Abstract
Cathelicidin is strongly expressed in lesional skin in psoriasis and may play an important role as both an antimicrobial peptide and as an autoinflammatory mediator in this chronic skin disease. The mechanism of increased cathelicidin in psoriatic keratinocytes is not known, but recent observations have found that psoriasis has abundant Th17 cells that produce IL-17A and IL-22. We found that human keratinocytes stimulated with supernatants from T cells isolated from lesional psoriatic skin increased expression of cathelicidin when stimulated in the presence of 1,25-dihydroxyvitamin D3 (1,25D3). This increase was signaled through the IL-17RA. In vitro, IL-17A, but not IL-22, enhanced cathelicidin mRNA and peptide expression in keratin-defensin ocytes dependent on the presence of 1,25D3. At the same time, coincubation with 1,25D3 blocked induction of human β-defension 2 (HBD2), IL-6, and IL-8, which are other target genes of IL-17A. Act1, an adaptor associated with IL-17RA and essential for IL-17A signaling, mediated cathelicidin induction, as its suppression by small interfering RNA inhibited HBD2 and cathelicidin. Both, 1,25D3 and IL-17A signaled cathelicidin induction through MEK-ERK. These results suggest that increased IL-17A in psoriatic skin increases cathelicidin through a vitamin D3-, Act1-, and MEK-ERK-dependent mechanism. Therapy targeting this cathelicidin-regulating system might be beneficial in patients suffering from psoriasis.
Psoriasis is a common, chronic inflammatory skin disease affecting ~2% of the general population. One of the characteristic symptoms of psoriasis is the appearance of thick, scaly patches overlying the inflamed skin. The specific cause for psoriasis is unknown but a large body of evidence has identified a dys-regulated interplay between keratinocytes and inflammatory cell infiltrates underlying cutaneous inflammation (1). Although considered an autoimmune disorder, the trigger for induction and perpetuation of inflammation in psoriasis has remained unidentified. Recently it was demonstrated that the human cathelicidin peptide LL-37 enables response to self-DNA by plasmacytoid dendritic cells (pDC)3 and therefore may participate in the activation of psoriasis (2). The pDC that densely populate psoriatic skin express TLR9, an intracellular receptor that recognizes viral or microbial nucleic acids within endosomal compartments (2). Human DNA is unable to activate pDC; however, in psoriasis this self-tolerance is broken. Cathelicidin LL-37 converts nonstimulatory self-DNA from apoptotic keratinocytes in psoriatic skin into a potent trigger of pDC activation by forming a LL-37/DNA complex that is delivered to early endocytic compartments of pDC to trigger TLR9 activation (2). In response, pDC produce IFN-α, which initiates the activation of autoimmune T cells, leading to the formation of skin lesions (3). Thus, a mechanism has been hypothesized by which pDC sense and respond to self-DNA coupled with cathelicidin peptide LL-37, which drives autoimmunity in psoriasis (2). These results indicate a fundamental role of cathelicidin in activating cutaneous inflammation in psoriasis.
Cathelicidins were initially identified as defense molecules against microbial pathogens in human and mammalian skin (4). Recently, several studies have identified additional functions for the cathelicidins that complement their role as antibiotics. Cathelicidins at nanomolar concentrations are chemotactic for distinct populations of leukocytes as well as some nonleukocytes (5). In addition, cathelicidins induce the release of various cytokines and chemokines from resident skin cells (6). On the basis of these unique activities, the term “alarmins” has been proposed to summarize the multiple functions of these antimicrobial peptides as activators of adaptive immune responses (7). Human cathelicidin is often referred to by one of its peptide forms LL-37, or by the nomenclature assigned to its precursor protein (hCAP18) (8). Peptide processing has emerged as a critical element in the control of cathelicidin activity. In its nascent form, hCAP18 is thought to be inactive. Upon cleavage by serine proteases, the generation of the mature peptide results in multiple potential activities (9). In initial observations, cathelicidin expression in skin followed a pattern that was expected for a molecule involved in defense function. Cathelicidin expression is high in bacterial skin infection and induced by cutaneous barrier disruption such as invasive bacterial infection or physical injury of the skin (10). In psoriasis, cathelicidin expression in keratinocytes is increased compared with healthy skin (11). This observation may explain in part the relative resistance to cutaneous infections seen in patients with psoriasis. However, in the light of the recent studies, cathelicidin may function more as an alarmin than an antimicrobial in psoriasis (2, 6).
The cause and signaling pathways of induced cathelicidin in psoriatic skin are unknown. Moreover, the mechanisms of cathelicidin transcription in keratinocytes were long unclear, as classic mediators of inflammation or infection did not influence expression (12). A breakthrough in the understanding of cathelicidin expression in skin came with the identification of a vitamin D responsive element in the promoter of the human cathelicidin antimicrobial peptide (CAMP) gene (13). As a consequence, expression of cathelicidin is directly controlled by 1,25-dihydroxyvitamin D3 (1,25D3), the hormonally active form of vitamin D3 (12, 13). Still, increased vitamin D3 metabolism or altered vitamin D3 levels, which could lead to increased cathelicidin, have not been observed in psoriasis.
Recent studies have highlighted a role for the Th17 cytokine network in mediating cutaneous skin inflammation in psoriasis. The proinflammatory cytokines IL-17A and IL-22 are effector molecules produced by Th17 cells, a new lineage of CD4+-producing Th cells. Skin samples from psoriatic lesions are highly enriched in Th17 cells and cytokines (14). Furthermore, in vitro activated Th17 cells secrete IL-17A, which induces the expression of several antimicrobial peptides in keratinocytes (14). Similarly, neutralization of IL-17A in supernatants of T cells isolated from psoriatic skin completely blocks the induction of genes encoding antimicrobial peptides in keratinocytes (14). In vitro, the Th17 cytokine IL-22 also induces many psoriatic features in cultured reconstituted human epidermis. For example, IL-22 up-regulates several proinflammatory molecules, induces keratinocyte migration in an in vitro injury model, and induces hyperplasia of reconstituted human epidermis (15). These results collectively indicate that Th17 signaling is important for control of innate epithelial immunity and is involved in human psoriasis.
Although the exact cause of psoriasis remains unclear, recent studies suggest that this disease is caused by pathologic activation of inflammatory cell infiltrates. This activation is initiated by increased cathelicidin LL-37 levels in psoriatic skin and results in the production of inflammatory cytokines and chemokines by pDCs (2). In this study we demonstrate that supernatants from T cells isolated from lesional psoriatic skin increase expression of cathelicidin in keratinocytes through a vitamin D3 dependent mechanism. IL-17A signaling is critically involved in this effect through activation of the IL-17RA. In cultured primary keratinocytes, IL-17A increases cathelicidin dependent on the presence of 1,25D3. Furthermore, IL-17A enhanced cathelicidin expression through activation of Act1, an adaptor protein involved in IL-17A signaling, and MEK-ERK. The findings presented in this study uncover a novel mechanism for IL-17A-driven inflammation in skin in psoriasis through increased cathelicidin.
Materials and Methods
Cell culture and stimuli
Normal human epidermal keratinocytes (NHEK) were grown in EpiLife cell culture medium (Cascade Biologics) containing 0.06 or 1.7 mM calcium and 1X EpiLife defined growth supplement at 37°C under standard tissue culture conditions. Stock cultures were maintained for up to six passages in this medium with the addition of 10 μg/ml gentamicin and 0.25 μg/ml amphotericin B. HaCaT keratinocytes were cultured in DMEM with 4.5 g/L glucose supplemented with 10% FBS, 50 U/ml penicillin, and 50 μg/ml streptomycin (PAA Laboratories). Cells at 40 – 60% confluence were stimulated for different time periods with 1,25D3 (10−11–10−8 M; Sigma-Aldrich), IL-17A (1–100 ng/ml; R&D Systems), IL-22 (10 –100 ng/ml; R&D Systems), IFN-γ(10 ng/ml; Biomol), or TNF-α (20 ng/ml; Biomol) or their combinations. For blockade of the IL-17RA, a monoclonal mouse anti-human IL-17RA Ab (5 μg/ml; R&D Systems) was added 2 h before stimulation with IL-17A. For neutralization of IL-17A bioactivity, a monoclonal mouse anti-human IL-17A Ab (10 μg/ml; R&D Systems) was added to medium containing IL-17A 2 h before stimulation. Mouse control IgG (10 μg/ml; DakoCytomation) served as a negative control in the blocking experiments with Abs against IL-17RA and IL-17A, respectively. For inhibition of transcriptional activity, actinomycin D (ActD, 5 μg/ml; Sigma-Aldrich) was added with 1,25D3 and/or IL-17A. For analyses of mRNA transcript stability ActD was added 24 h after stimulation. For inhibition of MEK-ERK the MEK1 inhibitor PD98059 (20 μM; Cell Signaling Technology) was added 1 h before stimulation with 1,25D3 and/or IL-17A. For analyses of p44/p42 phosphorylation cells were incubated without addition of EpiLife defined growth supplement for 24 h before stimulation.
Isolation of psoriatic T cells and stimulation of keratinocytes with T cell supernatants
Small, spindel-shaped skin specimens of ~1 cm length were taken from chronic psoriatic plaques of two patients with informed consent and approval of the local ethics committee. Dermis and epidermis were dissociated from each other following treatment with dispase (2.5 U/ml; Roche) for 12 h at 4°C as previously described (16). Epidermis and dermis were cut up into small fragments, and T cells were allowed to emigrate from the tissue. Dermal T cells were cultivated at a density of 105 cells per ml in RPMI 1640 medium (PAA Laboratories) in the presence of 5 U/ml recombinant human IL-2 (Roche) without additional stimuli. Supernatants were harvested after 7 days of cultivation as previously described (16). HaCaT keratinocytes were cultured as described and were stimulated with these supernatants in the presence of 1,25D3 (10−11 M) for 24 h. Cathelicidin induction was analyzed by quantitative real-time PCR as described below.
Quantitative real-time PCR
After cell stimulation, total RNA was extracted using TRIzol (Invitrogen), and 1 μg of RNA was reverse transcribed using iScript (Bio-Rad). The expression of cathelicidin, Act1, HBD2, CYP27B1, IL-8, IL-6, vitamin D receptor (VDR) and IL-17RA was evaluated using a LightCycler 2.0 system and the corresponding human Universal Probe Library Set (Roche). The primers were designed by an algorithm (www.universalprobelibrary.com), and porphobilinogen deaminase was used as a housekeeping gene in a duplex quantitative real-time PCR. Porphobilinogen deaminase was chosen because porphobilinogen, cathelicidin, and HBD2 belong to a low-abundance class of mRNAs, and expression levels in untreated keratinocytes are low. Preliminary tests verified that expression of porphobilinogen deaminase was not affected upon treatment of NHEK and HaCaT keratinocytes. Analyzed genes and corresponding primers are listed in Table I. All analyses were performed in triplicate from at least three independent cell stimulation experiments. Fold induction relative to the vehicle-treated control was calculated as previously described (17). Results were considered significant when at least a 2-fold difference in expression levels was detected and statistical analysis revealed p < 0.05.
Table I.
Target genes and corresponding primers for quantitative real-time PCR
| Primer Sequence |
||
|---|---|---|
| Target Gene | Forward | Reverse |
| Cathelicidin | 5′-tcggatgctaacctctaccg-3′ | 5′-acaggctttggcgtgtct-3′ |
| HBD2 | 5′-tcagccatgagggtcttgta-3′ | 5′-ggatcgcctataccaccaaa-3′ |
| CYP27B1 | 5′-cgcagctgtatggggaga-3′ | 5′-cacctcaaaatgtgttaggatctg-3′ |
| IL-8 | 5′-agacagcagagcacacaagc-3′ | 5′-atggttccttccggtggt-3′ |
| IL-6 | 5′-caggagcccagctatgaact-3′ | 5′-gaaggcagcaggcaacac-3′ |
| Act1 | 5′-cacagagagactgctcctttcc-3′ | 5′-ccaggagcaccagctctatta-3′ |
| VDR | 5′-cttctctggggactcctcct-3′ | 5′-tggacgagtccatcatgtct-3′ |
| IL-17RA | 5′-gtcatcctgctcatcgtctg-3′ | 5′-ttggtgtcatcactgtatttttcac-3′ |
Immunohistochemistry
Immunohistochemistry was performed to detect localization of cathelicidin and IL-17RA in skin from patients with psoriasis. Paraffin sections of lesional skin were incubated overnight with an hCAP18/LL-37 Ab (Innovagen) or an IL-17RA Ab (Santa Cruz Biotechnology). Infiltrating neutrophils were stained with an Ab against myeloperoxidase (DakoCytomation). The sections were then incubated with secondary Abs (DakoCytomation) 1 h before visualization reagent APAAP (DakoCytomation) was added. Positive staining was visualized with the SigmaFast Fast Red TR/Naphthol AS-MX Alkaline Phosphatase Substrate Tablets set (Sigma-Aldrich). Cells were counterstained with hematoxylin, and sections were analyzed with a TissueFAXS System microscope at a 100–200X magnification and the corresponding HistoQuest software (TissueGnostics).
Western blot
NHEK were stimulated with 1,25D3 (10−9–10−8 M) or IL-17A (10 –100 ng/ml) for 24 h and lysed in RIPA buffer (10 mM Tris-Cl, 1 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, 140 mM NaCl, 1 mM PMSF). Total protein was measured with the BCA Protein Assay kit (Pierce). Equivalent amounts were separated on a NuPAGE 10% Bis-Tris Gel (Invitrogen). After separation, proteins were blotted onto an Invitrolon PVDF Membrane (Invitrogen) and blocked in TBS diluted 1% Western Blocking Reagent (Invitrogen) for 1 h at room temperature. Membranes were stained with Abs detecting hCAP18/LL-37 (Innovagen), α-tubulin(Biozol), β-actin (Sigma-Aldrich), acetyl-histone H4 (Cell Signaling Technology), and phospho-p44/p42 MAPK (Cell Signaling Technology) overnight and reprobed with respective HRP-conjugated secondary Abs (DakoCytomation). Stained protein was visualized using the Amersham ECL Plus Western blotting Detection System (GE Healthcare).
RNA interference and transfection
NHEK at ~20% confluence were transfected twice (48 h apart) with 20 nM small interfering RNA (siRNA) oligonucleotides using Lipofectamine RNAiMAX Reagent (Invitrogen) to maximize the silencing effect. Cells were transfected with either a siRNA oligonucleotide against Act1 or a nontargeted control siRNA oligonucleotide and maintained at 37°C under standard tissue culture conditions. Four hours after the second transfection, cells were stimulated with 1,25D3 (10−9–10−8 M) or IL-17A (10 –100 ng/ml). Keratinocytes were then harvested and evaluated by quantitative real-time PCR or Western blot.
Immunofluorescence
NHEK were grown on chamber slides, fixed with 4% formaldehyde in PBS, and permeabilized with 0.2% Triton X-100 in PBS. Slides were blocked in 5% BSA in PBS for 1 h at room temperature. Staining was performed overnight at 4°C with an IL-17RA Ab (Santa Cruz Biotechnology). Slides were reprobed for 1 h at room temperature with a Northern-Lights-conjugated secondary Ab (R&D Systems). After subsequent washings, slides were mounted in ProLong Anti-Fade reagent (Molecular Probes) containing DAPI (4′,6-diamidino-2-phenylindol) and evaluated with a TissueFAXS System microscope at 200X magnification and the corresponding TissueFAXS software (TissueGnostics).
IL-8 ELISA
NHEK were stimulated for 24 h with 1,25D3 (10−8 M) or IL-17A (100 ng/ml), and cell culture medium was collected. IL-8 levels were determined by an IL-8 Eli-Pair (Diaclone) ELISA according to the manufacturer’s instructions.
Analysis of 1,25D3 concentrations in cell culture medium
NHEK were stimulated as described, and culture supernatants were collected and purified by column chromatography. The 1,25D3 level was determined by 1,25(OH)2-Vitamin D Elisa kit (Immundiagnostik) according to the manufacturer’s instructions.
Analysis of cathelicidin promoter activity
To analyze cathelicidin promoter activity after IL-17A stimulation, a 1500-bp fragment of the 5′ untranslated region of the human cathelicidin gene CAMP was cloned into a luciferase reporter plasmid (pGL-3–1500) as previously described (12). HaCaT cells were transfected with the indicated reporter plasmid and stimulated 6 h later with 1,25D3 (10−8 or 10−10M) or IL-17A (10 ng/ml) for 24 h. Firefly luciferase activity from the CAMP pGL3 reporter vector and Renilla luciferase activity were measured by the Dual Luciferase Assay system (Promega) in a luminometer. Promoter activity was reported as the ratio of pGL-3–1500 to pGL-3-basic (empty vector) activity in each sample.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 4.0. The Student t test was used to calculate statistical differences. Values of p < 0.05 were considered significant and all data are displayed as mean ± SD.
Results
T cell supernatants increase vitamin D3-induced cathelicidin
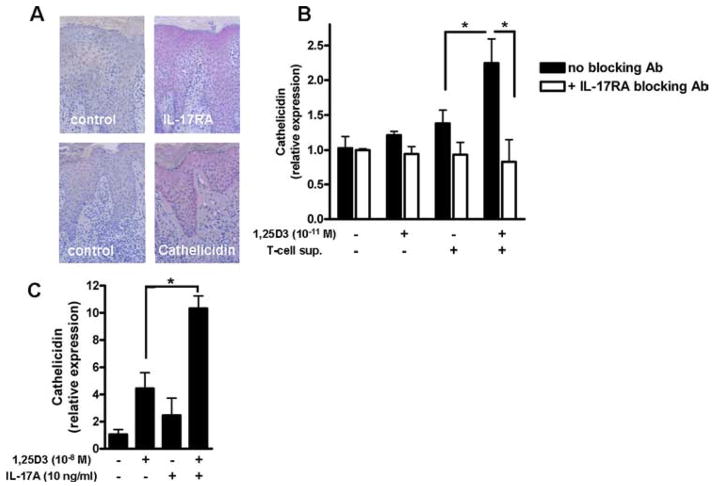
Th17 cytokines secreted by a specialized T cell subfamily and cathelicidin peptide LL-37 expressed by keratinocytes have both been suggested to contribute to cutaneous inflammation in psoriasis. To examine a possible interaction, we first confirmed coexpression of cathelicidin peptide and the IL-17RA in keratinocytes in lesional skin in psoriasis by immunohistochemistry. As displayed in (Fig. 1A) psoriatic keratinocytes strongly express both cathelicidin peptide and IL-17RA. Tissue infiltrating neutrophils that also express cathelicidin peptide were stained with an Ab against myeloperoxidase (see Supplemental Fig. 1).4 To then analyze whether T cells in psoriatic skin secrete factors that induce cathelicidin in human keratinocytes (HaCaT), T cells were isolated from lesional skin. Dermal T cells were cultured, and cell culture supernatants were used for keratinocyte stimulation. T cell culture supernatants alone or 1,25D3 at a very low dose (10−11 M) did not induce cathelicidin. In contrast, when keratinocytes were stimulated with T cell culture supernatants in the presence of 1,25D3, significant induction was observed (Fig. 1B). IL-17A signaling was involved in cathelicidin induction in keratinocytes as preincubation with a specific IL-17RA blocking Ab completely inhibited induction (Fig. 1B).
FIGURE 1.
1,25D3 enables supernatants from T cells isolated from psoriatic plaques to increase cathelicidin expression in human keratinocytes. A, Keratinocytes in lesional skin strongly express IL-17RA and cathelicidin peptide as demonstrated by staining of paraffin sections of skin from patients with psoriasis with an IL-17RA and hCAP18/LL-37 Ab, respectively. B, Human keratinocytes (HaCaT) were treated with supernatants from T cells isolated from psoriatic plaques in the presence or absence of 1,25D3 (10−11 M). To block IL-17A signaling, an IL-17RA blocking Ab was added 30 min before stimulation. Cells were harvested after 24 h and cathelicidin transcript levels were analyzed by quantitative real-time PCR. C, In a control experiment, HaCaT keratinocytes were treated for 24 h with 1,25D3 (10−8 M), IL-17A (10 ng/ml), or the combination. Cathelicidin transcript abundance was analyzed by quantitative real-time PCR. Data are mean ± SD of a single experiment performed in triplicate and are representative of three independent experiments *, p < 0.05, determined by Student’s t test.
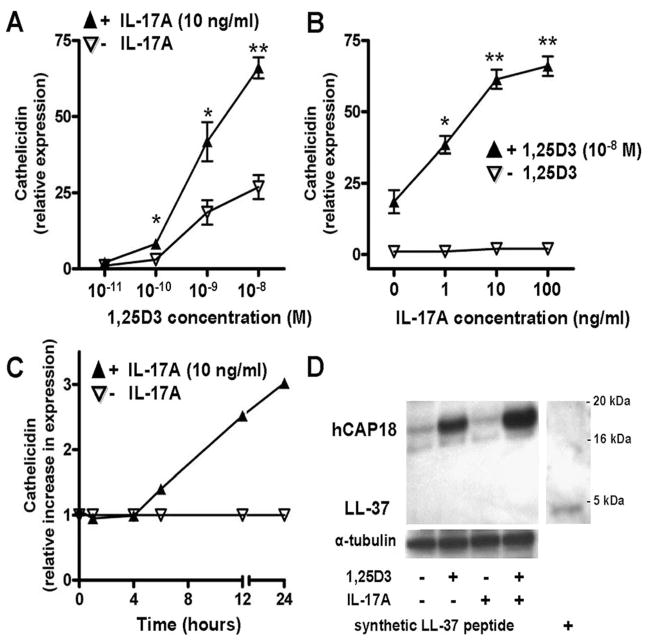
IL-17A enhances 1,25D3-induced cathelicidin expression and hCAP18 production in keratinocytes in vitro
To analyze whether the inducing effect of cell culture supernatants from psoriatic T cells could be reproduced with IL-17A alone, HaCaT keratinocytes were treated with recombinant human IL-17A or 1,25D3. Similar to T cell supernatants, IL-17A alone had no effect on cathelicidin expression. As expected, 1,25D3 (10−8M) induced cathelicidin mRNA expression, which was significantly enhanced by the addition of IL-17A (Fig. 1C). These results were confirmed using primary human keratinocytes (NHEK): again, IL-17A alone had no effect on cathelicidin expression, but in combination with increasing concentrations of 1,25D3 cathelicidin transcript abundance was enhanced in a dose-dependent manner (Fig. 2A). Similarly, the effect of IL-17A on cathelicidin was dose-dependent (Fig. 2B), as well as time-dependent (Fig. 2C) in the presence of constant 1,25D3 levels. Preincubation of NHEK with 1,25D3 for 24 h was sufficient to enable IL-17A to increase cathelicidin (data not shown). Western blot analyses confirmed increased cathelicidin peptide (Fig. 2D). Although 1,25D3 strongly induced cathelicidin peptide expression, the combination 1,25D3 with IL-17A further enhanced hCAP18. NHEK cells stimulated with 1,25D3 and 1,25D3 with IL-17A showed strong expression of precursor protein hCAP18. The processed active cathelicidin LL-37 could not be detected.
FIGURE 2.
IL-17A enhances the effect of 1,25D3 on cathelicidin mRNA and cathelicidin peptide hCAP18 in primary human keratinocytes. A, NHEK were treated with IL-17A (10 ng/ml) in the presence of increasing concentrations of 1,25D3 (10 −11–10−8 M) for 24 h. Cathelicidin transcript abundance was analyzed by quantitative real-time PCR. B, NHEK were treated for 24 h with 1,25D3 (10−8 M), and increasing concentrations of IL-17A (1–100 ng/ml). Cathelicidin was measured by quantitative real-time PCR. C, Keratinocytes were stimulated with IL-17A (10 ng/ml) in the presence of 1,25D3 (10−8 M). Cathelicidin induction as measured by quantitative real-time PCR at the indicated time points was calculated relative to the induction by 1,25D3 alone. Data are mean ±SD of a single experiment performed in triplicate and are representative of three independent experiments.*, p < 0.05 and **, p < 0.01, determined by Student’s t test. D, To evaluate cathelicidin peptide induction, NHEK were treated for 24 h with 1,25D3 (10−8 M), IL-17A (100 ng/ml), or the combination. Cathelicidin hCAP18/LL-37 protein expression was analyzed in NHEK lysates by Western blot using an Ab that detects hCAP18 and LL-37. Synthetic LL-37 peptide was used as a positive control (far right lane).
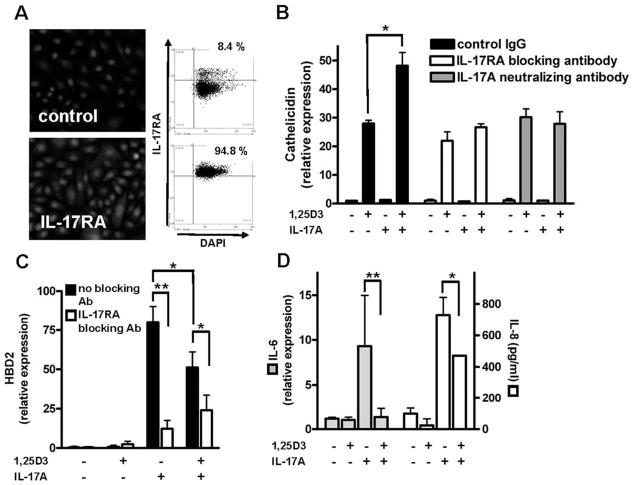
IL-17A signals its effect on cathelicidin and HBD2 through activation of IL-17RA
To analyze whether IL-17A enhanced expression of cathelicidin was mediated through activation of the IL-17RA additional stimulation experiments were performed. First we determined IL-17RA expression in primary human keratinocytes and found strong constitutive expression of IL-17RA by immunofluorescence (Fig. 3A). TissueFAXS analyses revealed that >94% of the primary human keratinocytes expressed IL-17RA. Preincubation of these cells with the IL-17RA Ab, which inhibited the effects of T cell supernatants in earlier experiments, completely blocked the effect of IL-17A on cathelicidin (Fig. 3B). Similar observations were made when IL-17A bioactivity was neutralized (Fig. 3B). Next we analyzed expression of HBD2, another antimicrobial peptide in keratinocytes and a known target gene of IL-17A. As expected, IL-17A strongly induced HBD2 through activation of IL-17RA, whereas 1,25D3 alone had no effect (Fig. 3C). In contrast, when NHEK were stimulated with IL-17A together with 1,25D3, the presence of 1,25D3 reduced HBD2 induction by IL-17A. Again, neutralization of IL-17A bioactivity strongly inhibited HBD2 induction by IL-17A (data not shown). Similar observations were made when IL-6 and IL-8 expression were determined after stimulation with IL-17A and 1,25D3 (Fig. 3D). IL-17A strongly induced IL-6 and IL-8 in human keratinocytes and the presence of 1,25D3 decreased this effect.
FIGURE 3.
NHEK express IL-17RA while 1,25D3 differentially affects IL-17A-induced gene expression in keratinocytes. A, Expression of the IL-17RA was confirmed in primary NHEK by immunofluorescence staining and TissueFAXS analyses. Cell nuclei were detected by DAPI. B, NHEK were stimulated with IL-17A (10 ng/ml) and 1,25D3 (10−8 M) for 24 h, and cathelicidin transcript abundance was measured by quantitative real-time PCR. In addition, cells were preincubated 2 h with an IL-17RA blocking Ab before stimulation. As an additional control, an IL-17A neutralizing Ab was added to culture medium containing IL-17A 2 h before the medium was applied to the cells. The 1,25D3 stimulation enabled IL-17A to increase cathelicidin, which was inhibited by the IL-17RA blocking Ab or the IL-17A neutralizing Ab. To evaluate the expression of other IL-17A regulated genes, NHEK were stimulated with IL-17A (10 ng/ml) and 1,25D3 (10−8 M) for 24 h and HBD2 (C) and IL-6 (D) transcript abundance was measured by quantitative real-time PCR. IL-8 expression (D) in cell culture supernatants was analyzed by ELISA. Cells were incubated with an IL-17RA blocking Ab 2 h before stimulation. HBD2 expression was induced by IL-17A alone, and induction was attenuated in the presence of 1,25D3. C, Further inhibition of IL-17A signaling inhibited HBD2 induction. D, Similar to HBD2 expression, IL-17A induced IL-6 (left) and IL-8 (right) expression that was decreased when 1,25D3 was present. Data are mean ± SD of a single experiment performed in triplicate and are representative of three independent experiments.*, p < 0.05 and **, p < 0.01, determined by Student’s t test.
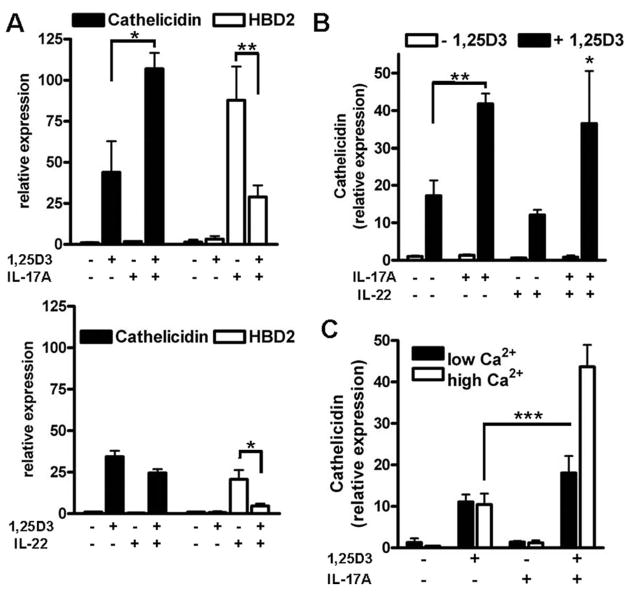
IL-17A and IL-22 differentially affect antimicrobial peptide expression in keratinocytes
To analyze whether only IL-17A or other known Th17 cytokines affect cathelicidin expression in keratinocytes, we compared the effect of the proinflammatory Th17 cytokines IL-17A and IL-22 on antimicrobial peptide expression. Both, IL-17A and IL-22 alone induced HBD2 but neither induced cathelicidin in NHEK (Fig. 4A). When cells were stimulated with IL-17A or IL-22 in the presence of 1,25D3, reduced HBD2 transcript levels were observed. Conversely, 1,25D3 induced cathelicidin was further increased by stimulation with IL-17A, but not with IL-22 (Fig. 4A). Coadministration of IL-17A and IL-22 with 1,25D3 confirmed increased cathelicidin expression by Th17 cytokines, but no difference was detected when IL-17A alone and the IL-17A/IL-22 combination were compared (Fig. 4B). To confirm the unique stimulatory activity of IL-17A on cathelicidin expression, additional proinflammatory cytokines relevant in psoriasis pathogenesis such as TNF-α and IFN-γ were tested. However, although both cytokines elicited an inflammatory response in keratinocytes, as measured by IL-8 induction, neither of these factors induced cathelicidin in the presence of 1,25D3 (see Supplemental Fig. 2)3.
FIGURE 4.
Th17 cytokines IL-17A and IL-22 differentially affect antimicrobial peptide expression. To compare the effects of the Th17 cytokines, IL-17A (top) and IL-22 (bottom) keratinocytes were treated with IL-17A (10 ng/ml) or IL-22 (10 ng/ml) in the presence of 1,25D3 (10−8 M). Cells were harvested 24 h after stimulation and mRNA levels for cathelicidin and HBD2 were analyzed by quantitative real-time PCR. A, 1,25D3 enabled IL-17A to increase cathelicidin, whereas IL-22 had no effect. Both, IL-17A and IL-22 induced HBD2 in keratinocytes as measured by quantitative real-time PCR and again 1,25D3 attenuated HBD2 induction. B, The combination of IL-17A and IL-22 showed the same effect on cathelicidin transcript abundance as IL-17A alone. C, To evaluate the influence of cell differentiation on cathelicidin induction, NHEK were grown in medium containing low (0.06 mM) or high (1.7 mM) concentrations of calcium for 24 h. Cells were then stimulated with IL-17A (10 ng/ml) in the presence or absence of 1,25D3 (10−8 M) for another 24 h. Cathelicidin transcript abundance was analyzed by quantitative real-time PCR. Induction of cathelicidin by 1,25D3 did not change with increasing calcium concentration, but the effect of IL-17A was significantly stronger in keratinocytes treated with 1.7 mM calcium. Data are mean ± SD of a single experiment performed in triplicate and are representative of three independent experiments.*, p < 0.05; **, p < 0.01; and ***, p < 0.001, determined using Student’s t test.
The effect of IL-17A on 1,25D3-induced cathelicidin expression depends on the differentiation status of primary NHEK
To analyze whether cathelicidin induction is affected by cell differentiation, NHEK were incubated in medium containing high calcium (1.7 mM). Cathelicidin transcript abundance did not change when cells were treated with 1,25D3 or IL-17A alone under these conditions (Fig. 4C). Still, the cellular differentiation status was relevant to cathelicidin induction because the effect of 1,25D3 and IL-17A combined was significantly stronger in keratinocytes treated in medium containing higher calcium concentrations (Fig. 4C). To analyze whether the enhancing effect of IL-17A on 1,25D3-induced cathelicidin expression was due to altered levels of the receptors VDR or IL-17RA, their expression was measured. No significant changes were observed (see Supplemental Fig. 3D).3
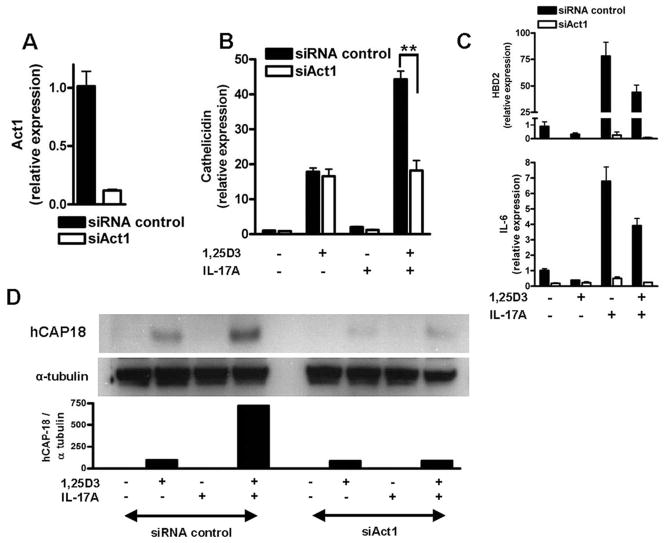
Act1 mediates the enhancing effect of IL-17A on cathelicidin and HBD2 expression
To examine further how IL-17A increases cathelicidin in keratinocytes, the role of the recently identified activator of NF-κB1 protein Act1 was analyzed. Keratinocytes express Act1 and expression is not changed in the presence of 1,25D3 (data not shown). To analyze the function of Act1 NHEK were transfected with siRNA oligonucleotides targeting Act1 to decrease expression. Silencing of Act1 was confirmed by quantitative real-time PCR (Fig. 5A). NHEK were subsequently transfected with siAct1, stimulated with IL-17A and 1,25D3 and cathelicidin expression was analyzed. Inhibition of Act1 expression had no effect on 1,25D3-induced cathelicidin in NHEK but completely blocked the effect of IL-17A (Fig. 5B). Induction of HBD2 and IL-6 by IL-17A was also completely inhibited in NHEK after Act1 silencing (Fig. 5C). Western blot analysis confirmed that Act1 was not only involved in increasing cathelicidin transcript abundance but also in hCAP18 protein expression (Fig. 5D). These findings confirm that Act1 is a critical mediator for the effect of IL-17A on antimicrobial peptide expression in human keratinocytes.
FIGURE 5.
Act1 mediates the effect of IL-17A on cathelicidin expression in keratinocytes. Act1 is a recently identified adaptor protein involved in IL-17RA signaling. To investigate the role of Act1 in IL-17A increased cathelicidin NHEK were transfected with siRNA to decrease Act1 expression before stimulation with IL-17A (10 ng/ml) and 1,25D3 (10−8 M). Silencing of Act1 was confirmed by quantitative real-time PCR. A, The siRNA suppression of Act1 blocked enhancement of cathelicidin by IL-17A in the presence of 1,25D3. B, 1,25D3-induced cathelicidin was not affected. C, NHEK were transfected with siRNA oligonucleotides for Act1 and stimulated with 1,25D3 (10−8 M), IL-17A (10 ng/ml), or the combination. Again, silencing of Act1 blocked induction of HBD2 (top) and IL-6 (bottom). Data are mean ± SD of a single experiment performed in triplicate and are representative of three independent experiments. **, p <0.01, determined by Student’s t test. D, The corresponding cathelicidin peptide hCAP18 expression levels are displayed as observed by Western blot. Similar to cathelicidin mRNA expression, silencing of Act1 blocked IL-17A-enhanced cathelicidin peptide.
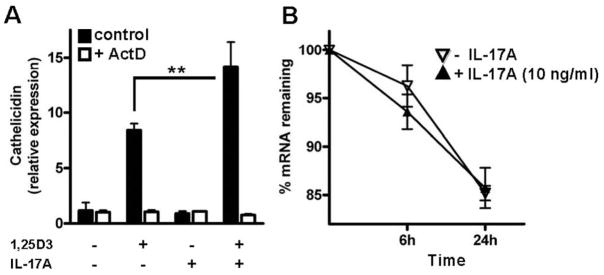
Augmentation of cathelicidin expression by IL-17A requires active gene transcription
We next sought to identify the mechanism of IL-17A-increased cathelicidin expression. Recently, it was shown that IL-17A enhances chemokine gene expression through mRNA stabilization (18). Therefore, ActD was used to inhibit active mRNA transcription and evaluate cathelicidin mRNA stability. Cathelicidin expression was completely blocked by ActD when cells were stimulated with 1,25D3 or IL-17A and ActD was present (Fig. 6A). To measure cathelicidin mRNA stability, cells were stimulated with IL-17A and 1,25D3 for 24 h before ActD was added. Cells were subsequently harvested at different time points, and cathelicidin transcript abundance was analyzed. Cathelicidin mRNA levels decreased over time, but no difference between 1,25D3-stimulated cells and cells stimulated with 1,25D3 and IL-17A was observed (Fig. 6B).
FIGURE 6.
IL-17A increases cathelicidin through activation of a transcriptional mechanism. To investigate the mechanism of IL-17A-enhanced cathelicidin, NHEK were stimulated with IL-17A (10 ng/ml) alone or in combination with 1,25D3 (10−8 M). ActD (5 μg/ml) was added to inhibit active mRNA transcription and cathelicidin was measured by quantitative real-time PCR. A, ActD completely blocked the effect of IL-17A on cathelicidin. To evaluate the effect of IL-17A on cathelicidin mRNA stability, 5 cells were treated with 1,25D3 and the combination of IL-17A with 1,25D3. ActD was added 24 h after stimulation and cells were harvested at different time points. Cathelicidin expression was analyzed by quantitative real-time PCR and calculated relative to the time of ActD addition. Data are mean ± SD of a single experiment performed in triplicate and are representative of three independent experiments. **, p < 0.01, determined by Student’s t test.
Increased cathelicidin in keratinocytes has recently been linked to increased CYP27B1 (1α-hydroxylase) and subsequently activated vitamin D3 metabolism in these cells (17). To determine whether IL-17A increased cathelicidin in keratinocytes by induction of vitamin D3 metabolism, CYP27B1 mRNA abundance and 1,25D3 levels in the cell culture medium were evaluated. CYP27B1 expression was not changed after 1,25D3 or IL-17A stimulation (see Supplemental Fig. 3A).3 Also, the levels of active 1,25D3 in the culture medium of NHEK 24 h after stimulation with 1,25D3 or with the combination IL-17A with 1,25D3 were equal (see Supplemental Fig. 3A).3
Recently, it was also demonstrated that the transcriptional activity of the VDR depends on coactivators such as the steroid receptor coactivator SRC3 (19). SRC3 recruits histone acetyltransferases, which by enhancing histone acetylation open up the chromatin, thus facilitating access of transcription factors. SRC3 is a critical factor for 1,25D3-induced cathelicidin and factors that increase histone acetylation such as butyrate enhanced cathelicidin transcription (19). To test whether IL-17A increases cathelicidin by enhancing histone acetylation keratinocytes were stimulated and cell lysates were evaluated by Western blot. However, IL-17A did not induce histone acetylation in these cells (see Supplemental Fig. 3B).3
To identify the relevant region in the cathelicidin promoter mediating the effect of IL-17A, a 1500-bp fragment of the 5′ untranslated region of the human cathelicidin gene CAMP was cloned into a luciferase reporter plasmid and transfected into HaCaT keratinocytes. The fragment included the identified vitamin D responsive element and transcriptional activity was strongly induced by 1,25D3 (see Supplemental Fig. 3C).3 However, IL-17A did not enhance transcriptional activity in this promoter region.
MEK-ERK signals cathelicidin expression by 1,25D3 and IL-17A
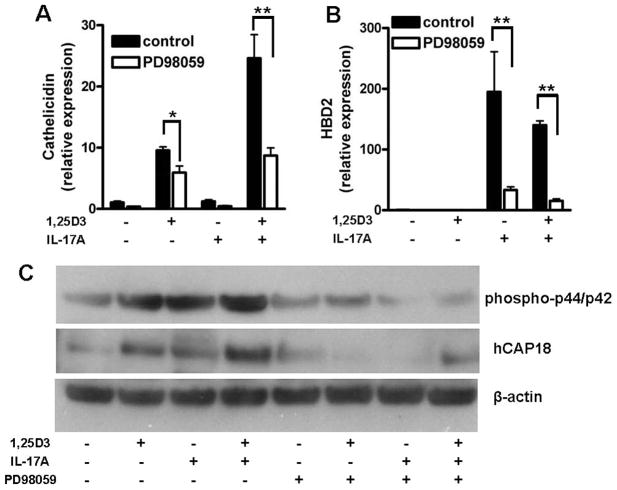
IL-17A activates multiple signaling pathways in target cells, among those MEK-ERK (20). To further characterize the signaling pathways involved in IL-17A increased cathelicidin expression in keratinocytes PD98059, a selective inhibitor of MEK-ERK signaling, was used. Preincubation of NHEK with PD98059 blocked the effect of IL-17A on cathelicidin and HBD2 expression (Fig. 7, A and B). IL-17A and 1,25D3 both activated MEK-ERK in keratinocytes as observed by p44/p42 phosphorylation (Fig. 7C). Stimulation of keratinocytes using the IL-17A with 1,25D3 combination increased p44/p42 phosphorylation, suggesting enhanced MEK-ERK activation (Fig. 7C). Inhibition of cathelicidin expression was confirmed on the protein level: PD98059 completely blocked the effect of IL-17A on hCAP18 expression (Fig. 7C). Interestingly, 1,25D3-induced cathelicidin was also decreased by PD98059, which indicates that MEK-ERK signaling is generally involved in cathelicidin regulation in keratinocytes (Fig. 7, A and C).
FIGURE 7.
IL-17A and 1,25D3 increase cathelicidin by activation of MEK-ERK. To investigate the role of MEK-ERK signaling in IL-17A-induced cathelicidin, NHEK were treated with the specific MEK inhibitor PD98059 (20 μM) before stimulation with IL-17A or 1,25D3. Inhibition of MEK-ERK blocked increased cathelicidin by IL-17A in the presence of 1,25D3. A, The 1,25D3-induced cathelicidin was also diminished. To investigate whether MEK-ERK was involved in induction of other innate immune genes by IL-17A, NHEK were treated with PD98059 and subsequently stimulated with 1,25D3 (10−8 M), IL-17A (10 ng/ml), or the combination. B, Again, inhibition of MEK-ERK blocked induction of HBD2. Data are mean ± SD of a single experiment performed in triplicate and are representative of three independent experiments.*, p < 0.05 and **, p < 0.01, determined by Student’s t test. C, The inhibitory effect of PD98059 on MEK-ERK was confirmed by Western blot analyses of phospho-p44/p42. Similar to cathelicidin mRNA expression, inhibition of MEK-ERK signaling blocked IL-17A with 1,25D3-enhanced cathelicidin peptide hCAP18.
Discussion
The expression and function of CAMP is essential for optimal host defense against infection (4). In addition, recent studies indicate that besides killing microbes cathelicidin peptide can also modulate host inflammatory responses (21). These observations are consistent with an evolving understanding of the functions of antimicrobial peptides. Several additional properties of cathelicidins and other antimicrobial peptides have been observed: they can stimulate cytokine release (22), chemotaxis (5), angiogenesis (23), and wound repair (24). The discovery of these functions explains how impaired cathelicidin expression or function contributes to the pathogenesis of inflammatory skin diseases: in atopic dermatitis induction of cathelicidin is disturbed, which increases the susceptibility of these patients to superinfections (11). Increased protease activity in the skin of patients with rosacea leads to abnormal cathelicidin processing and the peptide fragments generated induce several clinical symptoms such as skin inflammation and a vascular response (25). In the case of psoriasis, cathelicidin peptide LL-37 has been identified as a factor that may be responsible for the activation of cutaneous pDC (2). Although cathelicidin levels in intact skin are very low, lesional skin in psoriasis shows strong expression of cathelicidin. Increased cathelicidin in psoriatic skin functions as a trigger of an autoinflammatory cascade as LL-37 peptide forms complexes with self-DNA, which activate pDCs through TLR9 (2). Although this inflammatory cascade has been recently characterized, the mechanisms of increased cathelicidin in lesional skin in psoriasis were unclear. In this study we present evidence that IL-17A, a member of the Th17 family of cytokines involved in psoriasis pathogenesis, increases cathelicidin in human keratinocytes. Unexpectedly, induction of cathelicidin was dependent on the presence of active vitamin D3 (1,25D3), suggesting an interaction of IL-17A and vitamin D3 signaling in these cells.
IL-17A is secreted as a homodimer and activates its receptor IL-17RA, which leads to increased production of cytokines and chemokines, including TNF-α, IL-1β, IL-8, and IL-6 in target cells (26). When we stimulated human keratinocytes with supernatants from T cells isolated from lesional psoriatic skin, we observed an increase in cathelicidin expression dependent on the presence of 1,25D3 in the cell culture medium. Inhibition of IL-17RA blocked this effect, and in vitro experiments confirmed that cathelicidin expression was indeed controlled by IL-17A. In contrast to other antimicrobial peptides such as HBD2, S100A7, S100A8, and S100A9, which are cooperatively induced by IL-17A and IL-22, expression of cathelicidin was affected by IL-17A only (27). Also, in the presence of 1,25D3, induction of other known target genes of IL-17A such as HBD2 and IL-6 was reduced, whereas cathelicidin was increased.
When investigating the mechanism of IL-17A-enhanced cathelicidin we found that the signaling adaptor protein Act1 was critically involved. Act1 has recently been shown in vivo to be essential for IL-17A-mediated inflammation in the brain during experimental autoimmune encephalomyelitis and in gut epithelial cells during induced colitis (28). The primary human keratinocytes used in our study constitutively expressed Act1 and cathelicidin. HBD2 and IL-6 induction by IL-17A was strongly reduced after Act1 silencing. To further evaluate downstream signaling effects additional experiments were performed. Hartupee et al. (18) recently demonstrated that chemokine gene expression by IL-17A is increased through mRNA stabilization by an Act1-dependent mechanism. However, in our experiments IL-17A did not enhance cathelicidin expression by increased RNA stability. Cathelicidin expression was increased through an active transcriptional mechanism as treatment of the cells with ActD completely blocked induction. Still, no difference in cathelicidin promoter activity was observed after stimulation of keratinocytes with IL-17A, which might indicate that cis-acting elements are involved.
Unexpectedly, IL-17A augmented cathelicidin expression in keratinocytes only in the presence of 1,25D3. An interaction between vitamin D3 and IL-17A pathways has not been previously described. Therefore we asked whether IL-17A increased vitamin D3 signaling to enhance cathelicidin in keratinocytes. However, IL-17A did not affect vitamin D3 signaling events, which lead to increased cathelicidin in keratinocytes such as activation of 1,25D3 by CYP27B1 or enhanced acetylation of core histones (17, 19, 29, 30). However, we found that MEK-ERK signaling, which is activated in cells after stimulation with IL-17A and 1,25D3, was involved in cathelicidin induction (31, 32). Inhibition of MEK-ERK by a specific inhibitor blocked cathelicidin and HBD2 induction by IL-17A. Involvement of MEK-ERK in 1,25D3-induced cathelicidin in keratinocytes has been previously described (12).
Increased cathelicidin mRNA after IL-17A stimulation was accompanied by increased cathelicidin precursor protein hCAP18. Processing of hCAP18 to active LL-37 in skin is mediated by serine proteases of the kallikrein family such as KLK5 and KLK7 (33). However, in our experiments no increased LL-37 could be detected. This finding can either be explained by low and therefore undetectable peptide levels in our experimental system or by the fact that critical factors for activation of hCAP18 processing were missing. Cathelicidin expression and LL-37 release are rapidly activated upon injury or bacterial infection, such as situations in which the affected skin tissue is broken up and essential proteases might be released (10, 17). In psoriasis, keratinocyte turnover is high and dying cells will release an array of proteases that are then able to process hCAP18 to LL-37. LL-37 will then bind to self-DNA and initiate the autoinflammatory cascade (2).
Our data raise two further questions: first, Th17 cells are increased in peripheral blood and infiltrate in the skin of patients with atopic eczema (34). This should lead to higher tissue levels of IL-17A and induction of cathelicidin. Still, atopic eczema is characterized by low cutaneous cathelicidin expression and increased risk for superinfections (11). This discrepancy might be due to chemokines or cytokines present in skin in atopic eczema, which might interfere with signaling pathways leading to appropriate cathelicidin expression. Indeed, Th2 cytokines such as IL-4 and IL-13 have been identified to block cathelicidin induction in atopic skin (35). Secondly, vitamin D3 analogs are successfully used in the treatment of psoriasis. One would expect that activation of the VDR by these analogs will increase cathelicidin expression resulting in exacerbation of cutaneous inflammation. The opposite is true and a possible explanation might be that vitamin D3 analogs compete with 1,25D3 for binding to the VDR. Other explanations include an altered cytokine milieu in affected skin lesions resulting in a modified 1,25D3 response, differential effects of vitamin D3 analogs on already maximally induced cathelicidin in psoriatic skin, or other biological effects of vitamin D3 analogs beyond cathelicidin regulation such as its effects on keratinocyte differentiation.
Our results show for the first time to our knowledge an interaction between vitamin D3 and IL-17A signaling in the regulation of cathelicidin in keratinocytes. Active vitamin D3 enables IL-17A to increase cathelicidin in keratinocytes through activation of Act1 and MEK-ERK. As cathelicidin peptide is the principal trigger for IFN-α release from pDCs in psoriasis and IL-17A is high in psoriatic tissue, it will be important to investigate whether therapeutic targeting of this pathway might be beneficial in patients with psoriasis.
Supplementary Material
Footnotes
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (Emmy Noether Programm; Scha 979/3-1) and the Friedrich Baur Stiftung.
Abbreviations used in this paper: pDC, plasmacytoid dendritic cell; CAMP, cathelicidin antimicrobial peptide; HBD2, human β-defensin 2; 1,25D3, 1,25-dihydroxyvitamin D3; NHEK, normal human epidermal keratinocyte; VDR, vitamin D receptor; siRNA, small interfering RNA; ActD, actinomycin D.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Nickoloff BJ, Qin JZ, Nestle FO. Immunopathogenesis of psoriasis. Clin Rev Allergy Immunol. 2007;33:45–56. doi: 10.1007/s12016-007-0039-2. [DOI] [PubMed] [Google Scholar]
- 2.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, Cao W, Wang YH, Su B, Nestle FO, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 3.Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, Burg G, Liu YJ, Gilliet M. Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J Exp Med. 2005;202:135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol. 2008;122:261–266. doi: 10.1016/j.jaci.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, Kiessling R, Jornvall H, Wigzell H, Gudmundsson GH. The human antimicrobial and chemotactic peptides LL-37 and α- defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. [PubMed] [Google Scholar]
- 6.Schauber J, Gallo RL. Expanding the roles of antimicrobial peptides in skin: alarming and arming keratinocytes. J Invest Dermatol. 2007;127:510–512. doi: 10.1038/sj.jid.5700761. [DOI] [PubMed] [Google Scholar]
- 7.Oppenheim JJ, Tewary P, de la Rosa G, Yang D. Alarmins initiate host defense. Adv Exp Med Biol. 2007;601:185–194. doi: 10.1007/978-0-387-72005-0_19. [DOI] [PubMed] [Google Scholar]
- 8.Zaiou M, Gallo RL. Cathelicidins, essential gene-encoded mammalian antibiotics. J Mol Med. 2002;80:549–561. doi: 10.1007/s00109-002-0350-6. [DOI] [PubMed] [Google Scholar]
- 9.Braff MH, Hawkins MA, Di Nardo A, Lopez-Garcia B, Howell MD, Wong C, Lin K, Streib JE, Dorschner R, Leung DY, Gallo RL. Structure-function relationships among human cathelicidin peptides: dissociation of antimicrobial properties from host immunostimulatory activities. J Immunol. 2005;174:4271–4278. doi: 10.4049/jimmunol.174.7.4271. [DOI] [PubMed] [Google Scholar]
- 10.Dorschner RA, V, Pestonjamasp K, Tamakuwala S, Ohtake T, Rudisill J, Nizet V, Agerberth B, Gudmundsson GH, Gallo RL. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J Invest Dermatol. 2001;117:91–97. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- 11.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 12.Schauber J, Dorschner RA, Yamasaki K, Brouha B, Gallo RL. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology. 2006;118:509–519. doi: 10.1111/j.1365-2567.2006.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang TT, Nestel F, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JH, Mader S, White JH. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 14.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950 –957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 15.Boniface K, Guignouard E, Pedretti N, Garcia M, Delwail A, Bernard FX, Nau F, Guillet G, Dagregorio G, Yssel H, et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol. 2007;150:407–415. doi: 10.1111/j.1365-2249.2007.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prinz JC, Gross B, Vollmer S, Trommler P, Strobel I, Meurer M, Plewig G. T cell clones from psoriasis skin lesions can promote keratinocyte proliferation in vitro via secreted products. Eur J Immunol. 1994;24:593–598. doi: 10.1002/eji.1830240315. [DOI] [PubMed] [Google Scholar]
- 17.Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartupee J, Liu C, Novotny M, Li X, Hamilton T. IL-17 enhances chemokine gene expression through mRNA stabilization. J Immunol. 2007;179:4135–4141. doi: 10.4049/jimmunol.179.6.4135. [DOI] [PubMed] [Google Scholar]
- 19.Schauber J, Oda Y, Buchau AS, Yun QC, Steinmeyer A, Zugel U, Bikle DD, Gallo RL. Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-Dihydroxyvitamin D3. J Invest Dermatol. 2008;128:816–824. doi: 10.1038/sj.jid.5701102. [DOI] [PubMed] [Google Scholar]
- 20.Kao CY, Huang F, Chen Y, Thai P, Wachi S, Kim C, Tam L, Wu R. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK/NF-κ B-dependent signaling pathway. J Immunol. 2005;175:6676–6685. doi: 10.4049/jimmunol.175.10.6676. [DOI] [PubMed] [Google Scholar]
- 21.Di Nardo A, Braff MH, Taylor KR, Na C, Granstein RD, McInturff JE, Krutzik S, Modlin RL, Gallo RL. Cathelicidin antimicrobial peptides block dendritic cell TLR4 activation and allergic contact sensitization. J Immunol. 2007;178:1829–1834. doi: 10.4049/jimmunol.178.3.1829. [DOI] [PubMed] [Google Scholar]
- 22.Niyonsaba F, Ushio H, Nagaoka I, Okumura K, Ogawa H. The human β-defensins (-1, -2, -3, -4) and cathelicidin LL-37 induce IL-18 secretion through p38 and ERK MAPK activation in primary human keratinocytes. J Immunol. 2005;175:1776 –1784. doi: 10.4049/jimmunol.175.3.1776. [DOI] [PubMed] [Google Scholar]
- 23.Koczulla R, von Degenfeld G, Kupatt C, Krötz F, Zahler S, Gloe T, Issbrücker K, Unterberger P, Zaiou M, Lebherz C, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carretero M, Escámez MJ, García M, Duarte B, Holguín A, Retamosa L, Jorcano JL, Río MD, Larcher F. In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37. J Invest Dermatol. 2008;128:223–236. doi: 10.1038/sj.jid.5701043. [DOI] [PubMed] [Google Scholar]
- 25.Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, Dorschner RA, Bonnart C, Descargues P, Hovnanian A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975–980. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- 26.van Beelen AJ, Teunissen MB, Kapsenberg ML, de Jong EC. Interleukin-17 in inflammatory skin disorders. Curr Opin Allergy Clin Immunol. 2007;7:374 –381. doi: 10.1097/ACI.0b013e3282ef869e. [DOI] [PubMed] [Google Scholar]
- 27.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8:247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 29.Schauber J, Iffland K, Frisch S, Kudlich T, Schmausser B, Eck M, Menzel T, Gostner A, Luhrs H, Scheppach W. Histone-deacetylase inhibitors induce the cathelicidin LL-37 in gastrointestinal cells. Mol Immunol. 2004;41:847– 854. doi: 10.1016/j.molimm.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Schauber J, Svanholm C, Termen S, Iffland K, Menzel T, Scheppach W, Melcher R, Agerberth B, Luhrs H, Gudmundsson GH. Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: relevance of signaling pathways. Gut. 2003;52:735–741. doi: 10.1136/gut.52.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawaguchi M, Kokubu F, Odaka M, Watanabe S, Suzuki S, Ieki K, Matsukura S, Kurokawa M, Adachi M, Huang SK. Induction of granulocyte-macrophage colony-stimulating factor by a new cytokine, ML-1 (IL-17F), via Raf I-MEK-ERK pathway. J Allergy Clin Immunol. 2004;114:444 – 450. doi: 10.1016/j.jaci.2004.03.047. [DOI] [PubMed] [Google Scholar]
- 32.De Haes P, Garmyn M, Carmeliet G, Degreef H, Vantieghem K, Bouillon R, Segaert S. Molecular pathways involved in the anti-apoptotic effect of 1,25-dihydroxyvitamin D3 in primary human keratinocytes. J Cell Biochem. 2004;93:951–967. doi: 10.1002/jcb.20227. [DOI] [PubMed] [Google Scholar]
- 33.Yamasaki K, Schauber J, Coda A, Lin H, Dorschner RA, Schechter NM, Bonnart C, Descargues P, Hovnanian A, Gallo RL. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006;20:2068 –2080. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- 34.Koga C, Kabashima K, Shiraishi N, Kobayashi M, Tokura Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J Invest Dermatol. 2008;128:2625–2630. doi: 10.1038/jid.2008.111. [DOI] [PubMed] [Google Scholar]
- 35.Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, Leung DY. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006;24:341–348. doi: 10.1016/j.immuni.2006.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.