Abstract
Diffusion tensor imaging (DTI) studies have demonstrated abnormal anisotropic diffusion in schizophrenia. However, examining data with low spatial resolution and/or a low number of gradient directions and limitations associated with analysis approaches sensitive to registration confounds may have contributed to mixed findings concerning the regional specificity and direction of results. This study examined three major white matter tracts connecting lateral and medial temporal lobe regions with neocortical association regions widely implicated in systems-level functional and structural disturbances in schizophrenia. Using DTIstudio, a previously validated regions of interest tractography method was applied to 30 direction diffusion weighted imaging data collected from demographically similar schizophrenia (n=23) and healthy control subjects (n=22). The diffusion tensor was computed at each voxel after intra-subject registration of diffusion-weighted images. Three-dimensional tract reconstruction was performed using the Fiber Assignment by Continuous Tracking (FACT) algorithm. Tractography results showed reduced fractional anisotropy (FA) of the arcuate fasciculi (AF) and inferior longitudinal fasciculi (ILF) in patients compared to controls. FA changes within the right ILF were negatively correlated with measures of thought disturbance. Reduced volume of the left AF was also observed in patients. These results, which avoid registration issues associated with voxel-based analyses of DTI data, support that fiber pathways connecting lateral and medial temporal lobe regions with neocortical regions are compromised in schizophrenia. Disruptions of connectivity within these pathways may potentially contribute to the disturbances of memory, language, and social cognitive processing that characterize the disorder.
Keywords: diffusion tensor imaging, white matter, uncinate fasciculus, inferior longitudinal fasciculus, arcuate fasciculus, fractional anisotropy
1. Introduction
Some brain systems appear selectively vulnerable to disease processes in schizophrenia. Structural imaging studies have repeatedly demonstrated reductions of gray matter (Shenton et al., 2001; Narr et al., 2005b; Whitford et al., 2006) that appear most reproducible in lateral and medial temporal cortices, particularly the hippocampus (Narr et al., 2004; Goldman et al., 2008) and the superior temporal gyrus (Shenton et al., 2001; Honea et al., 2008). Disturbances of lateral and medial temporal lobe function including deficits in auditory sensory (Rabinowicz et al., 2000), language (DeLisi, 2001) memory (Saykin et al., 1994; Bilder et al., 2000; Harrison and Fowler, 2004; Harrison, 2004) and social cognitive processing (Green et al., 2005; Yamada et al., 2007) are also widely documented in schizophrenia. These behavioral disturbances may be associated with neuropathology in discrete brain regions and/or from disruptions of connectivity within functional networks (Frith et al., 1995; Walterfang et al., 2005). However, the effects of illness on the physiological connections linking fronto-limbic and other temporo-neocortical association areas are not fully understood.
With Diffusion tensor imaging (DTI) it is possible to address whether disturbances of white matter connectivity occur within particular cortical networks. That is, because the diffusion tensor approximating local diffusion is determined by the local tissue architecture, it can be used to generate an in vivo visualization of white matter tracts (Conturo et al., 1999; Ashburner and Friston, 2000; Catani et al., 2002a; Wakana et al., 2004). Tractography methods assemble the local diffusion tensor data into tracts where scalar metrics, such as fractional anisotropy (FA), along these tracts then allows for the precise localization of white matter abnormalities. Although some doubt exists that tractography may generate sufficiently reproducible neuroanatomical detail for use in quantitative analyses, growing evidence supports that tracking results agree well with postmortem definitions (Catani et al., 2002b; Jellison et al., 2004; Wakana et al., 2007). To investigate possible white matter abnormalities in schizophrenia, we examined three major white matter tracts: the arcuate fasciculus (AF), the inferior longitudinal fasciculus (ILF), and the uncinate fasciculus (UF). These tracts have been classically defined using postmortem studies and are easily identifiable using a multiple region of interest (ROI) approach in DTI data.
The AF (or temporal component of the superior longitudinal fasciculus [SLF]) connects the superior temporal gyrus with dorsal prefrontal cortex (Brodmann areas 8 and 46) (Petrides and Pandya, 1984; Makris et al., 2005). By linking frontal and temporal cortices, the AF may provide a means by which the prefrontal cortex receives and modulates auditory and audiospatial information (Leinonen et al., 1980; Petrides M, 2002). Several prior investigations have implicated fronto-temporal processing disruptions in schizophrenia (Frith et al., 1995; Liddle, 1996; Andreasen, 2000). Further, some prior voxel-based DTI studies suggest that white matter abnormalities occur in the vicinity of the AF (Burns et al., 2003; Kubicki et al., 2005; Douaud et al., 2007) and may be associated with the experience of hallucinations in the auditory modality (Hubl et al., 2004). To our knowledge, however, no prior studies have examined the AF using DTI tractography methods, which may provide a more powerful means to examine changes in the physiology of this tract in schizophrenia.
The ILF has been demonstrated by postmortem and DTI studies to be a major associative connection between the anterior temporal and occipital lobe and plays an important role in visual memory (Bauer and Trobe, 1984; Shinoura et al., 2007). It also connects the parahippocampal gyrus and amygdala (Catani et al., 2002a), regions implicated in the structural and functional neuropathology of schizophrenia (Benes and Berretta, 2000; Narr et al., 2001; Harrison, 2004; Surguladze et al., 2006). At least one prior schizophrenia DTI tractography study has reported reduced FA for the left ILF (Ashtari et al., 2007a) where negative associations between FA and visual hallucinations were further observed.
The UF links the temporal lobe with ventral, medial, and orbital frontal cortices (Ebeling and von Cramon, 1992). This tract plays an important role in the formation and retrieval of memories (Levine et al., 1998; Squire et al., 2004). Though patients with schizophrenia exhibit generalized cognitive impairment, declarative memory deficits appear particularly pronounced (Bilder et al., 2000). However, while some DTI studies have observed lower FA in the UF in schizophrenia (Nestor et al., 2008), other reports have been mixed (Jones et al., 2006).
In spite of the evidence suggesting disturbances of fiber connectivity in schizophrenia, the regional specificity of results remains unclear. Discrepancies in prior findings may be at least partially attributable to the methodological limitations associated with examining data with a low number of gradient directions and low spatial resolution in small cohorts, using voxel-based methods sensitive to registration confounds (Alexander et al., 2001), and the paucity of studies using methods that allow the precise mapping of tract anatomy within subjects (Kubicki et al., 2007). We thus examined the connectivity of the AF, ILF, and UF in 23 schizophrenia and 22 healthy subjects using 30 direction DTI data and sensitive fiber tracking methods that do not rely on registration procedures to align imaging data across subjects. We predicted that each of these fiber pathways would be disturbed in schizophrenia. We further explored whether positive and negative symptom cluster scores are associated with fiber tract abnormalities within medial and lateral temporal lobe systems in schizophrenia patients.
2. Methods
2.1 Subjects
23 patients with schizophrenia and 22 healthy control subjects participated in this study. Diagnostic groups did not differ significantly in age or sex (Table 1). All participants provided informed consent as approved by the University of California, Los Angeles (UCLA) Institutional Review Board. Schizophrenia patients were recruited from the UCLA Aftercare Research Program through the UCLA Family Study protocol. Control subjects were recruited through direct calls using a survey sampling methodology as well as community outreach via advertisement in newspapers and fliers. Exclusion criteria for all subjects included mental retardation, neurological disorder (e.g., temporal lobe epilepsy), and recent or past history of significant and habitual drug abuse or alcoholism. Control subjects were screened by clinical interview using the SCID-NP (First et al., 1994) to exclude schizophrenia and schizoaffective disorder and potential schizophrenia spectrum disorders. A radiologist reviewed any suspected abnormalities in the high-resolution T1-weighted data. However, using this sequence only, it was not possible to rule out the presence of all possible brain abnormalities. No incidental brain lesions were observed in the current sample, and no subjects were excluded from analysis. Schizophrenia diagnosis was confirmed by consensus of specially trained diagnosticians (Ventura et al., 1998) as determined by DSM-IV criteria using the Structured Clinical Interview for DSM-IV (SCID-I/P) (First et al., 1994; First et al., 1996; First et al., 2001) and by informant information. Clinical symptoms were assessed using the expanded 24-item Brief Psychiatric Rating Scale (BPRS; (Ventura et al., 1993; Ventura et al., 2000) and clustered into withdrawal and thinking disorder scores (Burger et al., 1997). Withdrawal (negative symptoms factor) scores were calculated as the mean of BPRS scores for emotional withdrawal, motor retardation, blunted affect, disorientation, and self-neglect. Thinking disorder (positive symptoms factor) scores were calculated as the mean of BPRS scores for conceptual disorganization, grandiosity, hallucinatory behavior, unusual thought content, and bizarre behavior. Handedness was determined based on a modified version of the Edinburgh Inventory. (Oldfield, 1971)
Table 1.
Subject demographic Information.
| Schizophrenia | Controls | Statistic | P Value | |
|---|---|---|---|---|
| Age (mean ± SD) | 34.65±9.17 | 30.87±9.52 | t=-1.36 | 0.18 |
| Gender (male/female) | 17/6 | 17/5 | χ2 =0.07 | 0.79 |
| Handedness (dextral/non-dextral) | 23/0 | 21/1 | χ2 = 1.07 | 0.30 |
| Years of Education | 14.17±1.85 | 15.31±2.05 | t=1.96 | 0.06 |
| Duration of Illness | 10.14±7.94 | - | - | - |
| Age of Onset | 21.93±4.22 | - | - | - |
| Total Brain Volume (cc) | 1442.6 | 1431.4 | t=-0.29 | 0.77 |
| White Matter Volume (cc) | 566.5 | 556.3 | t=0.61 | 0.54 |
| Anergia (mean ± SD)* | 1.69±0.65 | - | - | - |
| Thought Disturbance (mean ± SD)* | 1.63+0.60 | - | - | - |
Missing data for 2 patients.
2.2. Image Acquisition
DTI data was acquired on a 1.5T Siemens Sonata scanner (Erlangen, Germany) at the UCLA Ahmanson-Lovelace Brain Mapping Center using an 8-channel head coil. The DTI acquisition protocol included 3 averages of a whole brain sequence with 30 non-collinear diffusion directions and 55 brain slices oriented obliquely to the anterior/posterior commissure (AC-PC) line (TR=6400 ms, TE=83 ms, b=0, 1000 sec/mm2, FOV: 240×240 mm, matrix: 96×96, voxel size: 2.5 mm3, acquisition time: 3.52 min per average). Parallel imaging used GRAPPA reconstruction with an acceleration factor of 2. High-resolution T1-weighted MPRAGE sequences were also collected (TR=1900 ms; TE=4.38 ms; flip angle: 15°, FOV: 256×256; voxel size: 1 mm3; NEX=4; TI=1100, acquisition time: 8.08 min per average). The DTI sequence was designed to have minimal eddy current induced distortions (Reese et al., 2003), whereas parallel imaging was employed to substantially reduce EPI distortions (Heidemann et al., 2003).
2.3. Image Processing
DTI data processing was performed using the LONI Pipeline environment (Rex et al., 2003). After image reconstruction, the diffusion gradient table was corrected for slice prescription. To correct for eddy current induced distortions, diffusion-weighted images were aligned to a non-diffusion-weighted image (b=0 sec/mm2) using a nonlinear 2D registration (Jezzard et al., 1998). Motion artifacts were corrected by applying a 3D rigid body registration (Woods et al., 1998a; Woods et al., 1998b), using the non-diffusion-weighted image as the target image. The transformation files from the two registrations were combined and only applied to the data once in order to minimize interpolation. The non-diffusion-weighted images were skull stripped using FSL's Brain Extraction Tool (BET) (http://www.fmrib.ox.ac.uk/fsl/bet2/index.html), and used to mask all diffusion-weighted images (Smith, 2002). The diffusion tensor was estimated at each voxel using a linear least squares algorithm applied to the log-transformed signal intensities. Each diffusion tensor was diagonalized to obtain the eigenvalues that were then used to compute fractional anisotropy (FA) (Pierpaoli and Basser, 1996).
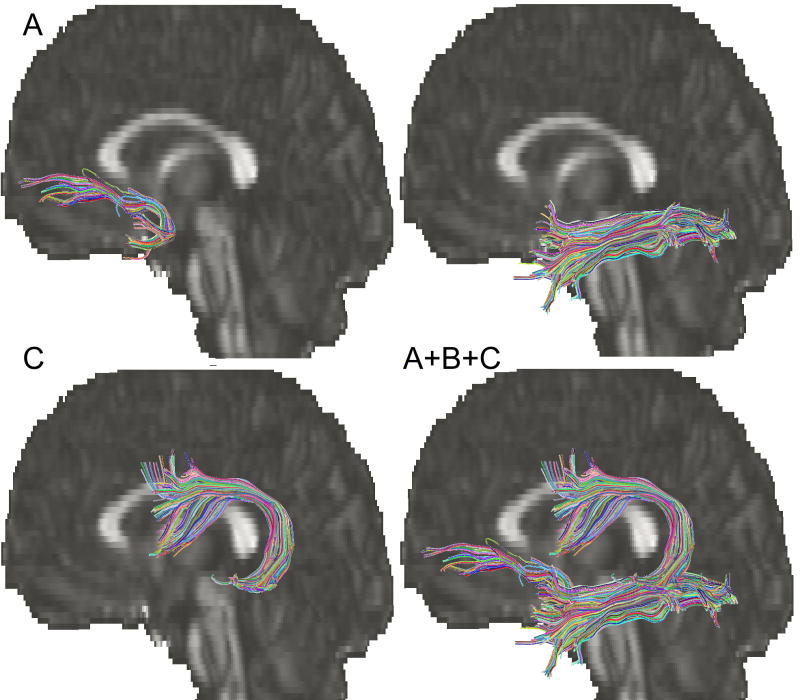
Three-dimensional tract reconstruction was performed using DTIstudio (Jiang et al., 2006). DTIstudio estimates fiber tracts using the Fiber Assignment by Continuous Tracking (FACT) algorithm, which has been shown to be a reliable method for identifying several neuroanatomically valid tracts (Mori et al., 1999; Xue et al., 1999; Mori and van Zijl, 2002; Wakana et al., 2004; Okada et al., 2006). In our study, an FA threshold of 0.2 and a turning angle threshold of 41° were used for each tract to restrict the algorithm to yield biologically plausible results (Wakana et al., 2007). ROIs were applied using the FA-weighted color maps for each subject and by following the protocols detailed in Wakana et al. (2007). Briefly, for the AF, the first ROI was identified by selecting the coronal slice at the middle of the posterior limb of the internal capsule where the AF is visible running anterior to posterior with a triangular shape. The second ROI was defined on the axial slice at the anterior commissure. For the ILF, a sagittal plane off midline was first identified at the level of the cingulum. The first ROI was selected at the posterior edge of the cingulum in the coronal plane encompassing the whole hemisphere. The second ROI included the entire temporal lobe in a coronal section towards the anterior pole of the temporal lobe. Finally, for the UF, the most posterior coronal slice in which the temporal lobe is separated from the frontal lobe was selected. The first ROI included the entire temporal lobe and the second ROI included the projections passing towards the frontal lobe in this plane (Wakana et al., 2007). Figure 1 illustrates the trajectories of each fiber pathway obtained using the ROIs described above. The locations of ROI placement for each tract are illustrated in supplementary Figures 1-3. If a tract showed a fiber that was clearly anatomically incorrect, the NOT function was used to remove the fiber from the bundle. Intra-rater reliability was estimated by identifying each of the tracts using ROI placement in 8 randomly chosen volumes duplicated in the dataset. To determine inter-rater reliability, fiber tracks were identified in 9 randomly chosen brain volumes by two independent raters. Excellent intra- and inter-rater reliability was achieved for ROI placement as determined by computing the inter-class correlation coefficients for tract volume, the volume for the number of voxels through which the tract passes, and mean FA, a value representing the average intra-voxel FA across the tract of interest in each subject (Table 2).
Figure 1.
Fiber tracts including the (a) arcuate fasciculus (AF), (b) inferior longitudinal fasciculus (ILF), and (c) uncinate fasciculus (UF) are shown for a representative control subject. Fiber pathways were obtained using multiple region of interest approach and tract statistics were collected across the whole tract. Colors represent individual fibers within the greater body of the tract.
Table 2.
Intra-class correlation coefficients (r) for ROI tracing. AF=arcuate fasciculus, ILF=inferior longitudinal fasciculus, UF=uncinate fasciculus.
| Intra-rater reliability | Inter-rater reliability | |||||||
|---|---|---|---|---|---|---|---|---|
| Tract | Mean FA | Volume | Mean FA | Volume | ||||
| Left | Right | Left | Right | Left | Right | Left | Right | |
| AF | 0.995 | 0.991 | 0.992 | 0.989 | 0.928 | 0.988 | 0.963 | 0.970 |
| ILF | 0.997 | 0.996 | 0.957 | 0.969 | 0.994 | 0.988 | 0.921 | 0.942 |
| UF | 0.998 | 0.994 | 0.942 | 0.962 | 0.978 | 0.986 | 0.993 | 0.971 |
Values in the table represent intraclass correlation coefficients (RI)
To obtain measures of intracranial brain volume and volumes of segmented brain tissue compartments, the T1-weighted MPRAGE images were processed as previously detailed (Narr et al., 2005b; Narr et al., 2005a). Briefly, image volumes were corrected for head tilt and alignment using six-parameter rigid-body transformations. To scalp edit the T1-weighted data, FSL's BET was used to generate a temporary mask that was then manually edited on a slice-by-slice basis to correct any small errors using MNI Display (http://www.bic.mni.mcgill.ca/software/Display/Display.html), (scalp editing inter-rater reliability, rI= .99). Edited brain volumes were then corrected for signal intensity inhomogeneities (Sled et al., 1998), and segmented into gray matter, white matter, and CSF using a partial volume classification method (Shattuck et al., 2001) after which whole brain and brain tissue volumes were computed.
2.4. Statistical Analysis
Mean FA and tract volume (the number of voxels the tract traverses multiplied by the 2.5 mm3 voxel size) were estimated for each fiber tract within subjects. A repeated measures analysis of variance (ANOVA) examined group differences in left and right mean FA, which were included as dependent variables in order to test fewer hypotheses and avoid potential Type 1 error while simultaneously assessing the presence of hemisphere effects for the AF, ILF, and UF. Diagnosis and sex were included as between-subject factors. To reduce potential bias due to less precise estimates in subjects contributing fewer voxels, mean FA measures were corrected for overall tract volume. That is, since hemisphere was a within-subject variable, left and right mean FA values were residualized for left and right tract volume respectively. Repeated measures ANOVAs were followed-up by univariate tests when appropriate.
As noted above, we controlled for tract volume when examining changes in mean FA between groups. However, to determine whether this measure itself was different across groups, the same statistical model was used to examine diagnostic group differences in fiber tract volume. For these comparisons, total brain white matter volume was included as a covariate to control bias in the overall number of white matter voxels expected in individuals with larger brain tissue volumes. Finally, correlations between mean FA and positive and negative symptom cluster scores were examined. Two patients were excluded from these analyses because BPRS data was unavailable. Demographic differences were assessed using chi-square or independent sample t-tests as appropriate.
3. Results
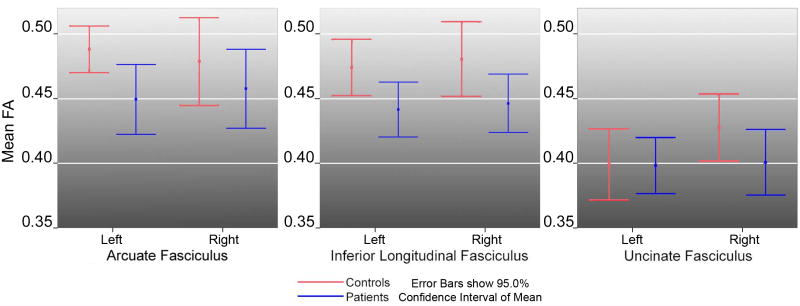
Patient and control groups did not significantly differ in age or gender distribution or in total brain or white matter volume (Table 1). The repeated measures ANOVA showed significantly lower FA values in patients compared to controls in the ILF [F(1,41)=6.55, p=0.014] and AF [F(1, 41)=4.78, p=0.035], but not in the UF, [F(1,41)=0.09, p=0.758]. There were no sex or asymmetry effects or interactions between diagnosis and sex or hemisphere. Follow-up univariate tests revealed significant effects of diagnosis for the Left ILF [F(1,44)=4.84, p=0.03] and Right ILF [F(1,44)=6.06, p=0.018] and the Left AF [F(1,45)=5.01, p=0.03]. Table 3 lists means and standard deviations (SDs) for mean FA values, and Figure 2 shows the 95% confidence intervals of the means within diagnostic groups. Follow-up tests performed after excluding the one left-handed control subject from the analysis produced similarly significant results.
Table 3.
Tract measurements for the arcuate fasciculus (AF), inferior longitudinal fasciculus (ILF), and uncinate fasciculus (UF).
| FA (Mean ± SD) | Volume (Mean ± SD), (cc) | |||
|---|---|---|---|---|
| Fiber Tract Measures | Controls | Patients | Controls | Patients |
| AF Left | 0.48±0.02* | 0.45±0.03* | 5.58±2.99* | 4.53±2.45* |
| AF Right | 0.48±0.04 | 0.46±0.03 | 4.32±2.90 | 3.24±2.15 |
| ILF Left | 0.47±0.02* | 0.45±0.02* | 10.67±4.07 | 9.76±3.02 |
| ILF Right | 0.47±0.02* | 0.45±0.02* | 11.34±3.13 | 10.39±3.55 |
| UF Left | 0.40±0.03 | 0.39±0.02 | 3.34±1.88 | 2.52±1.71 |
| UF Right | 0.42±0.03 | 0.40±0.04 | 2.66±1.2 | 3.11±2.13 |
Significant effects
Figure 2.
Means and the 95% confidence intervals for mean FA measures obtained from each of the three fiber tracts in patients with schizophrenia (blue) and control subjects (red).
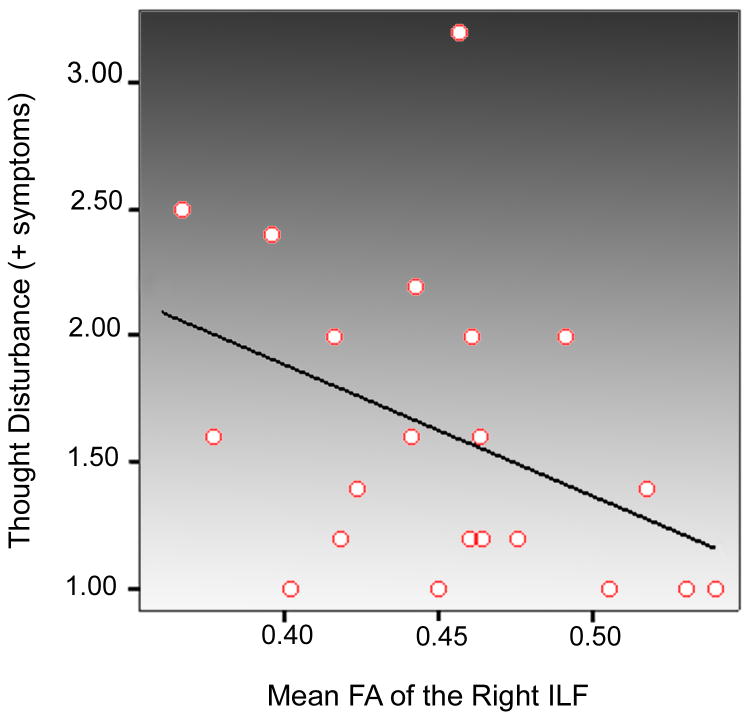
Analyses of fiber volume showed a significant reduction in left AF volume only [F(1,45)=7.725, p=0.008]. Means and SDs for AF, ILF, and UF volume are provided in Table 3. Spearman's correlation analyses between mean FA and positive (thinking disorder) and negative symptom (withdrawal) cluster scores revealed a significant negative association (r=-0.439) between thinking disorder (positive symptoms) and mean FA within the right ILF (p=0.046) (Figure 3).
Figure 3.
Correlation between right ILF mean FA and thinking disorder scores in schizophrenia patients.
4. Discussion
Using a validated ROI approach for identifying major fiber tracts (Wakana et al., 2007), we were able to examine highly localized changes of white matter integrity in brain systems widely implicated in the pathophysiology of schizophrenia. Specifically, we examined three major white matter tracts that form integral connections between lateral and medial temporal lobe regions with neocortical association areas. Results revealed significant reductions of mean FA within the ILF bilaterally and the left AF in schizophrenia patients compared to healthy controls. A significant negative association between thinking disorder and mean FA of the right ILF was further observed. Although the neurobiological mechanisms underlying altered FA are not known, prior human and animal studies of neural development and of related disturbances in brain function support that an increase in FA is related to an increase in the connectivity of white matter bundles (Dong et al., 2004). Our results are consistent with several previous diffusion imaging findings despite differences in patient populations (e.g. early onset, first episode, and chronic schizophrenia) and analysis approaches. However, our findings provide more detailed information concerning the localization of fiber tract abnormalities in the disorder that may be of particular functional relevance.
4.1. Inferior Longitudinal Fasciculus
Our observations of significantly reduced mean FA in patients in bilateral ILF support prior findings in adolescent and never-medicated first-episode schizophrenia patients (Ashtari et al., 2007b; Cheung et al., 2007) identified using tractography and voxel-based methods respectively. Our findings are also consistent with those of (Friedman et al., 2008) showing reduced FA in the left ILF in chronic patients and trend level FA reductions in first episode schizophrenia. Although the functions of the ILF are not fully characterized, this tract appears to mediate the fast transfer of visual signals to anterior temporal regions and neuromodulatory back-projections from the amygdala to early visual areas (Catani et al., 2003). The ILF, which is the major occipital-temporal white matter connection, is suggested to play a role in visual memory and in processing the emotional valence of a visual stimulus (Catani et al., 2003). Disturbances in the structural integrity of this tract may thus relate to observations that schizophrenia patients are impaired in their ability to recognize and respond appropriately to emotional facial expressions (Mueser et al., 1996; Penn et al., 1996; Brune, 2005) and contribute to developmental differences in social cognition as well as visual-spatial integration (Barnea-Goraly et al., 2005). Our finding of an association between mean FA in the right ILF and thinking disorder (positive symptoms) cluster scores, which includes a visual hallucination rating, is partially consistent with a report of a negative association between mean FA of the left ILF in early onset patients with a history of visual hallucinations (Ashtari et al., 2007a), although individual BPRS factor scores were not examined in our study.
4.2. Arcuate Fasciculus
Some diffusion studies have reported reduced FA in the vicinity of the left AF in adolescent onset (Douaud et al., 2007) and in adult schizophrenia patients (Burns et al., 2003; Kubicki et al., 2005; Douaud et al., 2007), consistent with our results. At least two prior studies using voxel-based methods further report positive associations between FA values and the symptom of auditory hallucinations, particularly in the left AF (Hubl et al., 2004; Shergill et al., 2007). Specifically, one study reported significantly greater FA values compared to controls in patients with current and past history of hallucinations (Hubl et al., 2004). However, a second study observing positive associations between FA and hallucination ratings still showed lower FA values in patients with a propensity for current or past hallucinations compared to healthy subjects (Shergill et al., 2007). A third study failed to observe AF associations, although positive relationships between the severity of auditory and tactile hallucinations and the inferior fronto-occipital fasciculus FA were detected (Szeszko et al., 2008). Finally, Seok et al., (2007) reported positive associations between auditory hallucinations and FA values in the frontal as opposed to the temporal component of the SLF. Unfortunately, we were not able clarify these relationships in the current investigation, since patients were not acutely ill at the time of scan, and symptom scores for auditory hallucinations were low and narrow in range. Regardless, our results suggest that reduced FA within the AF persist during periods of relative symptom stability. Our investigation also revealed a reduction in the overall size of the left AF in patients compared to controls. This finding may suggest that the length of AF fibers are less extensive in patients or indicate tract shape differences. Although tractography is sensitive to anatomical variations in fiber pathways (Wakana et al., 2007), it is possible that the extent of this fiber tract was not fully resolved with our choice of ROIs, which were devised based on the fiber anatomy of healthy individuals (Wakana et al., 2007).
4.3. Uncinate Fasciculus
The UF connects parahippocampal and prefrontal regions and is involved in declarative memory processing, for which patients exhibit pronounced deficits (Bilder et al., 2000). At least one investigation supports relationships between UF FA and memory functioning in schizophrenia specifically (Nestor et al., 2008). In this study, however, we failed to detect significant differences of mean UF FA in schizophrenia patients compared to controls. These results contrast with one prior tractography study (Price et al., 2008) and with some voxel-based investigations. Specifically, reduced FA of the UF has been documented in chronic schizophrenia (Seal et al., 2008) and in first episode patients bilaterally (Szeszko et al., 2008) and in the left UF exclusively (Szeszko et al., 2005), and at trend-level in a cluster at the border of the UF and the ILF (Burns et al., 2003). Still, several studies, like the present investigation, have failed to detect significant diagnostic group differences of FA within the UF (Jones et al., 2006). Some studies, however, have revealed altered FA asymmetries in schizophrenia (Kubicki et al., 2002; Park et al., 2004), although hemisphere by diagnosis effects in UF cross-sectional area and fiber numbers were not detected in post mortem data (Highley et al., 2002). In the absence of diagnostic group effects, another group reported a trend level age by group interaction for FA within the UF (Rosenberger et al., 2008). Inconsistencies in findings may be partially attributable to clinical heterogeneity amongst patient samples and to methodological differences, where it is worth noting that reported cluster coordinates locations are highly variable between voxel-based studies. Measurements for the UF may also be complicated by the tightly curved shape and smaller trajectory of this fiber bundle in relation to other larger tracts and/or could indicate that disturbances of UF connectivity in schizophrenia are subtler than those of other tracts.
4.4. Potential Limitations
A possible limitation to the study is the influence of patient medications on white matter connectivity and/or myelination. A positive association between FA and antipsychotic treatment has been observed in at least one prior study (Kuroki et al., 2006), although FA changes are also reported in never-medicated patients (Cheung et al., 2007). Whether other factors including intelligence, social economic status and past history of drug and alcohol use, which is shown to affect FA in non-schizophrenia samples e.g., (Pfefferbaum et al., 2006), influence fiber integrity warrants further study. However, some these factors may be more difficult to dissociate from other disease-related characteristics. In spite of excellent reliability for ROI placements, it is possible that the anatomic definitions imposed for ROI placement may have influenced results. Still, our method, which focused on well documented tracts from previous studies using anatomical constraints (multiple ROIs), made it possible to isolate and examine tracts based on the individual's morphology that do not appear influenced by ROI placement. Furthermore, this method does not require perfect anatomical registration across subjects and is thus not sensitive to registration-related confounds that continue to be an issue for the analysis of DTI data. Finally, it is possible that other diffusion measures such as radial, axial and mean diffusivity may reveal subtler cytoarchitectonic disturbances in schizophrenia. Future studies including larger sample sizes may address broader hypotheses relating to these diffusion measures.
4.5. Conclusion
Our observations of reduced mean FA in the AF and ILF support the hypothesis that abnormal white matter connectivity influences systems-level processing deficits involving fronto-limbic and temporo-neocortical networks in schizophrenia. Abnormal connectivity of the AF may contribute to language and auditory deficits in patients. Disturbances of ILF integrity may contribute to visual memory impairments and to deficits in social cognition. Furthermore, IFL connectivity may be influenced by clinical state as suggested by our observations of negative associations with positive symptom ratings. It remains unclear whether white matter abnormalities serve as primary or complicating mechanisms for impairments in specific brain systems in schizophrenia or whether these abnormalities are secondary to changes in brain morphology, particularly of gray matter. Future studies may clarify these questions and address whether FA changes are the result of disturbed white matter developmental trajectories.
Supplementary Material
Acknowledgments
Role of Funding Source: This work was supported by grants from the National Center for Research Resources (P41 RR13642), the National Institute of Mental Health (RO1 MH60374), the NIH Roadmap Initiative (P20 RR020750), the National Library of Medicine (R01 LM05639), the NIH Roadmap for Medical Research, Grant U54 RR021813 entitled Center for Computational Biology (CCB), and a Career Development Award (KO1 MH073990, to KLN). The NIMH grants MH066286 and MH037705 allowed recruitment of the subjects and MH049716 supported the MRIs. This study utilized the LONI Pipeline environment (http://pipeline.loni.ucla.edu), which was developed by the Laboratory of Neuro Imaging and partially funded by NIH grants P41 RR013642, R01 MH71940 and U54 RR021813. The above funding agencies had no further contributions to the study design, data collect, analysis, manuscript preparation, and in the decision to submit the paper for publication.
Footnotes
Conflict of Interest: None of the authors have any conflicts of interest to report.
Contributors: Owen R. Phillips was responsible for data acquisition and analysis and manuscript preparation. Keith H. Nuechterlein and Robert F. Asarnow provided the clinical expertise for this study. Liberty S. Hamilton assisted in preparation of the manuscript and data acquisition. Kristi A. Clark was responsible for software development and expertise regarding registration algorithms. Nathan S. Hageman provided expertise regarding diffusion measurements. Arthur W. Toga and Katherine L. Narr contributed to the conception and overall design of the study. Substantial scientific contributions were made by all authors of this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander DC, Pierpaoli C, Basser PJ, Gee JC. Spatial transformations of diffusion tensor magnetic resonance images. IEEE Trans Med Imaging. 2001;20:1131–1139. doi: 10.1109/42.963816. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Schizophrenia: the fundamental questions. Brain Res Brain Res Rev. 2000;31:106–112. doi: 10.1016/s0165-0173(99)00027-2. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Cottone J, Ardekani BA, Cervellione K, Szeszko PR, Wu J, Chen S, Kumra S. Disruption of white matter integrity in the inferior longitudinal fasciculus in adolescents with schizophrenia as revealed by fiber tractography. Arch Gen Psychiatry. 2007a;64:1270–1280. doi: 10.1001/archpsyc.64.11.1270. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Cervellione KL, Hasan KM, Wu J, McIlree C, Kester H, Ardekani BA, Roofeh D, Szeszko PR, Kumra S. White matter development during late adolescence in healthy males: a cross-sectional diffusion tensor imaging study. Neuroimage. 2007b;35:501–510. doi: 10.1016/j.neuroimage.2006.10.047. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Bauer RM, Trobe JD. Visual memory and perceptual impairments in prosopagnosia. J Clin Neuroophthalmol. 1984;4:39–46. doi: 10.3109/01658108409019494. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. Amygdalo-entorhinal inputs to the hippocampal formation in relation to schizophrenia. Ann N Y Acad Sci. 2000;911:293–304. doi: 10.1111/j.1749-6632.2000.tb06733.x. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, Pappadopulos E, Willson DF, Alvir JM, Woerner MG, Geisler S, Kane JM, Lieberman JA. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry. 2000;157:549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- Brune M. Emotion recognition, ‘theory of mind,’ and social behavior in schizophrenia. Psychiatry Res. 2005;133:135–147. doi: 10.1016/j.psychres.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Burger GK, Calsyn RJ, Morse GA, Klinkenberg WD, Trusty ML. Factor structure of the expanded Brief Psychiatric Rating Scale. J Clin Psychol. 1997;53:451–454. doi: 10.1002/(sici)1097-4679(199708)53:5<451::aid-jclp5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Burns J, Job D, Bastin ME, Whalley H, Macgillivray T, Johnstone EC, Lawrie SM. Structural disconnectivity in schizophrenia: a diffusion tensor magnetic resonance imaging study. Br J Psychiatry. 2003;182:439–443. [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002a;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Catani M, Mecocci P, Tarducci R, Howard R, Pelliccioli GP, Mariani E, Metastasio A, Benedetti C, Senin U, Cherubini A. Proton magnetic resonance spectroscopy reveals similar white matter biochemical changes in patients with chronic hypertension and early Alzheimer's disease. J Am Geriatr Soc. 2002b;50:1707–1710. doi: 10.1046/j.1532-5415.2002.50465.x. [DOI] [PubMed] [Google Scholar]
- Cheung V, Cheung C, McAlonan GM, Deng Y, Wong JG, Yip L, Tai KS, Khong PL, Sham P, Chua SE. A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychol Med. 2007:1–9. doi: 10.1017/S0033291707001808. [DOI] [PubMed] [Google Scholar]
- Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, McKinstry RC, Burton H, Raichle ME. Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci U S A. 1999;96:10422–10427. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi LE. Speech disorder in schizophrenia: review of the literature and exploration of its relation to the uniquely human capacity for language. Schizophr Bull. 2001;27:481–496. doi: 10.1093/oxfordjournals.schbul.a006889. [DOI] [PubMed] [Google Scholar]
- Dong Q, Welsh RC, Chenevert TL, Carlos RC, Maly-Sundgren P, Gomez-Hassan DM, Mukherji SK. Clinical applications of diffusion tensor imaging. J Magn Reson Imaging. 2004;19:6–18. doi: 10.1002/jmri.10424. [DOI] [PubMed] [Google Scholar]
- Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, James S, Voets N, Watkins K, Matthews PM, James A. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130:2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- Ebeling U, von Cramon D. Topography of the uncinate fascicle and adjacent temporal fiber tracts. Acta Neurochir (Wien) 1992;115:143–148. doi: 10.1007/BF01406373. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P) New York: Biometrics Research Department, New York State Psychiatric Institute; 2001. [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Patient. New York: Biometrics Research; 1996. [Google Scholar]
- First MB, Frances AJ, Pincus HA, Vettorello N, Davis WW. DSM-IV in progress. Changes in substance-related, schizophrenic, and other primarily adult disorders. Hosp Community Psychiatry. 1994;45:18–20. doi: 10.1176/ps.45.1.18. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Tang C, Carpenter D, Buchsbaum M, Schmeidler J, Flanagan L, Golembo S, Kanellopoulou I, Ng J, Hof PR, Harvey PD, Tsopelas ND, Stewart D, Davis KL. Diffusion tensor imaging findings in first-episode and chronic schizophrenia patients. Am J Psychiatry. 2008;165:1024–1032. doi: 10.1176/appi.ajp.2008.07101640. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston KJ, Herold S, Silbersweig D, Fletcher P, Cahill C, Dolan RJ, Frackowiak RS, Liddle PF. Regional brain activity in chronic schizophrenic patients during the performance of a verbal fluency task. Br J Psychiatry. 1995;167:343–349. doi: 10.1192/bjp.167.3.343. [DOI] [PubMed] [Google Scholar]
- Goldman AL, Pezawas L, Mattay VS, Fischl B, Verchinski BA, Zoltick B, Weinberger DR, Meyer-Lindenberg A. Heritability of brain morphology related to schizophrenia: a large-scale automated magnetic resonance imaging segmentation study. Biol Psychiatry. 2008;63:475–483. doi: 10.1016/j.biopsych.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Green MF, Olivier B, Crawley JN, Penn DL, Silverstein S. Social cognition in schizophrenia: recommendations from the measurement and treatment research to improve cognition in schizophrenia new approaches conference. Schizophr Bull. 2005;31:882–887. doi: 10.1093/schbul/sbi049. [DOI] [PubMed] [Google Scholar]
- Harrison CL, Fowler D. Negative symptoms, trauma, and autobiographical memory: an investigation of individuals recovering from psychosis. J Nerv Ment Dis. 2004;192:745–753. doi: 10.1097/01.nmd.0000144693.12282.11. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- Heidemann RM, Ozsarlak O, Parizel PM, Michiels J, Kiefer B, Jellus V, Muller M, Breuer F, Blaimer M, Griswold MA, Jakob PM. A brief review of parallel magnetic resonance imaging. Eur Radiol. 2003;13:2323–2337. doi: 10.1007/s00330-003-1992-7. [DOI] [PubMed] [Google Scholar]
- Highley JR, Walker MA, Esiri MM, Crow TJ, Harrison PJ. Asymmetry of the uncinate fasciculus: a post-mortem study of normal subjects and patients with schizophrenia. Cereb Cortex. 2002;12:1218–1224. doi: 10.1093/cercor/12.11.1218. [DOI] [PubMed] [Google Scholar]
- Honea RA, Meyer-Lindenberg A, Hobbs KB, Pezawas L, Mattay VS, Egan MF, Verchinski B, Passingham RE, Weinberger DR, Callicott JH. Is gray matter volume an intermediate phenotype for schizophrenia? A voxel-based morphometry study of patients with schizophrenia and their healthy siblings. Biol Psychiatry. 2008;63:465–474. doi: 10.1016/j.biopsych.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubl D, Koenig T, Strik W, Federspiel A, Kreis R, Boesch C, Maier SE, Schroth G, Lovblad K, Dierks T. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. 2004;61:658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- Jellison BJ, Field AS, Medow J, Lazar M, Salamat MS, Alexander AL. Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. AJNR Am J Neuroradiol. 2004;25:356–369. [PMC free article] [PubMed] [Google Scholar]
- Jezzard P, Barnett AS, Pierpaoli C. Characterization of and correction for eddy current artifacts in echo planar diffusion imaging. Magn Reson Med. 1998;39:801–812. doi: 10.1002/mrm.1910390518. [DOI] [PubMed] [Google Scholar]
- Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81:106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Jones DK, Catani M, Pierpaoli C, Reeves SJ, Shergill SS, O'sullivan M, Golesworthy P, McGuire P, Horsfield MA, Simmons A, Williams SC, Howard RJ. Age effects on diffusion tensor magnetic resonance imaging tractography measures of frontal cortex connections in schizophrenia. Hum Brain Mapp. 2006;27:230–238. doi: 10.1002/hbm.20179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, Jolesz FA, Shenton ME. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am J Psychiatry. 2002;159:813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Park H, Westin CF, Nestor PG, Mulkern RV, Maier SE, Niznikiewicz M, Connor EE, Levitt JJ, Frumin M, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage. 2005;26:1109–1118. doi: 10.1016/j.neuroimage.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki N, Kubicki M, Nestor PG, Salisbury DF, Park HJ, Levitt JJ, Woolston S, Frumin M, Niznikiewicz M, Westin CF, Maier SE, McCarley RW, Shenton ME. Fornix integrity and hippocampal volume in male schizophrenic patients. Biol Psychiatry. 2006;60:22–31. doi: 10.1016/j.biopsych.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen L, Hyvarinen J, Sovijarvi AR. Functional properties of neurons in the temporo-parietal association cortex of awake monkey. Exp Brain Res. 1980;39:203–215. doi: 10.1007/BF00237551. [DOI] [PubMed] [Google Scholar]
- Levine B, Black SE, Cabeza R, Sinden M, McIntosh AR, Toth JP, Tulving E, Stuss DT. Episodic memory and the self in a case of isolated retrograde amnesia. Brain. 1998;121(Pt 10):1951–1973. doi: 10.1093/brain/121.10.1951. [DOI] [PubMed] [Google Scholar]
- Liddle PF. Functional imaging--schizophrenia. Br Med Bull. 1996;52:486–494. doi: 10.1093/oxfordjournals.bmb.a011562. [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr, Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Mori S, van Zijl PC. Fiber tracking: principles and strategies - a technical review. NMR Biomed. 2002;15:468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Mueser KT, Doonan R, Penn DL, Blanchard JJ, Bellack AS, Nishith P, DeLeon J. Emotion recognition and social competence in chronic schizophrenia. J Abnorm Psychol. 1996;105:271–275. doi: 10.1037//0021-843x.105.2.271. [DOI] [PubMed] [Google Scholar]
- Narr KL, Toga AW, Szeszko P, Thompson PM, Woods RP, Robinson D, Sevy S, Wang Y, Schrock K, Bilder RM. Cortical thinning in cingulate and occipital cortices in first episode schizophrenia. Biol Psychiatry. 2005a;58:32–40. doi: 10.1016/j.biopsych.2005.03.043. [DOI] [PubMed] [Google Scholar]
- Narr KL, Thompson PM, Sharma T, Moussai J, Blanton R, Anvar B, Edris A, Krupp R, Rayman J, Khaledy M, Toga AW. Three-dimensional mapping of temporo-limbic regions and the lateral ventricles in schizophrenia: gender effects. Biol Psychiatry. 2001;50:84–97. doi: 10.1016/s0006-3223(00)01120-3. [DOI] [PubMed] [Google Scholar]
- Narr KL, Thompson PM, Szeszko P, Robinson D, Jang S, Woods RP, Kim S, Hayashi KM, Asunction D, Toga AW, Bilder RM. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage. 2004;21:1563–1575. doi: 10.1016/j.neuroimage.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Toga AW, Woods RP, Rex DE, Szeszko PR, Robinson D, Sevy S, Gunduz-Bruce H, Wang YP, DeLuca H, Thompson PM. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex. 2005b;15:708–719. doi: 10.1093/cercor/bhh172. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Niznikiewicz M, Gurrera RJ, McCarley RW, Shenton ME. Neuropsychological disturbance in schizophrenia: a diffusion tensor imaging study. Neuropsychology. 2008;22:246–254. doi: 10.1037/0894-4105.22.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Miki Y, Fushimi Y, Hanakawa T, Kanagaki M, Yamamoto A, Urayama S, Fukuyama H, Hiraoka M, Togashi K. Diffusion-tensor fiber tractography: intraindividual comparison of 3.0-T and 1.5-T MR imaging. Radiology. 2006;238:668–678. doi: 10.1148/radiol.2382042192. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Park HJ, Westin CF, Kubicki M, Maier SE, Niznikiewicz M, Baer A, Frumin M, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. White matter hemisphere asymmetries in healthy subjects and in schizophrenia: a diffusion tensor MRI study. Neuroimage. 2004;23:213–223. doi: 10.1016/j.neuroimage.2004.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn DL, Spaulding W, Reed D, Sullivan M. The relationship of social cognition to ward behavior in chronic schizophrenia. Schizophr Res. 1996;20:327–335. doi: 10.1016/0920-9964(96)00010-2. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol. 1984;228:105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- Petrides M, P D. Association pathways of the prefrontal cortex and functional observations. In: Struss DT, Knight RT, editors. Principles of frontal lobe functions. 2002. pp. 31–50. [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Supratentorial profile of white matter microstructural integrity in recovering alcoholic men and women. Biol Psychiatry. 2006;59:364–372. doi: 10.1016/j.biopsych.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Price G, Cercignani M, Parker GJ, Altmann DR, Barnes TR, Barker GJ, Joyce EM, Ron MA. White matter tracts in first-episode psychosis: A DTI tractography study of the uncinate fasciculus. Neuroimage. 2008;39:949–955. doi: 10.1016/j.neuroimage.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowicz EF, Silipo G, Goldman R, Javitt DC. Auditory sensory dysfunction in schizophrenia: imprecision or distractibility? Arch Gen Psychiatry. 2000;57:1149–1155. doi: 10.1001/archpsyc.57.12.1149. [DOI] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Rex DE, Ma JQ, Toga AW. The LONI Pipeline Processing Environment. Neuroimage. 2003;19:1033–1048. doi: 10.1016/s1053-8119(03)00185-x. [DOI] [PubMed] [Google Scholar]
- Rosenberger G, Kubicki M, Nestor PG, Connor E, Bushell GB, Markant D, Niznikiewicz M, Westin CF, Kikinis R, A JS, McCarley RW, Shenton ME. Age-related deficits in fronto-temporal connections in schizophrenia: a diffusion tensor imaging study. Schizophr Res. 2008;102:181–188. doi: 10.1016/j.schres.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994;51:124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- Seal ML, Yucel M, Fornito A, Wood SJ, Harrison BJ, Walterfang M, Pell GS, Pantelis C. Abnormal white matter microstructure in schizophrenia: a voxelwise analysis of axial and radial diffusivity. Schizophr Res. 2008;101:106–110. doi: 10.1016/j.schres.2007.12.489. [DOI] [PubMed] [Google Scholar]
- Seok JH, Park HJ, Chun JW, Lee SK, Cho HS, Kwon JS, Kim JJ. White matter abnormalities associated with auditory hallucinations in schizophrenia: a combined study of voxel-based analyses of diffusion tensor imaging and structural magnetic resonance imaging. Psychiatry Res. 2007;156:93–104. doi: 10.1016/j.pscychresns.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13:856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shergill SS, Kanaan RA, Chitnis XA, O'Daly O, Jones DK, Frangou S, Williams SC, Howard RJ, Barker GJ, Murray RM, McGuire P. A diffusion tensor imaging study of fasciculi in schizophrenia. Am J Psychiatry. 2007;164:467–473. doi: 10.1176/ajp.2007.164.3.467. [DOI] [PubMed] [Google Scholar]
- Shinoura N, Suzuki Y, Tsukada M, Katsuki S, Yamada R, Tabei Y, Saito K, Yagi K. Impairment of inferior longitudinal fasciculus plays a role in visual memory disturbance. Neurocase. 2007;13:127–130. doi: 10.1080/13554790701399254. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Surguladze S, Russell T, Kucharska-Pietura K, Travis MJ, Giampietro V, David AS, Phillips ML. A reversal of the normal pattern of parahippocampal response to neutral and fearful faces is associated with reality distortion in schizophrenia. Biol Psychiatry. 2006;60:423–431. doi: 10.1016/j.biopsych.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Ardekani BA, Ashtari M, Kumra S, Robinson DG, Sevy S, Gunduz-Bruce H, Malhotra AK, Kane JM, Bilder RM, Lim KO. White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. Am J Psychiatry. 2005;162:602–605. doi: 10.1176/appi.ajp.162.3.602. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robinson DG, Ashtari M, Vogel J, Betensky J, Sevy S, Ardekani BA, Lencz T, Malhotra AK, McCormack J, Miller R, Lim KO, Gunduz-Bruce H, Kane JM, Bilder RM. Clinical and Neuropsychological Correlates of White Matter Abnormalities in Recent Onset Schizophrenia. Neuropsychopharmacology. 2008;33:976–984. doi: 10.1038/sj.npp.1301480. [DOI] [PubMed] [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV(SCID-I/P) Psychiatry Research. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Ventura J, Nuechterlein KH, Subotnik KL, Gutkind D, Gilbert EA. Symptom dimensions in recent-onset schizophrenia and mania: a principal components analysis of the 24-item Brief Psychiatric Rating Scale. Psychiatry Res. 2000;97:129–135. doi: 10.1016/s0165-1781(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Ventura J, Lukoff D, Nuechterlein KH, Liberman RP, Green MF, Shaner A. Brief Psychiatric Rating Scale (BPRS) expanded version: Scales, anchor points, and administration manual. International Journal of Methods in Psychiatric Research. 1993;3:227–243. [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van Zijl P, Mori S. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walterfang M, Wood SJ, Velakoulis D, Copolov D, Pantelis C. Diseases of white matter and schizophrenia-like psychosis. Aust N Z J Psychiatry. 2005;39:746–756. doi: 10.1080/j.1440-1614.2005.01678.x. [DOI] [PubMed] [Google Scholar]
- Whitford TJ, Grieve SM, Farrow TF, Gomes L, Brennan J, Harris AW, Gordon E, Williams LM. Progressive grey matter atrophy over the first 2-3 years of illness in first-episode schizophrenia: a tensor-based morphometry study. Neuroimage. 2006;32:511–519. doi: 10.1016/j.neuroimage.2006.03.041. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr. 1998a;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. J Comput Assist Tomogr. 1998b;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- Xue R, van Zijl PC, Crain BJ, Solaiyappan M, Mori S. In vivo three-dimensional reconstruction of rat brain axonal projections by diffusion tensor imaging. Magn Reson Med. 1999;42:1123–1127. doi: 10.1002/(sici)1522-2594(199912)42:6<1123::aid-mrm17>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Yamada M, Hirao K, Namiki C, Hanakawa T, Fukuyama H, Hayashi T, Murai T. Social cognition and frontal lobe pathology in schizophrenia: a voxel-based morphometric study. Neuroimage. 2007;35:292–298. doi: 10.1016/j.neuroimage.2006.10.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.