzed using a 180-min gradient LC run. The buffer solutions used were 5% acetonitrile/0.1% formic acid (buffer A) and 80% acetonitrile/0.1% formic acid (buffer B). Buffer B changed between 0 and 100% during the 180-min gradient with the following profile: a 12-min gradient from 0 to 15%, 108-min gradient from 15 to 45%, 30-min gradient from 45 to 75%, 5-min gradient from 75 to 100%, 5-min at 100%, 1-min gradient from 100 to 0%, and finally for 19 min at 0%.
As peptides were eluted from the microcapillary column, they were electrosprayed directly into an LTQ mass spectrometer (ThermoFinnigan, Palo Alto, CA) with the application of a distal 2.4 kV spray voltage. A cycle of one full-scan mass spectrum (400–1800 m/z) followed by five data-dependent MS/MS spectra at a 35% normalized collision energy was repeated continuously throughout each step of the multidimensional separation. Application of mass spectrometer scan functions and HPLC solvent gradients were controlled by the Xcalibur data system (Xcalibur Data Systems, Pittsburgh, PA).
MS/MS spectra were analyzed using the following software analysis protocol. Poor quality spectra were removed from the dataset using an automated spectral quality assessment algorithm (Bern et al., 2004). MS/MS spectra remaining after filtering were searched with the SEQUEST algorithm (Eng et al., 1994) against the Saccharomyces Genome Database (http://seq.yeastgenome.org/cgi-bin/blast-fungal.pl; December 16, 2005) concatenated to a decoy database in which the sequence for each entry in the original database was reversed (Peng et al., 2003). No enzyme specificity was considered for any search. SEQUEST results were assembled and filtered using the DTASelect (version 2.0) program (Tabb et al., 2002; Cociorva et al., 2007). DTASelect 2.0 uses a linear discriminant analysis to dynamically set XCorr and DeltaCN thresholds for the entire dataset to achieve a user-specified false-positive rate (5% in this analysis). The false-positive rates are estimated by the program from the number and quality of spectral matches to the decoy database.
The resulting protein list was used to create a subset database to expedite SEQUEST differential modification searches. The MS/MS spectra were then researched four times against the subset database to consider modifications of +80 on lysine for phosphorylation and +88.12 on lysine for the cleaved cross-linker. The MS/MS spectra for the modified peptides were manually evaluated using criteria reported previously (Link et al., 1999). Modified peptide spectra exceeding these criteria were researched using SEQUEST against the National Center for Biotechnology Information (NCBI) nonredundant protein database. Confidence for modifications was estimated from overlapping modified peptides as described previously (MacCoss et al., 2002).
In Vitro Kinase Assay
In vitro kinase assays were done as previously described (Cheeseman et al., 2002). Dam1 complex was purified as previously described (Westermann et al., 2005). Ipl1-Sli15 and Ipl1-Sli15-Bir1-Nbl1 complexes were purified by TAP purification (see CPC purification in Materials and Methods) and by gel filtration using a Superose 6 column (GE Healthcare, Waukesha, WI). The amount of Ipl1p in each fraction was quantified by a SDS-PAGE gel stained with SYPRO Ruby (Molecular Probes, Eugene, OR) using a serial dilution of BSA as a standard. 0.15 pmol of Ipl1p was used to phosphorylate 10 pmol of Dam1 complex.
Fluorescence Microscopy
Indirect fluorescence microscopy was done as previously described (Cheeseman et al., 2002) with the following changes. Cells were synchronized in G1 using α-factor at 23°C and released at 37°C, and then samples were fixed every 10 min. Rabbit anti-green fluorescent protein (GFP) antibody (Torrey Pines Biolabs, San Diego, CA) was used at 1:2000. Images were acquired using an Olympus IX71 (Melville, NY) equipped with 100-Å NA 1.4 objectives and an Orca II camera (Hamamatsu, Bridgewater, NJ). Live cell microscopy and simultaneous imaging of mCherry and GFP were done as previously described (Kaksonen et al., 2005) using an Olympus IX81 microscope equipped with 100-Å, NA 1.4 objectives and an Orca II camera.
Images were processed using the following software: Huygens Deconvolution (Scientific Volume Imaging, Hilversum, The Netherlands), ImageJ (http://rsb.info.nih.gov/ij/), iVision (BioVision Technologies, Exton, PA), and Imaris (Bitplane, Zurich, Switzerland).
Yeast Two-Hybrid Assays
Constructs were made in pADC and pBDC (Millson et al., 2003) for expression of the yeast CPC subunits (Sli15p, Bir1p, and Nbl1p) fused (N- and/or C-terminally) to the activation and DNA-binding domains of Gal4p, respectively. In addition, partial constructs were made for SLI15 and BIR1 (Supplemental Table S1).
Screening was conducted as described elsewhere (Uetz et al., 2000) with the following changes. Growth assays were conducted on media without leucine, tryptophan, and adenine as well as on media without leucine, tryptophan, and histidine supplemented with 3 mM 3-amino-1,2,4-triazole. The reported growth results are based on consistent behavior on both these media for reciprocal crosses.
Nbl1p Sequence Analysis
To find remote Borealin homologues, we started with the human Borealin (Homsa_Bor) and used PSI-BLAST (http://www.ncbi.nlm.nih.gov/blast/) with default settings to collect a group of well-conserved homologues. This list was supplemented by using tBLASTn to search EST and genomic sequence databases with an E-value cutoff of 1e-05. When multiple nucleotide sequences were found, CAP3 (Huang and Madan, 1999) was used to assemble them via the Mobyle portal (http://mobyle.pasteur.fr/). WISE2 (http://www.ebi.ac.uk/Wise2/; Birney et al., 2004), AUGUSTUS (http://augustus.gobics.de/), and direct translation of ORFs were used to derive protein from nucleotide sequences. These predictions were validated using BLASTp (NCBI). MUSCLE (http://www.drive5.com/muscle/; Edgar, 2004) was then used to align these sequences. A HMM constructed from this alignment (Eddy, 1998) was used to find related sequences using an E-value cutoff of 0.1 (Mobyle portal). This process was iterated and CSC-1 (Gassmann et al., 2004) was added to the alignment after several rounds of searching and model building. This procedure identified likely homologues of Borealin/Dasra/CSC-1 in many fungi but not in Saccharomyces. An alignment of the six Saccharomyces Nbl1p sequences with their syntenous homologues from within Saccharomycotina was ultimately aligned to the Borealin/Dasra/CSC-1–related proteins found above using the –profile option in MUSCLE. The syntenous homologues were found based on the genes flanking NBL1 in Saccharomyces spp. by using the Yeast Gene Order Browser (http://wolfe.gen.tcd.i.e.,/browser/; Byrne and Wolfe, 2006).
Alignments and secondary structures were displayed using the ESPript 2.2 (http://espript.ibcp.fr/; Gouet et al., 1999) with similarity calculations based on BLOSUM62 (http://www.ncbi.nlm.nih.gov/Class/BLAST/BLOSUM62.txt; Henikoff and Henikoff, 1992) using a global score of 0.1 and a difference score of 0.2. Nuclear localization signals were predicted using WoLF PSORT (http://www.wolfpsort.org/; Horton et al., 2007).
Comparative Structure Modeling of Bir1p889–941–Nbl1p8–67–Sli15p3-46
Protein sequence alignments for Bir1p889-941 and Sli15p3-46 were conducted as described for Borealin above. These alignments were used to generate a model using the crystal structure of Survivin–Borealin10-109–INCENP1-58 complex (PDB ID: 2QFA; Jeyaprakash et al., 2007) using MODELLER (http://www.salilab.org/modeller; Sali and Blundell, 1993). Optimization was carried out for 10 models using the variable target function method (Braun and Go, 1985), and the process was repeated five times. The best model was chosen based on the Discrete Optimized Protein Energy (DOPE) and conditional probability density functions (pdf; Sali and Overington, 1994). The pdf and DOPE scores of the model are 376.9 and −14,656.2, respectively. The DOPE score for the region of template 2QFA that is aligned with the model is −20,029.0. Images were generated using PyMOL0.99 (DeLano Scientific, South San Francisco, CA).
RESULTS
Identification of a Novel CPC Subunit in Yeast
Although the role of the CPC in attachment of kinetochores to opposing spindle poles (chromosome biorientation) is established, the spatial and temporal regulation of this complex is not fully understood. To explore these regulatory mechanisms, we purified the complex from metaphase-arrested cells using TAP and identified all associated proteins by mass spectrometric analysis. Together with the known CPC components, we identified a 73-aa protein encoded by a hypothetical ORF, YHR199C-A (Cliften et al., 2003). We conducted RT-PCR to verify expression of this ORF and found that the product lacked its 66-base pair intron, suggesting the expression of a functional mRNA (Figure 1A). We have named this ORF NBL1 (N-terminal-Borealin-like protein 1) for reasons detailed below.
Figure 1.
Nbl1p is part of the CPC. (A) Confirmation of NBL1 expression by RT-PCR using duplicate cDNA samples. gDNA, genomic DNA (B) Growth phenotype of nbl1-6. Serial 10-fold dilutions of NBL1 and nbl1-6 cells on synthetic minimal medium plates incubated at 25 or 37°C for 48 h. (C) Nbl1p copurifies with the other CPC components. Sli15-TAP and Nbl1-TAP purified complexes. Top, silver-stained 12% discontinuous gel. Note that Nbl1p is too small to be seen on a discontinuous gel that is able to resolve Sli15p and Bir1p. † Bands that always copurify with Sli15-TAP (Cheeseman et al., 2002). * Contaminants. Bottom, Western blot of 4–20% continuous gel.
NBL1 Is Required for Viability and Encodes a Protein That Copurifies with the CPC
All known CPC components are essential for viability in yeast (Chan and Botstein, 1993; Biggins et al., 1999; Kim et al., 1999; Li et al., 2000). By deleting its ORF, we found that NBL1 is also required for cell viability. After 24 d, 27% of spores carrying nbl1Δ had formed slow-growing colonies with elongated and multibudded polyploid cells (Supplemental Figure S1A). Similar slow-growing spore colonies with these morphological characteristics were reported for bir1Δ (Li et al., 2000; Sandall et al., 2006). To explore NBL1 function further, we generated the temperature-sensitive nbl1-6 mutant that grows normally at 25°C, but has a severe growth defect at 37°C (Figure 1B). This allele also has a synthetic growth defect when combined with ipl1-2 at 25°C (Supplemental Figure S1B).
We biochemically tested the association of Nbl1p with the CPC using reciprocal TAPs. TAP-tagged Nbl1p (Nbl1-TAP) pulled down Ipl1p, Sli15p, and Bir1p, indicating that Nbl1p is a part of the CPC (Figure 1C). Nbl1-TAP and Sli15-TAP pull downs showed equivalent amounts of Bir1p and Sli15p. This is most clearly shown by Western blotting (Figure 1C, bottom); untagged Sli15p stains less efficiently with silver than Sli15-TAP. Purifications were done using the same number of cells, and these cells only contained a single copy of NBL1 or SLI15. The simplest explanation for this observation is that Nbl1p interacts with most, if not all, cellular Sli15p, and with the Sli15-associated Bir1p. In contrast, Nbl1-TAP pulled down less Ipl1p than Sli15-TAP, implying that Nbl1p and Ipl1p may not interact directly.
Nbl1p Is Essential for Chromosome Segregation and Metaphase Chromosome Alignment
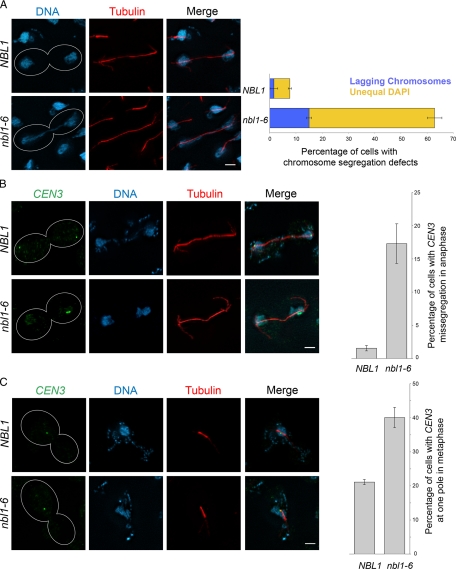
The CPC is required for bipolar chromosome orientation, proper metaphase chromosome alignment, and subsequent accurate chromosome segregation (reviewed in Ruchaud et al., 2007). To determine if Nbl1p has a role in these processes, we examined the DNA and spindle morphologies of nbl1 mutants. We also evaluated localization of a GFP-labeled centromere III proximal region (CEN3; Straight et al., 1996). In nbl1-6, 63% of anaphase cells showed an abnormal DNA distribution, 48% showed unequal DNA distribution, and 15% showed lagging chromosomes (Figure 2A). In addition, consistent with defects in chromosome condensation, the DNA mass was more diffuse and the CEN3 dots were larger in nbl1-6 cells (Figure 2, A–C). Moreover, 17% of the nbl1-6 cells missegregated CEN3 (Figure 2B). This phenotype is similar to the chromosome missegregation caused by mutations in IPL1 and SLI15 (Chan and Botstein, 1993; Biggins et al., 1999; Kim et al., 1999), and the proteins encoded by these genes are known to facilitate chromosome biorientation (Cheeseman et al., 2002; Tanaka et al., 2002). Although the missegregation rate observed for ipl1-2 or sli15-3 cells was higher (Chan and Botstein, 1993; Tanaka et al., 2002), those alleles are completely lethal at the restrictive temperature, whereas the nbl1-6 allele only causes a growth defect. We therefore expect that the different rates of chromosome missegregation are due largely to differences in allele strength.
Figure 2.
Nbl1p is required for faithful chromosome segregation. (A) DNA morphology of NBL1 and nbl1-6 in anaphase. Left, representative images of haploid cells fixed and stained with anti-tubulin antibody and DAPI. The nbl1-6 image shows an example of lagging chromosomes. Bar, 2 μm. Right, mean percentage and SD for three replicates (μ+σ) of cells (n = 100) with abnormal DNA morphology, lagging chromosomes (blue), and unequal DAPI staining (yellow). (B) Segregation of centromere III proximal region (CEN3) in NBL1 and nbl1-6 in anaphase. Left, representative images showing cells with GFP marked CEN3. Bar, 2 μm. Right, percentage (μ+σ) of cells (n = 100) with CEN3 missegregation. (C) CEN3 positioning relative to the metaphase spindle in NBL1 and nbl1-6. Left, representative images showing GFP marked CEN3, DNA localization, and the spindle. Right, percentage (μ+σ) of cells (n > = 80) showing CEN3 within 0.2 μm of one spindle pole. At metaphase, pairs of CEN3 markers are unseparated because this marker is 23 kb away from the centromere (Straight et al., 1996).
Because the animal CPC functions in chromosome alignment, we investigated Nbl1p's role in this process. In budding yeast, metaphase centromeres tend to cluster between the two spindle poles (Pearson et al., 2001). We therefore compared metaphase CEN3 localization in wild-type and nbl1-6 cells. Seventy-nine percent of wild-type cells showed unseparated CEN3 dots in the middle of the spindle (Figure 2C), whereas 21% showed CEN3 at or near one spindle pole. This is consistent with previous observations for the localization of this CEN3 marker (Straight et al., 1997; Pearson et al., 2001). In contrast, 40% of nbl1-6 cells showed unseparated CEN3 at or near one spindle pole (Figure 2C). This result indicates that Nbl1p facilitates proper chromosome positioning in metaphase.
Nbl1p Colocalizes with the CPC throughout the Cell Cycle
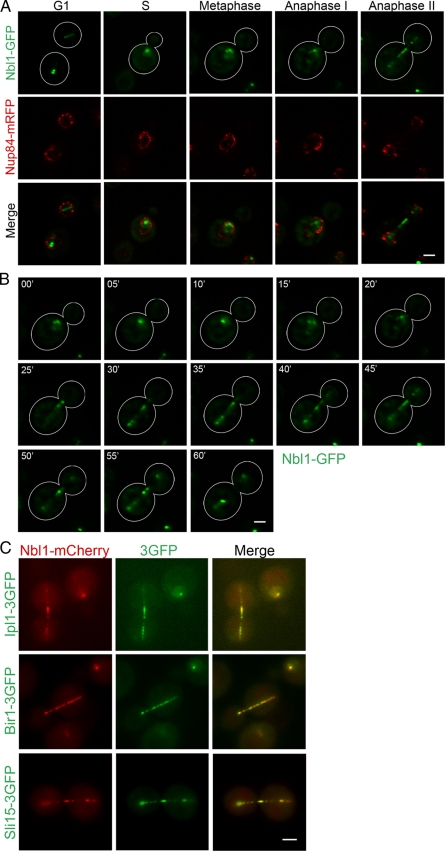
To explore the extent of Nbl1p colocalization with the CPC, we tagged Nbl1p with GFP at the endogenous NBL1 locus so that Nbl1-GFP was the only form of Nbl1p in the cells. Addition of the GFP tag caused no detectable change in growth at temperatures ranging from 16 to 39°C (unpublished data). Nbl1p localization changed throughout the cell cycle in a pattern reminiscent of that reported for the other CPC proteins (Buvelot et al., 2003). In G1, Nbl1p was observed along nuclear MTs and at spindle poles (Figure 3A, G1; Supplemental Figure S2B). In late S phase to metaphase, Nbl1p was seen as a few small nuclear foci and also localized diffusely within the nucleus (Figure 3A, S and Metaphase, and B, 0–15 min). This localization is different from that of most kinetochore proteins (Supplemental Figure S2A). Nbl1p was also found along the spindle in early anaphase (Figure 3A, Anaphase I, and B, 20–45 min), at the spindle midzone in late anaphase (Figure 3A, Anaphase II, and B:, 50–55 min), and finally concentrated at the plus ends of depolymerizing MTs before mitotic exit (Figure 3B, 60 min). We verified that Nbl1-mCherry colocalized with the known CPC components (Ipl1p, Sli15p, and Bir1p) tagged with 3GFP throughout the cell cycle (Figure 3C; unpublished data). This result combined with our biochemical analysis lead us to conclude that Nbl1p is part of the CPC.
Figure 3.
Nbl1p colocalizes with the CPC. (A) Representative images of diploid cells homozygous for NBL1-GFP and NUP84-mRFP from G1 to telophase. Images were acquired every 5 min with seven Z-sections separated by 0.5 μm and deconvolved. Bar, 2 μm. (B) Representative time-lapse images of Nbl1-GFP from the above cells. Images were acquired and processed as above. Bar, 2 μm. (C) Nbl1p colocalizes with CPC components. Representative images from diploid cells homozygous for NBL1-mCherry and SLI15-, IPL1-, or BIR1-3GFP. mCherry and GFP were simultaneously imaged using an optical beam splitter. Bar, 2 μm.
Nbl1p Functions in the Stability and Integrity of the CPC by Mediating the Sli15p-Bir1p Interaction
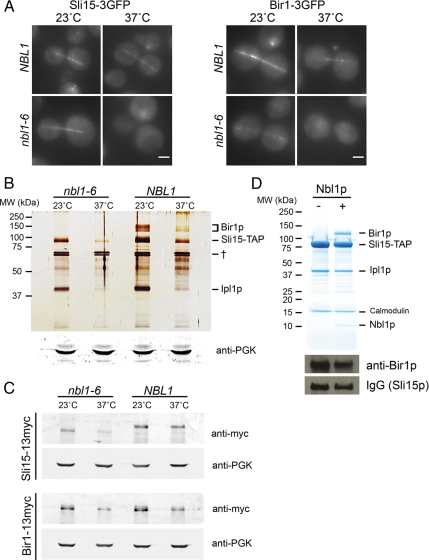
Because all four subunits are essential for proper CPC localization in animals (reviewed in Ruchaud et al., 2007), we tested whether Nbl1p is required for CPC localization in yeast by examining 3GFP-tagged Sli15p and Bir1p in nbl1-6 cells. At the restrictive temperature (37°C), the nbl1-6 mutation diminished the Sli15-3GFP signal and abolished that of Bir1-3GFP (Figure 4A). Although high temperature decreased the overall fluorescent signal in wild-type cells (see Supplemental Text for discussion), this effect was significantly more pronounced in the nbl1-6 mutant.
Figure 4.
Nbl1p is essential for CPC integrity, stability, and localization. (A) Localization of Sli15p and Bir1p depends on Nbl1p. Localization of Sli15-3GFP (left) and Bir1-3GFP (right) in NBL1 and nbl1-6 haploid strains in anaphase at 23 and 37°C. Bar, 2 μm. (B) CPC integrity and stability requires Nbl1p. Top, silver stain of a 12% discontinuous gel showing complexes purified using Sli15-TAP from NBL1 and nbl1-6 strains exposed to 23 or 37°C for 3 h. Bottom, Western blot of whole cell extracts used for the purification using anti-PGK antibody. (C) Protein levels of Sli15p and Bir1p in NBL1 and nbl1-6 cells. (top) Western blot of whole cell extracts from NBL1 and nbl1-6 cells bearing a single copy of BIR1-13myc at the BIR1 locus using an anti-myc antibody. The cells were treated as described in B. The same blot was reprobed with an anti-PGK antibody as a loading control. Bottom, Western blot of whole cell extracts from NBL1 and nbl1-6 cells bearing a single copy of SLI15-13myc at the SLI15 locus using an anti-myc antibody. The same blot was reprobed with an anti-PGK antibody as a loading control. (D) Interaction between Bir1p and the Sli15p-Ipl1p subcomplex requires Nbl1p. Top, colloidal blue–stained 4–20% continuous gel showing the CPC purified from insect cells transfected with virus expressing Ipl1p, Sli15p, and Bir1p in the presence (right lane) or absence (left lane) of virus expressing Nbl1p. Bottom, Western blot of whole cell extracts used for the purification (top) using anti-Bir1p antibody and purified rabbit IgG for Sli15-TAP detection.
To test whether this mislocalization was caused by the disruption of CPC integrity in nbl1-6 cells, we purified the CPC using TAP-tagged Sli15p from wild-type and from nbl1-6 cells that were incubated at either 23 or 37°C for 3 h (Figure 4B). While in wild-type cells Bir1p copurified with Sli15-TAP, no detectable Bir1p copurified with Sli15-TAP from nbl1-6 cells at 37°C. In these mutant cells, there was also a significant decrease in Bir1p association with Sli15p at 23°C that correlated with the decreased Bir1-3GFP signal at this temperature (Figure 4A). To explore if lack of the functional Nbl1p affected the protein stability of Bir1p or Sli15p, we assessed the Bir1p and Sli15p levels in wild-type and nbl1-6 cells. The amount of Bir1p was comparable between wild-type and mutant cells grown at the same temperature, whereas the amount of Sli15p was reduced in nbl1-6 cells (Figure 4C). Thus, at the restrictive temperature, the defect in nbl1-6p abolishes the association of Bir1p with Sli15p even though both proteins are still present. The markedly reduced amount of Sli15p purified from nbl1-6 compared with wild-type cells at both 23 and 37°C (Figure 4, B and C) implies that lack of functional Nbl1p reduces the stability of Sli15p. These data suggest a role for Nbl1p in the integrity of the CPC by mediating the association of Sli15p with Bir1p and that integrity of the CPC contributes to the stability of Sli15p. The decrease in the level of Sli15p and Bir1p seen at 37°C in wild-type cells is discussed in the Supplemental Text.
To investigate whether Nbl1p is required for the Sli15p-Bir1p interaction using a complementary approach, we used the baculovirus expression system. Sf9 cells were transfected with viruses expressing Ipl1p, Sli15p, and Bir1p in the presence or absence of virus expressing Nbl1p. All proteins were verified to be present in equivalent amounts (Figure 4D). The complex was then purified using the Sli15-TAP tag. No Bir1p copurified with Sli15-TAP in the absence of Nbl1p (Figure 4D), indicating that Nbl1p is sufficient to mediate the interaction between Sli15p and Bir1p.
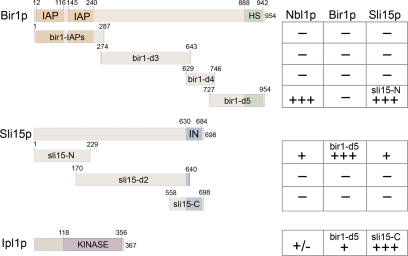
In humans, the N-terminus of Borealin/Dasra interacts with Survivin and the N-terminal domain of INCENP (Klein et al., 2006). Because Nbl1p is required for the Sli15p-Bir1p interaction in yeast in the same way that Borealin/Dasra/CSC-1 is required for the interaction of Survivin/BIR-1 with INCENP/ICP-1 in animals (Romano et al., 2003; Klein et al., 2006), we assessed whether the organization of the CPC is also conserved from Fungi to Animalia using yeast two-hybrid analysis of various domains of the yeast CPC components (Figure 5). Nbl1p strongly interacted with the C-terminal region of Bir1p (bir1-d5), which is homologous to the non-IAP (inhibitor of apoptosis) domain of Survivin (HS; Thomas and Kaplan, 2007). Nbl1p also interacted with the N-terminus of Sli15p (sli15-N). We also found that Nbl1p weakly interacts with Ipl1p, but this interaction could be mediated by the endogenous Sli15p because the conserved C-terminal domain of Sli15p/INCENP interacts with Ipl1p/Aurora (Adams et al., 2000; Kang et al., 2001).
Figure 5.
Nbl1p interacts with the C-terminus of Bir1p and the N-terminus of Sli15p. Yeast two-hybrid analysis of Nbl1p and various regions of the other CPC components. Relative strength of interactions is indicated: −, none; −/+, weak; and +++, very strong. All interactions occurred reciprocally among “bait” and “prey” constructs containing these domains. The known domains are shown in color: IAP, inhibitor of apoptosis domain; HS, homologous to Survivin (Thomas and Kaplan, 2007); IN, IN-box (Ipl1p/Aurora B interacting).
To assess Nbl1p's role in the regulation of Ipl1p kinase activity, we performed an in vitro kinase assay using either the binary (Ipl1p-Sli15p) or quaternary (Ipl1p-Sli15p-Bir1p-Nbl1p) complex, with a known in vivo substrate, the Dam1 complex (Cheeseman et al., 2002). The binary complex phosphorylated Dam1 complex components (Dam1p, Ask1p, and Spc34p) more extensively than the quaternary complex (Supplemental Figure S1), indicating that Nbl1p and Bir1p are not required for Dam1 complex phosphorylation in vitro, but possibly have a role in modulating the strength of the kinase activity.
Sequence Similarity of Nbl1p to Borealin/Dasra/CSC-1 and the Phylogenetic Distribution of Borealin-like Proteins
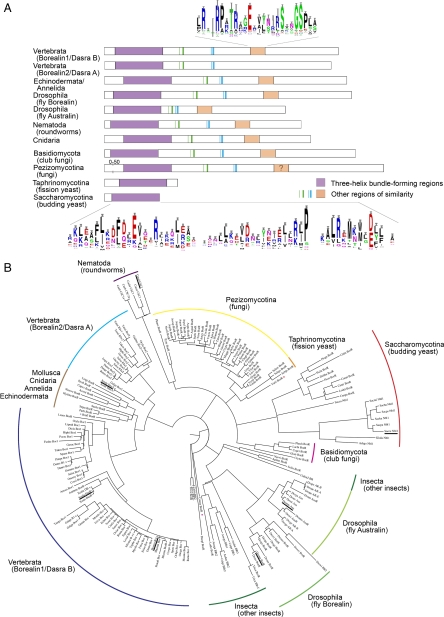
On the basis of the functional conservation between Nbl1p and Borealin/Dasra/CSC-1, we sought to determine whether these proteins are also homologous. Because no Nbl1p homologues outside of Saccharomyces were detectable using PSI-BLAST or the Saccharomyces Genome Database Fungal Genomes Search, we used a more sensitive method of remote homology detection. This entailed constructing a HMM of Borealin/Dasra/CSC-1 and related sequences. This HMM was used to find homologous sequences that were then used to refine the model. This iterative procedure (see Materials and Methods for details and references) led to the identification of nearly 200 likely homologues of Borealin/Dasra/CSC-1 from nearly 150 species throughout Animalia and Fungi, but not in Saccharomyces (Figure 6A). However, our results do contain a putative homolog in the parasitic protozoa Trichomonas vaginalis, which is in Parabasalidea, a sister taxa to the Fungi/Animalia group (Simpson et al., 2006). The phylogenetic distribution of the Borealin/Dasra/CSC-1–related proteins indicates that these proteins originated before the divergence of the Fungi and Animalia. In light of this observation and the strong functional overlap between Nbl1p and Borealin, the apparent lack of similarity between the putative Saccharomyces Borealin homolog, Nbl1p, and Borealin/Dasra/CSC-1 is quite intriguing.
Figure 6.
Nbl1p is a highly diverged homolog of Borealin/Dasra/CSC-1. (A) Block diagram summarizing features of sequences similar to Borealin/Dasra/CSC-1 from various fungal and animal taxa. Frequency of amino acid occurrence in conserved regions from the protein sequence alignment are shown as sequence logos (WebLogo: http://weblogo.berkeley.edu/; Crooks et al., 2004). (B) Phylogeny of Borealin/Dasra/CSC-1–related sequences. Consensus Parsimony tree with branch lengths estimated by Maximum Likelihood using PHYLIP v. 3.68 (http://evolution.genetics.washington.edu/phylip.html). Default settings were used and the sequence order was jumbled 10 times for the 1000 bootstrap replicates. The tree was based on the THB-forming domains from proteins listed in Supplemental Table S2A. Protein sequences missing more than 10% of this region were excluded. Borealin/Dasra/CSC-1–related sequences that have been characterized are underlined.
Protein sequence alignment of the Borealin/Dasra/CSC-1–related proteins found above did reveal a conserved region at or near the N-termini that corresponds to the α-helical region of Borealin/Dasra/CSC-1 known to participate in a THB with INCENP and Survivin in humans (Jeyaprakash et al., 2007). Because Nbl1p mediates interaction between the respective yeast orthologues, Sli15p and Bir1p, we investigated whether there might be conservation between Nbl1p and the N-termini of Borealin/Dasra/CSC-1. We aligned the six Saccharomyces Nbl1p sequences and their syntenous homologues to the Borealin/Dasra/CSC-1–related proteins found using the HMM procedure described above (Figure 6A, see Materials and Methods for details and references). The Nbl1p sequences aligned with the THB-forming domain and showed conservation along the entire length of the THB-forming domain with human Borealin/Dasra/CSC-1. The conserved residues are frequently hydrophobic, often with adjacent charged residues, and parallel many that have been shown to be important to the structure of the human CPC (discussed below). Furthermore, there is extensive overlap between the α-helical structures predicted for the Nbl1p proteins and those structurally determined for human Borealin (unpublished data). Nbl1p, however, terminates soon after the THB-forming domain with no further significant regions of similarity, as do the other proteins from the same subphylum (Saccharomycotina) and the fission yeasts (Schizosaccharomyces, Taphrinomycotina). In contrast, the sequences of the Borealin/Dasra/CSC-1 like proteins from Schizosaccharomyces, Taphrinomycotina continue with small regions of similarity to the corresponding portions of the animal proteins (Figure 6A; Supplemental Text).
Phylogenetic analysis places S. cerevisiae Nbl1p and its syntenous homologues within the group containing the other Saccharomycotina Borealin/Dasra/CSC-1-like proteins (Figure 6B); this analysis was based on the THB-forming domain of proteins found using our distant homology search. This grouping has 25% bootstrap support due to the short region of homology on which the analysis depends and the highly diverged nature of the Nbl1ps. Maximum likelihood analysis, however, also supports this grouping of these taxa (p < 0.01). This association indicates that the Nbl1ps are related to the other fungal Borealin/Dasra/CSC-1-like proteins. Interestingly, the other yeasts (Schizosaccharomyces) that lack the C-terminal region are in a different subphylum of Ascomycota (Taphrinomycotina). Because the Borealin/Dasra/CSC-1–related proteins from the third Ascomycota subphylum Pezizomycotina have a C-terminal region, this region has probably been lost independently in Saccharomycotina and Taphrinomycotina. Taken together with the functional conservation documented earlier in this study, the patterns of sequence similarity and phylogenetic relationship suggest that Nbl1p is likely to be a diverged homolog of Borealin that retains only the THB-forming domain.
Conservation of the THB-forming Domains of CPC Proteins across Fungi and Animalia
The protein sequence alignment of Borealin/Dasra/CSC-1–related proteins shows a clear pattern of hydrophobic residues at conserved positions that is consistent with conservation of the amphipathic helix seen in the human CPC structure (Jeyaprakash et al., 2007). To examine how well Nbl1p fits this structure and to explore further the possible role of the conserved elements in Nbl1p, Bir1p, and Sli15p, we built a structural model of Nbl1p complexed with the N-terminus of Sli15p and the C-terminus of Bir1p using the crystal structure of the human Survivin–Borealin10-109– INCENP1-58 complex as a template (Jeyaprakash et al., 2007; see Materials and Methods). Because of low sequence identity between yeast and human CPC proteins, we also created protein sequence alignments of Survivin/Bir1p and INCENP/Sli15p with the HMM-based method used to find putative Borealin homologues in Fungi. This procedure allowed us to have increased confidence in identifying homologous THB-forming regions and therefore enhanced the validity of our structural model (see Materials and Methods).
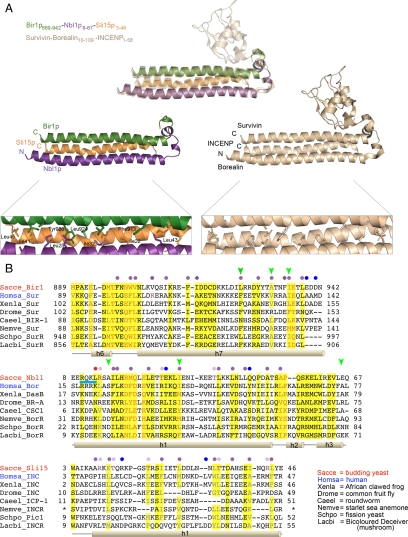
The resulting model for the yeast proteins (Bir1p889-941–Nbl1p8-67–Sli15p3-46) shows a bundle of three helices similar to what is seen in the crystal structure of the human CPC (Figure 7A). The THB made of the human Survivin, Borealin15-76, and INCENP3-47 is shown to be stabilized by interactions among interdigitated residues that can form hydrophobic interactions and face toward the center of the THB. The residues involved in hydrophobic interactions include lysines, arginines, and tyrosines whose charged or polar ends face away from the center of the THB, whereas the hydrophobic portions pass through and contribute to the hydrophobic core of the THB. Threonines are also occasionally seen at the hydrophobic positions. In these cases they appear to form a hydrogen bond with the peptide backbone, thereby masking their polarity and thus acting as a small aliphatic residue. These hydrophobic interactions occur among all three helices and are supplemented by seven electrostatic interactions linking each pair of proteins (Jeyaprakash et al., 2007). Our model shows that 84.8% of the residues at positions corresponding to those involved in hydrophobic interactions in the human CPC are also likely to be making hydrophobic contacts in the yeast THB (Figure 7B, purple and pale purple dots); note that we only analyzed the region of the main THB (Survivin helix 5, Borealin helix 1, and INCENP helix 1; see Figure 7B) because of the low confidence in correctly modeling loop regions. There is also one hydrophobic residue that faces the interior of the THB that is not seen in the human complex (Figure 7B, red dot). In addition to the hydrophobic interactions, there is one conserved electrostatic interaction (Bir1p K920-Sli15p D27) and four potential alternative electrostatic interactions among the helices based on the distance between the alpha carbons and length of the side chains.
Figure 7.
Fundamental design of the CPC is conserved from Fungi to Animalia. (A) (top) A comparative protein structure model of Bir1p889-941-Nbl1p8-67-Sli15p3-46 (green, purple, and orange, respectively) superimposed with the human Survivin–Borealin10-109–INCENP1-58 complex (pale brown; Jeyaprakash et al., 2007). The overall protein sequence identity between these regions is 13.4%. Information on model evaluation is in Materials and Methods. Middle, images of the structural model of Bir1p889-941-Nbl1p8-67-Sli15p3-46 (left) and the template crystal structure of the human Survivin–Borealin10-109–INCENP1-58 complex (right). Bottom, enlarged view showing the residues with hydrophobic properties that are directed toward the core of the THB. (B) Protein sequence alignment of the regions of Survivin/Bir1p, Borealin/Dasra/CSC-1/Nbl1p, and INCENP/Sli15p used for homology-based structure modeling. Residues showing conservation, based on the BLOSSUM62 matrix, in the multispecies alignment are shown with a yellow background; the most similar (global score cutoff = 0.2) are shown in orange, less similar (global score cutoff = 0.1) are shown in black, and those only partially conserved (conserved in more than two thirds of sequences, global score cutoff = 0.1) are shown in gray (see Materials and Methods for details). Secondary structure elements are shown below the protein sequences. Hydrophobic residues facing toward the interface between the helices are seen both in the crystal structure of the human Survivin–Borealin10-109–INCENP1-58 and in the homology-based model of Bir1p889-941–Nbl1p8-67–Sli15p3-46 (purple circles), only in the human Survivin–Borealin10-109–INCENP1-58 (blue circles), or only in the Bir1p889-941–Nbl1p8–67–Sli15p3-46 model (red circles). Arginines (R) and threonines (T) that are potentially involved in hydrophobic interactions in the structural model (pale purple text) and correlate with hydrophobic residues in the human structure (pale purple circles). Residues in Bir1p and Nbl1p whose mutation disrupts the integrity of the CPC (green arrowheads). Residues at the N-terminus of Borealin that were shown to be required for relocalization of the human CPC to the central spindle (blue line). Protein sequences from representative species of fungi, vertebrates, and invertebrates are shown: Sacce, S. cerevisiae; Homsa, Homo sapiens; Xenla, Xenopus laevis; Drome, Drosophila melanogaster; Caeel, Caenorhabditis elegans; Nemve, Nematostella vectensis; Schpo, Schizosaccharomyces pombe; Lacbi, Laccaria bicolor. Protein name abbreviations: Sur, Survivin; SurR, Survivin-related protein; Bor, Borealin; BorR, Borealin-related protein; Nbl1, N-terminal-Borealin-like protein; INC, INCENP; INCR, INCENP-related protein. * Unknown sequence position because of incomplete cDNA sequence.
The temperature-sensitive nbl1-6 allele caused chromosome missegregation and disrupted the interaction between Bir1p and Sli15p. The nbl1-6 protein contains the mutations A16V, I32N, and E66A. Nbl1p I32 is one of the conserved hydrophobic residues predicted to be involved in the interaction with the helices derived from Bir1p and Sli15p. Nbl1p A16 and E66 are at positions with little conservation among species (Figure 7B), though the position aligning with A16 is almost never valine in any species. Replacing the conserved hydrophobic residue with a polar amino acid (I32N), together with other minor effects from A16V and possibly E66A, may weaken the interaction among the helices leading to thermal instability of the THB and possibly its component proteins. This interpretation is consistent with the results shown in Figure 4B. Further supporting the homology-based structural model of the yeast CPC, a number of previously reported temperature-sensitive mutants in BIR1 also have alterations in hydrophobic residues that face toward the core of the helical bundle in our model (Figure 7A). First, mutation of two conserved hydrophobic residues at the C-terminus of Bir1p to glutamic acids [bir1 (A931E I935E)] was previously reported to disrupt its interaction with Sli15p and to increase chromosome missegregation and cell growth defects (Thomas and Kaplan, 2007). In addition, mutation from alanine to threonine of the residue corresponding to Bir1p-A931 in fission yeast results in temperature sensitivity (Morishita et al., 2001). Second, two temperature-sensitive bir1 alleles were found to contain mutations in L924 (to S and R; Shimogawa et al., 2009), suggesting that changing this conserved hydrophobic position to either a polar or charged residue also contributes to destabilization of the THB. Strikingly, 68.8% of the residues apparently involved in hydrophobic interactions among the helices (Jeyaprakash et al., 2007) are at positions conserved across the nearly 200 fungal and animal proteins in our analysis (Figure 7B, purple, pale purple, and blue dots above highlighted columns).
Our multispecies protein sequence alignment provides insight into the constraints on various regions of the THB. First, the C-terminal portion of the THB-forming domain in Borealin/Dasra/CSC-1–related proteins tolerates insertions and deletions of amino acids in a number of groups including Saccharomyces and a basal animal, Trichoplax adhaerens (Figure 7B; unpublished data). These variations map to the loop region between the main THB-forming domain α-helix and the small α-helical regions that wrap around the end of the bundle (Figure 7B) and thus are likely to minimally disrupt the structure. Second, the Caenorhabditis species are exceptional in having an insertion in the middle of the THB-forming domain (Figure 7B). This residue, which is either leucine or isoleucine, is predicted based on our multispecies alignment and THB crystal structure to be placed in toward the hydrophobic core where it would be expected to contribute to stability of the structure, which may counteract any destabilization caused by accommodating this change. Third, there are three basic residues (R17, R19, and K20) at the N-terminus of human Borealin whose simultaneous mutation interferes with targeting of the CPC to the central spindle (Figure 7B, blue line; Jeyaprakash et al., 2007). Our alignment reveals that although none of these positions is universally conserved as a basic residue (in Fungi these positions tend to be occupied by acidic residues), there is a bias toward basic amino acids in a 12-residue window around these positions (Figure 7B; unpublished data).
DISCUSSION
The Fundamental Design of the CPC Is Conserved from Fungi to Animals
Nbl1p is essential for viability and satisfies the criteria for being a member of the CPC: It copurifies and colocalizes with the other CPC components and is required for proper metaphase chromosome positioning and accurate chromosome segregation. Nbl1p also mediates the interaction between Sli15p and Bir1p and consequently is required for the stability, integrity, and proper localization of the CPC.
These results parallel those seen for Borealin/Dasra/CSC-1 in animals. First, Borealin/Dasra/CSC-1 was found to be the fourth member of the CPC holocomplex and Nbl1p was identified by copurification with the CPC as its fourth subunit. Second, inhibition of Borealin/Dasra/CSC-1 causes metaphase chromosome misalignment and subsequent chromosome missegregation (Romano et al., 2003; Gassmann et al., 2004) and mutation of NBL1 also causes metaphase chromosome misalignment and missegregation. Third, Borealin mediates the INCENP–Survivin interaction and this interaction allows the CPC to be targeted to centromeres and the central spindle (Romano et al., 2003; Klein et al., 2006; Jeyaprakash et al., 2007). Similarly, Nbl1p is required for the interaction between Sli15p and Bir1p and proper localization of these proteins. Lastly, both Borealin/Dasra/CSC-1 and Nbl1p have little effect on Aurora B kinase activity (Romano et al., 2003; Gassmann et al., 2004). In vitro, Nbl1p reduced Ipl1p activity toward subunits of the Dam1 complex. It is possible that Nbl1p, with Bir1p, increases substrate specificity.
Our results suggest that Nbl1p is a diverged Borealin/Dasra/CSC-1–related protein that retains only the N-terminal THB-forming domain. In addition, we have found putative homologues not only of Borealin/Dasra/CSC-1, but also of Survivin/BIR-1 and INCENP/ICP-1 throughout Fungi and Animalia with conserved regions corresponding to the THB-forming regions (sequence names, species of origin, and GenBank IDs are listed in Supplemental Table S2, A–C). Furthermore, domains containing these conserved regions from the S. cerevisiae proteins interact in yeast two-hybrid assays. Modeling of these peptides reveals a striking structural conservation with the human complex despite the low protein sequence identity. Given that AuroraB/AIR-2/Ipl1p and its interaction with INCENP/ICP-1/Sli15p is well conserved (Adams et al., 2000; Kang et al., 2001; Kaitna et al., 2002), we conclude that the fundamental structure and function of the CPC is conserved from fungi to animals and that this complex originated before the divergence of these groups.
Despite the conservation of the N-terminal THB domain, independent losses of the C-termini of the Borealin-related proteins from the Saccharomycotina (budding yeast) and from the Schizosaccharomyces (fission yeast in Taphrinomycotina) suggest that this region is not only dispensable but may be selected against in these organisms. It is possible that this is an indirect consequence of the growth constraints of being yeasts (single-celled fungi), although Cryptococcus neoformans, a pathogenic yeast in the Basidiomycota, retains an apparently complete Borealin/Dasra/CSC-1 protein. An alternative explanation might be that interacting with a kinetochore with fewer MT-attachment sites may have selected for an altered CPC geometry. In Saccharomycotina, both S. cerevisiae and Candida albicans have single MT-attachment sites (Winey et al., 1995; Joglekar et al., 2008), whereas Schizosaccharomyces pombe has only two to four MTs connected to its kinetochores (Ding et al., 1993). It should be noted, however, that Saccharomyces Nbl1p (even within the THB domain) and its interacting partners (Sli15p and Bir1p) are significantly diverged from their homologues in Schizosaccharomyces and all other organisms outside the Saccharomycotina. These differences indicate that there may be more factors affecting changes in the geometry and composition of the CPC.
Borealin's C-terminus is necessary for centromere targeting of the human CPC, whereas formation of the THB-containing Borealin's N-terminus is required for both central spindle and centromere localization (Jeyaprakash et al., 2007). Because Nbl1p lacks the region corresponding to Borealin's C-terminus, we propose that Nbl1p functions chiefly in formation of the THB and that this interaction is required for targeting the quaternary complex to both the central spindle and centromeres. The factor responsible for centromere targeting of the yeast CPC remains to be identified.
We identified nearly 200, mostly uncharacterized, putative homologues of Borealin/Dasra/CSC-1 from nearly 150 species throughout Animalia and Fungi including the model organism S. pombe and the human parasitic protozoa Trichomonas vaginalis. Analysis of the multispecies alignment of these Borealin/Dasra/CSC-1–related proteins revealed a number of sequence features. Future work comparing CPC function across a wide range of organisms should focus in part on the structural and functional consequences of the conservation or absence of these sequence features.
Supplementary Material
ACKNOWLEDGMENTS
We thank Rebecca Heald, Iain Cheeseman, Brian Young, and Christopher Toret for critical reading of this manuscript; Steven Ruzin and the CNR Biological Imaging Facility; Angela Brooks and the Brenner Lab for advice on remote homology detection; and Jeffery Woodruff (UC Berkeley, Berkeley, CA) for reagents, Ashard Desai (UC San Diego, La Jolla, CA) for CPC antibodies, Elmer Schiebel (ZMBH, Heidelberg, Germany) for a mCherry-Tub1 plasmid, and Barnes and Drubin Lab members for their assistance. This work was supported by National Institutes of Health (NIH) Grant R01 GM47842 from the National Institute of General Medical Sciences (G.B.) and NIH Grant P41 RR011823 (J.RY.).
Glossary
Abbreviations used:
- CPC
chromosomal passenger complex, aka Aurora/Ipl1 complex
- MT
microtubule
- THB
three-helix bundle of the CPC
- HMM
Hidden Markov Model.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-10-1011) on January 21, 2009.
REFERENCES
- Adams R. R., Wheatley S. P., Gouldsworthy A. M., Kandels-Lewis S. E., Carmena M., Smythe C., Gerloff D. L., Earnshaw W. C. INCENP binds the Aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle and cleavage furrow. Curr. Biol. 2000;10:1075–1078. doi: 10.1016/s0960-9822(00)00673-4. [DOI] [PubMed] [Google Scholar]
- Bern M., Goldberg D., McDonald W. H., Yates J. R., 3rd Automatic quality assessment of peptide tandem mass spectra. Bioinformatics. 2004;20(Suppl 1):i49–54. doi: 10.1093/bioinformatics/bth947. [DOI] [PubMed] [Google Scholar]
- Biggins S., Severin F. F., Bhalla N., Sassoon I., Hyman A. A., Murray A. W. The conserved protein kinase Ipl1 regulates microtubule binding to kinetochores in budding yeast. Genes Dev. 1999;13:532–544. doi: 10.1101/gad.13.5.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birney E., Clamp M., Durbin R. GeneWise and Genomewise. Genome Res. 2004;14:988–995. doi: 10.1101/gr.1865504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun W., Go N. Calculation of protein conformations by proton-proton distance constraints. A new efficient algorithm. J. Mol. Biol. 1985;186:611–626. doi: 10.1016/0022-2836(85)90134-2. [DOI] [PubMed] [Google Scholar]
- Buvelot S., Tatsutani S. Y., Vermaak D., Biggins S. The budding yeast Ipl1/Aurora protein kinase regulates mitotic spindle disassembly. J. Cell Biol. 2003;160:329–339. doi: 10.1083/jcb.200209018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne K. P., Wolfe K. H. Visualizing syntenic relationships among the hemiascomycetes with the Yeast Gene Order Browser. Nucleic Acids Res. 2006;34:D452–D455. doi: 10.1093/nar/gkj041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. S., Botstein D. Isolation and characterization of chromosome-gain and increase-in-ploidy mutants in yeast. Genetics. 1993;135:677–691. doi: 10.1093/genetics/135.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman I. M., Anderson S., Jwa M., Green E. M., Kang J., Yates J. R., 3rd, Chan C. S., Drubin D. G., Barnes G. Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell. 2002;111:163–172. doi: 10.1016/s0092-8674(02)00973-x. [DOI] [PubMed] [Google Scholar]
- Cheeseman I. M., Enquist-Newman M., Muller-Reichert T., Drubin D. G., Barnes G. Mitotic spindle integrity and kinetochore function linked by the Duo1p/Dam1p complex. J. Cell Biol. 2001;152:197–212. doi: 10.1083/jcb.152.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliften P., Sudarsanam P., Desikan A., Fulton L., Fulton B., Majors J., Waterston R., Cohen B. A., Johnston M. Finding functional features in Saccharomyces genomes by phylogenetic footprinting. Science. 2003;301:71–76. doi: 10.1126/science.1084337. [DOI] [PubMed] [Google Scholar]
- Cociorva D., Tabb D. L., Yates J. R. Validation of tandem mass spectrometry database search results using DTASelect. Curr. Protoc. Bioinformatics. 2007 doi: 10.1002/0471250953.bi1304s16. Chapter 13, Unit 13 14. [DOI] [PubMed] [Google Scholar]
- Crooks G. E., Hon G., Chandonia J. M., Brenner S. E. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding R., McDonald K. L., McIntosh J. R. Three-dimensional reconstruction and analysis of mitotic spindles from the yeast, Schizosaccharomyces pombe. J. Cell Biol. 1993;120:141–151. doi: 10.1083/jcb.120.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy S. R. Profile hidden Markov models. Bioinformatics. 1998;14:755–763. doi: 10.1093/bioinformatics/14.9.755. [DOI] [PubMed] [Google Scholar]
- Edgar R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng J. K., McCormack A. L., Yates J. R., 3rd An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- Gassmann R., Carvalho A., Henzing A. J., Ruchaud S., Hudson D. F., Honda R., Nigg E. A., Gerloff D. L., Earnshaw W. C. Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J. Cell Biol. 2004;166:179–191. doi: 10.1083/jcb.200404001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouet P., Courcelle E., Stuart D. I., Metoz F. ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics. 1999;15:305–308. doi: 10.1093/bioinformatics/15.4.305. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Henikoff J. G. Amino acid substitution matrices from protein blocks. Proc. Natl. Acad Sci. USA. 1992;89:10915–10919. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P., Park K. J., Obayashi T., Fujita N., Harada H., Adams-Collier C. J., Nakai K. WoLF PSORT: protein localization predictor. Nucleic Acids Res. 2007;35:W585–W587. doi: 10.1093/nar/gkm259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Madan A. CAP3, A DNA sequence assembly program. Genome Res. 1999;9:868–877. doi: 10.1101/gr.9.9.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaprakash A. A., Klein U. R., Lindner D., Ebert J., Nigg E. A., Conti E. Structure of a Survivin-Borealin-INCENP core complex reveals how chromosomal passengers travel together. Cell. 2007;131:271–285. doi: 10.1016/j.cell.2007.07.045. [DOI] [PubMed] [Google Scholar]
- Joglekar A. P., Bouck D., Finley K., Liu X., Wan Y., Berman J., He X., Salmon E. D., Bloom K. S. Molecular architecture of the kinetochore-microtubule attachment site is conserved between point and regional centromeres. J. Cell Biol. 2008;181:587–594. doi: 10.1083/jcb.200803027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaitna S., Pasierbek P., Jantsch M., Loidl J., Glotzer M. The aurora B kinase AIR-2 regulates kinetochores during mitosis and is required for separation of homologous Chromosomes during meiosis. Curr. Biol. 2002;12:798–812. doi: 10.1016/s0960-9822(02)00820-5. [DOI] [PubMed] [Google Scholar]
- Kaksonen M., Toret C. P., Drubin D. G. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell. 2005;123:305–320. doi: 10.1016/j.cell.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Kang J., Cheeseman I. M., Kallstrom G., Velmurugan S., Barnes G., Chan C. S. Functional cooperation of Dam1, Ipl1, and the inner centromere protein (INCENP)-related protein Sli15 during chromosome segregation. J. Cell Biol. 2001;155:763–774. doi: 10.1083/jcb.200105029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H., Kang J. S., Chan C. S. Sli15 associates with the ipl1 protein kinase to promote proper chromosome segregation in Saccharomyces cerevisiae. J. Cell Biol. 1999;145:1381–1394. doi: 10.1083/jcb.145.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U. R., Nigg E. A., Gruneberg U. Centromere targeting of the chromosomal passenger complex requires a ternary subcomplex of Borealin, Survivin, and the N-terminal domain of INCENP. Mol. Biol. Cell. 2006;17:2547–2558. doi: 10.1091/mbc.E05-12-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Flanary P. L., Altieri D. C., Dohlman H. G. Cell division regulation by BIR1, a member of the inhibitor of apoptosis family in yeast. J. Biol. Chem. 2000;275:6707–6711. doi: 10.1074/jbc.275.10.6707. [DOI] [PubMed] [Google Scholar]
- Link A. J., Eng J., Schieltz D. M., Carmack E., Mize G. J., Morris D. R., Garvik B. M., Yates J. R., 3rd Direct analysis of protein complexes using mass spectrometry. Nat. Biotechnol. 1999;17:676–682. doi: 10.1038/10890. [DOI] [PubMed] [Google Scholar]
- Longtine M. S., McKenzie A., 3rd, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., Pringle J. R. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- MacCoss M. J., Wu C. C., Yates J. R., 3rd Probability-based validation of protein identifications using a modified SEQUEST algorithm. Anal. Chem. 2002;74:5593–5599. doi: 10.1021/ac025826t. [DOI] [PubMed] [Google Scholar]
- Millson S. H., Truman A. W., Piper P. W. Vectors for N- or C-terminal positioning of the yeast Gal4p DNA binding or activator domains. Biotechniques. 2003;35:60–64. doi: 10.2144/03351bm06. [DOI] [PubMed] [Google Scholar]
- Morishita J., Matsusaka T., Goshima G., Nakamura T., Tatebe H., Yanagida M. Bir1/Cut17 moving from chromosome to spindle upon the loss of cohesion is required for condensation, spindle elongation and repair. Genes Cells. 2001;6:743–763. doi: 10.1046/j.1365-2443.2001.00459.x. [DOI] [PubMed] [Google Scholar]
- Pearson C. G., Maddox P. S., Salmon E. D., Bloom K. Budding yeast chromosome structure and dynamics during mitosis. J. Cell Biol. 2001;152:1255–1266. doi: 10.1083/jcb.152.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J., Elias J. E., Thoreen C. C., Licklider L. J., Gygi S. P. Evaluation of multidimensional chromatography coupled with tandem mass spectrometry (LC/LC-MS/MS) for large-scale protein analysis: the yeast proteome. J. Proteome. Res. 2003;2:43–50. doi: 10.1021/pr025556v. [DOI] [PubMed] [Google Scholar]
- Romano A., Guse A., Krascenicova I., Schnabel H., Schnabel R., Glotzer M. CSC-1, a subunit of the Aurora B kinase complex that binds to the survivin-like protein BIR-1 and the incenp-like protein ICP-1. J. Cell Biol. 2003;161:229–236. doi: 10.1083/jcb.200207117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchaud S., Carmena M., Earnshaw W. C. Chromosomal passengers: conducting cell division. Nat. Rev. Mol. Cell Biol. 2007;8:798–812. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- Sali A., Blundell T. L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- Sali A., Overington J. P. Derivation of rules for comparative protein modeling from a database of protein structure alignments. Protein Sci. 1994;3:1582–1596. doi: 10.1002/pro.5560030923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath S. C., Ohi R., Leismann O., Salic A., Pozniakovski A., Funabiki H. The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell. 2004;118:187–202. doi: 10.1016/j.cell.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Sanchatjate S., Schekman R. Chs5/6 complex: a multiprotein complex that interacts with and conveys chitin synthase III from the trans-Golgi network to the cell surface. Mol. Biol. Cell. 2006;17:4157–4166. doi: 10.1091/mbc.E06-03-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandall S., Severin F., McLeod I. X., Yates J. R., 3rd, Oegema K., Hyman A., Desai A. A Bir1-Sli15 complex connects centromeres to microtubules and is required to sense kinetochore tension. Cell. 2006;127:1179–1191. doi: 10.1016/j.cell.2006.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimogawa M. M., Widlund P. O., Riffle M., Ess M., Davis T. N. Bir1 is required for the tension checkpoint. Mol. Biol. Cell. 2009;20:915–923. doi: 10.1091/mbc.E08-07-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson A. G., Inagaki Y., Roger A. J. Comprehensive multigene phylogenies of excavate protists reveal the evolutionary positions of “primitive” eukaryotes. Mol. Biol. Evol. 2006;23:615–625. doi: 10.1093/molbev/msj068. [DOI] [PubMed] [Google Scholar]
- Straight A. F., Belmont A. S., Robinett C. C., Murray A. W. GFP tagging of budding yeast chromosomes reveals that protein-protein interactions can mediate sister chromatid cohesion. Curr. Biol. 1996;6:1599–1608. doi: 10.1016/s0960-9822(02)70783-5. [DOI] [PubMed] [Google Scholar]
- Straight A. F., Marshall W. F., Sedat J. W., Murray A. W. Mitosis in living budding yeast: anaphase A but no metaphase plate. Science. 1997;277:574–578. doi: 10.1126/science.277.5325.574. [DOI] [PubMed] [Google Scholar]
- Sun Y., Carroll S., Kaksonen M., Toshima J. Y., Drubin D. G. PtdIns(4,5)P2 turnover is required for multiple stages during clathrin- and actin-dependent endocytic internalization. J. Cell Biol. 2007;177:355–367. doi: 10.1083/jcb.200611011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabb D. L., McDonald W. H., Yates J. R., 3rd DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics. J. Proteome Res. 2002;1:21–26. doi: 10.1021/pr015504q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T. U., Rachidi N., Janke C., Pereira G., Galova M., Schiebel E., Stark M. J., Nasmyth K. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell. 2002;108:317–329. doi: 10.1016/s0092-8674(02)00633-5. [DOI] [PubMed] [Google Scholar]
- Thomas S., Kaplan K. B. A Bir1p Sli15p kinetochore passenger complex regulates septin organization during anaphase. Mol. Biol. Cell. 2007;18:3820–3834. doi: 10.1091/mbc.E07-03-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetz P., et al. A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature. 2000;403:623–627. doi: 10.1038/35001009. [DOI] [PubMed] [Google Scholar]
- Westermann S., Avila-Sakar A., Wang H. W., Niederstrasser H., Wong J., Drubin D. G., Nogales E., Barnes G. Formation of a dynamic kinetochore-microtubule interface through assembly of the Dam1 ring complex. Mol. Cell. 2005;17:277–290. doi: 10.1016/j.molcel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Winey M., Mamay C. L., O'Toole E. T., Mastronarde D. N., Giddings T. H., Jr, McDonald K. L., McIntosh J. R. Three-dimensional ultrastructural analysis of the Saccharomyces cerevisiae mitotic spindle. J. Cell Biol. 1995;129:1601–1615. doi: 10.1083/jcb.129.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J., et al. A protein interaction map of the mitotic spindle. Mol. Biol. Cell. 2007;18:3800–3809. doi: 10.1091/mbc.E07-06-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.