Abstract
Aims
Cryothermal energy (CTE) ablation via a balloon catheter (Arctic Front, Cryocath™) represents a novel technology for pulmonary vein isolation (PVI). However, balloon-based PVI approaches are associated with phrenic nerve palsy (PNP). We investigated whether ‘single big cryoballoon’-deployed CTE lesions can (i) achieve acute electrical PVI without left atrium (LA) imaging and (ii) avoid PNP in patients with paroxysmal atrial fibrillation (PAF).
Methods and results
After double transseptal punctures, one Lasso catheter and a big 28 mm cryoballoon catheter using a steerable sheath were inserted into the LA. PV angiography and ostial Lasso recordings from all PVs were obtained. Selective PV angiography was used to evaluate balloon to LA–PV junction contact. CTE ablation lasted 300 s, and the PN was paced during freezing at right-sided PVs. Twenty-seven patients (19 males, mean age: 56 ± 9 years, LA size: 42 ± 5 mm) with PAF (mean duration: 6.6 ± 5.7 years) were included. PVI was achieved in 97/99 PVs (98%). Median (Q1; Q3) procedural, balloon, and fluoroscopy times were 220 min (190; 245), 130 min (90; 170), and 50 min (42; 69), respectively. Three transient PNP occurred after distal PV ablations. No PV stenosis occurred. Total median (Q1; Q3) follow-up time was 271 days (147; 356), and 19 of 27 patients (70%) remained in sinus rhythm (3-month blanking period).
Conclusion
Using the single big cryoballoon technique, almost all PVs (98%) could be electrically isolated without LA imaging and may reduce the incidence of PNP as long as distal ablation inside the septal PVs is avoided.
Keywords: Catheter ablation, Atrial fibrillation, Cryoballoon, Pulmonary vein
Introduction
Muscular sleeves connecting the left atrium (LA) and pulmonary veins (PVs) can be ablated using either a segmental or a circumferential approach.1–3
Success rates for radiofrequency current (RFC) ablation have improved after the introduction of advanced mapping and ablation strategies.3 However, procedural complexity, if the endpoint was defined as PV isolation and procedure-related complications, has limited a widespread application.4–7 Balloon-based catheter ablation systems have the potential to isolate the PVs with a single application.8 Cryothermal energy (CTE) has been previously demonstrated to be safe.9,10 Using CTE for PV isolation (PVI), no PV stenosis and a low risk of thrombus formation have been reported.11,12 This is in agreement with experimental animal data.13,14 Moreover, PVI using the novel cryoballoon (Arctic Front, Cryocath™) could be successfully achieved in a canine model13 and in humans,15–18 however, with the use of different cryoballoon sizes and additional ‘touch-up’ freezes (Freezor Max, Cryocath™). Notably, phrenic nerve palsy (PNP) did occur particularly with the use of small balloons at the right superior PV (RSPV).15–18
We hypothesized that a cryoballoon-based PVI has the potential to become a straightforward, simple, and safe ablation procedure for paroxysmal atrial fibrillation (PAF) if only one ‘single big cryoballoon’ is used: (i) to acutely isolate all PVs, (ii) to create lesions at the antrum level (LA–PV junction), and (iii) to prevent complications, particularly PNP.
Methods
Inclusion and exclusion criteria
Between April 2006 and May 2007, a total of 27 consecutive patients with PAF were included in this study and provided written informed consent. The study subjects met the following inclusion criterion: a history of highly symptomatic PAF (≥1 episode/week) despite treatment with one or more anti-arrhythmic drugs (AADs), and both investigators (K.R.J.C. and K.H.K.) were present to perform the procedure. Exclusion criteria were defined as an LA diameter ≥55 mm, severe left ventricular hypertrophy (LV wall thickness ≥15 mm), LA thrombus, prior stroke, or decompensated heart failure. Importantly, no cardiac magnetic resonance imaging (MRI) or computed tomography scan had been obtained before the procedure. Therefore, no patient was excluded due to previously known PV anatomy.
Cryoballoon catheter
The cryoballoon catheter (Arctic Front, Cryocath™, 28 mm diameter, 10.5 F shaft) consists of an inner and outer lumen. The refrigerant N2O is delivered into the inner balloon where it undergoes a liquid-to-gas phase change, resulting in an inner balloon cooling temperature of approximately −80°C. The catheter is equipped with central lumina, which is used for (i) the insertion of the guide wire (Amplatz stiff wire) and (ii) injection of contrast medium (diluted 1:1 ratio with 0.9% saline) for PV angiograms. Using the ‘over-the-wire’ technique in conjunction with the steerable sheath (12 F, FlexCath, Cryocath™), the balloon can be navigated to each PV ostium. In addition, the cryoballoon itself is steerable through a pull wire mechanism integrated in the handle of the catheter.
Procedure
Vital parameters such as blood pressure and oxygen saturation were continuously monitored throughout the entire procedure. All procedures were performed under deep sedation using boluses of midazolam and fentanyl as well as a continuous infusion of propofol (1%). After placing 6 F decapolar catheters into the coronary sinus and the His bundle region, two transseptal punctures were performed using a modified Brockenbrough technique to introduce two 8 F sheaths (SL1; St Jude Medical) into the LA. One puncture site in the fossa ovalis was rather posterior, and the other more anterior. Thereafter, heparin boluses were repeatedly administered to maintain the activated clotting time between 250 and 300 s. Selective PV angiography was performed to identify the PV anatomy in standard angulations (RAO 30° and LAO 40°). Left-sided PVs were classified as separate or short trunk left common PVs (LCPV), according to Marom et al.19 A Lasso catheter (Biosense Webster) was placed at the PV ostium via the posterior sheath to record PV potentials (sinus rhythm and coronary sinus pacing) using a conventional computerized EP system (AXIOM Sensis, Siemens). The more anterior SL1 sheath was exchanged for the 12 F transseptal sheath (FlexCath®) in order to introduce the cryoballoon (28 mm) catheter into the LA. Both transseptal sheaths were constantly flushed with heparinized saline (500 IE/50 mL) (8 F: 10 mL/h and 12 F: 20 mL/h). The 28 mm balloon was manoeuvred to all PV ostia. To assess the exact position of the inflated balloon in relation to the PV–LA region, contrast medium (Imeron®) diluted with saline 0.9% (1:1 ratio) was injected from the distal lumen of the cryoballoon catheter.
Then, the freezing cycle (300 s) was started. Local temperature was continuously monitored from a sensor at the proximal part of the cryoballoon, which could only be recorded but not read out (Cryoconsole Gen 4). The PN was constantly paced (10 V, 2.9 ms) from the superior vein cava when freezing at the RPVs. In the case of loss of PN capture, freezing was immediately terminated. After each freeze, PV conduction was re-evaluated by positioning the Lasso catheter at the same location within the PV as before the CTE application. Based on the angiograms, the Lasso was pulled back as proximal as possible until it dropped into the LA after PVI to find the site of PVI occurrence. If the PV was not isolated, the cryoballoon was repositioned and balloon to LA–PV contact was re-evaluated by angiography. Ablation endpoint was the loss of all PV potentials. The position of the cryoballoon during the freeze was documented for all applications. After a waiting period of 30 min after the last PVI, persistence of the conduction block was re-checked with the Lasso catheter for all veins. Thereafter, a repeat angiography was performed to exclude PV stenosis. No intraprocedural imaging except for fluoroscopy was used in this study. Notably, all procedures were performed by the same investigators (K.R.J.C. and K.H.K.) with no experience with cryoballoon technology before the study was started.
Ablation techniques
For different PVs, special ablation techniques were developed.
Left pulmonary veins
‘Crosstalk’ technique
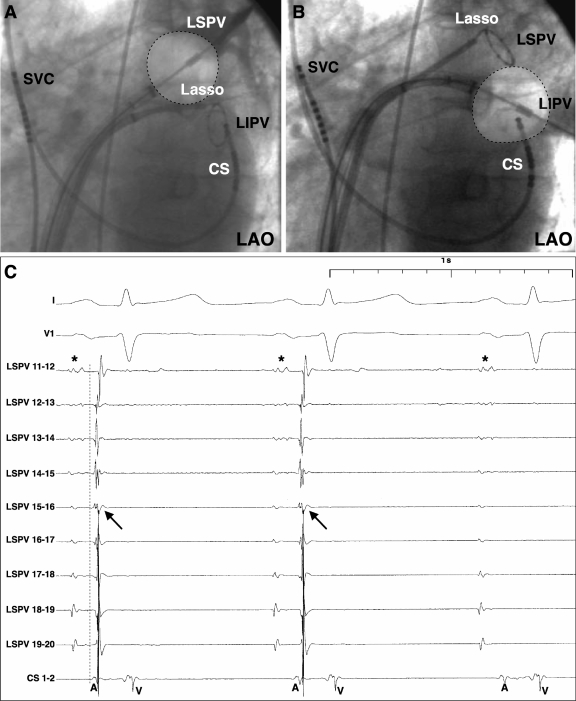
For the left-sided PVs, cryoablation was first deployed at the left superior PV (LSPV) with a Lasso catheter located in the left inferior PV (LIPV). After angiographic evaluation of PV occlusion by the balloon, CTE was applied. Thereafter, the Lasso was placed in the LSPV to check whether the PV was isolated or not. In the case of a gap along the inferior aspect of the LSPV, the cryoballoon was placed at the LIPV. After angiographic verification of the balloon position at the ostium of the LIPV, CTE was delivered (Figure 1A–C). During CTE application to the LIPV, simultaneous PV recordings from the Lasso catheter in the LSPV were obtained to assess either LSPV spike sequence changes or LSPV isolation (Figure 1C). Because of the interaction between the two veins, this approach has been termed as crosstalk ablation technique. Subsequent Lasso recordings from the LIPV documented either simultaneous PVI or guided closure of remaining conduction gaps by further balloon applications.
Figure 1.
The crosstalk technique: (A) big balloon ablation after occlusion of the left superior pulmonary vein (PV); the Lasso catheter is placed in the left inferior PV. (B) Left inferior PV ablation after exchanging balloon and Lasso positions. (C) Left superior PV Lasso recordings demonstrate remaining LA–PV conduction (arrow: PV spike, dotted line: earliest activation LSPV 15/16 indicating inferior conduction gap) and subsequent elimination of PV spike. A, atrium; CS, coronary sinus catheter; V, ventricle; LA, left atrium; LAO, left anterior oblique; PV, pulmonary vein. *Far field atrium.
Right superior pulmonary vein
‘Straightforward’ technique
The RSPV was always approached directly, and CTE was delivered after angiographic confirmation of PV occlusion. Special care was taken to avoid PN injury by constantly pacing the PN from a catheter located in the superior caval vein. In addition, the RSPV ostium diameter was measured from the angiographic information (RAO 30° and LAO 40°) to allow calculation of the ratio between the size of the PV and the size of the big balloon to assess the risk of PN injury.
Right and left inferior pulmonary veins
‘Direct approach’ technique
As for the superior PVs, a direct alignment of the catheter/balloon position and the PVs was always attempted initially. However, CTE was applied only when the cryoballoon could be positioned in such a way that complete PV occlusion was achieved. In all other cases, more sophisticated techniques were required.
‘Hockey stick’ technique
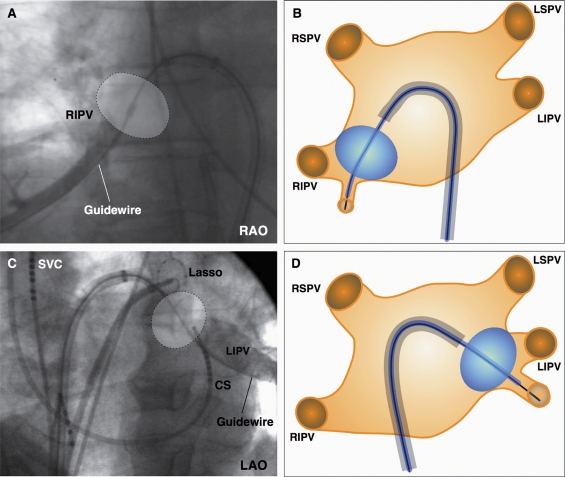
This strategy was used in patients with an early branching inferior PV to allow balloon to tissue contact at the inferior PV circumference. First, the guide wire was placed in the early branching inferior PV. Then, the sheath was advanced with maximal bend to the superior–posterior LA, allowing the balloon to be pushed into the inferior part of the PV ostium, which resulted in a hockey stick configuration (Figure 2).
Figure 2.
(A–D) The hockey stick technique: the guide wire is placed in an early branching inferior pulmonary vein (PV). The balloon is advanced over the wire, thereby resulting in a hockey stick configuration of the system. This technique enables right inferior PV (2A+B) and left inferior PV (2C+D) isolation. Both schematic drawings (B and D) illustrate the basic principle of the hockey stick technique. CS, coronary sinus catheter; LAO, left anterior oblique; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; RAO, right anterior oblique; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; SVC, superior caval vein.
‘Pull-down’ technique
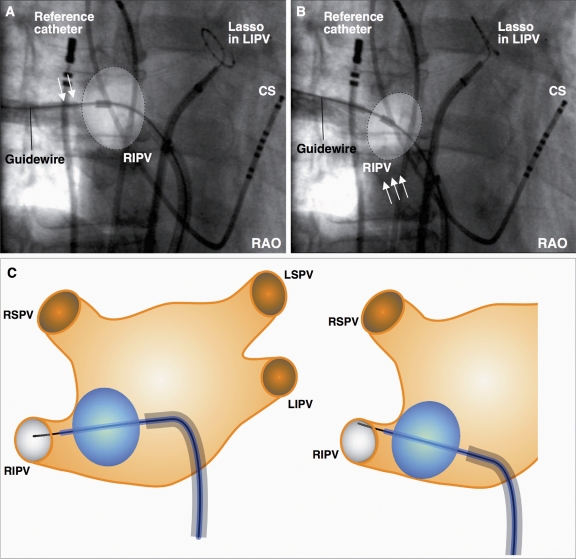
In patients with no or only a late branching inferior PV, the balloon was positioned parallel to the PV ostium. If angiography indicated only a perfect contact of the balloon at the superior circumference of the PV but not at the lower PV circumference, freeze was started regardless of the remaining leakage at the inferior PV circumference (Figure 3A). When full N2O flow into the inner balloon was reached, both the sheath and the frozen cryoballoon, which was attached to the superior PV circumference, were then pulled down to close the inferior gap (Figure 3B and C). Therefore, this technique was termed pull-down technique.
Figure 3.
Pull-down technique: the balloon is positioned parallel to the pulmonary vein (PV) ostium. (A) Although angiography indicates perfect contact of the balloon only at the superior circumference of the target PV (arrows) but not at the lower PV circumference, the freeze is started regardless of the remaining leakage at the inferior PV circumference. (B) Both the sheath and the frozen cryoballoon attached to the superior PV circumference are pulled down to close the inferior gap (arrows). (C) The schematic drawings illustrate the sequential steps of the pull-down technique. CS, coronary sinus catheter; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; RAO, right anterior oblique; reference catheter, CARTO backup (Biosense Webster); RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
The ‘big loop’ technique
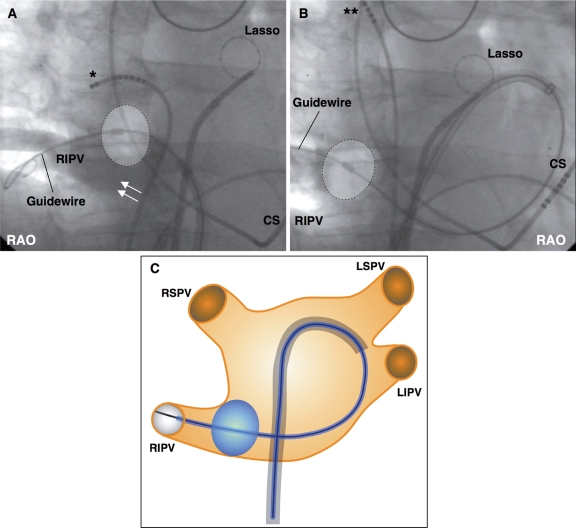
This technique was used in a case with an inferior RIPV ostium, when an early branch required for the hockey stick technique was lacking and the pull-down technique could not be used because of a larger inferior gap (>1 cm) (Figure 4A). To reach the PV ostium with the balloon, the sheath was bent and directed towards the lateral posterior LA, allowing the guide wire to be advanced along the posterior mitral annulus until the distal part of the RIPV was reached. Then, the sheath was further advanced to guide the balloon over the wire into the RIPV ostium (Figure 4B).
Figure 4.
The big loop technique: (A) right inferior pulmonary vein (RIPV) with a large inferior gap (arrows) lacking an early PV branch. (B) The sheath is directed towards the lateral posterior left atrium, allowing the guide wire to be advanced until the distal part of the RIPV is reached. The balloon is placed over the wire at the RIPV ostium. (C) Schematic drawing of the big loop technique. CS, coronary sinus catheter; LA, left atrium; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; RAO, right anterior oblique; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; *Dislodged multipolar catheter. **Repositioned multipolar catheter for phrenic nerve stimulation.
Post-ablation treatment and follow-up
In all patients, pericardial effusion and pneumothorax were ruled out (transthoracic echocardiogram and chest X-ray) after the procedure. After ablation, patients were treated with intravenous heparin (target PTT 50–70 s). Oral anticoagulation was started the next day. All patients received phenprocoumon, targeting an INR value of 2.0–3.0 for at least 3 months. AAD therapy was continued for 1 month and then discontinued if the patients were free of AF relapse. Follow-up included weekly telephonic interviews and outpatient clinic visits at 1, 3, 6, 9, and 12 months after ablation [interview, ECG, Holter ECG, and transoesophageal echocardiography (TEE)]. In addition, all patients were equipped with a portable tele ECG device (Vitaphone, Germany) and asked to transmit daily ECGs plus additional symptom-triggered tele ECGs for 6 months after the ablation monitored via IKKF (Institut für klinisch-kardiovaskuläre Forschung, Munich, Germany). All documented AF episodes lasting >30 s were counted as recurrences with and without a blanking period of 3 months. In all patients, Doppler flow measurement using TEE was performed 3 months after the procedure to assess late PV stenosis.20
Endpoints
The primary endpoint of the study was acute PVI. The secondary endpoints were procedure-related complications and AF recurrence.
Statistical analysis
An exploratory data analysis was performed. Data mean ± standard deviation (SD) was used to describe continuous variables with approximately normal distribution, or the median and the 25 and 75% percentiles (Q1; Q3) were presented. For categorical data, absolute and relative frequencies were given. Both tables show a subset of data of all 27 patients, including the case with the large type II atrial septal defect (ASD), which is marked.
Results
Patients
Twenty-seven patients (19 males, mean age: 56 ± 9 years) were enrolled in the study (Table 1). Patients had a history of PAF with a median (Q1; Q3) duration of 6.0 (3.0; 9.0) years and were refractory to a median number (Q1; Q3) of 3 (2; 3) AADs (including amiodarone in five patients), with a median (Q1; Q3) of 10 (8; 12) AF episodes per month. In 9 of 27 patients, arterial hypertension was present. In five patients, a concomitant heart disease was present, which was a significant type II ASD (maximum diameter 1.7 cm) in one patient (Table 1).
Table 1.
Patients demographics
| Patient no. | Gender | Age (years) | Concomitant heart disease | LA size (mm) | Duration of PAF (years) | Number of PAF episodes before PVI per months | Number of failed AAD |
|---|---|---|---|---|---|---|---|
| 1 | M | 64 | None | 42 | 8 | 8 | 2 |
| 2 | M | 62 | None | 37 | 30 | 15 | 3 |
| 3 | M | 55 | HTN | 45 | 7 | 4 | 3 |
| 4 | M | 49 | HTN | 39 | 5 | 3 | 3 |
| 5 | M | 55 | None | 32 | 6 | 12 | 2 |
| 6 | M | 59 | HTN, CAD | 49 | 3 | 2 | 2 |
| 7 | F | 41 | None | 40 | 4 | 10 | 3 |
| 8 | F | 58 | HTN | 42 | 10 | 10 | 3 |
| 9 | F | 76 | None | 39 | 7 | 8 | 5 |
| 10 | F | 59 | None | 30 | 2 | 12 | 2 |
| 11 | F | 45 | None | 36 | 7 | 12 | 2 |
| 12 | M | 46 | None | 45 | 3 | 10 | 2 |
| 13 | F | 58 | HTN, ASD II | 50 | 8 | 10 | 3 |
| 14 | M | 44 | None | 39 | 10 | 30 | 3 |
| 15 | M | 47 | None | 41 | 3 | 6 | 3 |
| 16 | F | 64 | HTN | 43 | 11 | 8 | 3 |
| 17 | M | 62 | HTN | 43 | 9 | 3 | 2 |
| 18 | M | 49 | None | 42 | 2 | 12 | 2 |
| 19 | M | 63 | HTN, CAD | 43 | 2 | 3 | 4 |
| 20 | M | 55 | DCM | 43 | 3 | 10 | 2 |
| 21 | M | 44 | None | 41 | 8 | 30 | 3 |
| 22 | M | 41 | None | 33 | 11 | 30 | 3 |
| 23 | M | 66 | None | 49 | 2 | 10 | 3 |
| 24 | F | 62 | HTN, MI | 51 | 1 | 30 | 2 |
| 25 | M | 63 | HTN | 40 | 10 | 10 | 3 |
| 26 | M | 67 | None | 44 | 2 | 10 | 3 |
| 27 | M | 35 | None | 44 | 5 | 10 | 3 |
AAD, anti-arrhythmic drugs; ASD, atrial septal defect; CAD, coronary artery disease; DCM, dilative cardiomyopathy; F, female; HTN, hypertension; LA, left atrium; M, male; PAF, paroxysmal atrial fibrillation; PVI, pulmonary vein isolation.
Procedural data
Median (Q1; Q3) procedure and fluoroscopy times including PN pace mapping were 220 min (190; 245) and 50 min (42; 69), respectively The total balloon time (defined as the time of cryoballoon inside the LA) was 130 min (90; 170). A mean of 174 ± 50 mL contrast medium was required for PV angiography.
Pulmonary vein angiography
PV angiography was used to identify PVs and to calculate mean PV diameters. Mean PV diameter was 19 ± 2 mm for LSPVs (n = 18), 18 ± 3 mm for LIPVs (n = 18), 30 ± 6 mm for LCPVs (n = 9), 18 ± 3 mm for RSPVs (n = 27), and 20 ± 4 mm for RIPVs (n = 27).
Pulmonary vein isolation
The primary endpoint of acute PVI was achieved in 98% of all PVs. In 27 patients, a total of 99 PVs including nine LCPVs were identified, all of which showed PV spikes. Ninety-seven of the 99 (98%) PVs were electrically isolated using the single big cryoballoon technique exclusively. After the waiting time (>30 min), no PV reconduction was observed. All LSPVs (n = 18), LIPVs (n = 18), LCPVs (n = 9), and RSPVs (n = 27) were successfully isolated requiring a median (Q1; Q3) of 2 (2; 2), 2 (2; 3), 5 (4; 8), and 2 (1; 2) cryo freezes, respectively. In 2/27 RIPVs, electrical isolation was futile, despite a median of 3 (2; 5) freezes (Table 2). Interestingly, in one patient (#13) with failed RIPV isolation, a pre-procedurally diagnosed large type II ASD was present. The other patient (# 10) with failed RIPV isolation had the smallest LA size (30 mm) (Table 1).
Table 2.
Ablation details, ablation techniques, and success rates of acute electrical pulmonary vein isolation using the single big cryoballoon technique
| Patient no. | Balloon size (mm) | No. of cryo-freezes |
PV isolation (%) | Special ablation technique (s) | ||||
|---|---|---|---|---|---|---|---|---|
| LSPV | LCPV | LIPV | RSPV | RIPV | ||||
| 1 | 28 | 7 | 2 | 3 | 100 | LCPV: crosstalk RIPV; hockey stick | ||
| 2 | 28 | 2 | 4 | 1 | 1 | 100 | RIPV: pull-down | |
| 3 | 28 | 1 | 1 | 1 | 2 | 100 | ||
| 4 | 28 | 3 | 4 | 2 | 100 | LCPV: crosstalk; LIPV: pull-down | ||
| 5 | 28 | 3 | 5 | 4 | 2 | 100 | RIPV: pull-down; LIPV: hockey stick | |
| 6 | 28 | 2 | 2 | 2 | 3 | 100 | RIPV: pull-down | |
| 7 | 28 | 2 | 2 | 2 | 8 | 100 | RIPV: hockey stick | |
| 8 | 28 | 1 | 2 | 2 | 2 | 100 | ||
| 9 | 28 | 2 | 2 | 2 | 100 | LCPV: crosstalk | ||
| 10 | 28 | 1 | 3 | 2 | 6 | 75 | ||
| 11 | 28 | 5 | 2 | 5 | 100 | LCPV: crosstalk; RIPV: pull-down | ||
| 12 | 28 | 1 | 2 | 1 | 3 | 100 | RIPV: pull-down | |
| 13 | 28 | 13 | 4 | 5 | 67 | LCPV: crosstalk | ||
| 14 | 28 | 5 | 2 | 2 | 100 | LCPV: crosstalk; RIPV: pull-down | ||
| 15 | 28 | 2 | 2 | 2 | 8 | 100 | RIPV: hockey stick | |
| 16 | 28 | 8 | 1 | 7 | 100 | LCPV: crosstalk; RIPV: pull-down; LIPV: hockey stick | ||
| 17 | 28 | 3 | 6 | 1 | 3 | 100 | LSPV: crosstalk; RIPV: hockey stick | |
| 18 | 28 | 2 | 2 | 1 | 1 | 100 | ||
| 19 | 28 | 4 | 1 | 9 | 100 | LCPV: crosstalk; RIPV: pull-down; LIPV: pull-down | ||
| 20 | 28 | 4 | 1 | 5 | 100 | LCPV: crosstalk; RIPV: pull-down; LIPV: pull-down | ||
| 21 | 28 | 2 | 3 | 1 | 8 | 100 | RIPV: pull-down | |
| 22 | 28 | 2 | 2 | 3 | 3 | 100 | RIPV: pull-down | |
| 23 | 28 | 3 | 2 | 1 | 3 | 100 | RIPV: pull-down | |
| 24 | 28 | 2 | 2 | 1 | 3 | 100 | RIPV: pull-down | |
| 25 | 28 | 2 | 3 | 3 | 4 | 100 | RIPV: pull-down | |
| 26 | 28 | 2 | 2 | 1 | 1 | 100 | RIPV: pull-down | |
| 27 | 28 | 2 | 2 | 2 | 2 | 100 | RIPV: big loop | |
| Median (Q1; Q3) | 2 (2; 2) | 5 (4; 8) | 2 (2; 3) | 2 (1; 2) | 3 (2; 5) | 98.0 ± 8% | ||
PV, pulmonary vein; LCPV, left common pulmonary vein; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
Left pulmonary veins
Left superior pulmonary vein
In 18/27 patients (67%), separated left-sided PVs were identified. In 9/27 patients (33%), a short trunk LCPV was identified. In 17/18 patients (94%) with separated LPVs, complete LSPV occlusion was achieved, resulting in subsequent PVI. In the remaining 10 patients (one separated LPV and nine LCPVs), freezing at the LSPV branch did not isolate the LSPV, but isolation was achieved in all cases using the crosstalk technique.
Left inferior pulmonary vein (cross)
In 22/27 patients (81%), complete LIPV occlusion was achieved, resulting in PVI. In 5/27 patients (19%), an inferior contrast leakage was present, which was closed using either the pull-down (n = 3) or the hockey stick technique (n = 2).
Right pulmonary veins
Right superior pulmonary vein
In 27/27 patients (100%), complete RSPV occlusion was achieved (‘straightforward’ approach), resulting in subsequent PVI.
Right inferior pulmonary vein
Twenty-five of 27 RIPVs were successfully isolated. In two of 27 patients (7%) (#10 and #13), no RIPV isolation was achieved. In five of 27 patients (19%), complete RIPV occlusion was achieved using the ‘direct approach’ resulting in PVI, whereas in 20 of 27 patients (74%), either the pull-down (n = 15), the hockey stick (n = 4) or the big loop technique (n = 1) was used to achieve PVI.
Procedural complications
Complications as a secondary endpoint occurred in 3/27 patients (11%). Despite continuous right-sided PN stimulation during ablation of the septal PVs, one PNP occurred after an unanticipated freeze in a distal position inside the RSPV: during freezing, the balloon pressure decreased, which lead to a more distal balloon position (patient # 16, PV size: 18.2 mm) (Figure 5). Two additional PN lesions occurred: one during freezing from the RSPV (Patient 18, PV size: 26.0 mm) and the other from the RIPV (Patient 24, PV size: 26.0 mm). The ratio of PV size (26 mm) to balloon size (28 mm) with PN damage was 0.93. CTE application was immediately terminated if PN capture was lost (Patient 16, duration: 120 s; Patient 18, duration: 223 s; Patient 24, duration: 59 s). The PN function recovered in Patient 24 during the procedure after 3 min (freeze at RIPV). In the remaining patients, the PNP persisted until the end of the procedure. No further complications including PV stenosis were observed.
Figure 5.
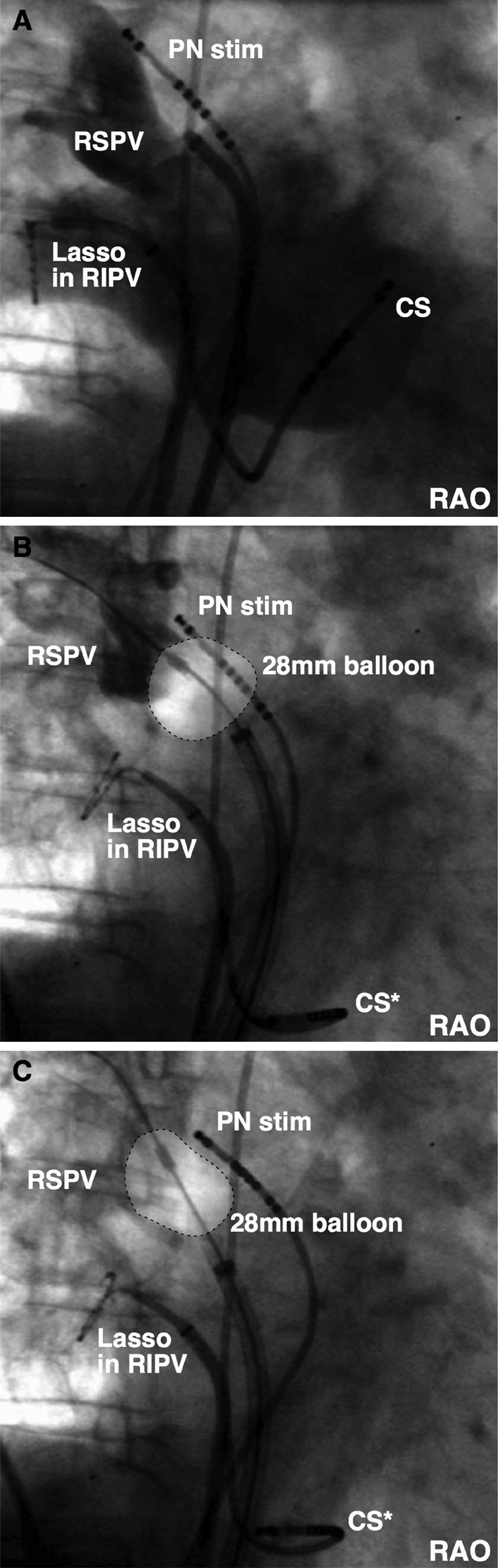
Phrenic nerve lesion: (A) baseline angiography of right superior pulmonary vein (RSPV) in right anterior oblique. (B) Ostial balloon position at the start of the freeze. (C) Unanticipated cryoballoon ablation inside the RSPV. CS, coronary sinus catheter; PN stim, phrenic nerve stimulation; RAO, right anterior oblique; RSPV, right superior pulmonary vein; RIPV, right inferior pulmonary vein.
Follow-up
AF recurrence defined as a secondary endpoint was observed in 8/27 patients (30%) during a median (Q1; Q3) follow up of 271 days (147; 356) with a blanking period of 3 months and in 13/27 patients (48%) without the blanking period. Interestingly, only AF, but no atrial tachycardia (AT), was observed during follow-up. Stable sinus rhythm was documented throughout the follow-up period in 19/27 patients (70%, 3-month blanking period) and in 14/27 patients (52%, no blanking period). In none of the patients, an increased flow velocity indicating PV stenosis could be found at the 3-month TEE.
In both patients with PNP at the end of the ablation procedure, the PN function has recovered within 28 days (Patient 18) and 384 days (Patient 16) of follow-up, respectively.
Discussion
Catheter ablation using RFC energy has been established as a curative treatment in patients with PAF.21 However, the creation of point by point RFC energy linear lesions requires the understanding of the individual LA anatomy, including the identification of the PV ostia,3,22 and may be associated with significant procedure-related complications.4–7 In contrast, CTE balloon-based ablation is designed to achieve complete circular lesions around the pulmonary veins, independent of the individual PV anatomy. Therefore, this novel ablation technology may facilitate PVI and improve the safety of the procedure.
Successful PVI using CTE delivered via a balloon approach requires perfect contact to the LA wall around the PVs. Incomplete contact would result in conduction gaps and prevent complete PVI. Complete contact can be rather easily achieved with small balloons, but they would isolate distal PV sites instead of the antrum. Importantly, the antrum has been shown to be not only responsible for AF initiation but also for AF maintenance.23,24 Furthermore, a distal balloon ablation in the septal PVs would particularly increase the risk of PNP.18
Therefore, in this study, we investigated the hypothesis of whether acute PVI of all PVs can be achieved using CTE and a single big balloon in consecutive patients without any prior or intraprocedural LA imaging except angiography. We tested this in the patient population with PAF, because it has been shown that PVI at the antrum level is highly effective.3,25
In this study, we have demonstrated that 98% of all PVs can be isolated using the single big cryoballoon technique only. No additional balloon sizes or catheter-based ‘touch-up’ freezes were used.
In this consecutive series of patients, only two RIPVs could not be isolated. It is well known that the RIPV is the most difficult PV to reach after transseptal puncture. Balloon positioning in the RIPV requires postero-inferior rotation of the whole sheath/balloon system. In a previous study analysing LA MRI data, the mean endoluminal distance from the fossa ovalis to the RIPV was 20.2 mm,26 explaining the difficulty in achieving stable contact and failure of RIPV isolation in the patient with the smallest LA diameter (30 mm). Interestingly, this patient still has remained free of AF episodes, which is in agreement with the observation that the RIPV is the least electrically active PV.27 In the second patient, the transseptal sheath and the balloon could not be stably positioned in the RIPV ostium due to a large type II ASD (1.7 cm).
To obtain PVI with the single big cryoballoon technique, different procedural approaches were developed. First, approaching the superior PVs was mostly straightforward as the superior PVs are in direct alignment with the catheter/balloon position following transseptal puncture. Therefore, a median (Q1; Q2) of only 2 (2; 2) and 2 (1; 2) freezes (RSPV and LSPV) was necessary to obtain PVI. Due to significant overlap of the big balloon with the ostia of the lateral veins, final PVI of the LSPV was usually achieved from the inferior PV after a first freeze was applied to the superior vein (crosstalk technique). This prevented multiple unnecessary applications to the LSPV for complete PVI. The inferior PVs, particularly the RIPV, were more difficult to isolate and required the development of a variety of ablation techniques depending on patients individual anatomy.
Side effects of cryothermal ablation using the single big cryoballoon technique
Phrenic nerve injury
PN injury is a rare (<1%) but severe complication when RFC energy is deployed at the RSPV.28 This complication is significantly higher (up to 10%) when balloon-based ablation strategies are used.15,17,29 Particularly, small balloons located inside the PVs may lead to a short distance to the PN and increased risk of PN injury.18,30 In our study, the single big cryoballoon strategy uses an intentionally oversized balloon covering the proximal LA antrum region with as much distance to the PN as possible. This explains why no PN palsies occurred as long as ablation inside the PVs was avoided. However, if CTE was unintentionally deployed inside the septal PVs, PNP also occurred, which was short-lasting (Patient 18: 28 days and Patient 24: 3 min) after freezing at two large septal PVs (RSPV and RIPV: both 26 mm) and long-lasting (Patient 16: 384 days) after freezing inside an RSPV (Patient 16, PV: 18.2 mm). These findings prove our concept that distal balloon ablation at the septal PVs must be avoided and emphasizes the need for a >28 mm cryoballoon for larger veins. The calculated ratio between PV and balloon sizes indicates that a ratio ≥0.93 should not lead to CTE balloon ablation since even with the 28 mm cryoballoon distal balloon positions and subsequent PNP cannot be avoided in all patients.
Cryothermal lesions
The primary endpoint of acute electrical PVI was achieved in almost all PVs. This study did not assess permanent PVI. The chronic course of CTE balloon lesions is not known. A previous study in patients with atrial flutter demonstrated permanent bidirectional block at the right atrial isthmus.31 However, several studies using CTE for catheter ablation of accessory pathways mediated tachycardias, and atrioventricular nodal re-entry tachycardias have shown a higher recurrence rate than with RFC.9,10
AF recurrences in this series may indicate recovered PV conduction. The relatively high recurrence rate (48%) compared with studies using RFC energy is biased by the fact that daily ECG recordings were also obtained within the first 3 months, a time that is generally not taken into consideration (blanking period). Applying a blanking period of 3 months, the rate of AF recurrences decreased (30%). Nevertheless, the chronic lesion assessment after big cryoballoon-induced PVI is mandatory in future studies. Interestingly, only AF recurrence, but not AT, has been observed. Whether this indicates rather large conduction gaps remains speculative.
Two interesting acute observations during CTE balloon ablation were made. First, the crosstalk phenomenon at the lateral PVs indicates that lesions were set at the LA antrum level with the potential limitation of a less chronic effect due to the thickness of the myocardium at this site. Secondly, acute PVI does not necessarily require complete PV occlusion at the initial balloon position, but may be achieved in a stepwise approach as demonstrated by the pull-down technique. This makes electrical isolation of the inferior PVs feasible using the big balloon technique.
Procedural costs
In the light of limited financial resources in many countries and the large number of patients with PAF, a simplified ablation strategy for PVI is desirable. The results of this study using only one single big balloon without the need for pre- or intraprocedural LA imaging could make such a strategy also attractive from an economic standpoint.
Limitations
In this study, only a rather small number of patients with paroxysmal AF have been studied. Nevertheless, we believe that the results for acute PVI are representative for this patient population and that PVI can be achieved in almost all patients. Identification of the site of PVI is limited to the fact that no three-dimensional reconstruction was performed at the end of the procedure. We do not know whether large common ostia can be isolated because these patients were not part of this study. Larger balloon sizes (>28 mm) would be necessary to successfully approach such an anatomy. Furthermore, daily ECG monitoring was only performed over the first 6 months after the procedure, followed by 24 h Holter monitoring at 9 and 12 months. Therefore, we do not know whether daily monitoring over 12 months would have changed the number of AF recurrences. Further studies will be required in larger patient populations to assess the incidence of permanent PVI and its effect on clinical outcome. Furthermore, it needs to be proven in larger series whether the suggested ratio between PV and balloon sizes (≥0.93) may be valuable to estimate the risk of PNP and whether an even bigger balloon could further reduce the risk of PNP. In addition, randomized studies comparing cryoballoon ablation with conventional RFC energy will be necessary to assess efficacy, complications, and cost-efficacy.
Conclusion
Using the single big cryoballoon technique, almost all PVs (98%) could be electrically isolated without LA imaging and may reduce the incidence of PNP as long as distal ablation inside the septal PVs is avoided.
Funding
Funding to pay the Open Access publication charges for this article was provided by ASKLEPIOS proresearch (Hamburg, Germany).
Conflict of interest: K.-R.J.C. and K.H.K. received educational honoraria from Cryocath.
References
- 1.Haissaguerre M, Shah DC, Jais P, Hocini M, Yamane T, Deisenhofer I, Chauvin M, Garrigue S, Clementy J. Electrophysiological breakthroughs from the left atrium to the pulmonary veins. Circulation. 2000;14:2463–2465. doi: 10.1161/01.cir.102.20.2463. [DOI] [PubMed] [Google Scholar]
- 2.Oral H, Scharf C, Chugh A, Hall B, Cheung P, Good E, Veerareddy S, Pelosi F, Jr, Morady F. Catheter ablation for paroxysmal atrial fibrillation: segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation. 2003;108:2355–2360. doi: 10.1161/01.CIR.0000095796.45180.88. [DOI] [PubMed] [Google Scholar]
- 3.Ouyang F, Bansch D, Ernst S, Schaumann A, Hachiya H, Chen M, Chun J, Falk P, Khanedani A, Antz M, Kuck KH. Complete isolation of left atrium surrounding the pulmonary veins: new insights from the double-Lasso technique in paroxysmal atrial fibrillation. Circulation. 2004;110:2090–2096. doi: 10.1161/01.CIR.0000144459.37455.EE. [DOI] [PubMed] [Google Scholar]
- 4.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Packer D, Skanes A. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100–1105. doi: 10.1161/01.CIR.0000157153.30978.67. [DOI] [PubMed] [Google Scholar]
- 5.Epstein MR, Knapp LD, Martindill M, Lulu JA, Triedmann JK, Calkins H, Huang SK, Walsh EP, Saul JP. Embolic complications associated with radiofrequency catheter ablation. Atakr Investigator Group. Am J Cardiol. 1996;77:655–658. doi: 10.1016/s0002-9149(97)89327-7. [DOI] [PubMed] [Google Scholar]
- 6.Pappone C, Oral H, Santinelli V, Vicedomini G, Lang CC, Manguso F, Torracca L, Benussi S, Alfieri O, Hong R, Lau W, Hirata K, Shikuma N, Hall B, Morady F. Atrio-esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation. 2004;109:2724–2726. doi: 10.1161/01.CIR.0000131866.44650.46. [DOI] [PubMed] [Google Scholar]
- 7.Robbins IM, Colvin EV, Doyle TP, Kemp WE, Loyd JE, McMahon WS, Kay GN. Pulmonary vein stenosis after catheter ablation of atrial fibrillation. Circulation. 1998;98:1769–1775. doi: 10.1161/01.cir.98.17.1769. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt B, Antz M, Ernst S, Ouyang F, Falk P, Chun JK, Kuck KH. Pulmonary vein isolation by high-intensity focused ultrasound: first-in-man study with a steerable balloon catheter. Heart Rhythm. 2007;4:575–584. doi: 10.1016/j.hrthm.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Zrenner B, Dong J, Schreieck J, Deisenhofer I, Estner H, Luani B, Karch M, Schmitt C. Transvenous cryoablation versus radiofrequency ablation of the slow pathway for the treatment of atrioventricular nodal re-entrant tachycardia: a prospective randomized pilot study. Eur Heart J. 2004;25:2226–2231. doi: 10.1016/j.ehj.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Friedman PL, Dubuc M, Green MS, Jackman WM, Keane DT, Marinchak RA, Nazari J, Packer DL, Skanes A, Steinberg JS, Stevenson WG, Tchou PJ, Wilber DJ, Worley SJ. Catheter cryoablation of supraventricular tachycardia: results of the multicenter prospective ‘frosty’ trial. Heart Rhythm. 2004;1:129–138. doi: 10.1016/j.hrthm.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Tse HF, Reek S, Timmermans C, Lee KL, Geller JC, Rodriguez LM, Ghaye B, Ayers GM, Crijns HJ, Klein HU, Lau CP. Pulmonary vein isolation using transvenous catheter cryoablation for treatment of atrial fibrillation without risk of pulmonary vein stenosis. J Am Coll Cardiol. 2003;42:752–758. doi: 10.1016/s0735-1097(03)00788-5. [DOI] [PubMed] [Google Scholar]
- 12.Khairy P, Chauvet P, Lehmann J, Lambert J, Macle L, Tanguay JF, Sirois MG, Santoianni D, Dubuc M. Lower incidence of thrombus formation with cryoenergy versus radiofrequency catheter ablation. Circulation. 2003;107:2045–2050. doi: 10.1161/01.CIR.0000058706.82623.A1. [DOI] [PubMed] [Google Scholar]
- 13.Sarabanda AV, Bunch TJ, Johnson SB, Mahapatra S, Milton MA, Leite LR, Bruce GK, Packer DL. Efficacy and safety of circumferential pulmonary vein isolation using a novel cryothermal balloon ablation system. J Am Coll Cardiol. 2005;46:1902–1912. doi: 10.1016/j.jacc.2005.07.046. [DOI] [PubMed] [Google Scholar]
- 14.van Oeveren W, Crijns HJ, Korteling BJ, Wegereef EW, Haan J, Tigchelaar I, Hoekstra A. Blood damage, platelet and clotting activation during application of radiofrequency or cryoablation catheters: a comparative in vitro study. J Med Eng Technol. 1999;23:20–25. doi: 10.1080/030919099294393. [DOI] [PubMed] [Google Scholar]
- 15.Reddy VY, Neuzil P, Pitschner HF, Kuniss M, Kralovec S, Kanova M, Laragy, Mihalik TA, Taborsky M, Ruskin JN. Clinical experience with a balloon cryoablation catheter for pulmonary vein isolation in patients with atrial fibrillation: one-year results. Circulation. 2005;Suppl II:112. Abstract 2630. [Google Scholar]
- 16.Pitschner HF, Reddy V, Kuniss M, Neuzil P, Laragy M, Taborsky M, Ruskin J, Hamm C. Pulmonary vein isolation in patients with atrial fibrillation using a novel balloon cryoablation catheter. Eur Heart J. 2005;(Suppl):131. Abstract 961. [Google Scholar]
- 17.Van Belle Y, Janse P, Rivero-Ayerza MJ, Thornton AS, Jessurun ER, Theuns D, Jordaens L. Pulmonary vein isolation using an occluding cryoballoon for circumferential ablation: feasibility, complications, and short-term outcome. Eur Heart J. 2007;28:2231–2237. doi: 10.1093/eurheartj/ehm227. [DOI] [PubMed] [Google Scholar]
- 18.Neumann T, Vogt J, Schumacher B, Dorszewski A, Kuniss M, Neuser H, Kurzidim K, Berkowitsch A, Koller M, Heintze J, Scholz U, Wetzel U, Schneider MA, Horstkotte D, Hamm CW, Pitschner HF. Circumferential pulmonary vein isolation with the cryoballoon technique results from a prospective 3-center study. J Am Coll Cardiol. 2008;52:273–278. doi: 10.1016/j.jacc.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 19.Marom EM, Herndon JE, Kim YH, McAdams HP. Variations in pulmonary venous drainage to the left atrium: implications for radiofrequency ablation. Radiology. 2004;230:824–829. doi: 10.1148/radiol.2303030315. [DOI] [PubMed] [Google Scholar]
- 20.Schneider C, Ernst S, Bahlmann E, Malisius R, Krumsdorf U, Boczor S, Lampe F, Hoffmann-Riem M, Kuck KH, Antz M. Transesophageal echocardiography: a screening method for pulmonary vein stenosis after catheter ablation of atrial fibrillation. Eur J Echocardiogr. 2006;7:447–456. doi: 10.1016/j.euje.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Natale A, Raviele A, Arentz T, Calkins H, Chen SA, Haïssaguerre M, Hindricks G, Ho Y, Kuck KH, Marchlinski F, Napolitano C, Packer D, Pappone C, Prystowsky EN, Schilling R, Shah D, Themistoclakis S, Verma A. Venice Chart international consensus document on atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2007;18:560–580. doi: 10.1111/j.1540-8167.2007.00816.x. [DOI] [PubMed] [Google Scholar]
- 22.Marrouche NF, Martin DO, Wazni O, Gillinov AM, Klein A, Bhargava M, Saad E, Bash D, Yamada H, Jaber W, Schweikert R, Tchou P, Abdul-Karim A, Saliba W, Natale A. Phased-array intracardiac echocardiography monitoring during pulmonary vein isolation in patients with atrial fibrillation: impact on outcome and complications. Circulation. 2003;107:2710–2716. doi: 10.1161/01.CIR.0000070541.83326.15. [DOI] [PubMed] [Google Scholar]
- 23.Ouyang F, Ernst S, Chun J, Bänsch D, Li Y, Schaumann A, Mavrakis H, Liu X, Deger FT, Schmidt B, Xue Y, Cao J, Hennig D, Huang H, Kuck KH, Antz M. Electrophysiological findings during ablation of persistent atrial fibrillation with electroanatomic mapping and double Lasso catheter technique. Circulation. 2005;112:3038–3048. doi: 10.1161/CIRCULATIONAHA.105.561183. [DOI] [PubMed] [Google Scholar]
- 24.Haïssaguerre M, Sanders P, Hocini M, Hsu LF, Shah DC, Scavée C, Takahashi Y, Rotter M, Pasquié JL, Garrigue S, Clémenty J, Jaïs P. Changes in atrial fibrillation cycle length and inducibility during catheter ablation and their relation to outcome. Circulation. 2004;109:3007–3013. doi: 10.1161/01.CIR.0000130645.95357.97. [DOI] [PubMed] [Google Scholar]
- 25.Ouyang F, Antz M, Ernst S, Hachiya H, Mavrakis H, Deger FT, Schaumann A, Chun J, Falk P, Hennig D, Liu X, Bansch D, Kuck KH. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation. 2005;111:127–135. doi: 10.1161/01.CIR.0000151289.73085.36. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt B, Ernst S, Ouyang F, Chun KR, Broemel T, Bansch D, Kuck KH, Antz M. External and endoluminal analysis of left atrial anatomy and the pulmonary veins in three-dimensional reconstructions of magnetic resonance angiography: the full insight from inside. J Cardiovasc Electrophysiol. 2006;17:957–964. doi: 10.1111/j.1540-8167.2006.00548.x. [DOI] [PubMed] [Google Scholar]
- 27.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 28.Lee BK, Choi KJ, Kim J, Rhee KS, Nam GB, Kim YH. Right phrenic nerve injury following electrical disconnection of the right superior pulmonary vein. Pacing Clin Electrophysiol. 2004;27:1444–1446. doi: 10.1111/j.1540-8159.2004.00652.x. [DOI] [PubMed] [Google Scholar]
- 29.Antz M, Chun KR, Ouyang F, Kuck KH. Ablation of atrial fibrillation in humans using a balloon-based ablation system: identification of the site of phrenic nerve damage using pacing maneuvers and CARTO. J Cardiovasc Electrophysiol. 2006;17:1242–1245. doi: 10.1111/j.1540-8167.2006.00589.x. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Quintana D, Cabrera JA, Climent V, Farre J, Weiglein A, Ho SY. How close are the phrenic nerves to cardiac structures? Implications for cardiac interventionalists. J Cardiovasc Electrophysiol. 2005;16:309–313. doi: 10.1046/j.1540-8167.2005.40759.x. [DOI] [PubMed] [Google Scholar]
- 31.Manusama R, Timmermans C, Limon F, Philippens S, Crijns HJ, Rodriguez LM. Catheter-based cryoablation permanently cures patients with common atrial flutter. Circulation. 2004;109:1636–1639. doi: 10.1161/01.CIR.0000124478.98343.00. [DOI] [PubMed] [Google Scholar]