Abstract
In spite of the tremendous advances in modern spectroscopic methods, organic synthesis continues to play a pivotal role in elucidating the full structures of complex natural products. This method has the advantage that even in the absence of a firm structural assignment, a combination of logic and spectroscopic comparison can arrive at the correct structure. Herein, we report execution of this strategy with respect to ushikulide A, a newly isolated and previously stereochemically-undefined member of the oligomycin-rutamycin family. To maximize synthetic efficiency, we envisioned chemoselective manipulation of orthogonally reactive functional groups, notably alkenes and alkynes as surrogates for certain carbonyl and hydroxyl functionalities. This approach has the dual effect of minimizing the number of steps and protecting groups required for our synthetic route. This strategy culminated in the efficient synthesis and stereochemical assignment of ushikulide A.
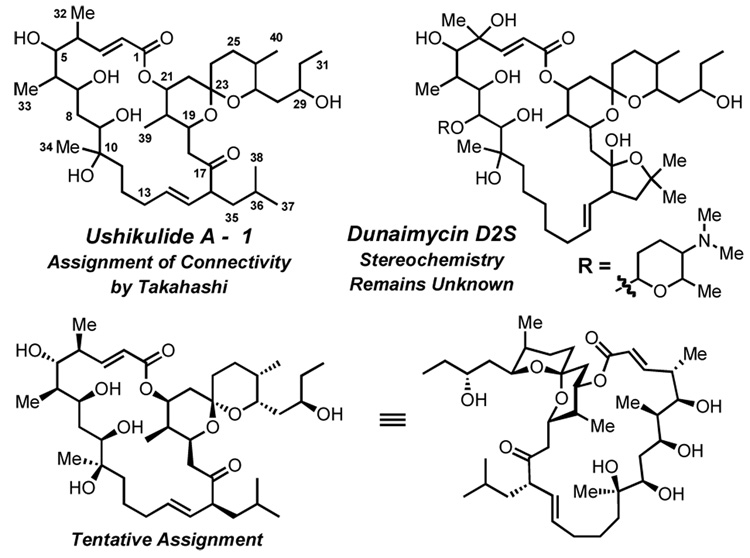
In 2005, Takahashi and co-workers reported the isolation of ushikulide A (1) from a culture broth of Streptomyces sp. IUK-102 (Figure 1).1 This compound exhibits potent activity against murine splenic lymphocyte proliferation (IC50 = 70 nM), rivaling that of the well-known cyclosporin A2 and FK506,3 which revolutionized organ transplant therapy by suppressing rejection. Takahashi’s structural assignment dealt with connectivity, not stereochemistry, as analysis of 1 by X-ray diffraction proved untenable in this particular case.4 Thus the complete structure remains unknown, along with several other members of this family.5,6 Interestingly, while some family members have potent antibiotic activity (cytovaricin), others exhibit immunosuppressant activity (ushikulide A, dunaimycin D2S). The exact cause of this divergent activity and in some cases the full structure of the bioactive natural product (e.g. dunaimycin D2S, Figure 1) remains unknown. Therefore, development of an effective and versatile synthetic strategy to such compounds would have the dual objective of establishing the full 3-dimensional structures of these natural products, while also providing a mechanism to explore the relationship of structure and function. To embark upon an ushikulide A synthesis, we made a tentative assignment based on analogy to cytovaricin,6 a structurally similar compound whose full structure is well established.
Figure 1. Tentative Assignment of Ushikulide A.
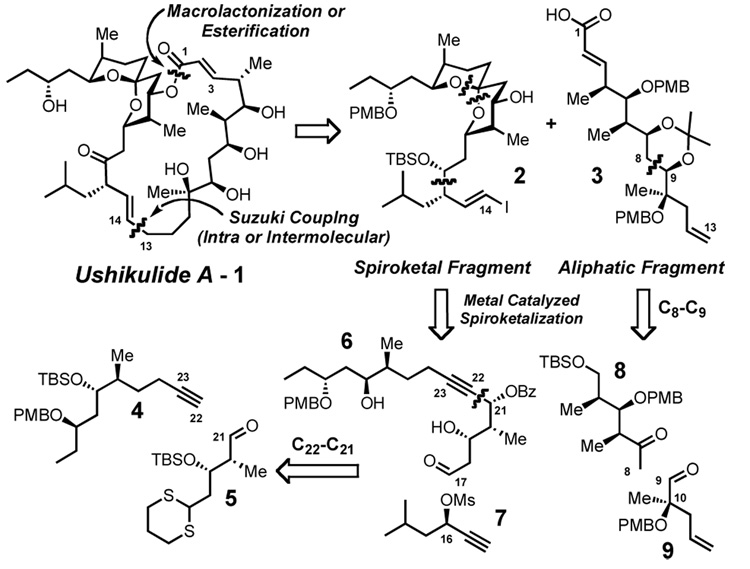
While sp2-sp2 Suzuki coupling and esterification are well-established in several related natural product syntheses,7,8 we envisioned a less common sp3-sp2 Suzuki coupling9 and esterification to be an attractive alternative to access the C-14, C-15 olefin with geometric control (Figure 1), thereby simplifying the synthetic problem to preparation of a spiroketal fragment (2) and an aliphatic fragment (3). By utilizing the reactivity of a carboxyl group in the former step, and then the reactivities of two C-C π-systems, we sequentially utilize more common carbonyl and hydroxyl disconnections with less utilized, but orthogonally reactive alkene and alkyne chemistry. Furthermore, these operations appear feasible in either order, allowing either the Suzuki coupling or a macrolactonization to close the 22-membered ring.
The desired spiroketal fragment (2) is rapidly assembled by utilizing two alkynes, which serve as functional equivalents to groups typically accessed via alcohols or ketones (e.g. the spiroketal and C-14, C-15 olefin, Scheme 1). Specifically, the alkyne 4 serves as a stabilized anion, which undergoes addition to aldehyde 5. The alkyne contained in 6 then functions as an electrophile in a metal catalyzed spiroketalization reaction. Utilization of the alkyne in this manner achieves efficiency by functioning as a carbonyl surrogate, which chemoselectively unveils the desired spiroketal (2). A novel aspect of this strategy is the recognition that the inherent polarization of the alkyne (6) would lead to the desired regioselectivity in the spirocyclization. By having an inductively electron-withdrawing benzoyloxy group at C-21, a positive charge at the neighboring acetylenic carbon (C-22) should be destabilized, directing the initial cyclization onto C-23 and leading to the desired spiroketal.10 A second alkyne building block (7) serves as a propargyl nucleophile under conditions developed by Tamaru11 and extended by Marshall,12 thus completing the spiroketal fragment (2).
Scheme 1. Retrosynthesis of Ushikulide A.
We envision that the aliphatic fragment (3) could be accessed in a convergent manner via disconnection of the C-8, C-9 bond, furnishing an aldol donor (8) and an acceptor (9). Given the similar size of the methyl and allyl groups at C-10, such a process is not expected to give acceptable diastereoselectivity. However, recent advances in our zinc aldol methodology13 cause us to envision that such a reaction could be made diastereoselective through catalyst control.
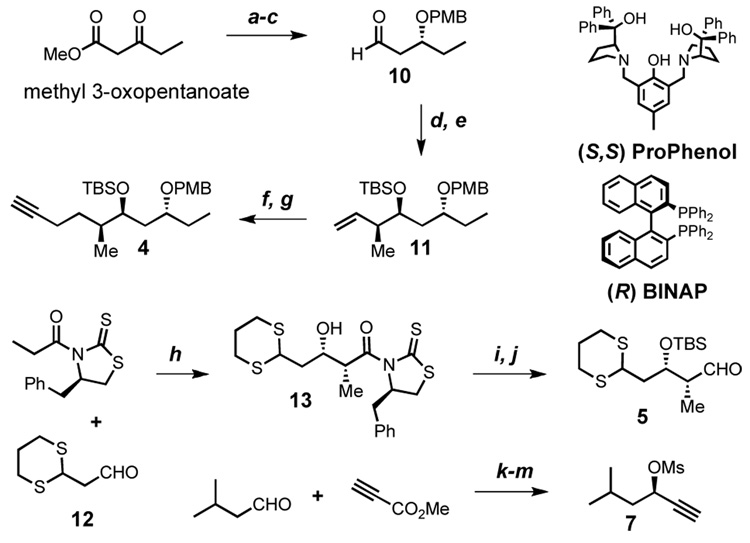
Preparation of the spiroketal (2) begins with the known reduction of methyl 3-oxopentanoate,14 followed by protection of the secondary alcohol as a p-methoxybenzyl ether and mono-reduction with DIBAL-H to yield aldehyde 10 in good yield and excellent optical activity (Scheme 2). A brief survey of crotylations led us to the method of Brown15, which after TBS protection provides the alkene 11 as a pure diastereomer (d.r. > 20:1). Hydroboration/iodination is followed by nucleophilic substitution yielding the desired alkyne 4 in seven linear steps.
Scheme 2. Toward the Synthesis of the Spiroketal Fragmenta.
aConditions: (a) 0.1 mol% (R)-BINAP, 0.05 mol% [RuCl2(C6H6)]2, MeOH, H2 1800 psi, 92%, 99% ee (b) PMBO(C=NH)CCl3, 1 mol% Cu(OTf)2, toluene, 85% (c) DIBAL-H, CH2Cl2, 82% (d) i.) cis-2-butene, n-BuLi, KOt-Bu, THF ii.) (+) Ipc2BOMe iii.) BF3·OEt2 then 10, >20:1 d.r. (e) TBSCl, imid., DMF, 87% over 2 Steps (f) 9-BBN, THF then I2, NaOCH3, MeOH, 85% (g) THF, DMPU, LiCCTMS, then KOH, MeOH, 81% (h) TiCl4, EtN(i-Pr)2, NMP, CH2Cl2, single diastereomer, 90%, (i) TBSOTf, 2,6-lutidine, CH2Cl2, 96% (j) DIBAL-H, CH2Cl2, 87% (k) 10 mol% (S,S) ProPhenol, Me2Zn, toluene, 88%, 95% ee (l) LiOH, THF, H2O then CuCl, CH3CN, 75% (m) MsCl, Et3N, CH2Cl2, 95%.
The known aldehyde 1216 participates in an aldol reaction under the conditions of Crimmins17 yielding the aldol adduct 13 in excellent yield as a single diastereomer. Protection and DIBAL-H reduction affords the aldehyde 5.
For synthesis of propargyl mesylate 7, we turned to our ProPhenol zinc alkynylation methodology,18 which affords the adduct of isovaleraldehyde and methyl propiolate in good yield and excellent stereoselectivity (Scheme 2). Following saponification of the methyl ester, exposure of the crude acid to cuprous chloride induces decarboxylation,19 leaving only mesylation of the secondary propargyl alcohol to complete the propargyl mesylate (7).
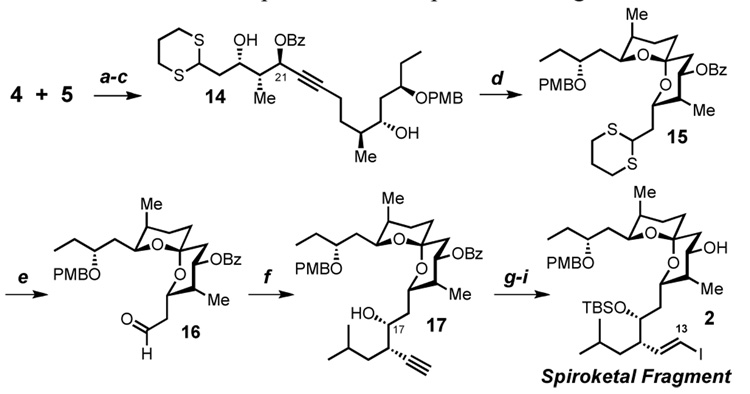
Union of aldehyde 5 and alkyne 4 (Scheme 3) by metalation of alkyne (n-BuLi, −78 °C) followed by addition of the aldehyde, affords a mixture of diastereomers at C-21 (~1.5:1 d.r.). Addition of bidentate Lewis acids (Et2AlCl, MgBr2·OEt2 or Ti(Oi-Pr)Cl3) to favor the desired anti 1,3 diol are unsuccessful due to low conversion and modest stereoselectivity. However, these epimers (not shown) are easily separable on silica gel and both epimers converge to the desired diol 14, one via ester formation by Mitsunobu inversion and the other via standard esterification (BzCl, pyridine). Utilization of a PdII catalyzed spiroketalization20,21,22 results in low conversion in this case. On the other hand, warming a THF solution containing diol 14, gold (I) chloride, and PPTS23,24 proceeds smoothly to yield spiroketal 15. Hydrolysis of the 1,3 dithiane in the presence of methyl iodide yields the aldehyde 16, which is successfully propargylated under the conditions of Marshall12 furnishing a 3.8:1 distribution of separable C-17 epimers. As this center will be oxidized, both epimers are of equal value in our synthesis, however for practical reasons, we continue forward with the major compound 17. Three additional steps produce the desired spiroketal fragment (2) in a total of 16 linear steps from methyl 3-oxopentanoate.
Scheme 3. Completion of the Spiroketal Fragmenta.
aConditions: (a) n-BuLi, THF, 90%, 1.5:1 syn:anti (b) for syn: DEAD, PhCOOH, PPh3, toluene, 92%, for anti: BzCl, pyridine, 95% (c) HCl, H2O, THF (d) 10 mol % AuCl, PPTS, THF, 63% (e) MeI, NaHCO3, CH3CN, H2O, 80% (f) 10 mol% Pd(OAc)2, ZnEt2, PPh3, THF, 7, 90%, 3.8:1 d.r. (g) TBSOTf, 2,6-lutidine, CH2Cl2 (h) K2CO3, MeOH (i) Bu3SnH, 5 mol% PdCl2(PPh3)2, THF, then I2, 90% over 3 steps.
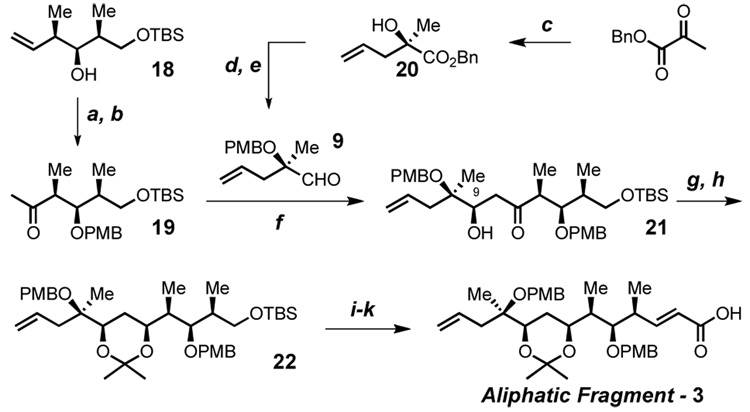
Our approach toward the aliphatic fragment (3) begins with the known homoallyl alcohol 18 (Scheme 4).25 Wacker oxidation10 unveils the desired ketone 19. Preparation of the aldehyde partner 9 utilizes a tartrate modified SnII mediated addition of allyl bromide to benzyl pyruvate, as described by Mukaiyama,26 to give tertiary alcohol 20 in excellent chemical yield and optical purity. Protection and DIBAL-H reduction affords the aldehyde 9.
Scheme 4. Synthesis of the Aliphatic Fragmenta.
aConditions: (a) PMBO(C=NH)CCl3, 0.5 mol% Sc(OTf)3, toluene, 88% (b) 10 mol% PdCl2, CuCl, O2, DMF, H2O, 60% (c) allyl bromide, tin (II) catecholate, DBU, CuI, (−)-diisopropyl tartrate, CH2Cl2, 92%, 99% ee (d) PMBO(C=NH)CCl3, 1 mol% Sc(OTf)3, toluene, 85% (e) DIBAL-H, CH2Cl2, 90% (f) 60 mol% Et2Zn, t-BuOH, dioxane, 30 mol% (S,S) ProPhenol, 65% b.r.s.m. (g) NaBH4, MeOBEt2, MeOH, THF (h) p-TsOH, CH2Cl2, 2,2-dimethoxypropane, 71% over 2 steps (i) n-Bu4NF, THF (j) Dess-Martin reagent, CH2Cl2, (k) n-BuLi, (EtO)2P(O)CH2CO2TMS, THF, 92% over 3 steps.
Enolate formation (19, LiN(i-Pr)2, −78 °C) prior to addition of aldehyde 9 reveals the expected low stereoselectivity (~1:1 d.r.) for the direct aldol reaction. Addition of chelating Lewis acids (e.g. ZnCl2) failed to increase this selectivity. Worse, separation of the resulting C-9 aldol epimers is prohibitively difficult on preparative scale. An attempted Mukaiyama aldol reaction after formation of the silyl enol ether (19, LiN(i-Pr)2, Me3SiCl, −78 °C) with several bidentate Lewis acids (ZnCl2,MgBr2·OEt2, GaCl3 TiCl4 or Ti(Oi-Pr)2Cl2) results in only slow hydrolysis of the silyl enol ether. Therefore, our attention turned to catalyst control as a means to overcome this stereochemical quandary. Gratifyingly, the (S,S) ProPhenol dinuclear zinc complex promotes the aldol process, wherein the desired diastereomer is formed with >20:1 d.r.13 A syn reduction of the aldol 21 is followed by formation of acetonide 22. The aliphatic fragment (3) is completed in three additional steps and a total of 11 linear steps from the (S)-Roche ester.
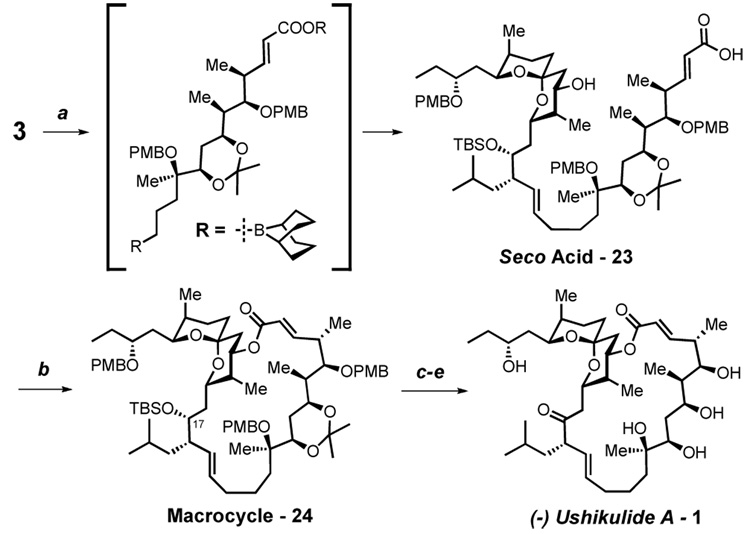
Suzuki coupling9 unites the spiroketal and aliphatic fragments yielding seco-acid 23 directly (Scheme 5). Yonemitsu’s modification of the Yamaguchi esterification,7 fails to give any product when applied to seco-acid 23. Applying the Wasserman-Kita esterification to macrolaconization27 affords the desired macrocycle 24 in ~30% yield. However, the method of Shiina28 improves this yield to 65%.
Scheme 5. Completion of the Synthesisa.
aConditions: (a) 3, 9-BBN, THF; then 2, DMF, H2O, 10 mol% PdCl2(dppf), 10 mol% Ph3As, Cs2CO3, 67% (b) 2-methyl-6-nitrobenzoic anhydride, 4-DMAP, DCE, 65% (c) HF·pyridine, pyridine, THF (d) Dess-Martin reagent, CH2Cl2 (e) DDQ, H2O, CH2Cl2 then dilute with 3:2 AcOH, H2O, 52% over 3 steps.
Deprotection of the C-17 TBS group of 24 is accomplished under buffered HF·pyridine conditions (Scheme 5). Dess-Martin oxidation unveils the sensitive β, γ unsaturated ketone, which is cleanly deprotected of its three p-methoxylbenzyl ethers upon exposure to DDQ. The resulting triol (not-shown) is dissolved in a 3:2 mixture of acetic acid and water to remove the acetonide. As the reaction progresses, TLC analysis shows the formation of a more polar spot of indistinguishable Rf compared to an authentic sample of ushikulide A. Upon isolation, this compound exhibits identical 1H, 13C, IR, HRMS & HPLC properties when compared to the same authentic sample. Measurement of the optical rotation {[α]24 D = −12° (c 0.28, MeOH)} confirms that the absolute stereochemistry also matches that of natural ushikulide A1 {[α]25 D = −13° (c 0.50, MeOH)}. In total, these data prove our tentative assignment to be correct and lead us to assign the complete structure of (−)-ushikulide A as depicted below.
In summary, we report the first synthesis of (−)-ushikulide A which establishes its relative and absolute stereochemistry. Our route requires 21 linear and 41 total steps (2.2% yield over the linear sequence), which is gratifying given the complexity of our target (40 carbon atoms and 14 stereogenic centers). The efficiency of this strategy is in no small measure due to the utilization of alkenes and alkynes as orthogonally reactive surrogates for hydroxyl and carbonyl functionalities. Further novel features of this synthesis include: a new and highly regioselective gold catalyzed spiroketalization reaction, utilization of (S,S) ProPhenol in enantio-and diastereoselective alkynylation and aldol reactions, and establishment of the full structure of ushikulide A via total synthesis. Further efforts to optimize this route and apply it to other members of the family are underway and will be reported in due course.
Supplementary Material
Detailed experimental procedures, full characterization of all products, and comparison NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
We gratefully acknowledge K. Takahashi for providing an authentic sample of ushikulide A. We also acknowledge S. Lynch for his invaluable help in the acquisition of 2-D NMR data. We thank the Stanford Graduate Fellowship Program and NIH (GM 13598) for their financial support of our program, Johnson Matthey for their supply of precious metal salts and Aldrich for ProPhenol.
References
- 1.Takahashi K, Yoshihara T, Kurosawa K. J. Antibiot. 2005;58:420. doi: 10.1038/ja.2005.55. [DOI] [PubMed] [Google Scholar]
- 2.Dreyfuss M, Harri E, Hoffmann H, Kobel H, Pache W, Tscherter H. Eur. J. Appl. Microbiol. 1976;3:125. [Google Scholar]
- 3.Kino T, Hatanaka H, Hashimoto M, Nishiyama M, Goto T, Okuhara M, Kohsaka M, Aoki H, Imanaka H. J. Antibiot. 1987;40:1249. doi: 10.7164/antibiotics.40.1249. [DOI] [PubMed] [Google Scholar]
- 4.Attempts to prepare crystals of ushikulide A suitable for X-ray diffraction were unsuccessful in this case. Takahashi K. 2008 July 22; personal communication.
- 5.Kirst HA, Mynderse JS, Martin JW, Baker PJ, Paschal JW, Steiner JL, Lobkovsky E, Clardy J. J. Antibiot. 1996;49:162. doi: 10.7164/antibiotics.49.162. [DOI] [PubMed] [Google Scholar]
- 6.Evans DA, Kaldor SW, Jones TK, Clardy J, Stout TJ. J. Am. Chem. Soc. 1990;112:7001. [Google Scholar]
- 7.Evans DA, Ng HP, Rieger DL. J. Am. Chem. Soc. 1993;115:11446. [Google Scholar]
- 8.White JD, Hanselmann R, Jackson RW, Porter WJ, Ohba Y, Tiller T, Wang S. J. Org. Chem. 2001;66:5217. doi: 10.1021/jo0104429. [DOI] [PubMed] [Google Scholar]
- 9.Chemler SR, Trauner D, Danishefsky SJ. Angew. Chem. Int. Ed. 2001;40:4544. doi: 10.1002/1521-3773(20011217)40:24<4544::aid-anie4544>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 10.Kang SK, Jung KY, Chung JU, Namkoong EY, Kim TH. J. Org. Chem. 1995;60:4678. [Google Scholar]
- 11.Tamaru Y. Activation of allyl alcohols as allyl cations, allyl anions, and amphiphilic allylic species by palladium. Eur. J. Org. Chem. 2005;13:2647–2656. [Google Scholar]
- 12.Marshall JA, Schaaf GM. J. Org. Chem. 2001;66:7825. doi: 10.1021/jo015936k. [DOI] [PubMed] [Google Scholar]
- 13.Trost BM, Silcoff ER, Ito H. Org. Lett. 2001;3:2497. doi: 10.1021/ol0161211. [DOI] [PubMed] [Google Scholar]
- 14.Kitamura M, Tokunaga M, Ohkuma T, Noyori R. Org. Syn. 1998;71:589. [Google Scholar]
- 15.Brown HC, Bhat KS. J. Am. Chem. Soc. 1986;108:5919. doi: 10.1021/ja00279a042. [DOI] [PubMed] [Google Scholar]
- 16.Hatakeyama S, Kawamura M, Shimanuki E, Saijo K, Takano S. Synlett. 1992;2:114. [Google Scholar]
- 17.Crimmins MT, She J. Synlett. 2004;8:1371. [Google Scholar]
- 18.Trost BM, Weiss AH, von Wangelin AJ. J. Am. Chem. Soc. 2006;128:8. doi: 10.1021/ja054871q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trost BM, Weiss AH. Org. Lett. 2006;8:4461. doi: 10.1021/ol0615836. [DOI] [PubMed] [Google Scholar]
- 20.Utimoto K. Pure & Appl. Chem. 1983;55:1845. [Google Scholar]
- 21.Trost BM, Weiss AH. Angew. Chem. Int. Ed. 2007;46:7664. doi: 10.1002/anie.200702637. [DOI] [PubMed] [Google Scholar]
- 22.Trost BM, Horne DB, Woltering MJ. Chem. Eur. J. 2006;12:6607. doi: 10.1002/chem.200600202. [DOI] [PubMed] [Google Scholar]
- 23.Antoniotti S, Genin E, Michelet V, Genet JP. J. Am. Chem. Soc. 2005;127:9976. doi: 10.1021/ja0530671. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Zhou F, Forsyth C. J. Angew. Chem. Int. Ed. 2007;46:279. doi: 10.1002/anie.200601963. [DOI] [PubMed] [Google Scholar]
- 25.Keck GE, Abbott DE, Boden EP, Enholm EJ. Tetrahedron Lett. 1984;25:3927. [Google Scholar]
- 26.Yamada K, Tozawa T, Nishida M, Mukaiyama T. Bull. Chem. Soc. Jpn. 1997;70:2301. [Google Scholar]
- 27.Trost BM, Chisholm JD. Org. Lett. 2002;4:3743. doi: 10.1021/ol026726c. [DOI] [PubMed] [Google Scholar]
- 28.Shiina I, Kubota M, Oshiumi H, Hashizume M. J. Org. Chem. 2004;69:1822. doi: 10.1021/jo030367x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed experimental procedures, full characterization of all products, and comparison NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.