Abstract
Background
Abnormally high CSF 3-OMD occurs frequently for RLS patients indicating either increased L-dopa synthesis, limitations in L-dopa decarboxylation or increased MAT/COMT activity, or some combination of these. Increased tyrosine hydroxylase activity was found on both RLS autopsy and the rodent iron-deprivation model of RLS, suggesting increased DA synthesis in RLS. We, therefore, hypothesized elevated 3-OMD in RLS results from increased DA synthesis and that this should occur accordingly with increased HVA. It would then also reflect both more severe iron insufficiency pathology of RLS and greater clinical severity, shown by the objective measure of PLMS/hr.
Methods
Patients off RLS medications and matched controls had lumbar punctures at either 10 AM or 10PM. RLS patients were grouped by normal or abnormally high 3-OMD (>10 nmol/l).
Results
49 RLS patients (30 high, 19 normal 3-OMD) and 36 age- and gender-matched controls, analyzed separately by time of CSF collection, did not significantly differ in age or gender. RLS patients with High-3-OMD had significantly higher CSF HVA, while those with normal 3-OMD had consistently lower CSF HVA than controls. CSF ferritin was consistently lower compared to controls for the high 3-OMD but not the normal 3-OMD RLS patients. The PLMS/hr was significantly higher for RLS patients with high compared to normal 3-OMD, indicating high 3-OMD patients had more severe RLS.
Conclusions
Abnormal elevation in 3-OMD for RLS patients may reflect increased dopamine synthesis for more severe but perhaps not mild RLS. These differences in the putative dopamine pathology of RLS may indicate different phases or expression of RLS biology or different underlying disease processes.
Keywords: Restless legs Syndrome, Dopamine, CSF, 3-OMD, HVA, Tyrosine hydroxylase
Introduction
The excellent symptomatic response to levodopa experienced by patients with restless legs syndrome (RLS) has provided the primary evidence supporting involvement of the dopaminergic system in the pathology of RLS. While PET and SPECT imaging of the striatum held initial promise of linking dopamine abnormities to RLS, they have failed to reveal a consistent pattern of abnormalities. The dopamine-2 receptor binding potentials of RLS patients have been found to be increased, not changed, and even decreased (1-6). The strongest support for dopaminergic pathology in RLS comes from recent autopsy data that somewhat unexpectedly found increases in both total and phosphorylated tyrosine hydroxylase (TH) in the putamen from RLS patients. This occurred with low iron in the substantia nigra and putamen (7). This increase in TH, particularly phosphorylated TH (pTH), runs counter to the commonly held view that the response to a dopamine agonist indicates a decrease in dopamine in RLS. Moreover, since iron is a co-factor for TH iron, it had been assumed decreased iron would result in decreased TH, contrary to these empirical results. Given these concerns, the authors in that study looked at the effects of decreased iron on TH in cell culture models (PC12) and in iron deficient (ID) animals. They found that ID conditions in both cell culture models and in animals, produced this unexpected increase in TH and pTH (7). Moreover, ID animals also have increased extra-cellular dopamine in the striatum during the end of the inactive and start of the active circadian cycle (8). The combined results from the autopsy and from both the animal and cell studies suggest significantly low cellular iron in RLS increases TH and dopamine synthesis.
The normal increase in CSF tetrahydrobiopterin (BH4) for 10 AM compared to 10 PM samples was much greater for RLS patients than controls. This large AM increase of BH4 is consistent with the concept of abnormally increased circadian activity of TH in RLS patients. These CSF studies also showed markedly significant increases in 3-O-methyldopa (3-OMD) for RLS compared to controls for both AM and PM samples with marginally, non-significant diurnal differences (9, 10). Significant elevations in 3-OMD is also reported for Parkinson patients on levodopa where the 3-OMD values were 10 times that reported in the RLS studies (11). The increase in 3-OMD with levodopa treatment could occur from extra-cellular metabolism of exogenous levodopa to 3-OMD using COMT (catechol-O-methyltransferase). The studies showing increased 3-OMD in RLS patients (9, 10) were, however, conducted on patients free of any pharmacological treatment for RLS for at least 2-weeks prior to the CSF collection. Moreover, only 2 of the 36 patients in those studies were treated with levodopa prior to the study. There was, therefore, no exogenous increase in l-dopa, and an alternative explanation is required to account for the increased 3-OMD in RLS.
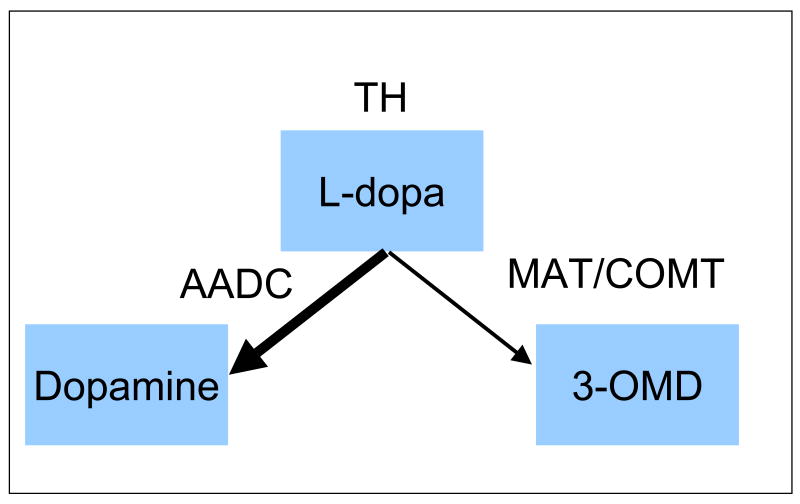
Under physiological conditions, increased 3-OMD could occur for 3 reasons. It could occur as shown in figure 1: reflect decreased AAD activity or increased COMT activity, both of which appear to be regulated. It could also indicate an increase in TH activity to a level where the L-dopa synthesis rates exceed immediate AAD capacity and the amount metabolized to 3-OMD increases (see figure 1). If the 3-OMD increases result primarily from increased TH activity, and thus increased levodopa and dopamine synthesis, then we would expect the increased 3-OMD would relate to increased dopamine and higher values of CSF homovanillic acid (HVA). If, however, the 3-OMD increases result primarily from decreased AAD or increased COMT, then we would expect no difference or even a decrease in HVA, reflecting a shunting of l-dopa to 3-OMD, decreasing dopamine synthesis. (See figure1.) Thus, there are two different types of explanations for the 3-OMD increases without exogenous L-dopa: 1) decreased dopamine synthesis caused by either increased COMT or decreased AAD versus 2) increased TH activity producing increased synthesis of both dopamine and 3-OMD. These would have opposite effects on the relation between CSF HVA and 3-OMD. If increased 3-OMD reflects decreased DA synthesis there should be either no relation or an inverse relation between CSF values of HVA and 3-OMD. If the increased 3-OMD reflects increased TH activity with increased dopamine synthesis, then CSF HVA and 3-OMD should both be increased. The RLS autopsy, ID animal and cellular iron chelation studies support increased TH activity. We, therefore, hypothesized abnormally high levels of 3-OMD defined a group of RLS patients who had abnormally increased TH activity and dopamine synthesis that would produce abnormally high values of CSF HVA.
Figure 1.
Pathways for producing dopamine or alternatively 3-OMD in dopamine producing cells
Moreover, since the animal and cellular data indicate increased TH activity results from decreased iron it seems likely that this will also be seen in RLS patients. Thus, if the high 3-OMD for RLS patients reflects increased TH activity, then it may also reflect a significant decrease in brain iron, producing the increase in TH. We, therefore, also made a secondary hypothesis that the high 3-OMD RLS patients would show significantly decreased CSF ferritin when compared to controls or RLS patients with normal 3-OMD. Since decreased iron status generally indicates greater clinical severity of RLS we would also expect primary RLS symptoms would be greater for patients with higher 3-OMD. Given the relation shown between dopamine and periodic limb movements (PLM) suggested by the opposite effects on PLM of dopamine agonists (12, 13) and antagonists (14) and the PLMS occurrence in a range of dopaminergic related disorders (15), we hypothesize that higher 3-OMD would be associated with higher PLMS. This could also produce generally more severe RLS symptoms as indicated by a subjective clinical rating of severity. The subjective clinical scales, however, are affected by many environmental and subject factors not related to RLS severity. This is most clearly shown by the large placebo responses to treatment for subjective scales (16) but not for the objective PLMS/hr measurements (12) (17). Thus, subjective report of symptoms on clinical scale may not be sensitive enough to show differences, given the small sample sizes of these studies in which CSF measures are obtained.
Methods
Data were analyzed from two separate samples of RLS patients and matched controls. As previously reported for both of these samples (9, 18), the RLS patients were withdrawn from all RLS related medications for at least 2 weeks prior to the study. CSF was obtained from a lumbar puncture at 10 AM for group 1 and 10 PM for group 2, following procedures previously described (10). On the day of admission to the study, all patients were evaluated for RLS severity on the Johns Hopkins Restless Legs Severity Scale that ranged from 0 for no RLS symptoms to 3 for severe symptoms (19). Group 1 was permitted to return home about 1 hour after the lumbar puncture with instructions to limit activities. Group 2 was admitted to the clinical research unit and before obtaining the CSF sample had 2 consecutive nights of standard clinical polysomnography (PSG). These included bilateral recording of anterior tibialis EMG, C3-A2 EEG, submental EMG, left and right EOG, air pressure changes in a nasal cannula measuring airflow, and abdominal and thoracic respiratory effort. Subjects with greater than 15 sleep disordered breathing events per hour on the first night were excluded from these analyses. The PLMS/hr of sleep was determined for the second night based on standard criteria (20). Two RLS patients in group 2 were participating in ongoing studies related to treatment issues that were conducted after the close of the data analysis presented in our prior publication (10). These had exactly the same procedures as subjects studied before them, including no medication treatment for RLS for at least 2 weeks before the CSF collection. They were included to provide complete analyses of all our subjects who met the criteria for inclusion in this study.
The RLS patients for each time of day (morning and evening) were divided into two groups based on normal CSF 3-OMD(<= 10 nmol/l) and those with abnormally high CSF 3-OMD (>10 nmol/l). This criterion for usual maximum normal was set prior to starting our CSF studies, based on our initial experience with the morning CSF samples. For the assay we were using 10 nmol/l was also considered the lowest reliable measurement, and all values below that were therefore set at 9 for these analyses. None of the 14 control subjects in our CSF study with morning samples exceeded the expected maximum normal value of 10 nmol/l, and only three of the 22 controls from our second CSF study with night samples exceeded that value. The following results included these three as part of the normal controls, but removing them did not change any of the significant results reported below. The division of the RLS patients into a normal and abnormally elevated 3OMD range produced 3 groups for each time of day. The morning CSF group included 14 normal controls (average ± sd age of 51.4 ±14.8), four RLS with normal 3-OMD (average ± sd age of 58.0 ± 14.2) and 13 RLS with abnormally high 3-OMD (average age ± sd of 65.3 ± 9.4). The night CSF group included 22 normal controls (average ± sd age of 59.2 ± 8.3), 15 RLS with normal 3-OMD (average ± sd age of 59.7 ± 9.4) and 17 RLS with high 3-OMD (average ± sd age of 65.6 ± 9.7). The male-to-female ratio did not differ between the groups within the morning or night populations. The average ± sd 3-OMD values in the abnormally high 3-OMD groups were 30.9 ±20.1 nmol/l and 37.2 ±20.8 nmol/l for night and day samples respectively.
Statistical Analyses
Given the limited and uneven sample sizes for the morning CSF data, the two sets of data (morning and night) were analyzed separately. An ANOVA was used to evaluate the 3 groups (Controls, normal-3-OMD RLS (Normal3OMD), abnormally high 3-OMD RLS (High3OMD)) for our primary hypothesis of differences for CSF HVA and our secondary hypotheses of differences for CSF ferritin. The PLS/hr and clinical ratings of severity were similarly analyzed for our secondary hypothesis, but only for high- vs. Normal3OMD groups since subject selection criteria ensured controls were lower than RLS patients. Exploratory analyses include the correlation between 3-OMD values and that of CSF HVA, 5HIAA and CSF ferritin. The ages did not differ significantly between the groups. The nighttime High3OMD group, however, had a somewhat older age range than the controls. The ages of 3 RLS patients in the High3OMD group exceeded the oldest age in the control group (age 74). Additional analyses, therefore, were made limiting the age ranges to that for the normal controls.
Results
Primary hypotheses
The analyses of night and day samples both showed CSF HVA values for the High3OMD group were significantly higher than either the nomal3OMD or control groups (see tables 1 and 2). The CSF HVA for Normal 3OMD groups was, in contrast, slightly lower than the controls for the night samples and significantly lower for the day samples.
Table 1.
Night CSF sample group: means ± standard deviations and statistical comparisons between groups. * indicates significant difference (p<0.05) from control and ** indicate significant difference between High and normal 3OMD. ANOVA is used for the comparison for all groups with pair-wise comparisons using FPLSD. The PLMS/hr and JHRLSS are only compared between normal and high 3OMD using t test.
| Normal controls | RLS | ANOVA/ t test | ||
|---|---|---|---|---|
| Normal 3OMD | High 3OMD | F/t, p | ||
| Sample size | 22 | 15 | 17 | |
| age | 59.2 ± 8.3 | 59.7 ± 9.4 | 65.6 ±9.7* | F= 2.7, p=0.07 |
| Male:Female | 11:11 | 6:9 | 10:7 | ChiSqr=1.1, p=0.29 |
| CSF HVA (nmol/l) | 216.9 ±88.3 | 177.7 ±106.7 | 296.8 ±126.4*, ** | F=5.3, p=0.008 |
| CSF 5HIAA (nmol/l) | 98.0 ±33.7 | 85.1 ±41.1 | 136.4 ±47.8 *, ** | F=7.2, p=0.002 |
| CSF ferritin (mcg/l) | 2.89 ±0.91 | 2.65 ±0.77 | 2.31 ±0.69 * | F=2.39, p=0.10 |
| PLMS/hr | 5.4 ±5.0 | 66.8 ±49.9 | 106.6 ±56.9 ** | t=1.95, p=0.03 |
| JHRLSS | ------ | 1.73 ±0.46 | 1.82 ±0.73 | t=0.43, p=ns |
Table 2.
Day CSF sample group: means ± standard deviations and statistical comparisons between groups. * indicates significant difference (p<0.05) from control and ** indicate significant difference between High and normal 3-OMD. ANOVA is used for the comparison for all groups with pair-wise comparisons using FPLSD. The JHRLSS are only compared between normal and high 3OMD groups using t test. Gender information was unavailable for normal controls.
| Normal controls | RLS | ANOVA/ t test | ||
|---|---|---|---|---|
| Normal 3OMD | High 3OMD | F/t, p | ||
| Sample size | 14 | 4 | 13 | |
| age | 51.4 ± 14.8 | 58.0 ± 14.2 | 65.3 ± 9.6* | t=1.2, p=0.25 |
| Male:Female | *** | 4:0 | 10:3 | ChiSqr=1.2, p=0.29 |
| CSF HVA (nmol/l) | 233.3 ±49.2 | 152.5 ± 64.6* | 246.4 ± 71.1*, ** | F=3.7, p=0.038 |
| CSF 5HIAA (nmol/l) | 107.22 ± 22.6 | 55.6 ± 43.8* | 99.9 ± 33.5** | F=4.6, p=0.019 |
| CSF ferritin (mcg/l) | 3.5 ± 1.56 | 0.61 ± 0.24* | 1.36 ±1.11* | F=10.6, p=0.0006 |
| JHRLSS | 2.0 ±1.16 | 2.3 ±0.77 | t=0.78, p=0.45 | |
Secondary hypotheses
The High3OMD groups showed significantly lower CSF ferritin than the controls for both the day and night samples. The Normal3OMD groups showed lower average CSF ferritin than controls; this was significant for the morning but not the night samples. The CSF ferritin values of the Normal3OMD groups did not differ significantly from the High3OMD groups for either the morning or night samples. The PLMS/hr was significantly greater for the High3OMD than the Normal3OMD group for the night samples. PLMS were not measured for the morning studies. The scores on the JHRLSS clinical rating scale were greater for the High3OMD than the Normal3OMD groups for both the day and night samples, but these differences were not statistically significant. (See tables 1 and 2).
A separate age-adjusted analysis excluding those three patients over 74 in the High3OMD night group produced the same significant results as in table 1 except that there were no indications of age differences between the three night groups.
Exploratory analyses
The CSF 5HIAA showed the same significant differences between the groups found for HVA except that the relationships were generally not as highly significant (See tables 1 and 2).
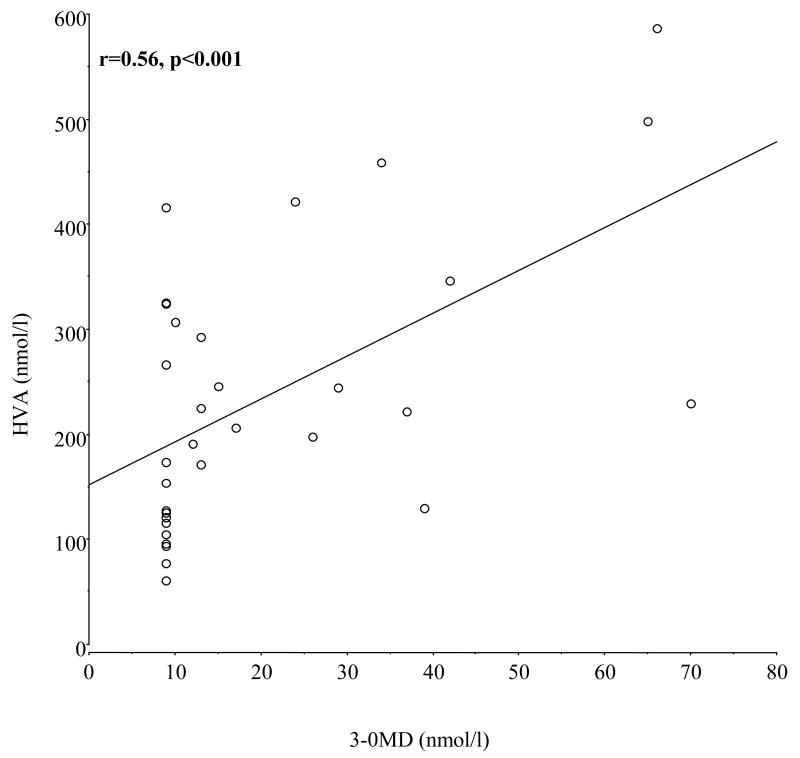
The night 3-OMD values from RLS patients correlated significantly with HVA for both High3OMD and Normal3OMD samples combined (r=0.56, p=0.0008) (see figure 2) and for the High3OMD group alone (r=0.51, p=0.038). The night CSF 5HIAA values also showed a significant correlation with 3-OMD for all RLS patients (r==0.37, p=0.037) but not for only the high3OMD RLS patients (r=0.11, p=0.67). None of the CSF ferritin samples correlated significantly with CSF 3-OMD for either all RLS patients or only the High3OMD patients. Similarly, the PLMS/hr did not correlate significantly with 3-OMD for either the High3OMD or combined high and Normal3OMD samples. (These correlation values were less than 0.27, p>0.3).
Figure 2.
Linear regression for night-time samples of CSF HVA as a function of CSF 3-OMD for all RLS patients.
The daytime CSF measures did not show any significant correlations with 3-OMD values.
Discussion
The data strongly confirmed our primary hypotheses that RLS patients with abnormally increased CSF 3-OMD also have abnormally increased CSF HVA. 3-OMD is product of a minor alternative pathway for the metabolism of levodopa. Levodopa is generally not present in the extra-cellular space; 3-OMD is not made there unless levodopa is provided from concurrent use of medications. None of our patients were on levodopa within the past two weeks before the lumbar puncture. The increased 3-OMD could, therefore, only reflect either a relative deficiency in the amino-acid decarboxylase activity, an excessive COMT and MAT activity, an excessive production of levodopa by tyrosine hydroxylase (TH), or some combination of these factors (See figure 1). But only the increased TH activity with increased DA production would account for the remarkably close association relation between increased CSF 3-OMD and increased HVA. Moreover, the autopsy data indicting increased TH and increased phosphorylated TH (7) with RLS further supports our hypothesis that the increased CSF 3-OMD with RLS results from increased TH activity, producing increased levodopa and dopamine as well as 3-OMD synthesis.
The significant correlation between the CSF 3-OMD and HVA for the night sample further supports our hypothesized relation between increased 3-OMD and the TH increase observed with iron deficiency. The failure to find this correlation for the morning sample may relate to their somewhat more extreme 3-OMD values compared to the night samples, or it may reflect effects of circadian variation on the underlying patterns of dopaminergic activity in RLS.
The secondary hypotheses were partly confirmed. The High3OMD group compared to the Normal3OMD group showed greater PLMS/hr in the night sample consistent with more severe disease. The clinical ratings of disease severity showed a similar trend but that was not statistically significant. Similarly, for the night CSF samples, ferritin was lower for the High3OMD group than for the Normal3OMD groups, and only the High3OMD group showed statistically significant difference from controls. Thus, the clinical and CSF iron status patterns were consistent with our hypothesis that the elevated 3-OMD reflects a more severe RLS disorder. Some of the hypothesized relationships were not statistically significant, but all were in the expected direction, except for the morning CSF ferritin that was actually somewhat lower for the Normal3OMD group than for the High3OMD group. These morning CSF ferritin values are, however, very low and we may have a floor effect obscuring factors affecting the low values for this measure. This difference may also reflect abnormalities in the circadian changes in RLS possibly also related to circadian variation in symptom expression.
Although significantly increased 3-OMD in RLS CSF has now been found in our two separate studies, no significant 3-OMD elevation was reported in an independent study by Karin Stiasny-Kolster et al. (21). Her data, however, show remarkably high CSF 3-OMD for RLS patients compared to controls (31.0 ±42.9 vs. 17.3 ±8.2 mmol/L, respectively). This almost 2-fold increase in the average over control values is the largest relative absolute difference in any of the values reported in that study. The large variance for the RLS patients indicates a very skewed distribution that may include significantly more abnormally elevated values than was observed for the controls. This possibility was not evaluated in that study.
Overall the autopsy data and these CSF findings suggest that elevated 3-OMD reflects excessive levodopa production consistent with increased TH activity and increased levodopa and dopamine production. The close correlation with the dopamine metabolite HVA not matched by a similar relation for the serotonin metabolite 5HIIAA further supports our hypothesized increased levodopa production for more severe RLS patients. It deserves note that milder patients not showing any increase in 3-OMD appear to have, if anything, decreased levodopa production, as shown by the HVA values decreased below control values. There may be a biphasic pattern of the dopamine status, in relation to RLS status, with dopamine production increased for those with more PLM and more CNS iron loss, but possibly decreased for those with less PLM and less CNS iron loss. This might reflect differing effects of the disease on the dopamine system related to phase or severity of the disease or possibly to differing biological or even genetic bases for the disease. Certainly, if true, this presents a major complication for understanding the dopamine pathology in RLS that may explain in part the conflicting results from dopamine related imaging studies and the vexing problem of RLS augmentation with dopamine treatments of RLS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Turjanski N, Lees AJ, Brooks DJ. Striatal dopaminergic function in restless legs syndrome: 18F-dopa and 11C-raclopride PET studies. Neurology. 1999;52(5):932–7. doi: 10.1212/wnl.52.5.932. [DOI] [PubMed] [Google Scholar]
- 2.Michaud M, Soucy JP, Chabli A, Lavigne G, Montplaisir J. SPECT imaging of striatal pre- and postsynaptic dopaminergic status in restless legs syndrome with periodic leg movements in sleep. J Neurol. 2002 Feb;249(2):164–70. doi: 10.1007/pl00007859. [DOI] [PubMed] [Google Scholar]
- 3.Ruottinen HM, Partinen M, Hublin C, Bergman J, Haaparanta M, Solin O, et al. An FDOPA PET study in patients with periodic limb movement disorder and restless legs syndrome. Neurology. 2000;54(2):502–4. doi: 10.1212/wnl.54.2.502. [DOI] [PubMed] [Google Scholar]
- 4.Cervenka S, Palhagen SE, Comley RA, Panagiotidis G, Cselenyi Z, Matthews JC, et al. Support for dopaminergic hypoactivity in restless legs syndrome: a PET study on D2-receptor binding. Brain. 2006 Jul 1; doi: 10.1093/brain/awl163. [DOI] [PubMed] [Google Scholar]
- 5.Tribl GG, Asenbaum S, Klosch G, Mayer K, Bonelli RM, Auff E, et al. Normal IPT and IBZM SPECT in drug naive and levodopa-treated idiopathic restless legs syndrome (letter to editor) Neurology. 2002 Aug 27;59(4):649–50. doi: 10.1212/wnl.59.4.649. [DOI] [PubMed] [Google Scholar]
- 6.Eisensehr I, Wetter TC, Linke R, Noachtar S, von Lindeiner H, Gildehaus FJ, et al. Normal IPT and IBZM SPECT in drug-naive and levodopa-treated idiopathic restless legs syndrome. Neurology. 2001 Oct 9;57(7):1307–9. doi: 10.1212/wnl.57.7.1307. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Allen RP, Earley CJ, Beard JL, Connor JR. Altered Dopaminergic Expression in Restless Leg Syndrome. Society for Neuroscience; 2005; Washington, DC: 2005. [Google Scholar]
- 8.Nelson C, Erikson K, Pinero DJ, Beard JL. In vivo dopamine metabolism is altered in iron-deficient anemic rats. J Nutr. 1997 Dec;127(12):2282–8. doi: 10.1093/jn/127.12.2282. [DOI] [PubMed] [Google Scholar]
- 9.Earley CJ, Hyland K, Allen RP. Circadian changes in CSF dopaminergic measures in restless legs syndrome. Sleep Med. 2006 Apr;7(3):263–8. doi: 10.1016/j.sleep.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Earley CJ, Connor JR, Beard JL, Malecki EA, Epstein DK, Allen RP. Abnormalities in CSF concentrations of ferritin and transferrin in restless legs syndrome. Neurology. 2000;54(8):1698–700. doi: 10.1212/wnl.54.8.1698. [DOI] [PubMed] [Google Scholar]
- 11.Tohgi H, Abe T, Kikuchi T, Takahashi S, Nozaki Y. The significance of 3-O-methyldopa concentrations in the cerebrospinal fluid in the pathogenesis of wearing-off phenomenon in Parkinson's disease. Neurosci Lett. 1991 Oct 28;132(1):19–22. doi: 10.1016/0304-3940(91)90422-p. [DOI] [PubMed] [Google Scholar]
- 12.Allen R, Becker PM, Bogan R, Schmidt M, Kushida CA, Fry JM, et al. Ropinirole decreases periodic leg movements and improves sleep parameters in patients with restless legs syndrome. Sleep. 2004 Aug 1;27(5):907–14. doi: 10.1093/sleep/27.5.907. [DOI] [PubMed] [Google Scholar]
- 13.Partinen M, Hirvonen K, Jama L, Alakuijala A, Hublin C, Tamminen I, et al. Efficacy and safety of pramipexole in idiopathic restless legs syndrome: A polysomnographic dose-finding study-The PRELUDE study. Sleep Med. 2006 Jun 30; doi: 10.1016/j.sleep.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Winkelmann J, Schadrack J, Wetter TC, Zieglgansberger W, Trenkwalder C. Opioid and dopamine antagonist drug challenges in untreated restless legs syndrome patients. Sleep Med. 2001 Jan;2(1):57–61. doi: 10.1016/s1389-9457(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 15.Montplaisir J, Michaud M, Denesle R, Gosselin A. Periodic leg movements are not more prevalent in insomnia or hypersomnia but are specifically associated with sleep disorders involving a dopaminergic impairment. Sleep Med. 2000;1(2):163–7. doi: 10.1016/s1389-9457(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 16.Trenkwalder C, Garcia-Borreguero D, Montagna P, Lainey E, De Weerd AW, Tidswell P, et al. Ropinirole in the treatment of restless legs syndrome: results from the TREAT RLS 1 study, a 12 week, randomised, placebo controlled study in 10 European countries. J Neurol Neurosurg Psychiatry. 2004 Jan;75(1):92–7. [PMC free article] [PubMed] [Google Scholar]
- 17.Partinen M, Hirvonen K, Jama L, Alakuijala A, Hublin C, Tamminen I, et al. Efficacy and safety of pramipexole in idiopathic restless legs syndrome: a polysomnographic dose-finding study--the PRELUDE study. Sleep Med. 2006 Aug;7(5):407–17. doi: 10.1016/j.sleep.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Earley CJ, Hyland K, Allen RP. CSF dopamine, serotonin, and biopterin metabolites in patients with restless legs syndrome. Mov Disord. 2001 Jan;16(1):144–9. doi: 10.1002/1531-8257(200101)16:1<144::aid-mds1009>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 19.Allen RP, Earley CJ. Validation of the Johns Hopkins restless legs severity scale. Sleep Med. 2001;2:239–42. doi: 10.1016/s1389-9457(00)00080-0. [DOI] [PubMed] [Google Scholar]
- 20.Atlas Task Force of the American Sleep Disorders Association. Recording and scoring leg movements. Sleep. 1993;16(8):748–59. [PubMed] [Google Scholar]
- 21.Stiasny-Kolster K, Moller JC, Zschocke J, Bandmann O, Cassel W, Oertel WH, et al. Normal dopaminergic and serotonergic metabolites in cerebrospinal fluid and blood of restless legs syndrome patients. Mov Disord. 2004 Feb;19(2):192–6. doi: 10.1002/mds.10631. [DOI] [PubMed] [Google Scholar]