Abstract
Aims
To determine whether risk stratification tests can predict serious arrhythmic events after acute myocardial infarction (AMI) in patients with reduced left ventricular ejection fraction (LVEF ≤ 0.40).
Methods and results
A total of 5869 consecutive patients were screened in 10 European centres, and 312 patients (age 65 ± 11 years) with a mean LVEF of 31 ± 6% were included in the study. Heart rate variability/turbulence, ambient arrhythmias, signal-averaged electrocardiogram (SAECG), T-wave alternans, and programmed electrical stimulation (PES) were performed 6 weeks after AMI. The primary endpoint was ECG-documented ventricular fibrillation or symptomatic sustained ventricular tachycardia (VT). To document these arrhythmic events, the patients received an implantable ECG loop-recorder. There were 25 primary endpoints (8.0%) during the follow-up of 2 years. The strongest predictors of primary endpoint were measures of heart rate variability, e.g. hazard ratio (HR) for reduced very-low frequency component (<5.7 ln ms2) adjusted for clinical variables was 7.0 (95% CI: 2.4–20.3, P < 0.001). Induction of sustained monomorphic VT during PES (adjusted HR = 4.8, 95% CI, 1.7–13.4, P = 0.003) also predicted the primary endpoint.
Conclusion
Fatal or near-fatal arrhythmias can be predicted by many risk stratification methods, especially by heart rate variability, in patients with reduced LVEF after AMI.
Keywords: Sudden cardiac death, Heart rate, Variability, Implantable cardioverter-defibrillator
Introduction
Survivors of an acute myocardial infarction (AMI) who have impaired left ventricular ejection fraction (LVEF ≤ 0.40) are at high risk of dying suddenly due to cardiac arrhythmias.1–4 Prophylactic implantation of a cardioverter-defibrillator (ICD) has been shown to reduce all cause mortality among these patients, when the ICD was implanted late after AMI.5,6 The benefit is reportedly less marked in patients who received an ICD early after the acute event.3,7 There is, therefore, a need to identify other risk markers beyond the LVEF for patients who are at risk of sudden arrhythmic deaths and who could also benefit from implantation of an ICD early after AMI, a time period which accounts for a large cumulative number of sudden deaths worldwide.2
Several non-invasive and invasive risk stratification tests are able to predict all-cause mortality after AMI.8–14 All-cause mortality, however, in combination with other endpoints,8–15 is not considered a reliable surrogate for life-threatening or fatal arrhythmic events.15 Previous observational risk stratification studies have not adequately estimated the need of an ICD in view of such endpoint limitation and incomplete event documentation.15 The Cardiac Arrhythmias and Risk Stratification after AMI (CARISMA) trial was designed to investigate the power of various invasive and non-invasive risk markers to predict the occurrence of arrhythmias that could probably be treated by an ICD after AMI. An implantable ECG loop-recorder was used to document fatal or near-fatal ventricular tachyarrhythmias as a primary endpoint of the study.
Methods
Study population and protocol
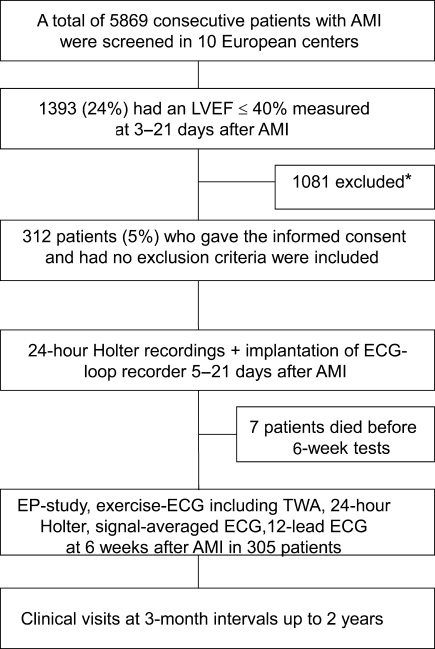
A total of 5869 consecutive patients were screened in the acute phase of AMI (2–7 days after the event) in 10 European centres between August 2001 and November 2004. Of these, 1393 (23%) patients had a LVEF ≤ 0.40. After exclusions, 312 patients were included in the study. The inclusion and exclusion criteria are shown in Table 1 and a flow chart of the study design is shown in Figure 1. A consecutive series of patients with an ECG and enzyme defined AMI were screened and patients with a LVEF ≤0.40, measured 3–5 days post-AMI, were included in the study. Day 0 was the time when the AMI was diagnosed. Major reasons for exclusion from the study among the patients with a LVEF≤0.40 were refusal of the patient or the attending physician treating the patient for participation in the study (n = 380), the inability of the patient to participate in the study due to other serious illness (n = 312), planned coronary bypass graft surgery (n = 184) or death (n = 89) before the implantation of a loop-recorder and/or the risk stratification tests. The patients included in the study received an implantable ECG loop-recorder 5–21 days after AMI to document arrhythmic events and were followed-up for 24 months to document arrhythmic events. The patients had clinical visits at 3-month intervals up to 2 years after AMI. The last follow-up was planned to be at 24 months after AMI. The appropriate ethics review boards approved the protocol and the study was conducted in accordance with the Declaration of Helsinki.
Table 1.
Inclusion and exclusion criteria of the patients
| Inclusion criteria |
| AMI, verified by enzyme analyses or elevated troponin and associated with typical chest pain and/or ECG changes according to the European Society of Cardiology and American College of Cardiology consensus documents |
| LVEF ≤ 40%, measured between Days 3 and 21 post-AMI, preferably before Day 7 |
| Exclusion criteria |
| Index AMI older than 21 days |
| Death before the implantation of loop-recorder |
| Patient unwilling or unable to give written informed consent |
| Insertable loop-recorder cannot be implanted within 3 weeks after AMI for any reason except planned pacemaker implantation |
| NYHA class IV despite appropriate treatment of heart failure |
| Planned or previous ICD implantation; planned CABG; severe valvular disease |
| Participation in a competing clinical trial |
| Psychologically or physically (due to any other illness) unfit for participation in the study according to the opinion of the investigator; Patient compliance doubtful |
| Patients who are geographically or otherwise inaccessible for follow-up |
| Pregnancy; life expectancy <1 year for non-cardiac cause; age below 18 |
AMI, acute myocardial infarction; CABG, coronary artery bypass grafting; LVEF, ejection fraction; ICD, implantable cardioverter-defibrillator; ILR, insertable loop-recorder.
Figure 1.
Study Flow Chart. *Major reasons for exclusions from the study were refusal of the patient or the attending physician treating the patient for participation in the study (n = 380), the inability of the patient to participate in the study due to other serious illness (n = 312), planned coronary bypass graft surgery (n = 184) or death (n = 89) before the implantation of a loop-recorder and/or the risk stratification tests. AMI, acute myocardial infarction; EP, electrophysiological; ILR, implantable loop-recorder; SAECG, signal-averaged electrocardiogram; TWA, T-wave alternans.
Endpoints of the study
The primary endpoint of the study was ECG-documented fatal or near-fatal cardiac arrhythmia, adjudicated as ‘most probably treatable’ by an ICD according to a consensus of the five-member endpoint committee blinded to the test results. This included resuscitated cardiac arrest due to documented primary arrhythmia, symptomatic sustained ventricular tachycardia (VT), or documented arrhythmic death. Each arrhythmia had to be ECG-documented, either by the implanted loop-recorder, ICD, pacemaker, Holter recording, or telemetry strip. An arrhythmic event was excluded as a primary endpoint when considered by any member of the endpoint committee, that it could not have been successfully treated by an ICD. For example, documented arrhythmic events did not meet the definition of the primary endpoint if they occurred in end-stage chronic heart failure or during refractory ischaemia. All-cause mortality and cardiac death were secondary endpoints.
Implantation and programming of the loop-recorder
An implantable loop-recorder (Reveal Plus, Medtronic Inc., Minneapolis, MN, USA) with automatic ECG storage was used to document the primary endpoint.16,17 The device was implanted subcutaneously under local anaesthesia in the left parasternal area 5–21 days after an AMI and programmed automatically to store tachyarrhythmias at heart rates ≥125 beat/min that persist for at least 16 consecutive beats, and to automatically store bradyarrhythmias at heart rates ≤30 beats/min, or asystole for at least 4.5 s.16
During follow-up, the memory of the loop-recorder was interrogated 6 weeks after implantation and thereafter at 3-month intervals. The sensitivity of arrhythmia detection was adjusted individually according to the amount of false events at each visit. Over the course of the study, some patients were implanted with other cardiac devices, such as pacemaker (n = 22, 17 due to intermittent or permanent II–III degree AV-block, and 5 due to sinus node disease) or ICDs (n = 57, 2 due to resuscitated cardiac arrest, 10 due to sustained ventricular tachycardia, and 45 due to prophylactic indications), which were used to document the arrhythmic events as evidence of primary endpoint. The ICD programming was left at the discretion of the primary physicians. In order to differentiate between non-sustained and sustained VT, however, the ICDs were required to be programmed with no antitachycardia pacing or ICD shock delivered within the first 30 s delay, i.e. with therapy ‘off’ and with monitoring ‘on’ for >76 beats between the cycle lengths of 480 and 310 ms. In cases with fast VT or ventricular fibrillation (VF), defined as rates from 194 to 220 bpm and >220 bpm, respectively, the therapy was typically programmed to be delivered after detection of 18 intervals.
Description of the methods in risk stratification
Echocardiography
A 2-dimensional echocardiogram was performed twice, 2–5 days after index AMI, and at 6 weeks post-AMI. The LVEF was measured locally by the investigators using standard techniques.18,19
Holter monitoring
All patients underwent a 24 h ambulatory two or three-channel ECG recording, immediately after the index AMI (between Days 5 and 21) and at Week 6. The analysis methods of various measures of heart rate variability/turbulence have been described elsewhere.18–23 The following variables were analysed: average heart rate, total number and frequency of premature ventricular beats, episodes of non-sustained ventricular tachycardia (≥3 consecutive beats), time and frequency domain measures along with short-term fractal exponent of heart rate variability, the onset and slope of heart rate turbulence. Holter recordings were analysed locally by an experienced technician in each centre, and the heart rate variability/turbulence measures were analysed centrally at the University of Oulu (Finland) using a previously described custom-made software.18–23
Microvolt T-wave alternans
Exercise tests and microvolt T-wave alternans analysis were performed at 6 weeks post-AMI.21 Maximal work load, heart rate, blood pressure, and ST-T changes were analysed from the symptom-limited exercise ECG. The presence of microvolt T-wave alternans was assessed using a CH2000 system (Cambridge Heart, Inc., Bedford, MA, USA) and high-resolution electrodes with standard 12-lead ECG and three orthogonal (X,Y,Z) ECG lead positions. Initially, the test was performed using a bicycle exercise protocol.21 Test results were defined as positive, negative, or incomplete/indeterminate.21,24 The goal of the exercise test was to achieve a target heart rate at least 105 beats/min and to maintain a slowly increasing heart rate between 105 and 110 beats/min for 2–3 min. The exercise stress test was then continued as symptom-limited test until exhaustion. For patients with an incomplete or indeterminate result, the microvolt T-wave alternans was re-analysed during the electrophysiological study by relying on atrial or simultaneous ventricular and atrial pacing. Earlier pilot work describes these pacing methods as well as the concordance between exercise test and pacing in the analysis of T-wave alternans.24 The tests were interpreted locally by the investigators and re-analysed independently by a Cambridge Heart Inc. expert review board blinded to the outcome. The results are reported both for the exercise TWA testing and the TWA testing obtained from exercise+pacing.
Signal-averaged electrocardiogram
Signal-averaged ECGs were performed at 6 weeks post-AMI by devices capable of measuring averaged high-frequency QRS-complexes.17,20 Filtered QRS duration >120 ms, the root mean square voltage of the terminal 40 ms of the filtered QRS complex <20 µV or the duration of the terminal portion >38 ms were predefined criteria for abnormal signal-averaged ECG.18,21 All signal-averaged ECGs were analysed locally by the investigators.
Electrophysiological study
A programmed electrical stimulation (PES) was performed 6 weeks post-AMI during a standard electrophysiological study with up to three extra stimuli (minimum of 200 ms) and up to two basic drive cycle lengths (600 and 400 ms) from the right ventricular apex and outflow tract. Induction of sustained monomorphic ventricular tachycardia and induction of any sustained ventricular tachyarrhythmia, including ventricular fibrillation and sustained polymorphic ventricular tachycardia, were analysed separately.
Standard 12-lead electrocardiogram
QT interval, QTc interval, QT dispersion, and QRS duration were analysed from a standard 12-lead ECG.18
Statistical analysis
In sample size calculation, the study was designed to have a 90% power to detect at least a three-fold risk of primary endpoint of any of the risk variables, assuming that 10–30% of the patients would have abnormal test results. The rate of primary endpoints was estimated to be 10% based on prior literature.
Hazard ratios (HR) obtained by the Cox proportional hazard models were fitted to identify predictors of primary and secondary endpoints. Analysis were conducted using risk variables as continuous variables when it was possible. HR were also calculated on dichotomized test variables at thresholds predefined before the study onset, based on prior experience18–23 after adjustments for common risk variables, such as age, prior AMI, history of congestive heart failure, and diabetes. The time to development of the primary endpoint was displayed by constructing Kaplan–Meier curves and the significance estimated by a log-rank test. All analyses were performed in SAS v. 9.1. Two-sided P-values <0.05 were considered significant.
Results
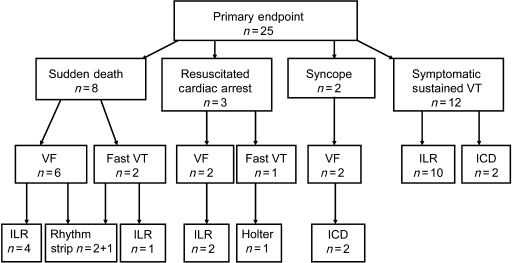
Of the 312 enrolled patients, 25 (8.0%) experienced a primary endpoint during the follow-up. Twelve of these were symptomatic sustained monomorphic VTs, eight were sudden deaths, three aborted cardiac arrests, and two patients had syncope before termination of VF by ICD shock. The clinical presentation, type of documented arrhythmia, and the mode of diagnosing the primary endpoints are shown in Figure 2. Ten out of the 12 sustained VTs were documented by the ILR and two by the ICD. Six sudden deaths were due to VF, four documented by the ILR, and two by ECG strip obtained by telemetry. Two sudden deaths were preceded by fast VT, one documented by the ILR, and one by telemetry strip. Two patients with resuscitated cardiac arrest had VF and one had fast VT. The arrhythmia was terminated by the external DC shock among these three patients. Two of these were documented by the ILR and one occurred during the Holter recording. VT (n = 3) or VF (n = 4) was documented by the ILR in seven patients, in whom the documented arrhythmias were not considered as primary endpoints. Five of these occurred among patients hospitalized due to terminal heart failure, one occurred in a patient dying for acute heart failure, and one occurred in a hospitalized patient with a recurrent AMI associated with frequent VF episodes not responding to DC shocks and resuscitation attempts.
Figure 2.
Clinical presentation, type of documented arrhythmia, and the mode of documentation of primary endpoints. ICD, implantable cardioverter-defibrillator; ILR, implantable loop-recorder; VT, ventricular tachycardia; VF, ventricular fibrillation.
During the median follow-up of 2.02 years (quartiles from 1.97 to 2.06 years) (mean 1.85 ± 0.5 years), 38 patients (12.2%) died, with 28 of the deaths (9.0%) classified as cardiac deaths. None of the patients with an implanted ICD died suddenly during the follow-up. Seven patients had died before the 6-week risk stratification tests. The characteristics of the whole study cohort are described in Table 2.
Table 2.
Characteristics of patients at baseline (n = 312)
| Age (years) | 65 ± 11 |
| Male gender | 239 (77%) |
| Prior MI | 116 (37%) |
| Prior CHF | 33 (11%) |
| Diabetes | 62 (20%) |
| Hypertension | 136 (44%) |
| Blood pressure (systolic) (mmHg) | 122 ± 22 |
| Beta-blocker | |
| At discharge | 299 (96%) |
| At 6 weeks after AMI | 279 (89%) |
| ACE-inhibitor/AT blocker | |
| At discharge | 279 (89%) |
| At 6 weeks after AMI | 260 (83%) |
| Statin | |
| At discharge | 256 (82%) |
| At 6 weeks after AMI | 241 (77%) |
| Antiplatelet agent | |
| At discharge | 297 (95%) |
| At 6 weeks after AMI | 274 (88%) |
| Characteristics of AMI | |
| Q-wave AMI | 184 (59%) |
| Anterior location | 174 (56%) |
| Maximum troponin T (mmol/l) | 7.0 ± 25.5 |
| Maximum troponin I (mmol/l) | 377 ± 644 |
| Treatment of index MI primary PCI | 45 (14%) |
| Thrombolysis | 106 (34%) |
| Ejection fraction (%) | |
| 3–7 days after index MI | 31 ± 6 |
| 6 weeks after index MI | 35 ± 10 |
Values reported as n patients (% of population) or as mean ± SD. ACE, angiotensin converting enzyme; AMI, acute myocardial infarction; AT, angiotensin; CHF, congestive heart failure; PCI, primary coronary intervention.
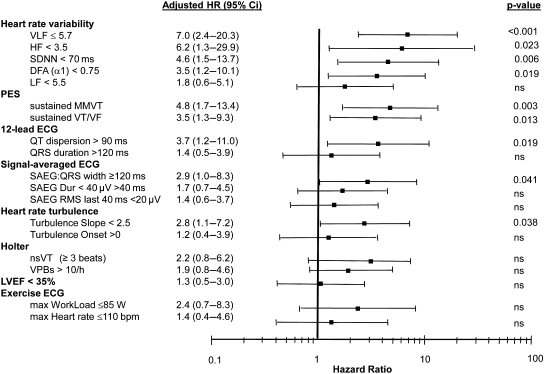
Fractal heart rate variability index measured from Holter recordings 1 week post-AMI was a significant predictor of primary endpoint. No other heart rate variability index or LVEF, were able to predict the primary endpoint when measured very early after AMI (Table 3). At 6 weeks post-AMI, however, standard deviation of N–N intervals, very-low frequency and high-frequency power spectral components, fractal scaling exponent of heart rate variability as well as heart rate turbulence slope predicted the primary endpoint, as evidenced by a relatively high AUC values and adjusted HR of pre-defined dichotomized values (Table 3 and Figure 3). In the total study cohort, a per-patient LVEF increased from baseline value of 31 ± 6 to 35 ± 10% at 6 weeks (P < 0.001), while the change was not significant in the subgroup of patients with a primary endpoint (30 ± 6 to 32 ± 6%, P = 0.7). However, the HR of LVEF as a continuous variable at 6 weeks (HR = 0.96, 95% CI: 0.92–1.006, P = 0.09) or the change of LVEF from baseline to 6 weeks (HR = 0.95, 95% CI: 0.91–1.00, P = 0.06) did not reach significance as predictors of the primary endpoint.
Table 3.
Arrhythmia risk variables as predictors of primary and secondary endpoints
| Number of patients | Primary endpoint: HR (95% CI), P-value | AUC ± SD, P-value | Overall death: HR (95% CI), P-value | Cardiac Death: HR (95% CI), P-value | |
|---|---|---|---|---|---|
| LVEF | |||||
| 3–21 days after MI | 312 | 0.97 (0.92–1.03), P = 0.36 | 0.57 ± 0.06, P = 0.28 | 0.96 (0.92–1.01), P = 0.09 | 0.96 (0.91–1.01), P = 0.10 |
| 6 weeks after AMI | 291 | 0.96 (0.92–1.01), P = 0.09 | 0.62 ± 0.05, P = 0.07 | 0.97 (0.94–1.01), P = 0.12 | 0.96 (0.92–1.01), P = 0.08 |
| Heart rate variability | |||||
| SDNN | |||||
| 1 week | 252 | 0.99 (0.97–1.00), P = 0.07 | 0.65 ± 0.07, P = 0.03 | 0.99 (0.97–1.00), P = 0.08 | 0.99 (0.97–1.00), P = 0.09 |
| 6 weeks | 239 | 0.98 (0.96–0.99), P = 0.007 | 0.68 ± 0.07, P = 0.01 | 0.97 (0.96–0.99), P = 0.002 | 0.97 (0.95–0.99), P = 0.01 |
| VLF spectral component | |||||
| 1 week | 252 | 0.77 (0.51–1.17), P = 0.22 | 0.59 ± 0.08, P = 0.18 | 0.66 (0.47–0.94), P = 0.02 | 0.68 (0.45–1.02), P = 0.06 |
| 6 weeks | 239 | 0.44 (0.29–0.67), P < 0.001 | 0.73 ± 0.07, P = 0.002 | 0.39 (0.26–0.58) P < 0.001 | 0.40 (0.24–0.66) P < 0.001 |
| LF spectral component | |||||
| 1 week | 251 | 0.98 (0.67–1.44), P = 0.91 | 0.49 ± 0.08, P = 0.92 | 0.73 (0.54–0.99), P = 0.04 | 0.71 (0.51–1.01), P = 0.06 |
| 6 weeks | 239 | 0.55 (0.37–0.82), P = 0.003 | 0.66 ± 0.08, P = 0.03 | 0.47 (0.32–0.68), P < 0.001 | 0.49 (0.31–0.79), P = 0.003 |
| HF spectral component | |||||
| 1 week | 251 | 1.21 (0.77–1.89), P = 0.41 | 0.44 ± 0.07, P = 0.36 | 0.85 (0.55–1.30), P = 0.44 | 0.79 (0.48–1.32), P = 0.37 |
| 6 weeks | 239 | 0.76 (0.44–1.30), P = 0.31 | 0.58 ± 0.09, P = 0.28 | 0.55 (0.33–0.92), P = 0.02 | 0.56 (0.29–1.05), P = 0.07 |
| Fractal scaling exponent (α1) | |||||
| 1 week | 250 | 0.14 (0.03–0.62), P < 0.009 | 0.69 ± 0.06, P = 0.009 | 0.15 (0.04–0.60), P = 0.007 | 0.23 (0.05–1.14), P = 0.07 |
| 6 weeks | 236 | 0.06 (0.01–0.29), P < 0.001 | 0.75 ± 0.06, P = 0.001 | 0.05 (0.01–0.60), P = 0.007 | 0.23 (0.05–1.14), P = 0.07 |
| Heart rate turbulence | |||||
| TO | |||||
| 1 week | 243 | 1.08 (0.88–1.33), P = 0.45 | 0.56 ± 0.07, P = 0.39 | 1.10 (0.91–1.33), P = 0.31 | 1.09 (0.88–1.35), P = 0.45 |
| 6 weeks | 234 | 0.95 (0.80–1.13), P = 0.56 | 0.51 ± 0.07, P = 0.92 | 0.95 (0.80–1.12), P = 0.52 | 0.92 (0.75–1.12), P = 0.39 |
| TS | |||||
| 1 week | 243 | 0.97 (0.90–1.04), P = 0.44 | 0.57 ± 0.07, P = 0.31 | 0.97 (0.91.1.03), P = 0.33 | 0.94 (0.85–1.03), P = 0.19 |
| 6 weeks | 234 | 0.92 (0.84–1.00), P = 0.04 | 0.67 ± 0.07, P = 0.02 | 0.94 (0.87–1.01), 0.10 | 0.90 (0.82–1.00), P = 0.05 |
| Signal-averaged ECG | |||||
| QRS width | 236 | 1.04 (1.02–1.06), P < 0.001 | 0.70 ± 0.07, P = 0.005 | 1.03 (1.01–1.06), P = 0.004 | 1.04 (1.02–1.07), P < 0.001 |
| Duration <40 µV/ms | 236 | 1.03 (1.00–1.05), P = 0.08 | 0.58 ± 0.08, P = 0.24 | 1.03 (1.00–1.05), P = 0.05 | 1.03 (1.00–1.07), P = 0.03 |
| rms last 40 ms | 236 | 1.00 (0.98–1.02), P = 0.88 | 0.56 ± 0.09, P = 0.43 | 0.97 (0.94–1.00), P = 0.04 | 0.96 (0.93–1.00), P = 0.07 |
| 12-lead ECG | |||||
| QRS duration | 308 | 1.01 (1.00–1.02), P = 0.15 | 0.56 ± 0.07, P = 0.37 | 1.02 (1.01–1.03), P < 0.001 | 1.02 (1.01–1.03), P < 0.001 |
| QT dispersion | 251 | 1.01 (1.00–1.03), P = 0.02 | 0.63 ± 0.08, P = 0.07 | 1.00 (0.99–1.02), P = 0.73 | 1.00 (0.98–1.02), P = 0.97 |
| Exercise ECG | |||||
| Maximum work load | 226 | 0.99 (0.97–1.00), P = 0.07 | 0.65 ± 0.09, P = 0.06 | 0.97 (0.96–0.99), P = 0.001 | 0.97 (0.95–0.99), P = 0.002 |
| Maximum heart rate | 227 | 0.98 (0.95–1.01), P = 0.13 | 0.64 ± 0.07, P = 0.07 | 0.98 (0.95–1.00), P = 0.06 | 0.98 (0.95–1.01), P = 0.14 |
| Holter | |||||
| No of VPBs/h | 287 | 1.62 (1.00–2.63), P = 0.05 | 0.62 ± 0.07, P = 0.08 | 1.66 (1.10–2.48), P = 0.01 | 1.51 (0.93–2.45). P = 0.09 |
| nsVT | 287 | 1.95 (0.71–5.36), P = 0.20 | — | 1.23 (0.47–3.25), P = 0.67 | 1.43 (0.48–4.27), P = 0.52 |
| T-wave alternans | |||||
| TWA by exercise | 215 | 1.28 (0.37–4.37), P = 0.70 | — | 1.88 (0.67–5.27), P = 0.23 | 1.62 (0.50–5.26), P = 0.42 |
| TWA by exercise + pacing | 225 | 1.53 (0.54–4.29), P = 0.42 | — | 1.36 (0.57–3.24), P = 0.48 | 0.90 (0.33–2.47), P = 0.83 |
| Programmed electrical stimulation | 282 | 7.18 (2.77–18.36), P < 0.001 | — | 1.55 (0.59–4.09), P = 0.38 | 1.93 (0.64–5.87), P = 0.25 |
| Induction of sustained MMVT | 3.95 (1.52–10.24), P = 0.005 | — | 1.54 (0.69–3.39), P = 0.29 | 1–75 (0.70–4.39), P = 0.23 | |
1 week is tested 5–14 days after AMI. Six week is tested 42–56 days after AMI. Values are means ± standard deviation. AUC, area under receiver operating characteristics curve; CI, confidence interval; ECG, electrocardiogram; HF, high frequency; HR, hazard ratio; LF, low frequency; LVEF, left ventricular ejection fraction; MMVT, monomorphic ventricular tachycardia; nsVT, non-sustained ventricular tachycardia; PES, programmed electrical stimulation; rms, root mean square; TO, turbulence onset; TS, turbulence slope; TWA, T-wave alternans; VLF, very-low frequency; VPBs, ventricular premature beats; VT, ventricular tachycardia; VF, ventricular fibrillation.
Figure 3.
Adjusted hazard ratios (HR) with 95% confidence intervals of the variables as predictors of primary endpoint. HR are calculated from pre-defined threshold values of continuous variables. HR are adjusted for age, prior myocardial infarction, history of congestive heart failure and diabetes The variables are listed in descending order starting with the highest HR for each risk stratification method. Abbreviations: see in Table 3.
QRS duration measured from the signal-averaged ECG was a significant predictor of the primary endpoint, as was induction of sustained monomorphic VT (Table 3). However, when adjusted to clinical variables, the predictive power of signal-averaged ECG was only of borderline significance (P = 0.04) (Figure 2). Prolonged QT dispersion also predicted the primary endpoint (Table 3 and Figure 3). Microvolt T-wave alternans, analysed either from the exercise test or exercise+pacing, non-sustained ventricular tachycardia or frequency of premature beats on Holter and QRS duration measured from the 12-lead ECG were not significant predictors of the primary endpoint.
All-cause mortality and cardiac death were also predicted by several heart rate variability measures, QRS-duration, and maximal exercise capacity but not by PES (Table 3).
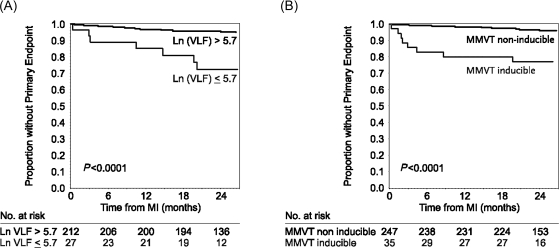
Table 4 shows the sensitivity, specificity, and predictive values of significant predictors of the primary endpoint. These values illustrate the relatively high accuracy of some heart rate variability indexes, especially the very-low frequency spectral component, as well as inducibility of sustained monomorphic VT in predicting the primary endpoint. The Kaplan–Meier representation of time to primary endpoint is shown in Figure 4, stratified by inducibility of sustained monomorphic VT or by reduced very-low frequency spectral component of heart rate variability.
Table 4.
Sensitivity, specificity, and predictive accuracy of predefined values of individual variables in predicting primary endpoint
| Name of variable | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | PPV (%) (95% CI) | NPV (%) (95% CI) |
|---|---|---|---|---|
| LVEF < 0.35 | 57 (36–78) | 53 (47–59) | 9 (4–13) | 94 (90–98) |
| SDNN <70 ms | 35 (13–58) | 90 (86–94) | 21 (6–35) | 95 (92–98) |
| VLF spectral component ln ≤5.7 ms2 | 41 (18–65) | 91 (87–95) | 26 (9–42) | 95 (92–98) |
| Fractal scaling exponent <0.75 | 65 (42–87) | 71 (65–77) | 15 (7–23) | 96 (93–99) |
| Heart rate turbulence slope <2.5 ms/RRi | 53 (29–77) | 74 (68–80) | 14 (5–22) | 95 (92–99) |
| QRS-width on SAECG >120 ms | 44 (21–67) | 85 (80–90) | 20 (7–32) | 95 (92–98) |
| Induction of sustained MMVT by PES | 47 (23–71) | 90 (86–93) | 23 (9–37) | 96 (95–99) |
| Induction of VT/VF by PES | 53 (29–77) | 78 (73–83) | 14 (5–22) | 96 (94–99) |
NPV, negative predictive value; PPV, positive predictive value. For other abbreviations see Table 3.
Figure 4.
Kaplan–Meier estimate of the time from MI to primary endpoint, stratified by inducible sustained monomorphic ventricular tachycardia (MMVT) (panel A) and by reduced very-low frequency spectral component (VLF) of heart rate variability (panel B).
Discussion
The present prospective, multicenter study showed that several measures of heart rate variability analysed at 6 weeks after AMI among patients with impaired LVEF are powerful predictors of the occurrence of ECG-documented fatal or near-fatal arrhythmic events that could have probably been treated by an ICD. Induction of monomorphic sustained VT during PES and prolonged QRS duration measured from SAECG also predicted the arrhythmic events.
Our primary endpoint was distinctly different compared with previous observational risk stratification studies. Earlier studies have reported on all-cause mortality, alone or in combination with other serious events, such as cardiac death, sudden death, or resuscitation from cardiac arrest.9–14 Yet, it is now recognized that many sudden cardiac deaths are not due to arrhythmia as many other clinical conditions can evolve rapidly and result in sudden death.25,26 Similarly, many deaths defined as non-sudden may be due to cardiac arrhythmia.25,26 We used an implantable ECG-loop-recorder or other ECG recording system to document the fatal or near-fatal arrhythmic events to be able to explore these distinctions. Using this endpoint definition, the study gives direct information on the performance of risk stratification tests to identify patients that could potentially benefit from implantation of a prophylactic ICD early after AMI. It should be noted that ICD may not always document reliably the fatal arrhythmia because some tachyarrhythmias may terminate spontaneously after the pre-defined delay setting of onset therapy of the devices.27,28 In this study, we had two such primary endpoints, where ICD terminated the ventricular fibrillation.
Heart rate variability and turbulence have been shown to predict all-cause mortality and sudden cardiac death in several previous post-AMI populations.8,9,22,23 In recent studies, these indexes of heart rate variability or turbulence have not been shown to be predictors of sudden death among the patients with impaired left ventricular function, when measured early after the AMI.18,23 Similarly, a randomized ICD trial, using depressed heart rate variability and reduced LVEF measured at an early phase after AMI, as enrolment criteria, did not show the benefit of ICD treatments on all-cause mortality.3 These results are partly confirmed by our trial results, where indexes of heart rate variability measured early after AMI were not as powerful predictors of arrhythmic events as the same parameters established 6 weeks after AMI.
Similar to previous observational studies,11,15 QRS width from the signal-averaged ECG predicted the occurrence of arrhythmic events, although its predictive value decreased after adjustments with clinical variables. Induction of sustained monomorphic VT during an electrophysiological study was also a predictor of primary endpoint. These findings were not surprising, since both of these risk stratification tests describe the presence of an electrical substrate for re-entrant ventricular tachyarrhythmias of the infarcted myocardium increasing the vulnerability to sustained ventricular tachycardia.
LVEF did not provide significant additional information on the risk of future arrhythmic events in this population. Reduced LVEF measured early after AMI is not a specific indicator for the occurrence of ventricular tachyarrhythmias during the subsequent months and this has been reported in previous prophylactic ICD studies.3,7 The power of this study cannot exclude the significance of the LVEF as predictors of serious arrhythmic events, however, because reduced LVEF itself was an inclusion criterion resulting in a relatively narrow range of LVEFs.
While microvolt T-wave alternans has been shown to be a risk stratifier in patients with chronic ischaemic heart failure,29,30 the present study did not confirm its clinical benefit early after the AMI. There are a few key differences in our T-wave alternans results compared with prior studies that deserve attention. First, we observed a greater percentage of positive test results,29 because additional pacing was performed in patients with incomplete or indeterminate test result. Secondly, prior studies have combined incomplete and indeterminate test results with the positives, thereby including poor exercise tolerance and presence of premature beats together with positive T-wave alternans to indicate increased risk.29,30 However, when T-wave alternans was analysed only from exercise tests without pacing, it did not provide prognostic information in this study, suggesting that this test is not useful in predicting arrhythmic events in the early post-infarction phase. Finally, concurrent with the present study, a recent MASTER I study31 showed that TWA did not predict life-threatening ventricular arrhythmias or ICD shocks in 575 post-infarction patients with depressed LVEF.
The implantation rates of prophylactic ICD vary enormously from one country to another in western societies.15 The reasons are partly economical and perhaps related to a lack of definite scientific evidence for the clinical benefits of ICDs in different clinical settings. In particular, evidence for the benefits of prophylactic ICD among patients with a recent AMI and reduced LVEF is sparse.3,7 The relatively small sample size of our study may limit the generalization of the results, yet the data clearly suggest that indications relying exclusively on low LVEF can further benefit from additional risk stratification tests. Using the pre-defined cut-off values of many risk variables, the negative predictive value exceeded 95%, suggesting that these methods could become useful in excluding the patients who need the ICD after AMI.
The risk stratification tests were performed relatively late after AMI and the measures of heart rate variability predicted the primary endpoint better when measured late than early after AMI. Therefore, the present study does not provide information on prediction of arrhythmia events occurring very early (<6 weeks) after AMI.
Many previous observational studies have assessed the ability of one or two risk variables in prediction of sudden death or all-cause mortality after AMI. This study is, as far as we know, the only observational study comparing the predictive value of electrophysiological testing and several non-invasive risk stratification tests in predicting the arrhythmic events in the same study. Therefore, the results can be used in the design of future randomized ICD trials of post-AMI patients. We have shown here that a generally available, simple, and inexpensive measurement of 24 h heart rate variability, performed 6 weeks after AMI, could provide useful information for selecting patients who might benefit from implantation of an ICD. While the results are promising, prospective validation of this approach will be needed in larger patient population, using randomized interventional study design, before recommendations can be given regarding widespread screening of the post-AMI patients with Holter recordings.
Funding
Supported by research grants from the Medtronic Bakken Research Center, Maastricht, Netherlands and Cambridge Heart Inc., Massachusetts, USA. Funding to pay the Open Access publication charges for this article was provided by Medtronic's Bakken Research Center, The Netherlands.
Conflict of interest: Marc Messier is a full time employee of the sponsor of the study, Medtronics Bakken Research Center (Maastricht, The Netherlands). We declare that none of the other authors have any significant conflicts of interest.
Acknowledgements
Previous presentation: Presented in part in the ‘Late-Breaking Trials’ session of the Heart Rhythm Society in 15 May 2007, Denver, USA. Additional Contributions: We gratefully acknowledge Daniel Becker for statistics and Hindrik Robbe for his sustained commitment to the study, Dr Lars Køber who was the director of the ECHO Core laboratory in Rigshospitalet, Department of Cardiology B, Copenhagen, Denmark, the Endpoint Review Committee including Prof Dietrich Andresen, Klinikum am Urban, Berlin, Germany; Prof Martin Rosenqvist, Karolinska Institutet-Södersjukhuset, Stockholm, Sweden and Dr Michele Brignole, Ospedale Riuniti V. Leonardi E. Riboli, Lavagna, Italy, Pirkko Huikuri RN and Päivi Karjalainen RN, in the Holter Core Laboratory, University of Oulu, Department of Internal Medicine, Oulu, Finland.
ClinicalTrials.gov Identifier: NCT00145119.
References
- 1.Camm AJ, Pratt CM, Schwartz PJ, Al-Khalidi HR, Spyt MJ, Holroyde MJ, Karam R, Sonnenblick EH, Brum JM AzimiLide post Infarct surVival Evaluation (ALIVE) Investigators. Mortality in patients after a recent myocardial infarction: a randomized, placebo-controlled trial of azimilide using heart rate variability for risk stratification. Circulation. 2004;109:990–996. doi: 10.1161/01.CIR.0000117090.01718.2A. [DOI] [PubMed] [Google Scholar]
- 2.Solomon SD, Zelenkofske S, McMurray JJ, Finn PV, Velazquez E, Ertl G, Harsanyi A, Rouleau JL, Maggioni A, Kober L, White H, Van de Werf F, Pieper K, Califf RM, Pfeffer MA Valsartan in Acute Myocardial Infarction Trial (VALIANT) Investigators. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. 2005;352:2581–2588. doi: 10.1056/NEJMoa043938. [DOI] [PubMed] [Google Scholar]
- 3.Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, Fain E, Gent M, Connolly SJ DINAMIT Investigators. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–2488. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 4.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 5.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 6.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 7.Wilber DJ, Zareba W, Hall WJ, Brown MW, Lin AC, Andrews ML, Burke M, Moss AJ. Time dependence of mortality risk and defibrillator benefit after myocardial infarction. Circulation. 2004;109:1082–1084. doi: 10.1161/01.CIR.0000121328.12536.07. [DOI] [PubMed] [Google Scholar]
- 8.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt G, Malik M, Barthel P, Schneider R, Ulm K, Rolnitzky L, Camm AJ, Bigger JT, Jr, Schomig A. Heart-rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet. 1999;353:1390–1396. doi: 10.1016/S0140-6736(98)08428-1. [DOI] [PubMed] [Google Scholar]
- 10.Bigger JT, Jr, Fleiss JL, Kleiger R, Miller JP, Rolnitzky LM. The relationships among ventricular arrhythmias, left ventricular dysfunction, and mortality in the 2 years after myocardial infarction. Circulation. 1984;69:250–258. doi: 10.1161/01.cir.69.2.250. [DOI] [PubMed] [Google Scholar]
- 11.Gomes JA, Cain ME, Buxton AE, Josephson ME, Lee KL, Hafley GE. Prediction of long-term outcomes by signal-averaged electrocardiography in patients with unsustained ventricular tachycardia, coronary artery disease, and left ventricular dysfunction. Circulation. 2001;104:436–441. doi: 10.1161/hc2901.093197. [DOI] [PubMed] [Google Scholar]
- 12.Wilber DJ, Garan H, Finkelstein D, Kelly E, Newell J, McGovern B, Ruskin JN. Out-of-hospital cardiac arrest. Use of electrophysiologic testing in the prediction of long-term outcome. N Engl J Med. 1988;318:19–24. doi: 10.1056/NEJM198801073180105. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda T, Sakata T, Takami M, Kondo N, Tezuka N, Nakae T, Noro M, Enjoji Y, Abe R, Sugi K, Yamaguchi T. Combined assessment of T-wave alternans and late potentials used to predict arrhythmic events after myocardial infarction. A prospective study. J Am Coll Cardiol. 2000;35:722–730. doi: 10.1016/s0735-1097(99)00590-2. [DOI] [PubMed] [Google Scholar]
- 14.Exner DV, Kavanagh KM, Slawnych MP, Mitchell LB, Ramadan D, Aggarwal SG, Noullett C, Van Schaik A, Mitchell RT, Shibata MA, Gulamhussein S, McMeekin J, Tymchak W, Schnell G, Gillis AM, Sheldon RS, Fick GH, Duff HJ REFINE Investigators. Noninvasive risk assessment early after a myocardial infarction. the REFINE study. J Am Coll Cardiol. 2007;50:2275–2284. doi: 10.1016/j.jacc.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 15.Huikuri HV, Mäkikallio TH, Raatikainen MJP, Perkiömäki J, Castellanos A, Myerburg RJ. Prediction of sudden cardiac death. Appraisal of the studies and methods assessing the risk of arrhythmic death. Circulation. 2003;108:110–115. doi: 10.1161/01.CIR.0000077519.18416.43. [DOI] [PubMed] [Google Scholar]
- 16.Huikuri HV, Mahaux V, Bloch-Thomsen PE. Cardiac arrhythmias and risk stratification after myocardial infarction: results of the CARISMA pilot study. Pacing Clin Electrophysiol. 2003;26:416–419. doi: 10.1046/j.1460-9592.2003.00061.x. [DOI] [PubMed] [Google Scholar]
- 17.Krahn AD, Klein GJ, Yee R, Takle-Newhouse T, Norris C. Use of an extended monitoring strategy in patients with problematic syncope. Reveal Investigators. Circulation. 1999;99:406–410. doi: 10.1161/01.cir.99.3.406. [DOI] [PubMed] [Google Scholar]
- 18.Huikuri HV, Tapanainen JM, Lindgren K, Raatikainen P, Makikallio TH, Juhani Airaksinen KE, Myerburg RJ. Prediction of sudden cardiac death after myocardial infarction in the beta-blocking era. J Am Coll Cardiol. 2003;42:652–658. doi: 10.1016/s0735-1097(03)00783-6. [DOI] [PubMed] [Google Scholar]
- 19.Tapanainen JM, Thomsen PE, Kober L, Torp-Pedersen C, Makikallio TH, Still AM, Lindgren KS, Huikuri HV. Fractal analysis of heart rate variability and mortality after an acute myocardial infarction. Am J Cardiol. 2002;90:347–352. doi: 10.1016/s0002-9149(02)02488-8. [DOI] [PubMed] [Google Scholar]
- 20.Bauer A, Kantelhardt JW, Barthel P, Schneider R, Makikallio T, Ulm K, Hnatkova K, Schomig A, Huikuri H, Bunde A, Malik M, Schmidt G. Deceleration capacity of heart rate as a predictor of mortality after myocardial infarction: cohort study. Lancet. 2006;367:1674–1681. doi: 10.1016/S0140-6736(06)68735-7. [DOI] [PubMed] [Google Scholar]
- 21.Tapanainen JM, Still AM, Airaksinen KE, Huikuri HV. Prognostic significance of risk stratifiers of mortality, including T wave alternans, after acute myocardial infarction: results of a prospective follow-up study. J Cardiovasc Electrophysiol. 2001;12:645–652. doi: 10.1046/j.1540-8167.2001.00645.x. [DOI] [PubMed] [Google Scholar]
- 22.Huikuri HV, Makikallio TH, Peng CK, Goldberger AL, Hintze U, Moller M. Fractal correlation properties of R-R interval dynamics and mortality in patients with depressed left ventricular function after an acute myocardial infarction. Circulation. 2000;101:47–53. doi: 10.1161/01.cir.101.1.47. [DOI] [PubMed] [Google Scholar]
- 23.Makikallio TH, Barthel P, Schneider R, Bauer A, Tapanainen JM, Tulppo MP, Schmidt G, Huikuri HV. Prediction of sudden cardiac death after acute myocardial infarction: role of Holter monitoring in the modern treatment era. Eur Heart J. 2005;26:762–769. doi: 10.1093/eurheartj/ehi188. [DOI] [PubMed] [Google Scholar]
- 24.Raatikainen MJ, Jokinen V, Virtanen V, Hartikainen J, Hedman A, Huikuri HV CARISMA Investigators. Microvolt T-wave alternans during exercise and pacing in patients with acute myocardial infarction. Pacing Clin Electrophysiol. 2005;28(Suppl. 1):S193–S197. doi: 10.1111/j.1540-8159.2005.00110.x. [DOI] [PubMed] [Google Scholar]
- 25.Epstein AE, Carlson MD, Fogoros RN, Higgins SL, Venditti FJ., Jr Classification of death in antiarrhythmia trials. J Am Coll Cardiol. 1996;27:433–442. doi: 10.1016/0735-1097(95)00482-3. [DOI] [PubMed] [Google Scholar]
- 26.Pratt CM, Greenway PS, Schoenfeld MH, Hibben ML, Reiffel JA. Exploration of the precision of classifying sudden cardiac death. Implications for the interpretation of clinical trials. Circulation. 1996;93:519–524. doi: 10.1161/01.cir.93.3.519. [DOI] [PubMed] [Google Scholar]
- 27.Wathen MS, DeGroot PJ, Sweeney MO, Stark AJ, Otterness MF, Adkisson WO, Canby RC, Khaligi K, Machado C, Rubenstein DS, Volosin KJ and for the PainFREE Rx II Investigators. Prospective randomized multicenter trial of empirical antitachycardia pacing versus shocks for spontaneous rapid ventricular tachycardia in patients with implantable cardioverter-defibrillators: Pacing Fast Ventricular Tachycardia Reduces Shock Therapies (PainFREE Rx II) trial results. Circulation. 2004;110:2591–2596. doi: 10.1161/01.CIR.0000145610.64014.E4. [DOI] [PubMed] [Google Scholar]
- 28.Wilkoff BL, Williamson BD, Stern RS, Moore SL, Lu F, Lee SW, Bigersdotter-Green UM, Wathen MS, Van Gelder IC, Heubner BM, Brown ML, Holloman KK for the PREPARE Study Investigators. Strategic programming of detection and therapy parameters in implantable cardioverter-defibrillators reduces shocks in primary prevention patients: results from the PREPARE (Primary Prevention Parameters Evaluation) study. J Am Coll Cardiol. 2008;52:541–550. doi: 10.1016/j.jacc.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Gehi AK, Stein RH, Metz LD, Gomes JA. Microvolt T-wave alternans for the risk stratification of ventricular tachyarrhythmic events: a meta-analysis. J Am Coll Cardiol. 2005;46:75–82. doi: 10.1016/j.jacc.2005.03.059. [DOI] [PubMed] [Google Scholar]
- 30.Hohnloser SH, Ikeda T, Bloomfield DM, Dabbous OH, Cohen RJ. T-wave alternans negative coronary patients with low ejection and benefit from defibrillator implantation. Lancet. 2003;362:125–126. doi: 10.1016/s0140-6736(03)13865-2. [DOI] [PubMed] [Google Scholar]
- 31.Chow T, Kereiakes DJ, Onufer J, Woelfel A, Gursoy S, Peterson BJ, Brown ML, Pu W, Benditt DG on behalf of the MASTER Trial Investigators. Does microvolt T-wave alternans testing predict ventricular tachyarrhythmias in patients with ischemic cardiomyopathy and prophylactic defibrillators? J Am Coll Cardiol. 2008;52:1607–1615. doi: 10.1016/j.jacc.2008.08.018. [DOI] [PubMed] [Google Scholar]