Abstract
Virtually all individuals with Down syndrome (DS) develop neurofibrillary tangles, a characteristic brain lesion of Alzheimer’s disease (AD), when they reach the fourth decade of life. In AD, neurofibrillary tangles are thought to result from abnormal hyperphosphorylation of tau protein, which, in turn, can result from down-regulation of protein phosphatase (PP) 2A, a major brain tau phosphatase. The abnormal hyperphosphorylation of tau in DS had not yet been characterized, and its causes were not understood. In this study, by using quantitative Western blot analysis, we found that the level of the catalytic subunit of PP2A, but not of PP1, PP2B or PP5, was dramatically decreased. The decrease of PP2A level correlated negatively to tau level and tau phosphorylation at several abnormal hyperphosphorylation sites, including Ser199, Thr205, Thr212, Ser262, Ser396 and Ser422. Our results indicate that PP2A is down-regulated in DS brain and suggest that this down-regulation might be involved in the abnormal hyperphosphorylation and accumulation of tau.
Keywords: Protein phosphatases, Down syndrome, tau, hyperphosphorylation, Alzheimer disease
1. Introduction
Down syndrome (DS), caused by partial or complete trisomy 21, is the most frequent congenital chromosomal abnormality and represents the most common genetic cause of mental retardation. Virtually all individuals with DS develop the typical brain pathologies of Alzheimer disease (AD), i.e., extracellular amyloid plaques and intracellular neurofibrillary tangles (NFTs), when they reach the fourth decade of life [21,36,37]. Accompanying these histopathological changes is the occurrence in DS patients of age-related cognitive impairment that progresses to dementia resembling AD [17,27]. The amyloidosis in DS brain is believed to result from overexpression of amyloid-β protein precursor (AβPP), the gene for which is located on chromosome 21 [4,28]. However, why adults with DS always develop neurofibrillary pathology is hardly understood.
NFTs are composed of paired helical filaments and straight filaments, which consist of microtubule-associated protein tau in an abnormally hyperphosphorylated form [13,14]. Many studies have been carried out on the phosphorylation sites and their functional roles in the abnormally hyperphosphorylated tau from AD brain. However, the same aspects have not yet been investigated in DS brain.
It has been demonstrated that the abnormal hyperphosphorylation of tau is critical to neurofibrillary pathology (reviewed in [18]). Theoretically, hyperphosphorylation of tau could be the result of over-activation of tau kinases, down-regulation of tau phosphatases, and/or alterations of tau molecule itself. Previous studies have shown that protein phosphatase (PP) 2A is the major tau phosphatase in the brain [7,9,11,23,30] and that down-regulation of this phosphatase is partially responsible for the abnormal tau phosphorylation in AD brain [8,10,23,24,32,35,39]. Whether PP2A is also down-regulated in adult DS brain had not yet been investigated before the present study.
To study the abnormal hyperphosphorylation of tau and its possible causes in adult DS brains, we determined the site-specific tau phosphorylation and the levels of the catalytic subunits of major brain PPs. We found that the level of the catalytic subunit of PP2A (PP2Ac) was dramatically decreased in adult DS brain, and this decrease correlated negatively with tau level and phosphorylation at several abnormal hyperphosphorylation sites.
2. Methods
2.1. Human brain tissue
Tissue from the temporal cortex of 6 adults with DS and 6 normal controls (Table 1) were obtained from the Brain Bank for Developmental Disabilities and Aging of our institute. Diagnosis of DS was confirmed genetically. Histopathological examinations confirmed Alzheimer-type brain pathology in all the DS cases and lack of such pathology in the control cases. The brain tissue samples were stored at −70 °C until use. Frozen human brain tissues were used in accordance with the U.S. National Institutes of Health guidelines and approved by the institutional review committee of the New York State Institute for Basic Research in Developmental Disabilities.
Table 1.
Human brain tissue used in this study
| Group | Case# | Gender | Age at death | *PMI (h) |
|---|---|---|---|---|
| DS | 1 | M | 65 | 4.5 |
| 2 | F | 58 | 5 | |
| 3 | F | 55 | 5 | |
| 4 | M | 55 | 6 | |
| 5 | F | 59 | 6 | |
| 6 | M | 61 | 3 | |
| Mean ± DS | 58.8 ± 3.8 | 4.9 ± 1.1 | ||
| Control | 1 | F | 67 | 2.5 |
| 2 | M | 86 | 1.5 | |
| 3 | F | 61 | 7 | |
| 4 | F | 68 | 3 | |
| 5 | F | 67 | 4 | |
| 6 | M | 59 | 6 | |
| Mean ± DS | 68.0 ± 9.5 | 4.0 ± 2.1 | ||
PMI (h), postmortem interval in hours.
2.2. Antibodies
All primary antibodies used in this study are listed in Table 2. Peroxidase-conjugated anti-mouse and anti-rabbit IgG was obtained from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). ECL kit was from Amersham Pharmacia Biotech (Piscataway, NJ, USA).
Table 2.
Primary antibodies employed in this study
| Antibody | Type | Specificity | Phosphorylation site* | Source/References |
|---|---|---|---|---|
| Anti-PP1 | Monoclonal | PP1c | BD Bioscience, Palo Alto, CA, USA | |
| Anti-PP2A | Monoclonal | PP2Ac | Upstate, Lake Placid, NY, USA | |
| Anti-PP2A | Polyclonal | PP2A-A | Upstate | |
| Anti-PP2A | Monoclonal | PP2A-B | Upstate | |
| R126d | Polyclonal | PP2Bc | [25] | |
| Anti-PP5 | Polyclonal | PP5 | [1] | |
| Anti-actin | Monoclonal | Actin | Sigma-Aldrich, St. Louis, MO, USA | |
| pT181 | Polyclonal | P-tau† | pT181 | Biosource, Camerillo, CA, USA |
| pS199 | Polyclonal | P-tau | pS199 | Biosource |
| pS202 | Polyclonal | P-tau | pS202 | Biosource |
| pT205 | Polyclonal | P-tau | pT205 | Biosource |
| pT212 | Polyclonal | P-tau | pT212 | Biosource |
| pS214 | Polyclonal | P-tau | pS214 | Biosource |
| pT217 | Polyclonal | P-tau | pT217 | Biosource |
| pS262 | Polyclonal | P-tau | pS262 | Biosource |
| pS396 | Polyclonal | P-tau | pS396 | Biosource |
| pS404 | Polyclonal | P-tau | pS404 | Biosource |
| pS422 | Polyclonal | P-tau | pS422 | Biosource |
| R134d | Polyclonal | Tau | [34] |
Numbered according to the largest isoform of human brain tau [6].
P-tau, phosphorylated tau
2.3. Western blots
The frozen temporal cortices were homogenized in cold buffer containing 50 mM Tris-HCl (pH 7.4), 2.0 mM EDTA, 10 mM β-mercaptoethanol and 8.5% sucrose. The homogenates were then immediately mixed at 1:1 ratio with 2-fold concentrated Laemmli sample buffer (125 mM Tris-HCl, pH 6.8, 4.0% SDS, 20% glycerol, 2.0% β-mercaptoethanol and 0.005% bromophenol blue) and heated in boiling water for 5 min, followed by assayfor protein concentrations with modified Lowry methods [2]. The levels of specific proteins and the site-specific phosphorylation of tau in the homogenates were determined by quantitative Western blots using 10% SDS-PAGE and standard procedure, and the blots were developed by the corresponding primary antibodies and enhanced chemiluminescence kit (Pierce Biotechnology, Rockford, IL). The immunoreactivities of the blots were then quantified densitometrically. All data concerning the level of specific proteins were normalized by the level of actin, and all data concerning phosphorylation levels of tau were normalized by the level of total tau protein. For densitometric quantitation of tau immunoreactivity of AD cases, we included both tau bands between 45 to 70 kDa and tau smears because these antibodies does not cross-react to any other proteins in human brain and it is well known that these smears are aggregated tau proteins.
2.4. Correlation analysis
Linear correlation between the levels of PP2A catalytic subunit and the level of tau or tau phosphorylation at individual phosphorylation sites of brain homogenates from six DS cases was analysed by using Microsoft Excel 2003. The correlation coefficients (r values) and p value were calculated. p < 0.05 was regarded to have a significant correlation.
3. Results
3.1. Accumulation and site-specific hyperphosphorylation of tau in DS brain
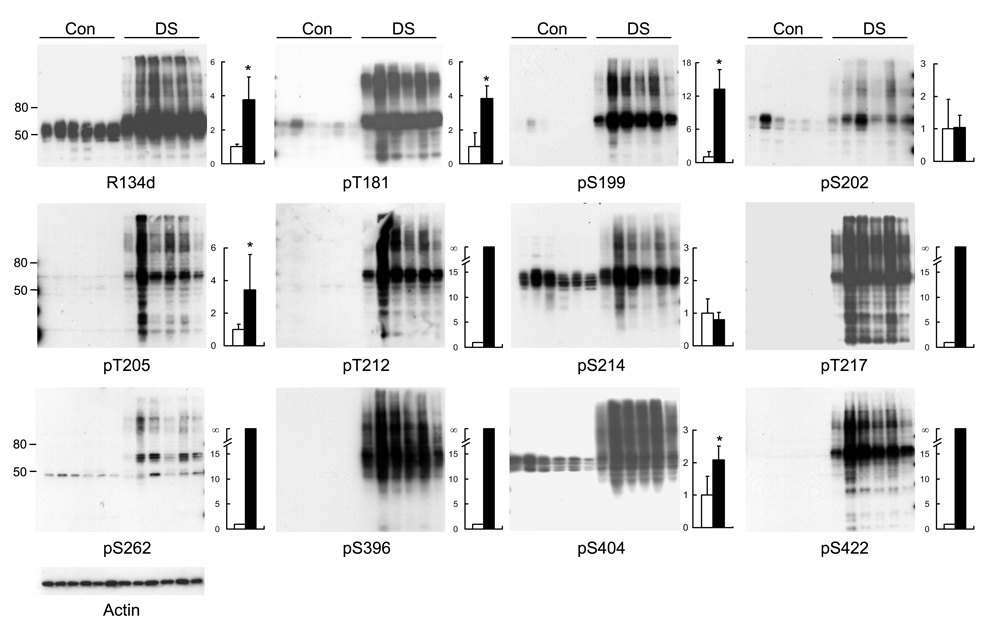
Neurofibrillary pathology has been seen in the brains of all adult DS cases, but the accumulation and site-specific hyperphosphorylation of tau have not been studied. We, therefore, studied brain homogenates from 6 cases of adult DS and 6 cases of adult controls by quantitative Western blots. We found that the total tau level in DS cases was nearly 4-fold greater than that in the controls (Fig. 1). In control brain homogenates, tau displayed as several bands between 45 to 60 kDa, whereas in DS, these bands had an upshift of gel mobility, and many higher molecular smears were also seen. These changes in tau in DS brain resembled those seen in AD brain [20]. When phosphorylation-dependent tau antibodies were used, we found a marked increase in tau phosphorylation at these detected sites, except at Ser202 and Ser214 (Fig. 1). The increase in immunoreactivity in the blots of pS202 and pS214 resulted from the increased tau level in DS brain, because the levels between DS and controls were similar after being normalized with the total tau levels (Fig. 1 bar graphs).
Fig. 1.
Level and site-specific phosphorylation of tau in DS brain. Homogenates of the temporal cortices from 6 cases each of adult DS and non-neurological controls (Con) were analyzed by Western blots developed with antibodies indicated under each blot. R134d is a phosphorylation-independent tau antibody for determining the total tau level. The other tau antibodies are phosphorylation-dependent and site-specific and were used to determine the phosphorylation level of tau at the corresponding individual phosphorylation sites. Actin blot was included as a loading control. Molecular weight markers in kDa are indicated on the left side of the blots. Densitometric quantitation of the blots is shown on the right side of each blot. The values were calculated after normalization with actin blot (for calculation of the total tau) or with R134d blot (for calculation of the site-specific phosphorylation), and the immunoreactivities of the DS group were expressed in relation to those of the control group. Because no phosphorylation of tau was detectable at Thr212, Thr217, Ser262, Ser396 or Ser422 in the control group, the increase in the DS group at these sites became infinity (∞). Data are presented as mean ± SD. Open bars, control; close bars, DS; *p < 0.05 as compared to controls.
3.2. Levels of protein phosphatases in DS brain
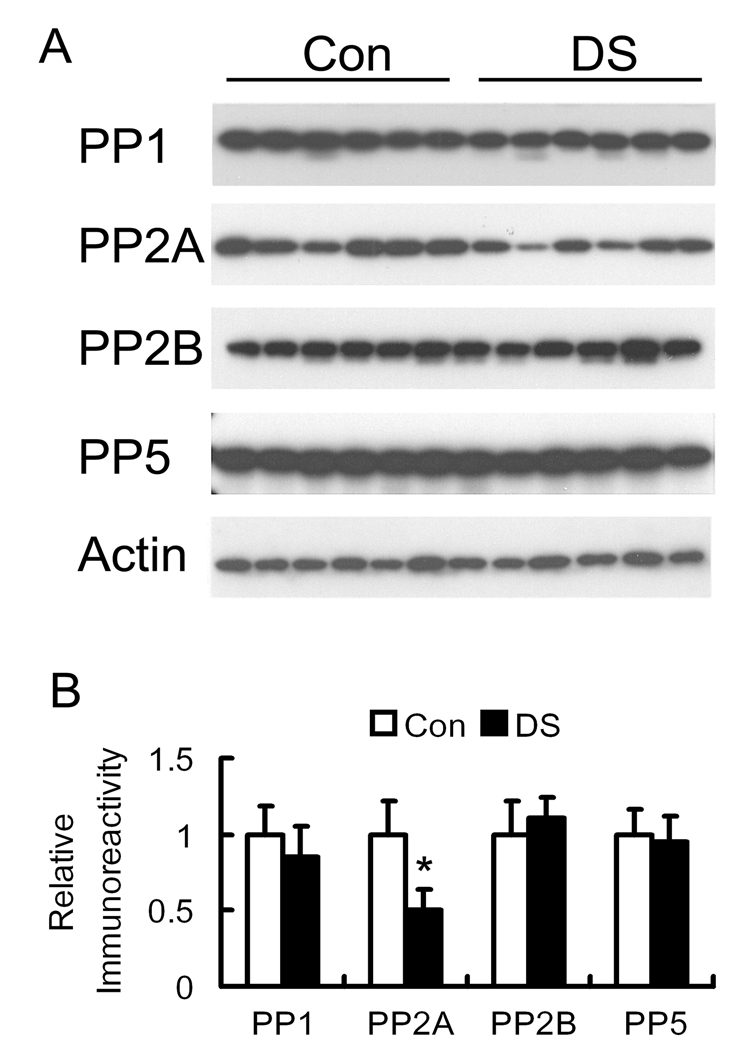
Tau phosphorylation level is largely regulated by PP2A and, to a lesser degree, by PP1, PP2B and PP5 in the brain [7,11,23]. To investigate whether the abnormal hyperphosphorylation of tau could be caused by dysregulation of these tau phosphatases, we determined the levels of the catalytic subunits of these phosphatases by quantitative Western blots. We found that the level of PP2Ac in DS brain homogenates was reduced to nearly half of that of controls, whereas the levels of other phosphatases were not altered in DS brain (Fig. 2).
Fig. 2.
Levels of protein phosphatases in DS and control brains. (A) Homogenates of the temporal cortices from 6 cases each of adult DS and non-neurological controls (Con) were analyzed by Western blots developed with antibodies to the catalytic subunit of PP1, PP2A, PP2B or PP5. Actin blot was also included as a loading control. (B) The blots shown in panel A were quantified densitometrically, and the relative immunoreactivities (mean ± SD) were normalized with the actin blot. *p < 0.05, as compared to controls.
PP2A is composed of the catalytic subunit and one or two regulatory subunits (subunit A and subunit B). We, therefore, determined the levels of A and B subunits of PP2A in the brain homogenates, but did not find any significant differences between DS and control brains (data not shown).
3.3. Correlation of PP2A level with tau accumulation and phosphorylation in DS brain
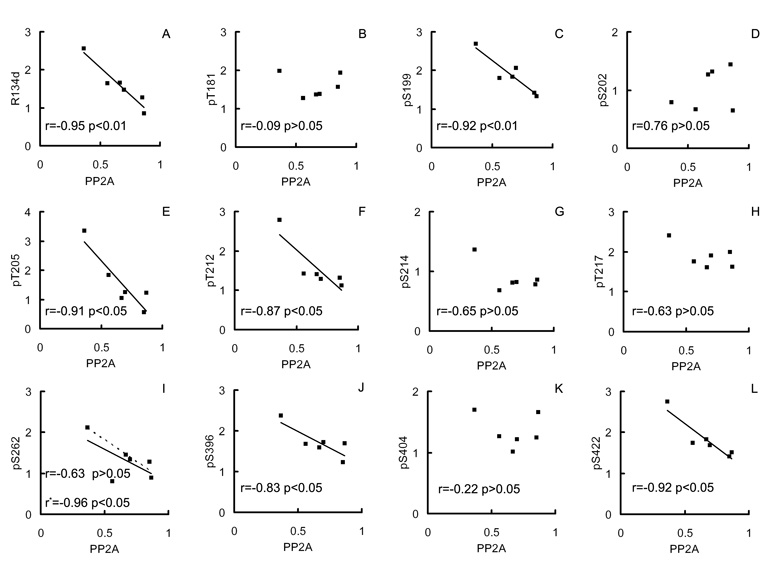
To study whether the decreased PP2Ac contributes to accumulation and hyperphosphorylation of tau, we carried out linear correlation analyses between the PP2A levels and the levels and the site-specific phosphorylation of tau in DS brains. We found that the levels of tau (R134d immunoreactivity) correlated negatively to the levels of PP2Ac (Fig. 3A; r = −0.95, p < 0.01). Among the 11 phosphorylation sites studied, tau phosphorylation levels at six of the sites were also correlated negatively to the levels of PP2Ac (Fig. 3B–L). These six phosphorylation sites include pSer262, where a very strong negative correlation (r = −0.96) was obtained when one case that was apparently out of the data range was excluded from the calculation. However, if this case was included, the negative correlation did not reach statistical significance, with the small number of cases.
Fig. 3.
Correlation analysis between levels of PP2A and of total or phosphorylated tau in DS brains. Levels of total (immunoreactivity of R134d) or phosphorylated tau at specific sites (immunoreactivity of the phosphorylation-dependent and site-specific tau antibodies), as determined by quantitative Western blots, in 6 DS brains were plotted against the level of the catalytic subunit of PP2A. A linear regression line is shown when the correlation reached statistical significance. The dotted line and the r* in the case of pS262 is the linear regression line and correlation coefficient, respectively, calculated when one case that was apparently out of the data range was excluded.
4. Discussion
Neurofibrillary pathology in adult DS brain not only resembles that in AD brain, but also occurs 20–30 years earlier than in AD. The major protein component of NFTs is the abnormally hyperphosphorylated tau protein [13,14]. Nearly 40 phosphorylation sites have been identified in tau isolated from AD brain [12,16], and the abnormal hyperphosphorylation is believed to be the major cause of neurofibrillary degeneration (reviewed in [18]). Although NFTs in DS brain are morphologically indistinguishable from those in AD brain and also consist of hyperphosphorylated tau [5,15], the abnormal phosphorylation sites of tau in DS brain had not been investigated. In the present study, we employed Western blots developed with a battery of site-specific and phosphorylation-dependent tau antibodies and studied a subset of the abnormal hyperphosphorylation sites in adult DS brain. We found that, as seen in AD brain, tau is also accumulated and hyperphosphorylated at most of these phosphorylation sites studied. To limit the ages to a small range and the post-mortem intervals to a short period, only 6 DS cases were included in this study, but these cases had a very small range of age (58 ± 3.8 years old) and short post-mortem intervals (4.9 ± 1.1 hrs). Since almost all DS patients develop NFTs in their brains before the age of 40 years old [21,36,37], the DS cases included in this study had had neurofibrillary pathology for approximately 20 years, which were equivalent to the late stage of AD. However, unlike in brains with the late stage of AD, we did not find increased tau phosphorylation at Ser202 and Ser214 in DS brain, suggesting that the phosphorylation pattern of tau in DS might be different from that of AD.
The molecular mechanism leading to the abnormal hyperphosphorylation of tau and the consequent neurofibrillary pathology in DS brain is not understood. The present study is the first to demonstrate a selective and dramatic decrease in the level of the catalytic subunit of PP2A in DS brain. Because PP2A is the major brain phosphatase regulating tau phosphorylation level and can dephosphorylate all the phosphorylation sites that were examined in this study [7,9,11,23,30,31], our findings suggest the intriguing possibility that the hyperphosphorylation of tau in DS brain might be caused by down-regulation of PP2A. This possibility was reinforced by the correlation analysis that demonstrated a strong negative correlation between the PP2Ac levels and the levels of total tau and its phosphorylation at several abnormal hyperphosphorylation sites in DS brain. The lack of such negative correlation between PP2A level and tau phosphorylation at Ser202 or Ser214, the latter of which was not altered in DS brain, further supports the specificity of the association between PP2A down-regulation and the hyperphosphorylation of tau at the sites found to be increased in DS brain. Inhibition of PP2A has been shown to induce hyperphosphorylation and accumulation of tau as well as axonopathy and memory deficits in animals of several in vivo models [19,33,38,39].
Our observations that the phosphorylation levels of tau at Thr181, Thr217 and Ser404 did not significantly correlate with PP2A levels suggest that other mechanisms in addition to PP2A down-regulation might also contribute to abnormal phosphorylation of tau in DS brain. For example, we recently found that a protein kinase named Dyrk1A, which is over-expressed as a result of trisomy 21 in DS brain, can phosphorylate tau at several phosphorylation sites, including the three sites above, and that the overexpression of this kinase may partially underlie the abnormal hyperphosphorylation of tau and neurofibrillary degeneration in DS (unpublished observations). In addition, overproduction of AβPP and accumulation of Aβ, as a result of trisomy 21, may also contribute to abnormal phosphorylation of tau and neurofibrillary pathology in DS brain, since recent studies have shown that duplication of AβPP gene can cause early-onset AD that has both amyloid plaques and NFTs in the brain [26,29].
Currently, we do not know the mechanism by which the level of PP2Ac is down-regulated in DS brain. One possibility is the selective increase in the degradation of PP2Ac, as up-regulation of some proteases has been reported in DS brain [3,22]. The other possibility could be that less PP2A catalytic subunit is expressed in adult DS brain. The ultimate approach to verify this possibility would be to compare the mRNA levels between adult DS and control brains, but it is difficult to reliably determine mRNA levels from post-mortem human brain tissue.
In conclusion, we have demonstrated in adult DS brain that (1) tau protein is accumulated and hyperphosphorylated at multiple phosphorylation sites; (2) the level of the catalytic subunit of PP2A is selectively decreased to nearly half of the controls; and (3) the decrease in PP2Ac levels correlates negatively to the levels of tau and its phosphorylation at several abnormal phosphorylation sites. These findings provide a likely mechanism of neurofibrillary pathology in DS and suggest that neurofibrillary degeneration in adult DS could be caused, at least in part, by PP2A down-regulation. Correction of the PP2A down-regulation could be a potential therapeutic target to inhibit neurofibrillary degeneration and, hence, treat dementia in adult DS patients.
Acknowledgements
We thank Ms. J. Murphy for secretarial assistance and Ms. M. Marlow for editorial suggestions. This work was supported in part by the New York State Office of Mental Retardation and Developmental Disabilities, National Institutes of Health Grants (R01 AG 027429 to CXG and R01 HD43960 to JW), Alzheimer Association Grant (IIRG-05-13095 to CXG), and the Li Foundation, NY (to CXG).
References
- 1.Bahl R, Bradley KC, Thompson KJ, Swain RA, Rossie S, Meisel RL. Localization of protein Ser/Thr phosphatase 5 in rat brain. Brain Res Mol Brain Res. 2001;90:101–109. doi: 10.1016/s0169-328x(01)00089-4. [DOI] [PubMed] [Google Scholar]
- 2.Bensadoun A, Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976;70:241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- 3.Engidawork E, Juranville JF, Fountoulakis M, Dierssen M, Lubec G. Selective upregulation of the ubiquitin-proteasome proteolytic pathway proteins, proteasome zeta chain and isopeptidase T in fetal Down syndrome. J Neural Transm Suppl. 2001;61:117–130. doi: 10.1007/978-3-7091-6262-0_10. [DOI] [PubMed] [Google Scholar]
- 4.Engidawork E, Lubec G. Protein expression in Down syndrome brain. Amino Acids. 2001;21:331–361. doi: 10.1007/s007260170001. [DOI] [PubMed] [Google Scholar]
- 5.Flament S, Delacourte A, Mann DM. Phosphorylation of Tau proteins: a major event during the process of neurofibrillary degeneration. A comparative study between Alzheimer's disease and Down's syndrome. Brain Res. 1990;516:15–19. doi: 10.1016/0006-8993(90)90891-e. [DOI] [PubMed] [Google Scholar]
- 6.Goedert M, Spillantini MG, Potier MC, Ulrich J, Crowther RA. Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. EMBO J. 1989;8:393–399. doi: 10.1002/j.1460-2075.1989.tb03390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goedert M, Jakes R, Qi Z, Wang JH, Cohen P. Protein phosphatase 2A is the major enzyme in brain that dephosphorylates tau protein phosphorylated by proline-directed protein kinases or cyclic AMP-dependent protein kinase. J Neurochem. 1995;65:2804–2807. doi: 10.1046/j.1471-4159.1995.65062804.x. [DOI] [PubMed] [Google Scholar]
- 8.Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K. Phosphoprotein phosphatase activities in Alzheimer disease brain. J Neurochem. 1993;61:921–927. doi: 10.1111/j.1471-4159.1993.tb03603.x. [DOI] [PubMed] [Google Scholar]
- 9.Gong CX, Grundke-Iqbal I, Iqbal K. Dephosphorylation of Alzheimer's disease abnormally phosphorylated tau by protein phosphatase-2A. Neuroscience. 1994;61:765–772. doi: 10.1016/0306-4522(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 10.Gong CX, Shaikh S, Wang JZ, Zaidi T, Grundke-Iqbal I, Iqbal K. Phosphatase activity toward abnormally phosphorylated tau: decrease in Alzheimer disease brain. J Neurochem. 1995;65:732–738. doi: 10.1046/j.1471-4159.1995.65020732.x. [DOI] [PubMed] [Google Scholar]
- 11.Gong CX, Lidsky T, Wegiel J, Zuck L, Grundke-Iqbal I, Iqbal K. Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. Implications for neurofibrillary degeneration in Alzheimer's disease. J Biol Chem. 2000;275:5535–5544. doi: 10.1074/jbc.275.8.5535. [DOI] [PubMed] [Google Scholar]
- 12.Gong CX, Liu F, Grundke-Iqbal I, Iqbal K. Post-translational modifications of tau protein in Alzheimer's disease. J Neural Transm. 2005;112:813–838. doi: 10.1007/s00702-004-0221-0. [DOI] [PubMed] [Google Scholar]
- 13.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261:6084–6089. [PubMed] [Google Scholar]
- 14.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanger DP, Brion JP, Gallo JM, Cairns NJ, Luthert PJ, Anderton BH. Tau in Alzheimer's disease and Down's syndrome is insoluble and abnormally phosphorylated. Biochem J. 1991;275(Pt 1):99–104. doi: 10.1042/bj2750099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanger DP, Byers HL, Wray S, Leung KY, Saxton MJ, Seereeram A, Reynolds CH, Ward MA, Anderton BH. Novel phosphorylation sites in tau from Alzheimer brain support a role for casein kinase 1 in disease pathogenesis. J Biol Chem. 2007;282:23645–23654. doi: 10.1074/jbc.M703269200. [DOI] [PubMed] [Google Scholar]
- 17.Holland AJ, Hon J, Huppert FA, Stevens F, Watson P. Population-based study of the prevalence and presentation of dementia in adults with Down's syndrome. Br J Psychiatry. 1998;172:493–498. doi: 10.1192/bjp.172.6.493. [DOI] [PubMed] [Google Scholar]
- 18.Iqbal K, del A, Alonso C, Chohan MO, El-Akkad E, Gong CX, Khatoon S, Liu F, Grundke-Iqbal I. Molecular basis of tau protein pathology: role of abnormal hyperphosphorylation. In: Dawbarn D, Allen SJ, editors. Neurobiology of Alzheimer's Disease. New York: Oxford University Press; 2007. pp. 111–131. [Google Scholar]
- 19.Kins S, Crameri A, Evans DR, Hemmings BA, Nitsch RM, Gotz J. Reduced protein phosphatase 2A activity induces hyperphosphorylation and altered compartmentalization of tau in transgenic mice. J Biol Chem. 2001;276:38193–38200. doi: 10.1074/jbc.M102621200. [DOI] [PubMed] [Google Scholar]
- 20.Kopke E, Tung YC, Shaikh S, Alonso AC, Iqbal K, Grundke-Iqbal I. Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J Biol Chem. 1993;268:24374–24384. [PubMed] [Google Scholar]
- 21.Lai F, Williams RS. A prospective study of Alzheimer disease in Down syndrome. Arch Neurol. 1989;46:849–853. doi: 10.1001/archneur.1989.00520440031017. [DOI] [PubMed] [Google Scholar]
- 22.Lemere CA, Munger JS, Shi GP, Natkin L, Haass C, Chapman HA, Selkoe DJ. The lysosomal cysteine protease, cathepsin S, is increased in Alzheimer's disease and Down syndrome brain. An immunocytochemical study. Am J Pathol. 1995;146:848–860. [PMC free article] [PubMed] [Google Scholar]
- 23.Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci. 2005;22:1942–1950. doi: 10.1111/j.1460-9568.2005.04391.x. [DOI] [PubMed] [Google Scholar]
- 24.Liu GP, Zhang Y, Yao XQ, Zhang CE, Fang J, Wang Q, Wang JZ. Activation of glycogen synthase kinase-3 inhibits protein phosphatase-2A and the underlying mechanisms. Neurobiol Aging. 2007 Apr 11; doi: 10.1016/j.neurobiolaging.2007.03.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Pei J-J, Gong CX, Iqbal K, Grundke-Iqbal I, Wu Q, Winblad B, Cowburn RF. Subcellular distribution of protein phosphatases and abnormally phosphorylated tau in the temporal cortex from Alzheimer's disease and control brains. J Neural Transm. 1998;105:69–83. doi: 10.1007/s007020050039. [DOI] [PubMed] [Google Scholar]
- 26.Rovelet-Lecrux A, Hannequin D, Raux G, Le Meur N, Laquerrière A, Vital A, Dumanchin C, Feuillette S, Brice A, Vercelletto M, Dubas F, Frebourg T, Campion D. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet. 2006;38:24–26. doi: 10.1038/ng1718. [DOI] [PubMed] [Google Scholar]
- 27.Schapiro MB, Haxby JV, Grady CL. Nature of mental retardation and dementia in Down syndrome: study with PET, CT, and neuropsychology. Neurobiol Aging. 1992;13:723–734. doi: 10.1016/0197-4580(92)90096-g. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro BL. The Down syndrome critical region. J Neural Transm Suppl. 1999;57:41–60. doi: 10.1007/978-3-7091-6380-1_3. [DOI] [PubMed] [Google Scholar]
- 29.Sleegers K, Brouwers N, Gijselinck I, Theuns J, Goossens D, Wauters J, Del-Favero J, Cruts M, van Duijn CM, Van Broeckhoven C. APP duplication is sufficient to cause early onset Alzheimer's dementia with cerebral amyloid angiopathy. Brain. 2006;129:2977–2983. doi: 10.1093/brain/awl203. [DOI] [PubMed] [Google Scholar]
- 30.Sontag E, Nunbhakdi-Craig V, Lee G, Bloom GS, Mumby MC. Regulation of the phosphorylation state and microtubule-binding activity of Tau by protein phosphatase 2A. Neuron. 1996;17:1201–1207. doi: 10.1016/s0896-6273(00)80250-0. [DOI] [PubMed] [Google Scholar]
- 31.Sontag E, Nunbhakdi-Craig V, Lee G, Brandt R, Kamibayashi C, Kuret J, White CL, Mumby MC, Bloom GS. Molecular interactions among protein phosphatase 2A, tau, and microtubules. Implications for the regulation of tau phosphorylation and the development of tauopathies. J Biol Chem. 1999;274:25490–25498. doi: 10.1074/jbc.274.36.25490. [DOI] [PubMed] [Google Scholar]
- 32.Sontag E, Luangpirom A, Hladik C, Mudrak I, Ogris E, Speciale S, White CL. Altered expression levels of the protein phosphatase 2A ABalphaC enzyme are associated with Alzheimer disease pathology. J Neuropathol Exp Neurol. 2004;63:287–301. doi: 10.1093/jnen/63.4.287. [DOI] [PubMed] [Google Scholar]
- 33.Sun L, Liu SY, Zhou XW, Wang XC, Liu R, Wang Q, Wang JZ. Inhibition of protein phosphatase 2A- and protein phosphatase 1-induced tau hyperphosphorylation and impairment of spatial memory retention in rats. Neuroscience. 2003;118:1175–1182. doi: 10.1016/s0306-4522(02)00697-8. [DOI] [PubMed] [Google Scholar]
- 34.Tatebayashi Y, Iqbal K, Grundke-Iqbal I. Dynamic regulation of expression and phosphorylation of tau by fibroblast growth factor-2 in neural progenitor cells from adult rat hippocampus. J Neurosci. 1999;19:5245–5254. doi: 10.1523/JNEUROSCI.19-13-05245.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogelsberg-Ragaglia V, Schuck T, Trojanowski JQ, Lee VM. PP2A mRNA expression is quantitatively decreased in Alzheimer's disease hippocampus. Exp Neurol. 2001;168:402–412. doi: 10.1006/exnr.2001.7630. [DOI] [PubMed] [Google Scholar]
- 36.Wegiel J, Wisniewski HM, Dziewiatkowski J, Popovitch ER, Tarnawski M. Differential susceptibility to neurofibrillary pathology among patients with Down syndrome. Dementia. 1996;7:135–141. doi: 10.1159/000106868. [DOI] [PubMed] [Google Scholar]
- 37.Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer's disease in Down's syndrome. Ann Neurol. 1985;17:278–282. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y, Yang XF, Wang YP, Tian Q, Wang XQ, Li HL, Wang Q, Wang JZ. Inhibition of protein phosphatases induces transport deficits and axonopathy. J Neurochem. 2007;102:878–886. doi: 10.1111/j.1471-4159.2007.04603.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhang CE, Tian Q, Wei W, Peng JH, Liu GP, Wang Q, Wang DW, Wang JZ. Homocysteine induces tau hyperphosphorylation by inactivating protein phosphatase-2A. Neurobiol Aging. 2007 May 28; doi: 10.1016/j.neurobiolaging.2007.04.015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]