Abstract
Activation of rod photoreceptors by light induces a massive redistribution of the heterotrimeric G-protein transducin. In darkness, transducin is sequestered within the membrane-enriched outer segments of the rod cell. In light, it disperses throughout the entire neuron. We show here that redistribution of rod transducin by light requires activation, but it does not require ATP. This observation rules out participation of molecular motors in the redistribution process. In contrast to the light-stimulated redistribution of rod transducin in rods, cone transducin in cones does not redistribute during activation. Remarkably, when cone transducin is expressed in rods, it does undergo light-stimulated redistribution. We show here that the difference in subcellular localization of activated rod and cone G-proteins correlates with their affinity for membranes. Activated rod transducin releases from membranes, whereas activated cone transducin remains bound to membranes. A synthetic peptide that dissociates G-protein complexes independently of activation facilitates dispersion of both rod and cone transducins within the cells. This peptide also facilitates detachment of both G-proteins from the membranes. Together, these results show that it is the dissociation state of transducin that determines its localization in photoreceptors. When rod transducin is stimulated, its subunits dissociate, leave outer segment membranes, and equilibrate throughout the cell. Cone transducin subunits do not dissociate during activation and remain sequestered within the outer segment. These findings indicate that the subunits of some heterotrimeric G-proteins remain associated during activation in their native environments.
Keywords: retina, rod, cone, transducin, signal transduction, diffusion
Introduction
The physiological state of a neuron is influenced by the distribution of signaling proteins within it. Some processes, e.g., axonal transport and mitochondrial import, use energy from ATP hydrolysis to distribute proteins, but simple diffusion and protein–protein or protein–lipid interactions also can determine distribution. For instance, scaffolding proteins influence the distributions of cAMP signaling enzymes (McConnachie et al., 2006), and protein–membrane interactions determine the subcellular distribution of Ras (Feig, 2006).
Photoreceptors are highly polarized neurons. On one end of the cell is a membrane-enriched dendritic structure known as the outer segment (OS). There, activation of rhodopsin by light facilitates binding of GTP to the Gα subunit of transducin, a heterotrimeric G-protein. On the opposite end of the photoreceptor is an axon leading to a glutaminergic synapse. Photoactivation of the G-protein cascade in the OS leads to rapid hyperpolarization of the entire neuron, which suppresses glutamate release.
A striking example of signal-induced protein redistribution occurs in rod photoreceptor cells. In darkness, transducin is sequestered within the OS. In light, it disperses throughout the entire cell, reaching the synapse, in <30 min after the onset of illumination. Two other photoreceptor proteins, arrestin and a Ca2+-binding protein, recoverin, also distribute differently in light and darkness (Sokolov et al., 2002; Mendez et al., 2003; Elias et al., 2004; Strissel et al., 2005). The redistribution of proteins within rods correlates with a 10-fold decrease in the gain of phototransduction (Sokolov et al., 2002; Kassai et al., 2005).
Arguments favoring either active transport or diffusion as the mechanism for protein migration in photoreceptors have been discussed (Marszalek et al., 2000; Lee et al., 2003; Lee and Montell, 2004; Nair et al., 2005a; Strissel et al., 2006). In a previous report, we showed that movement of arrestin does not require molecular motors; it occurs even without ATP (Nair et al., 2005). Here we show that redistribution of transducin also requires no ATP.
The widely held view that heterotrimeric G-protein complexes dissociate during activation (Gilman, 1987) primarily derives from early studies of rod transducin, whose subunits dissociate and detach from membranes during activation (Kuhn, 1980; Fung et al., 1981). In a recent review, Calvert et al. (2006) hypothesized a link between transducin subunit dissociation, diffusion, and redistribution. Here we provide direct experimental evidence that dissociation and diffusion of subunits are all that is required for redistribution of transducin in rods.
In cones, transducin remains in the OS even under intense illumination (Elias et al., 2004; Kennedy et al., 2004; Coleman and Semple-Rowland, 2005). Rods and cones express different visual pigments and transducins. It has been suggested that a shorter lifetime of light-activated cone visual pigment or activated cone transducin may explain the different distributions of transducin in cones versus rods (Lobanova et al., 2007). We show here that factors influencing the rate of transducin activation and inactivation are not crucial. Instead, transducin in cones does not redistribute in light simply because its subunits do not dissociate even during complete activation.
Materials and Methods
Animals and tissue preparation.
Animal research was conducted in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committee. After the required period of dark or light adaptation, mice were anesthetized with isoflurane and killed by cervical dislocation, and the eyes were enucleated. The eyecups or retinas were prepared by removing the cornea and lens in a dark room under a dissection microscope as described previously (Nair et al., 2005a) and maintained in culture in DMEM supplemented with or depleted of certain ingredients as required by a particular experiment.
Immunofluorescence.
The tissues were fixed with 4% paraformaldehyde, embedded in agar, sliced, and immunostained as described previously (Nair et al., 2005a). Antibodies against rod and cone Gα subunits were from Santa Cruz Biotechnology (Santa Cruz, CA). The secondary antibody was cyanine 3-conjugated affinity-purified donkey anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA). The preparations were then mounted on a coverslip using Antifade reagent (Invitrogen, Carlsbad, CA) and visualized first in the open field with a Nikon (Tokyo, Japan) TE3000 fluorescence microscope, and then the images were taken on a Zeiss (Oberkochen, Germany) LSM 510 laser scanning confocal microscope. Quantification of the relative amount of transducin in the OS and the inner compartments was determined using MetaMorph software (Universal Imaging, Downingtown, PA). Briefly, an area of the visual field was selected (see Fig. 2C) to include the entire photoreceptors length, from the OS to synaptic termini. The total fluorescence, Ft, was measured. Within the same selected area, we recorded the fluorescent signal from the regions corresponding only to the OS or the inner compartments [inner segment (IS), outer nuclear layer (ONL), outer plexiform layer (OPL)], which we designated Fo and Fi, respectively. To normalize to data between independent experiments, we determined the Fi/Ft or Fo/Ft ratio. For every image, three separate areas were selected, and the mean and SD were determined. The exposure time and other parameters were set so that none of the areas within the field was saturated. For rod photoreceptors, analysis of a single confocal plane or the collapsed series of confocal images produced essentially the same results. For image presentation purposes only, so that the relatively dim fluorescence in the inner compartments could be clearly visible in the print, the OS areas had to be saturated. It should be noted here also that, although it may appear that the immunofluorescence intensity in the inner compartments is low compared with the OS, the combined area of IS, ONL, and OPL is larger than that of OS, and so the total amount of the antigen in the inner compartments is higher than it may appear from the visual assessment of image intensity.
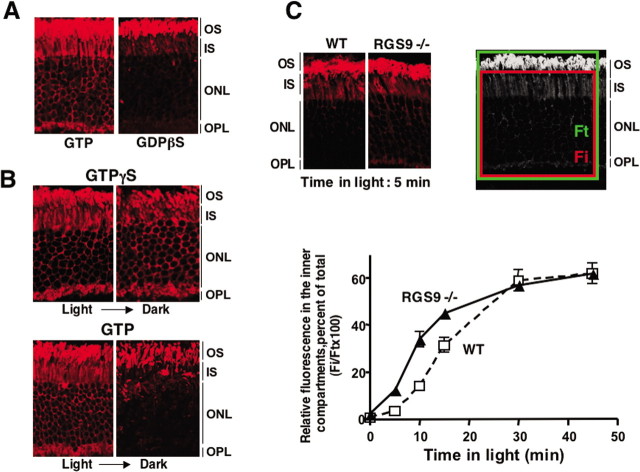
Figure 2.
Relocalization of rod transducin is governed by GTP binding and hydrolysis. A, Retinas were permeabilized in dark and incubated with 100 μm GTP in glucose-free DMEM or 100 μm GDPβS in DMEM supplemented with 1 mm ATP. Retinas were then illuminated, fixed, and probed for rod Gαt localization. B, Two retinas were permeabilized in ambient light in the presence of GTPγS and another pair in the presence of GTP. One retina from each pair was subsequently transferred to the dark for additional 2 h, whereas the control retina remained in light, after which they were fixed and analyzed for rod Gαt localization. These are representative of three independent experiments. C, The eyecups from dark-adapted wild-type and RGS9 knock-out (RGS9−/−) mice were prepared under dim red light and transferred to 500 lux illumination. At the indicated times, the tissue was fixed and subsequently sliced and stained with anti-Gαt antibody. The confocal images were analyzed as described in Materials and Methods. The two side-by-side images exemplify the difference between wild type (WT) and RGS9−/− mice at a single time point (5 min) after the onset of light. The grayscale panel illustrates how the fluorescence in the inner compartments (Fi, red box) and total fluorescence (Ft, green box) are selected to determine the relative distribution of Gαt (Fi/Ft). The LSM software color codes pixels outside the dynamic range of the camera, and so the images subjected to quantitative analysis remained within the linear range of fluorescent intensities. The graph below shows the mean ± SD Fi/Ft ratio for wild-type (white squares) and RGS9−/− (black triangles) mice from three independent experiments.
Ex vivo experiments.
Eyecups or retinas were dissected under the dim red light and incubated in DMEM as described previously (Nair et al., 2005a). ATP depletion was performed by incubating the specimen for 15 min in DMEM free of glucose and sodium pyruvate and supplemented with 2 mm deoxyglucose and 5 mm KCN, pH 7.5. For permeabilization, retinas were incubated with 50 μg/ml α-toxin (Hemolysin; Sigma, St. Louis, MO) for 45 min at room temperature. The nucleotides were added at the concentration of 0.1 mm. GDP, GTP, GDPβS, and GTPγS were from Sigma, and BODIPY (4,4-difluoro-4-bora-3a,4a-diaza-s-indacene fluorophore)–GTPγS was from Invitrogen. The sample incubated with BODIPY–GTPγS was washed for at least 1 min in the medium or isotonic buffer to remove excess fluorescence before fixing.
Biochemical assays.
SDS-PAGE, Western blot, and preparation of bovine and mouse OS were performed as described previously (Nair et al., 2004). For transducin membrane association assay, the OS were resuspended in 100 μl of buffer (20 mm Tris-HCl, pH 7.5, 100 mm KCl, and 1 mm DTT) with or without the nucleotide as required by a specific experiment, incubated in light or dark for 15 min, and centrifuged. The pellet was resuspended to the same volume as the supernatant, and both samples were analyzed by Western blot.
For trypsin protection assay, the OS membranes were incubated with GTPγS, and then trypsin was added at a concentration (100–500 ng), as required by the experiment. After 15 min incubation at 20°C, the reactions were stopped by addition of SDS-containing sample buffer for SDS-PAGE and Western blot analysis.
To study subunit dissociation by size-exclusion chromatography, bovine OS membranes were incubated in light in the presence or absence of GTPγS, and then sodium cholate was added to the final concentration of 1%. The slurry was centrifuged at 40,000 rpm for 30 min, and the extract was resolved on a 1 × 25 cm Superdex 75 column. The column was equilibrated with the buffer containing 1% cholate, and the collected fractions were analyzed by Western blot to detect rod or cone Gαt subunits. In the experiments with mSIRK (myristoylated Gβγ-binding peptide) (see Fig. 8B) and to fractionate the extracts from dissected mouse retinas (see Fig. 5B), 4-mm-diameter columns were used to reduce the volume. The volume of collected fractions was 0.3 ml for the 1 cm column and 60 μl for the 4 mm columns. Blue dextran, hemoglobin, and cytochrome c (Sigma) were used as internal molecular weight standards.
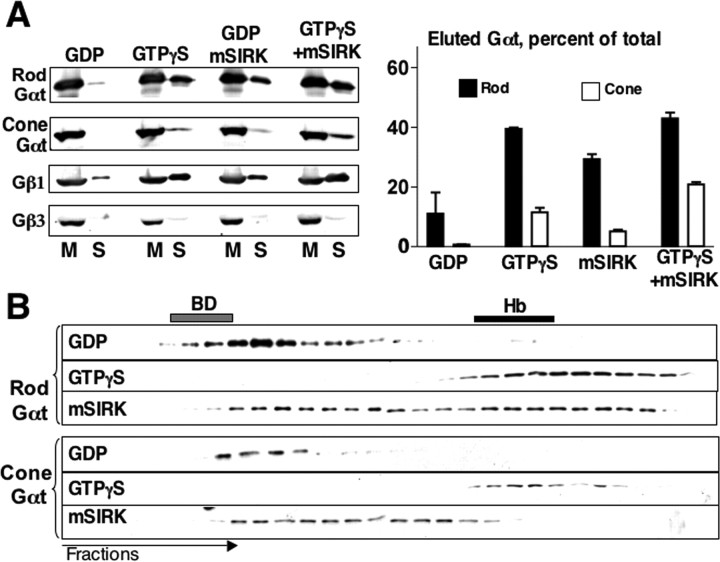
Figure 8.
Subunit dissociation underlies the difference between rod and cone transducins in membrane release and dispersion from OS. A, OS membranes isolated from dark-adapted retinas were resuspended in a buffer containing 100 μm GDP, GTPγS, 100 μm mSIRK, or the mixture of mSIRK and GTPγS. After a 10 min incubation at room temperature in light (1000 lux or sunlight), the suspension was centrifuged to obtain the supernatant (S) and membrane (M) fractions. These samples were analyzed by Western blot with antibodies against rod and cone Gαt, Gβ1, and Gβ3 subunits. The bar graph shows the amount of solubilized Gαt, as percentage of total, as mean ± SD from at least three independent experiments, in which the Western blots were scanned and analyzed by Scion (Frederick, MD) software. B, Bovine OS membranes were treated with 50 μm mSIRK and solubilized with 1% cholate. Fifty microliters of the extract were resolved by gel filtration on a 0.4 × 25 cm Superdex 75 column, which was preequilibrated with the buffer containing 25 μm mSIRK. The distribution of rod and cone Gαt in the collected fractions was determined by Western blot. The extracts from GDP- and GTPγS-treated membranes were resolved on the same column to determine the location of the heterotrimers and dissociated Gα subunits, respectively. BD, Blue dextran; Hb, hemoglobin.
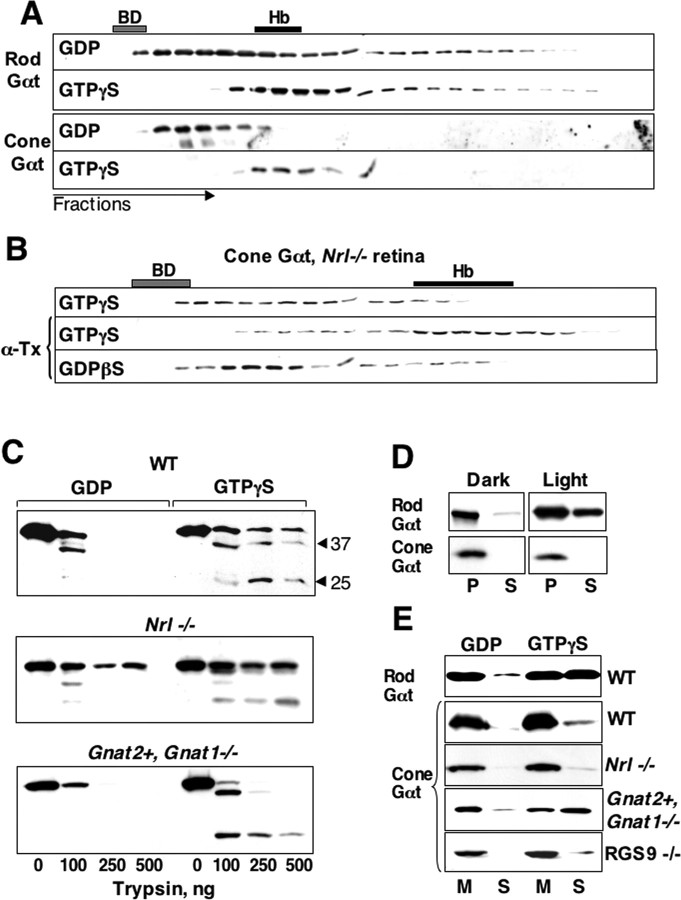
Figure 5.
Cone transducin binds GTPγS but does not dissociate from the membranes. A, Bovine OS membranes were incubated in light with GDP or GTPγS and solubilized with 1% sodium cholate. Blue dextran (BD) and hemoglobin (Hb; ∼60 kDa as the tetramer) were added to the extracts before fractionation on a 1 × 25 cm Superdex 75 size exclusion column. The fractions were probed for rod and cone Gαt subunits. Note that the Stokes radius of the G-proteins is increased relative to the theoretical size because of the bound cholate. B, Freshly dissected retinas from Nrl−/− mice were permeabilized with α-toxin (α-Tx) in the dark in the presence of GTPγS or GDPβS, then illuminated for 5 min at 1000 lux, and rinsed in 5 ml of medium to wash out free nucleotide. In a control testing the integrity of nonpermeabilized cell membranes, α-toxin was omitted from the media before the subsequent incubation with GTPγS. The retinas were then homogenized, solubilized with 1% cholate, and fractionated on a 0.4 × 24 cm Superdex 75 column. C, Limited trypsinolysis assay. The OS membranes from wild-type (WT), Nrl−/−, and Gnat2+,Gnat1−/− mice were incubated with the increasing amounts of trypsin, in the presence of GDP or GTPγS. The reaction was stopped by addition of SDS, and the samples were analyzed by Western blot. D, Whole retinas were dissected from dark-adapted wild-type mice and either kept in the dark or illuminated. The retinas were homogenized and centrifuged, and the pellet (P) and supernatant (S) were probed with antibodies for rod and cone Gα subunits. Shown is the result of a 15 min illumination at 1000 lux; the exposure of the retinas to direct sunlight resulted in the same distribution of cone and rod transducins. E, OS membranes were isolated in the dark from wild-type (WT), Nrl−/−, Gnat2+,Gnat1−/−, and RGS9−/− mice. Membrane suspension was illuminated at 1000 lux by a fluorescent lamp or by direct sunlight in the presence of GDP or GTPγS and then centrifuged to obtain membrane (M) and soluble (S) fractions. The pellet (P) and supernatant (S) were probed with antibodies for rod and cone Gα subunits.
Retinoid analyses.
Eyecups prepared in culture medium in darkness were exposed to 5 min of additional darkness, 5 min of direct sunlight, or 5 min of illumination from a hand-held UV illuminator placed 0.5 cm directly above the eyecup in a culture dish. The power output of the UV illuminator is described in the text. After 5 min of dark or illumination, eyecups were immediately frozen on dry ice. All subsequent steps were performed in dim red light. The frozen eyecups (four per sample) were thawed, homogenized, extracted, and analyzed by normal-phase HPLC as described previously (Garwin and Saari, 2000). An internal standard, Etretinate (all-trans-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7,-dimethyl-2,4,6,8-nonatetraen-1-ethyl ester), was used as described previously (Saari et al., 2002).
Analysis of N-myristoylation.
Samples for mass spectrometry analysis were prepared using the method developed by the Crabb laboratory (http://www.lerner.ccf.org/eye/crabb/protocols/). The membrane and soluble fractions obtained from bovine or mouse OS were resolved by SDS-PAGE and stained by Coomassie blue. The bands corresponding to rod or cone Gα subunits were excised, washed with ethanol/water solutions, and dried using a Speedvac. The dried gel slices were rehydrated overnight in a Tris-HCl, pH 7.4, buffer containing 0.01 mg/ml trypsin. The hydrolizate was extracted in a 60:40 acetonytryl solution, separated from the residual gel, lyophilized, and stored at −20°C. They were then dissolved in water (HPLC purity) with 5–10% of acetonitrile (HPLC purity) and subjected to reverse-phase liquid chromatography followed by mass spectrometry as described previously (Kennedy et al., 2001; Lee et al., 2002). The synthetic N-myristoylated (C14:0) peptides and N-terminal tryptic peptides (GAGASAEEK and GSGISAEDK for rod and cone Gαt, respectively) were purchased from Alpha Diagnostic (Austin, TX) and were used as standards to optimize the HPLC gradient, mass spectrometer settings, and collisionally induced dissociation (CID) tandem mass spectrometry conditions. Selected ion monitoring strongly improves the detection sensitivity of small amount of peptides in a digestion mixture. Data from the mass spectrometer were filtered through narrow mass windows (1 Da) centered on the predicted masses of the various fatty acylated N-terminal peptides of the rod and cone transducin being analyzed. To obtain relative amounts of the various fatty acyl species in a given sample, we integrated the area under peaks confirmed by CID to be transducin N termini. The relative amount of each species was calculated as a fraction of the total amount of rod or cone transducin peptide in membrane-bound or GTPγS-solubilized fraction.
Results
Transducin redistribution does not require ATP, but it is controlled by guanine nucleotides
In a previous study, we deprived mouse retinas of energy to show that ATP is not needed for redistribution of arrestin. We depleted ATP from freshly dissected mouse retinas by incubating them in glucose-free medium in the presence of deoxyglucose and KCN. ATP depletion did not interfere with redistribution of arrestin, but it blocked light-induced redistribution of rod transducin (Nair et al., 2005a). Initially, the significance of that finding was unclear. ATP could be needed to either support active transport or make GTP to activate transducin.
To distinguish these roles of ATP, we supplied GTP analogs to the cytoplasm of ATP-depleted photoreceptors. The analogs were delivered into photoreceptors using staphylococcal α-toxin, a polypeptide that makes pores that allow molecules <2000 Da to enter cells (Bhakdi et al., 1996; Otto-Bruc et al., 1998). Figure 1A shows that α-toxin allows entry of a fluorescently tagged nonhydrolyzable analog of GTP, BODIPY-GTPγS, but proteins such as rod transducin and recoverin do not escape via the α-toxin pores. Green fluorescent protein (GFP) expressed in rods of a transgenic mouse also is retained in the cells treated with α-toxin. BODIPY-GTPγS accumulates in photoreceptors when they are illuminated (Fig. 1B) evidently because of the rhodopsin-catalyzed exchange of endogenous GDP for the fluorescent nucleotide. Most importantly, BODIPY-GTPγS treatment in light induces dispersion of rod transducin α and β subunits to all compartments of the photoreceptor cell even in ATP-depleted rods (Fig. 1C). This shows that transducin must be activated for redistribution to occur but that ATP and active transport are not required.
Figure 1.
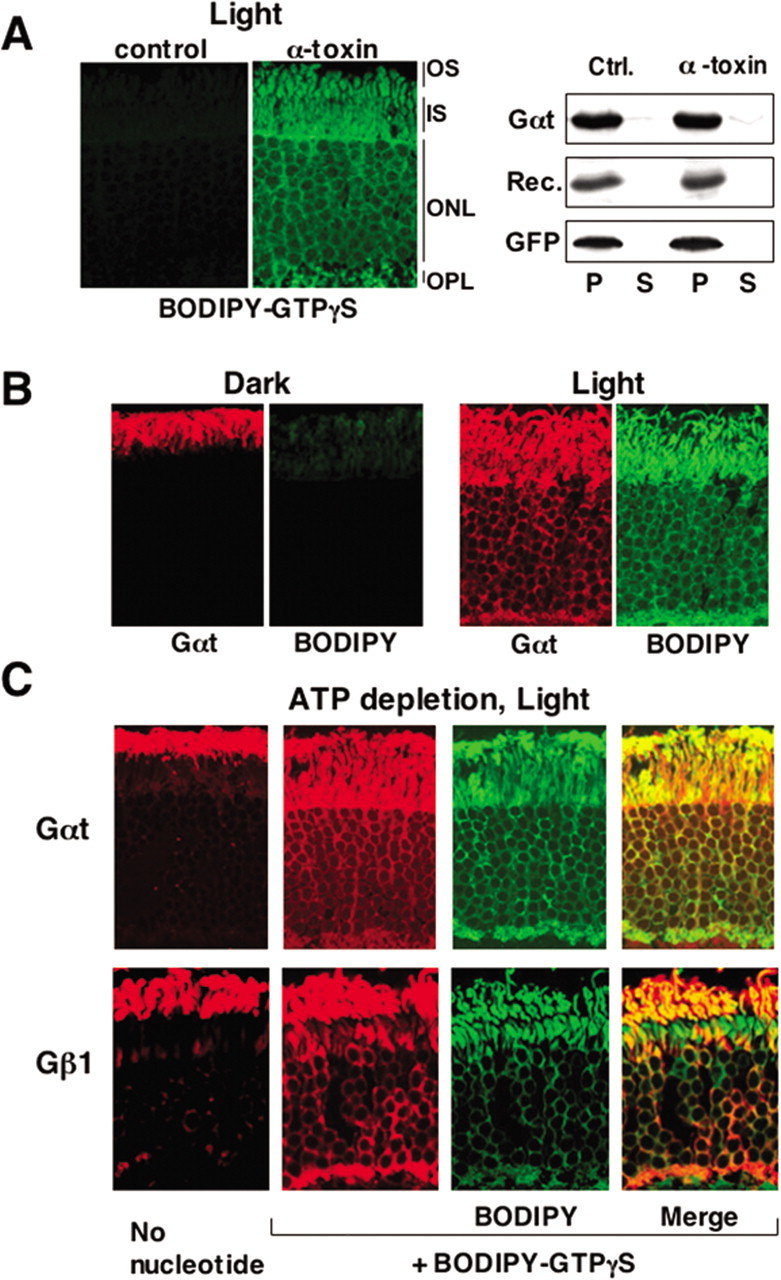
Light-dependent movement of rod transducin does not require ATP. A, Mouse retinas were incubated in DMEM in ambient light in the presence of 100 μm BODIPY-GTPγS with or without α-toxin, then fixed, sectioned, and visualized by fluorescence microscopy as described in Materials and Methods. A parallel set of retinas was tested for the potential loss of protein from the permeabilized cells. After the incubation with α-toxin, the retinas were separated from the medium by centrifugation at 500 × g for 30 s. The supernatant (S) containing the medium and the pellet (P) were analyzed by Western blot using antibodies to Gαt (39 kDa) and recoverin (24 kDa). To test the distribution of a marker cytosolic protein, GFP (27 kDa), retinas from GFP-expressing mice were also subjected to this assay. B, Retinas were permeabilized with α-toxin in the presence of BODIPY-GTPγS in the dark and then either illuminated or left in the dark for additional 30 min. Rod Gαt immunofluorescence is shown in red, and BODIPY fluorescence is green. C, Retinas were permeabilized with α-toxin in glucose-free DMEM supplemented with 2 mm deoxyglucose and 5 mm KCN to deplete ATP. They were then incubated in the presence or absence of BODIPY-GTPγS and subsequently illuminated for 30 min at 500 lux before fixing. Localization of rod transducin subunits and BODIPY-GTPγS was determined by fluorescence microscopy.
Nonfluorescent GTP or GTPγS exerted a similar effect on rod transducin localization (Fig. 2A,B), and GDPβS blocked light-induced redistribution (Fig. 2A). Both GTP and GTPγS dispersed transducin from the OS in light. However, return to the OS after the subsequent incubation in darkness only occurred when GTP was used. GTPγS prevented resequestration of transducin in the OS in darkness (Fig. 2B). These results show that transducin must return to its GDP-bound state to become once again sequestered within the OS. To confirm this conclusion in live animals, we studied the kinetics of light-induced transducin redistribution in mice lacking RGS9, a member of regulator of G-protein signaling (RGS) family that acts as the GTPase-activating protein for transducin (He et al., 1998; Chen et al., 2000). We expected that activated transducin would accumulate faster than normal because the rate of its inactivation in RGS9−/− rods is slowed. Consistent with this, redistribution of rod transducin occurs faster in RGS9−/− rods than in wild-type retinas (Fig. 2C).
Cone transducin in wild-type and genetically manipulated photoreceptors
In agreement with previous observations (Elias et al., 2004; Kennedy et al., 2004; Coleman and Semple-Rowland, 2005), we found that light does not stimulate redistribution of cone transducin in cones (Fig. 3A). In retinas lacking the Nrl (neural retina leucine zipper) transcription factor (Nrl−/−), all photoreceptors have morphological, biochemical, and physiological characteristics of cones (Mears et al., 2001; Daniele et al., 2005). We examined cone transducin in Nrl−/− cones and found that it does not redistribute to inner compartments in light, similar to its behavior in wild-type cones (Fig. 3, compare A, B). Cones express higher levels of RGS9 than rods (Cowan et al., 1998). Because RGS9 attenuates the relocalization of rod transducin (Fig. 2C), we hypothesized that the high level of RGS9 activity in cones might be responsible for the permanent sequestration of cone transducin in the OS of cones. However, the absence of RGS9 does not alter the localization of cone transducin in cones (Fig. 3C). Next, we tested whether the irreversible binding of GTPγS can cause redistribution of cone transducin in cones. We found that GTPγS introduced into illuminated cones using α-toxin does induce ∼10% of cone Gαt to move to the cone inner compartments (Fig. 3D).
Figure 3.
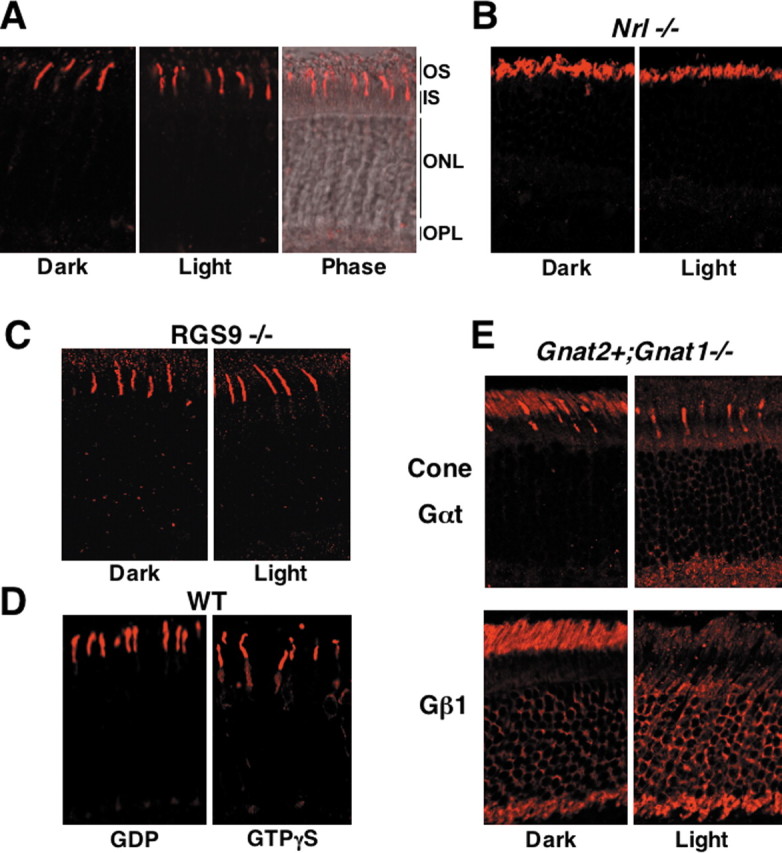
Behavior of cone Gαt in cones and rods. A, Wild-type mice were dark adapted for 12 h and killed, either immediately (Dark) or after an exposure to light (Light). Retinal sections were stained with the antibody against the α subunit of cone Gαt. The right shows the merged image with the phase contrast of the same (light-adapted) sample. Shown are the results obtained during 3 h illumination at ambient light (500 lux). An increase in light intensity to up to 100,000 lux (direct sunlight, dilated eyes) did not affect the distribution of cone transducin. B, Localization of cone Gαt in the dark- and light-adapted Nrl−/− mice. C, Localization of cone Gαt in the RGS9−/− mice. D, Retinas of wild-type mice were permeabilized with α-toxin in the dark in the presence of GDP or GTPγS (as in Figs. 1, 2), illuminated for 10 min at 5000 lux and then for 30 min at 500 lux, and subsequently fixed and stained for cone Gαt. Exposure of retinas to direct sunlight for up to 30 min or irradiation with 385 nm light source from a distance of 0.5 cm resulted in the similar distribution of cone Gαt. E, Localization of cone Gαt and rod Gβ subunit (Gβ1) in the dark- and light-adapted (500 lux, 30 min) Gnat2+,Gnat1−/− mice.
The dramatic difference in spatial redistribution of cone and rod transducins could be determined by either the type of Gαt protein or the context of the cell in which it is expressed. To resolve this, we used Gnat2+,Gnat1−/− (for guanine nucleotide binding protein, α transducin) mice in which cone Gαt is expressed in rods in a rod transducin-deficient background (Calvert et al., 2000). In striking contrast to its behavior in cones, cone Gαt undergoes light-dependent redistribution in Gnat2+,Gnat1−/− rods (Fig. 3E). Furthermore, the presence of cone Gαt in rods supports the light-dependent translocation of endogenous rod Gβγ. We conclude that the ability of transducin to change its distribution is determined by the cellular environment in the rod rather than by intrinsic properties of the α subunit.
Activation of cone transducin
Cones are less sensitive to light than rods, so the apparent inability of cone transducin to effectively redistribute in light could be explained by either inefficient activation of cone opsin or inefficient receptor–G-protein coupling. Nrl−/− retinas are dominated by UV opsin, so we used two types of light sources that have a strong UV output to ensure that cone transducin was fully activated in these experiments. One source was a 360 nm UV illuminator, and the other was direct sunlight. The UV illuminator was placed within 0.5 cm of the dissected eyecups. Its output peaked at 350 ± 20 nm, and its power was 1.5 mW/cm2. This corresponds to ∼3 × 107 350 nm photons per seconds per square micrometer. We evaluated the extent of bleaching of cone opsin using retinas from Nrl−/− mice exposed to the UV illuminator. Because the spectral characteristics of UV opsin and all-trans retinal are very similar, it was necessary to evaluate the extent of UV pigment bleaching by analyzing retinoids by HPLC (Garwin and Saari, 2000). Approximately 75% of the 11-cis-retinal chromophore in Nrl−/− retinas is converted into all-trans retinal, retinol, or retinyl esters after 5 min of exposure to either the UV illuminator or direct sunlight (Fig. 4A). We also evaluated the extent of cone pigment activation by measuring the extent to which the UV opsin C terminus is phosphorylated during its active lifetime. We measured the extent of phosphorylation of the UV opsin C terminus (Lee et al., 2002) after exposure to 1, 5, and 10 min of UV illumination or sunlight. With both sources of illumination, 30–35% of UV opsin was phosphorylated with one, two, or three phosphates in a steady state reached within 1 min exposure to the illumination (data not shown). These results show that, in the steady-state cycle of activation and inactivation in Nrl−/− cones, 75% of cone pigment is bleached and at least 30% have undergone phosphorylation during its active lifetime. Cone transducin did not relocalize under any of the tested illumination conditions.
Figure 4.
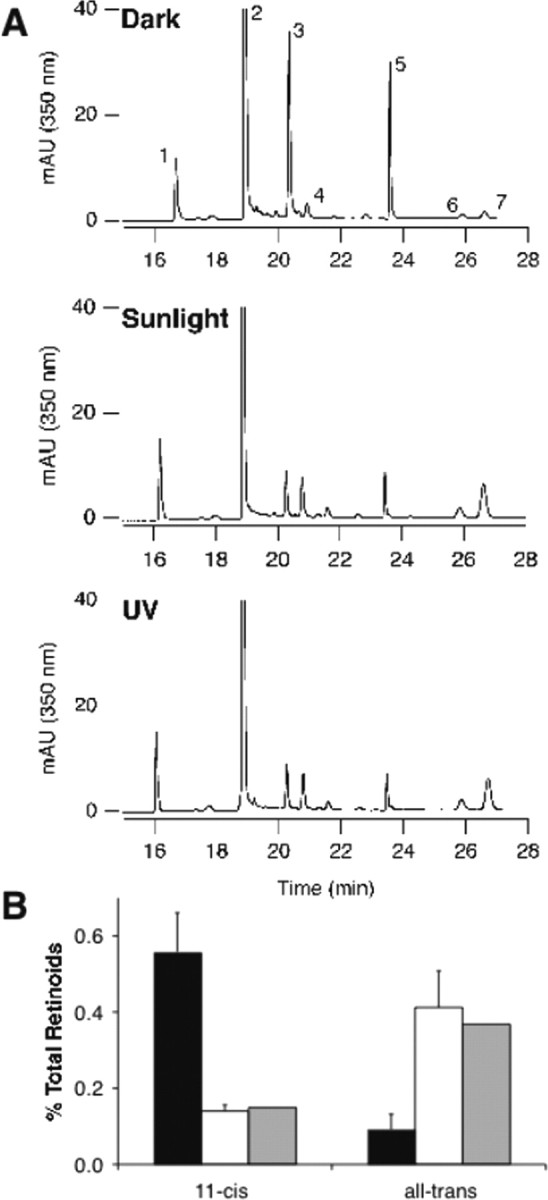
Retinoid analysis in Nrl−/− retinas under UV or sunlight illumination. Nearly complete bleaching of cone visual pigment in Nrl−/− retinas. A, HPLC traces showing retinoid analyses from three conditions. Eyecups were prepared from dark-adapted mice and exposed to 5 min darkness, 5 min direct sunlight, or 5 min of illumination from a 350 nm UV lamp. Retinoids were extracted and analyzed by HPLC (Garwin and Saari, 2000). Note the significant reduction of peaks 3 and 5 (syn and anti-11-cis-retinal oxime) and the corresponding increases in all-trans retinoids (peaks 4, 6, 7). Component 1, retinyl esters; 2, internal standard; 3, syn 11-cis-retinal oxime; 4, syn all-trans-retinal oxime; 5, anti-11-cis-retinal oxime; 6, all-trans-retinol; 7, anti-all-trans-retinal oxime. B, Quantification of the results shown in A. “11-cis-” refers to peaks 3 and 5, and “all-trans” refers to peaks 4, 6, and 7. The black bars represent dark-adapted eyecups (n = 2 samples, four eyes each); the white bars represent eyecups illuminated with sunlight (n = 2 samples, 4 eyes each), and the gray bars represent eyecups illuminated with UV light (n = 1 sample, 4 eyes).
To establish whether the high level of cone opsin stimulation under our illumination conditions translates into efficient activation of cone transducin, we also directly evaluated cone transducin activation. We used GTPγS binding as an integrator of activation events to evaluate the extent of cone transducin activation under the same experimental conditions we used to determine cone transducin localization (Fig. 3C). Size-exclusion chromatography can be used to detect a reduction in the apparent molecular weight of a G-protein that indicates dissociation of activated Gα and Gβγ subunits. Activated cone transducin binds tightly to membranes (Fig. 5D,E). Therefore, as with all other heterotrimeric G-proteins, except rod transducin, analysis of cone transducin by chromatography requires solubilization with mild detergent, typically 1% sodium cholate (Northup et al., 1983). Dissected retinas from Nrl−/− mice were treated with α-toxin in the presence of either GDP or GTPγS, exposed to direct sunlight, rinsed to remove unbound nucleotide, and then solubilized with cholate. We then analyzed the extracts by gel filtration. Our results showed a dramatic reduction in the apparent molecular weight of GTPγS-treated cone transducin characteristic of fully activated G-proteins (Fig. 5B). When the retinas were incubated with GTPγS in the absence of α-toxin or when they were incubated with GDP instead of GTPγS, or when they were incubated in darkness (data not shown), cone transducin remained heterotrimeric. In another set of experiments, we also compared the behavior of rod and cone transducin in isolated bovine OS membranes. The membranes were illuminated in the presence of either GDP or GTPγS, extracted with 1% cholate, and fractionated by gel filtration (Fig. 5A). Treatment with GTPγS caused a complete shift of the elution volume of both rod and cone transducin, demonstrating that both G-proteins bound GTPγS. The similarity of the results with permeabilized retinas and with isolated membranes indicates that, in both preparations, the bulk of cone transducin was stimulated to bind GTPγS.
We also used an independent method, a detergent-free protease protection assay, to confirm that GTPγS binds to cone transducin. Membranes from wild-type, Nrl−/−, or Gnat2+,Gnat1−/− mice were incubated with GDP or GTPγS in light and subsequently treated with increasing concentrations of trypsin (Fig. 5C). Cone Gαt undergoes proteolytic fragmentation, consistent with it being bound to GTPγS.
Altogether, our analyses of cone opsin activation (Fig. 4) and the nucleotide-binding state of the G-protein (Fig. 5) show that cone transducin is fully activated in our experiments, yet it remains sequestered in the OS.
Membrane association of rod and cone transducins
We reasoned that, if rod transducin moves between the cell compartments by diffusion, the prerequisite for its exit from the OS would be release from membrane to cytosol. To account for the different behaviors of cone and rod transducins, rod transducin would dissociate from membranes more effectively than cone transducin. To test this idea, we compared the distributions of rod and cone transducins between soluble and particulate fractions from homogenates of either acutely cultured live mouse retinas (Fig. 5D) or purified OS membranes (Fig. 5E). As expected, rod transducin was solubilized readily from either whole retina or OS membranes by GTPγS and light. In contrast, the bulk of cone transducin remained in the particulate fractions in wild-type, Nrl−/−, and RGS9−/− photoreceptors (Fig. 5E). Importantly, we found that cone Gαt can be eluted readily by GTPγS from membranes prepared from Gnat2+,Gnat1−/− mice. This is consistent with the ability of cone transducin to redistribute when expressed in rods (Fig. 3E). Thus, the ability of cone transducin to leave the OS correlates with its ability to dissociate from membranes.
N-acylation of rod and cone Gα subunits
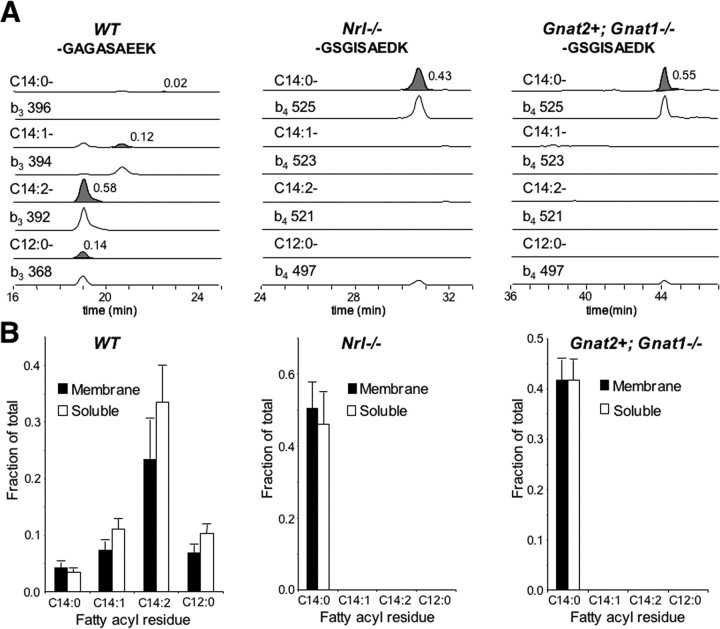
Unlike other heterotrimeric G-proteins, which are acylated at their N termini with a myristoyl (C14:0) residue, rod transducin is acylated heterogeneously with C14:0, C14:1, C14:2, and C12:0 fatty acyl residues (Kokame et al., 1992; Neubert et al., 1992). We hypothesized that the difference between membrane association of cone and rod transducins could be caused by differences in their lipid modifications. To examine N-acylation of rod and cone Gα subunits, we isolated them using SDS-PAGE, digested the excised protein bands with trypsin, and subjected the N-terminal peptides to reverse-phase chromatography and tandem mass spectrometry (Fig. 6) (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). We eluted rod transducin with light and GTPγS and compared the fatty acid compositions of the soluble and membrane-bound forms of Gαt. Both the soluble and membrane fractions contained all four species of rod Gαt: C14:0, C14:1, C14:2, and C12:0. There was only a slight enrichment of the membrane-bound Gαt with more hydrophobic acyl groups and only a slight enrichment of the soluble Gαt with the less hydrophobic species. To obtain sufficient amounts of cone Gαt, we took advantage of its relatively high expression in the Nrl−/− and Gnat2+,Gnat1−/− animals. We found that cone Gαt is modified exclusively with C14:0 in Nrl−/− mice. Surprisingly, cone Gαt in Gnat2+,Gnat1−/− retinas, in which it can be solubilized, is also modified only by C14:0. These findings show that (1) the distribution of fatty acids is determined by the amino acid sequence of Gαt and not by its environment and (2) the membrane affinity of Gαt in cones versus rods is determined by a factor other than differential N-myristoylation.
Figure 6.
Distinct N-acylation of rod and cone transducin α subunits. OS membranes were isolated from dark-adapted wild-type (WT), Nrl−/−, and Gnat2+,Gnat1−/− mice, treated with 100 μm GTPγS, and centrifuged to obtain the soluble and membrane fractions. The fractions were resolved on SDS-PAGE and stained with Coomassie blue. The bands corresponding to rod and cone Gαt were excised, and the slices were digested with trypsin and extracted. The resulting mixture of peptides was analyzed by HPLC and mass spectrometry to determine the amount of the N-acylated N-terminal peptides. A, Ion chromatograms monitoring narrow mass windows (1 Da) corresponding to rod and/or cone transducin N-terminal peptides with fatty acyl residues from the soluble fractions obtained from wild-type, Nrl−/−, and Gnat2+,Gnat1−/− retinas, respectively. The peptides were detected in their singly charged state with the following mass-to-charge ratio: rod Gαt, C14:0-GAGASAEEK, 1029.5; C14:1-GAGASAEEK, 1027.5; C14:2-GAGASAEEK, 1025.4; C12:0-GAGASAEEK, 1001.5; cone Gαt, C14:0-GSGISAEDK, 1072.5; C14:1-GSGISAEDK, 1070.5; C14:2-GSGISAEDK, 1068.5; C12:0-GSGISAEDK, 1044.5. Each peptide was sequenced by CID to confirm its identity (ion chromatograms for b3 or b4 shown above). The y-axis for each chromatogram represents the relative abundance of each peak and is normalized to the amplitude of the maximum signal in the whole ion chromatogram (molecular and fragment ion chromatograms have individual maxima). The x-axis represents the elution time from the C18 reversed-phase column. The numbers to the right of each peak represent the relative fraction of each species calculated by integrating the area under each peak. B, The four fatty acyl N-terminal transducin peptides in membrane and soluble fractions from wild-type, Nrl−/−, and Gnat2+,Gnat1−/− retina were quantified by integrating the areas under peaks corresponding to the peptides. The level of each species is expressed as the fraction of the rod or cone Gα peptide detected in the membrane (M) or soluble (S) fractions, respectively. Each data point is the average of at least four separate determinations. The error bars represent SD.
Subunit dissociation facilitates dissociation of transducin from membranes and its dispersion throughout photoreceptor cells
The tight association of cone Gαt with membranes could be attributable to its association with a cone-specific membrane protein. An obvious candidate for this protein would be RGS9, which is more abundant in cones than in rods (Cowan et al., 1998), but our finding that cone Gαt still associates with membranes in RGS9−/− retinas (Fig. 5E) rules out this possibility. Another binding partner that plays a major role in membrane association of Gαt is the Gβγ complex. To dissect the role of Gβγ in membrane binding and redistribution of transducin, we performed a set of experiments in which dissociation of Gα and Gβγ subunits was facilitated by synthetic myristoylated peptides, mSIRK and mSIGK. These novel reagents interfere with G-protein subunit association by binding directly to Gβ at the same site in which Gβ binds to Gα (Davis et al., 2005; Bonacci et al., 2006). The effects of these two peptides in our study were indistinguishable. Therefore, we will refer to either peptide as “mSIRK” in the following discussion. As a negative control in our studies, we used a mutant form of mSIRK, L9A, which has a single amino acid substitution and is ineffective at binding to Gβγ.
Addition of mSIRK alone to permeabilized retinas induced dispersion of rod Gαt and Gβγ even in the absence of nucleotides and in darkness (Fig. 7A). In contrast, mSIRK alone does not affect localization of cone Gαt in wild-type or Nrl−/− mice in light or darkness. Remarkably, the combination of mSIRK and GTPγS causes cone Gαt to disperse and equilibrate throughout the cytoplasm of wild-type (Fig. 7B,C) and Nrl−/− (Fig. 7E) cones. Up to 60% of total cone Gαt immunofluorescence was detected in the inner compartments in the presence of GTPγS and mSIRK, whereas GTPγS alone or in combination with L9A resulted in translocation of no more than 15% (Fig. 7B,C). We also monitored the localization of the cone Gβγ complex Gβ3γ8 and found that Gβ3 remained in cone OS under all tested conditions, in both wild-type and Nrl−/− retinas (Fig. 7D,E).
Figure 7.
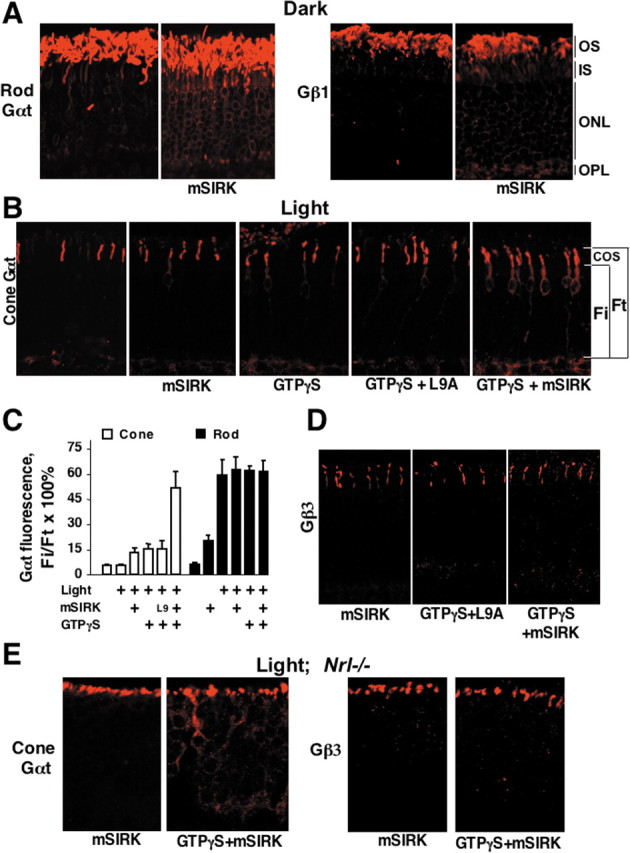
Translocation of rod and cone transducins is facilitated by the synthetic Gβ-binding peptide mSIRK. A, Dark-adapted retinas were permeabilized with α-toxin and incubated in the dark in the presence or absence of 50 μm mSIRK. After 1 h, the retinas were fixed, and the localization of rod Gαt and Gβ1 was determined. B, Dark-adapted retinas were permeabilized with α-toxin and treated with 50 μm mSIRK or its inactive mutant (L9A) peptide in the presence or absence of 100 μm GTPγS. Retinas were then exposed to sunlight for 15 min, fixed, sectioned, and stained with antibodies against cone Gαt. C, Quantification of the data from a series of experiments including one shown in B. Rod or cone Gαt immunofluorescence in the entire photoreceptor (Ft) and immunofluorescence from the inner compartments (Fi) were determined as described in Figure 2 and Materials and Methods. The average Fi/Ft ratio (mean ± SD) in each experiment was determined within three areas of the retina. The data were collected in at least six independent batches of retinas (groups of mice). Retinas were dissected under dim red light and permeabilized in the presence of GTPγS, mSIRK, L9A mutant, or mSIRK with GTPγS as indicated. For L9A plus GTPγS, n = 3. The concentration of mSIRK or L9A was 50 μm. Light intensities varied from 500 lux to direct sunlight, providing identical results. Control permeabilized retinas were kept in the dark. D, Localization of cone Gβγ (Gβ3 immunoreactivity) in permeabilized wild-type mouse retinas treated with mSIRK, L9 with GTPγS, and mSIRK with GTPγS, in light. This is representative of three independent experiments. E, Retinas of Nrl−/− mice were permeabilized and treated with mSIRK or mSIRK with GTPγS, in light (1000 lux or direct sunlight for 5 or 30 min produced identical results). Localization of cone Gαt and Gβ3 was determined by immunofluorescence microscopy.
Consistent with its effects on subcellular localization of rod and cone subunits, mSIRK promoted elution of rod Gα and Gβγ subunits from the membrane in the absence of GTPγS and facilitated solubilization of cone Gαt but not cone Gβ3 (Fig. 8A). The effects of mSIRK on subunit dissociation of rod and cone G-proteins were consistent with the effects of this peptide on transducin localization in cells and membrane association. In the gel filtration assay, mSIRK caused the shift of at least 50% of the GDP-bound rod transducin toward the size corresponding to the dissociated subunits (Fig. 8B). In contrast, mSIRK caused only some broadening of the cone transducin peak, so that the bulk of cone transducin remained in its heterotrimeric form.
In summary, we found that (1) subunit dissociation, not GTP binding per se, is the event that determines localization of transducin and (2) the α and βγ subunits of cone transducin associate with each more tightly than the subunits of rod transducin.
Discussion
This study shows that light-induced redistribution of transducin does not require active transport. It also answers two fundamental questions about transducin localization: (1) why does the distribution of transducin in rods change in light versus darkness?, and (2) why does the distribution of transducin in cones not depend on light?
Three experimental approaches were key to this study. (1) To deplete photoreceptors of energy, we incubated retinas without glucose and with KCN. This treatment causes loss of essentially all ATP in the retina, and it blocks rhodopsin phosphorylation (Nair et al., 2005a), a biochemical reaction with a low Km (∼2 μm) for ATP (Palczewski et al., 1988). (2) We permeabilized photoreceptors with α-toxin. α-Toxin-treated cells take up nucleotides (BODIPY-GTPγS) and retain proteins (transducin and recoverin) (Fig. 1). (3) To promote dissociation of transducin subunits in photoreceptors, we used mSIRK, a small peptide that competes with Gα for a binding site on Gβ (Figs. 7, 8).
The dissociate/disperse model
Classic in vitro studies of phototransduction suggest a simple explanation for the light-dependent localization of transducin in rods. Activation lowers the affinity of transducin α for βγ (Fung et al., 1981), and it weakens the affinity of rod transducin for membranes (Kuhn, 1980). Recent evidence indicates transducin subunits also dissociate under physiological conditions (Sokolov et al., 2002).
It is reasonable to propose (Calvert et al., 2006) a link between the dissociation state of transducin and its distribution. Immediately after activation, the subunits dissociate, leave OS membranes, and enter the cytoplasm. Cytosolic proteins equilibrate quickly throughout all compartments of a photoreceptor (Peet et al., 2004; Nair et al., 2005). If all of the transducin in a rod is activated, e.g., in photoreceptors containing GTPγS, its subunits will dissociate and disperse so that cytoplasmic concentrations of transducin in all compartments of the cell become equal.
The aim of our study was to test this hypothesis. We simplified the system by stabilizing either the active or inactive forms of transducin using specific guanine nucleotides introduced into photoreceptors permeabilized by α-toxin. GTPγS stabilizes the activated state, and GDPβS stabilizes the inactive state. GTPγS causes transducin to disperse from the OS (Figs. 1, 2), and GDPβS keeps it sequestered in the OS (Fig. 2A). These findings provide simple and direct experimental support for the dissociate/disperse hypothesis. They also provided us with an experimental framework to investigate the reason for the different localization of activated transducin in rods and cones.
Subunit association, membrane binding, and localization
Transducin localization in photoreceptors correlates with its membrane affinity. Rod transducin readily detaches from membranes (Figs. 5E, 8A) and disperses throughout the cell. Cone transducin in normal and Nrl−/− cones remains bound to membranes (Fig. 5D,E, 8A), and it stays mostly in the OS (Figs. 3, 7), even when fully activated (Figs. 4, 5A–C). The different membrane affinities and distributions of transducins in rods versus cones could reflect differences inherent in either the type of G-protein or the type of cell in which it is expressed. To resolve this, we examined membrane binding and localization of cone Gαt expressed in rods in place of rod Gαt. We found that activated cone Gαt in rods has a weak affinity for membranes, and it disperses throughout the rod cell when activated (Figs. 3E, 5E). Therefore, the ability to disperse transducin is a property of the cellular environment, not the type of Gαt. What could be the factors responsible for the strikingly different behavior of Gαt in rods versus in cones?
Lipid modification of Gαt
The affinity of a G-protein for membranes is influenced by N-acylation of its α subunit (Bigay et al., 1994; Wang et al., 1999; Chen and Manning, 2001). Rod transducin is modified by a mixture of fatty acyl residues, including C14:2, C14:1, and C12:0. We hypothesized that Gαt in cones is modified only with the more hydrophobic C14:0, but, when expressed in rods, it is acylated with the less hydrophobic residues. Indeed, cone Gαt in Nrl−/− cones is modified exclusively by C14:0 (Fig. 6) (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). This appeared to be a reasonable explanation for the enhanced membrane affinity of activated cone Gαt. However, two findings argue against that explanation. First, C14:0 rod transducin also partitions to the cytosol and is only slightly enriched in membranes (Fig. 6). Second, cone Gαt is still modified exclusively by C14:0 when expressed in rods (Figs. 3, 5, 6). These findings show that the type of N-terminal acylation on Gαt is a characteristic of the type of transducin, and it does not determine either the affinity of Gαt for membranes or the localization of Gαt.
Prenylation of Gγ subunits
Prenylation of Gγ influences the localization of rod Gβγ. Replacing the farnesyl (C15) modification normally present at the C terminus of the rod Gγ1 with a geranylgeranyl (C20) residue prevents light-induced dispersal of Gβ1γ1 from the OS (Kassai et al., 2005). However, the type of modification on Gγ1 does not affect the distribution of rod Gαt in rods (Kassai et al., 2005). It is, therefore, unlikely that differences in prenylation of rod Gγ1 and cone Gγ8 are responsible for the different distributions of cone Gαt when expressed in rods versus when it is expressed in cones.
The type of Gβγ complex
The Gβγ complex in rods is assembled from Gβ1 and Gγ1. In cones, it is Gβ3 and Gγ8 (Fung et al., 1992; Ong et al., 1995). A careful analysis of the data in Figures 7 and 8 reveals a relationship between localization of activated Gαt and its affinity for βγ. When either rod or cone Gαt is fully and stably activated by GTPγS, an equilibrium must occur in which the cytosolic concentration of soluble Gαt is equal in all compartments of the photoreceptor cell (Figs. 1–3). This equilibrium is obvious in rods (Fig. 7C), but, in cones under these conditions, there is an excess of cone Gαt in the outer segment (Fig. 7C). We hypothesized that this excess must represent a complex of GTPγS-activated cone Gαt with Gβ3γ8 bound to membranes (Fig. 7D). To test this, we used mSIRK, a peptide that promotes G-protein dissociation (Smrcka and Scott, 2002; Davis et al., 2005). If the excess cone Gαt in the OS is associated with Gβγ, mSIRK, a peptide that competes with Gα for binding to Gβγ, would disrupt the complex and Gαt would disperse. If excess cone Gαt were in the OS for a different reason, it would not be affected by mSIRK. mSIRK does disperse excess cone Gαt from the OS (Fig. 7B,C,E). Therefore, the excess cone Gαt in the OS does represent the fraction of GTPγS-bound cone Gαt associated with Gβ3γ8 (Figs. 7D, 8A). In rods, the equilibrium state is different: nearly all Gαt–GTPγS is dissociated from Gβ1γ1 so the subunits are dispersed throughout the rod. Consistent with this, mSIRK does not affect the distribution of activated rod Gαt (Fig. 7C).
Transducin redistribution
In darkness, the membrane-rich OS of rods and cones retain nearly all of the inactive heterotrimeric transducin (supplemental Fig. S4, available at www.jneurosci.org as supplemental material). Light stimulates transducin to bind GTP and assume its active conformation. The affinity of activated rod Gαt for its partner, Gβ1γ1, is weak. Dissociated rod Gαt and Gβγ subunits have a low affinity for membranes so they disperse throughout the cytoplasm of all compartments of the rod. The affinity of activated cone Gαt for its partner Gβ3γ8 is strong. Activated cone transducin essentially remains heterotrimeric. It is retained in the OS because the heterotrimer has a high affinity for membranes and because Gβ3γ8 itself has a high membrane affinity.
In a photoreceptor operating in constant light under physiological conditions, activation of transducin is neither complete nor stable. Instead of equilibrium, a steady state is established throughout the photoreceptor. The subcellular distribution of rod G-protein in this steady state and the rate at which this state is reached depend on relative rates of activation, deactivation, and diffusion. Impairment of GTP hydrolysis, by GTPγS binding, by RGS9 gene inactivation, or by the Q200L mutation in Gαt (Kerov et al., 2005a), favors the GTP-bound dissociated and dispersed state. Accordingly, GDPβS (Fig. 2A) or overexpression of RGS9 (Lobanova et al., 2007) shifts transducin toward the associated state, which concentrates in the OS. Transducin migration throughout the rod cell requires tens of minutes (Sokolov et al., 2002; Elias et al., 2004), which is much slower than unimpeded diffusion of a soluble protein in rods (Nair et al., 2005a) and cones (supplemental Fig. S3, available at www.jneurosci.org as supplemental material). Transducin binding partners, such as phosducin (Sokolov et al., 2004), centrin (Wolfrum et al., 2002), Leu-Gly-Asn repeat (LGN) protein (Kerov et al., 2005b; Nair et al., 2005b), and cytoskeletal elements (Peterson et al., 2005), may act as diffusion barriers that impede relocalization.
G-protein dissociation
Dissociation of rod transducin has served for many years as a general model for G-protein activation (Gilman, 1987; Levitzki and Klein, 2002). We have shown that the propensity of activated cone transducin to dissociate is so low that a significant proportion of it remains heterotrimeric and bound to membranes even when it is fully activated in cone cells. We infer that many G-proteins, like cone transducin, remain associated during activation in their cellular environment.
Footnotes
This work was supported by National Institutes of Health Grants GM 060019 (V.Z.S.), EY 06641 (J.B.H.), EY12008 (J.L.), EY13811 (C.-K.C.), EY11115 (A.S.), and GM60286 (A.V.S.), American Heart Association Florida Affiliate Fellowships (K.S.N., D.H.R.), and a grant from the Hope-for-Vision Foundation (V.Z.S.). We are grateful to Alison Scott and Zhogyan Wang for technical assistance. We also thank Valery Shestopalov (Bascom Palmer Eye Institute, Miami, FL) for invaluable help with confocal microscopy.
References
- Bhakdi S, Bayley H, Valeva A, Walev I, Walker B, Kehoe M, Palmer M. Staphylococcal alpha-toxin, streptolysin-O, and Escherichia coli hemolysin: prototypes of pore-forming bacterial cytolysins. Arch Microbiol. 1996;165:73–79. doi: 10.1007/s002030050300. [DOI] [PubMed] [Google Scholar]
- Bigay J, Faurobert E, Franco M, Chabre M. Roles of lipid modifications of transducin subunits in their GDP-dependent association and membrane binding. Biochemistry. 1994;33:14081–14090. doi: 10.1021/bi00251a017. [DOI] [PubMed] [Google Scholar]
- Bonacci TM, Mathews JL, Yuan C, Lehmann DM, Malik S, Wu D, Font JL, Bidlack JM, Smrcka AV. Differential targeting of Gbetagamma-subunit signaling with small molecules. Science. 2006;312:443–446. doi: 10.1126/science.1120378. [DOI] [PubMed] [Google Scholar]
- Calvert PD, Krasnoperova NV, Lyubarsky AL, Isayama T, Nicolo M, Kosaras B, Wong G, Gannon KS, Margolskee RF, Sidman RL, Pugh EN, Jr, Makino CL, Lem J. Phototransduction in transgenic mice after targeted deletion of the rod transducin alpha-subunit. Proc Natl Acad Sci USA. 2000;97:13913–13918. doi: 10.1073/pnas.250478897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert PD, Strissel KJ, Schiesser WE, Pugh EN, Jr, Arshavsky VY. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol. 2006;16:560–568. doi: 10.1016/j.tcb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Chen CA, Manning DR. Regulation of G proteins by covalent modification. Oncogene. 2001;20:1643–1652. doi: 10.1038/sj.onc.1204185. [DOI] [PubMed] [Google Scholar]
- Chen CK, Burns ME, He W, Wensel TG, Baylor DA, Simon MI. Slowed recovery of rod photoresponse in mice lacking the GTPase accelerating protein RGS9–1. Nature. 2000;403:557–560. doi: 10.1038/35000601. [DOI] [PubMed] [Google Scholar]
- Coleman JE, Semple-Rowland SL. GC1 deletion prevents light-dependent arrestin translocation in mouse cone photoreceptor cells. Invest Ophthalmol Vis Sci. 2005;46:12–16. doi: 10.1167/iovs.04-0691. [DOI] [PubMed] [Google Scholar]
- Cowan CW, Fariss RN, Sokal I, Palczewski K, Wensel TG. High expression levels in cones of RGS9, the predominant GTPase accelerating protein of rods. Proc Natl Acad Sci USA. 1998;95:5351–5356. doi: 10.1073/pnas.95.9.5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele LL, Lillo C, Lyubarsky AL, Nikonov SS, Philp N, Mears AJ, Swaroop A, Williams DS, Pugh EN., Jr Cone-like morphological, molecular, and electrophysiological features of the photoreceptors of the Nrl knockout mouse. Invest Ophthalmol Vis Sci. 2005;46:2156–2167. doi: 10.1167/iovs.04-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TL, Bonacci TM, Sprang SR, Smrcka AV. Structural and molecular characterization of a preferred protein interaction surface on G protein betagamma subunits. Biochemistry. 2005;44:10593–10604. doi: 10.1021/bi050655i. [DOI] [PubMed] [Google Scholar]
- Elias RV, Sezate SS, Cao W, McGinnis JF. Temporal kinetics of the light/dark translocation and compartmentation of arrestin and alpha-transducin in mouse photoreceptor cells. Mol Vis. 2004;10:672–681. [PubMed] [Google Scholar]
- Feig LA. The odyssey of k-ras. Mol Cell. 2006;21:447–449. doi: 10.1016/j.molcel.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Fung BK, Hurley JB, Stryer L. Flow of information in the light-triggered cyclic nucleotide cascade of vision. Proc Natl Acad Sci USA. 1981;78:152–156. doi: 10.1073/pnas.78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung BK, Lieberman BS, Lee RH. A third form of the G protein beta subunit. 2. Purification and biochemical properties. J Biol Chem. 1992;267:24782–24788. [PubMed] [Google Scholar]
- Garwin GG, Saari JC. High-performance liquid chromatography analysis of visual cycle retinoids. Methods Enzymol. 2000;316:313–324. doi: 10.1016/s0076-6879(00)16731-x. [DOI] [PubMed] [Google Scholar]
- Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- He W, Cowan CW, Wensel TG. RGS9, a GTPase accelerator for phototransduction. Neuron. 1998;20:95–102. doi: 10.1016/s0896-6273(00)80437-7. [DOI] [PubMed] [Google Scholar]
- Kassai H, Aiba A, Nakao K, Nakamura K, Katsuki M, Xiong WH, Yau KW, Imai H, Shichida Y, Satomi Y, Takao T, Okano T, Fukada Y. Farnesylation of retinal transducin underlies its translocation during light adaptation. Neuron. 2005;47:529–539. doi: 10.1016/j.neuron.2005.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MJ, Lee KA, Niemi GA, Craven KB, Garwin GG, Saari JC, Hurley JB. Multiple phosphorylation of rhodopsin and the in vivo chemistry underlying rod photoreceptor dark adaptation. Neuron. 2001;31:87–101. doi: 10.1016/s0896-6273(01)00340-3. [DOI] [PubMed] [Google Scholar]
- Kennedy MJ, Dunn FA, Hurley JB. Visual pigment phosphorylation but not transducin translocation can contribute to light adaptation in zebrafish cones. Neuron. 2004;41:915–928. doi: 10.1016/s0896-6273(04)00086-8. [DOI] [PubMed] [Google Scholar]
- Kerov V, Chen D, Moussaif M, Chen YJ, Chen CK, Artemyev NO. Transducin activation state controls its light-dependent translocation in rod photoreceptors. J Biol Chem. 2005a;280:41069–41076. doi: 10.1074/jbc.M508849200. [DOI] [PubMed] [Google Scholar]
- Kerov VS, Natochin M, Artemyev NO. Interaction of transducin-alpha with LGN, a G-protein modulator expressed in photoreceptor cells. Mol Cell Neurosci. 2005b;28:485–495. doi: 10.1016/j.mcn.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Kokame K, Fukada Y, Yoshizawa T, Takao T, Shimonishi Y. Lipid modification at the N terminus of photoreceptor G-protein alpha-subunit. Nature. 1992;359:749–752. doi: 10.1038/359749a0. [DOI] [PubMed] [Google Scholar]
- Kuhn H. Light- and GTP-regulated interaction of GTPase and other proteins with bovine photoreceptor membranes. Nature. 1980;283:587–589. doi: 10.1038/283587a0. [DOI] [PubMed] [Google Scholar]
- Lee KA, Craven KB, Niemi GA, Hurley JB. Mass spectrometric analysis of the kinetics of in vivo rhodopsin phosphorylation. Protein Sci. 2002;11:862–874. doi: 10.1110/ps.3870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Montell C. Light-dependent translocation of visual arrestin regulated by the NINAC myosin III. Neuron. 2004;43:95–103. doi: 10.1016/j.neuron.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Xu H, Kang LW, Amzel LM, Montell C. Light adaptation through phosphoinositide-regulated translocation of Drosophila visual arrestin. Neuron. 2003;39:121–132. doi: 10.1016/s0896-6273(03)00390-8. [DOI] [PubMed] [Google Scholar]
- Levitzki A, Klein S. G-protein subunit dissociation is not an integral part of G-protein action. Chembiochem. 2002;3:815–818. doi: 10.1002/1439-7633(20020902)3:9<815::AID-CBIC815>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Lobanova ES, Finkelstein Song, Tsang Chen, Sokolov Skiba, Arshavsky Transducin translocation in rods is triggered by saturation of the GTPase-activating complex. J Neurosci. 2007;27:1151–1160. doi: 10.1523/JNEUROSCI.5010-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek JR, Liu X, Roberts EA, Chui D, Marth JD, Williams DS, Goldstein LS. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102:175–187. doi: 10.1016/s0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- McConnachie G, Langeberg LK, Scott JD. AKAP signaling complexes: getting to the heart of the matter. Trends Mol Med. 2006;12:317–323. doi: 10.1016/j.molmed.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Mears AJ, Kondo M, Swain PK, Takada Y, Bush RA, Saunders TL, Sieving PA, Swaroop A. Nrl is required for rod photoreceptor development. Nat Genet. 2001;29:447–452. doi: 10.1038/ng774. [DOI] [PubMed] [Google Scholar]
- Mendez A, Lem J, Simon M, Chen J. Light-dependent translocation of arrestin in the absence of rhodopsin phosphorylation and transducin signaling. J Neurosci. 2003;23:3124–3129. doi: 10.1523/JNEUROSCI.23-08-03124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair KS, Hanson SM, Kennedy MJ, Hurley JB, Gurevich VV, Slepak VZ. Direct binding of visual arrestin to microtubules determines the differential subcellular localization of its splice variants in rod photoreceptors. J Biol Chem. 2004;279:41240–41248. doi: 10.1074/jbc.M406768200. [DOI] [PubMed] [Google Scholar]
- Nair KS, Hanson SM, Mendez A, Gurevich EV, Shestopalov VI, Vishnivetskiy SA, Kennedy MJ, Chen J, Hurley JB, Gurevich VV, Slepak VZ. Light-dependent re-distribution of arrestin in vertebrate rods is an energy-independent process governed by protein-protein interactions. Neuron. 2005a;46:555–567. doi: 10.1016/j.neuron.2005.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair KS, Mendez A, Blumer JB, Rozenzveig D, Slepak VZ. The presence of Leu-Gly-Asn repeat enriched protein, LGN, a putative binding partner for transducin, in photoreceptors. Invest Ophthalmol Vis Sci. 2005b;46:383–389. doi: 10.1167/iovs.04-1006. [DOI] [PubMed] [Google Scholar]
- Neubert TA, Johnson RS, Hurley JB, Walsh KA. The rod transducin alpha subunit amino terminus is heterogeneously fatty acylated. J Biol Chem. 1992;267:18274–18277. [PubMed] [Google Scholar]
- Northup JK, Smigel MD, Sternweis PC, Gilman AG. The subunits of the stimulatory regulatory component of adenylate cyclase. Resolution of the activated 45,000-dalton (alpha) subunit. J Biol Chem. 1983;258:11369–11376. [PubMed] [Google Scholar]
- Ong OC, Yamane HK, Phan KB, Fong HK, Bok D, Lee RH, Fung BK. Molecular cloning and characterization of the G protein gamma subunit of cone photoreceptors. J Biol Chem. 1995;270:8495–8500. doi: 10.1074/jbc.270.15.8495. [DOI] [PubMed] [Google Scholar]
- Otto-Bruc AE, Fariss RN, Van Hooser JP, Palczewski K. Phosphorylation of photolyzed rhodopsin is calcium-insensitive in retina permeabilized by alpha-toxin. Proc Natl Acad Sci USA. 1998;95:15014–15019. doi: 10.1073/pnas.95.25.15014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, McDowell JH, Hargrave PA. Purification and characterization of rhodopsin kinase. J Biol Chem. 1988;263:14067–14073. [PubMed] [Google Scholar]
- Peet JA, Bragin A, Calvert PD, Nikonov SS, Mani S, Zhao X, Besharse JC, Pierce EA, Knox BE, Pugh EN., Jr Quantification of the cytoplasmic spaces of living cells with EGFP reveals arrestin-EGFP to be in disequilibrium in dark adapted rod photoreceptors. J Cell Sci. 2004;117:3049–3059. doi: 10.1242/jcs.01167. [DOI] [PubMed] [Google Scholar]
- Peterson JJ, Orisme W, Fellows J, McDowell JH, Shelamer CL, Dugger DR, Smith WC. A role for cytoskeletal elements in the light-driven translocation of proteins in rod photoreceptors. Invest Ophthalmol Vis Sci. 2005;46:3988–3998. doi: 10.1167/iovs.05-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saari JC, Nawrot M, Garwin GG, Kennedy MJ, Hurley JB, Ghyselinck NB, Chambon P. Analysis of the visual cycle in cellular retinol-binding protein type I (CRBPI) knockout mice. Invest Ophthalmol Vis Sci. 2002;43:1730–1735. [PubMed] [Google Scholar]
- Smrcka AV, Scott JK. Discovery of ligands for beta gamma subunits from phage-displayed peptide libraries. Methods Enzymol. 2002;344:557–576. doi: 10.1016/s0076-6879(02)44740-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, Govardovskii VI, Pugh EN, Jr, Arshavsky VY. Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron. 2002;34:95–106. doi: 10.1016/s0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- Sokolov M, Strissel KJ, Leskov IB, Michaud NA, Govardovskii VI, Arshavsky VY. Phosducin facilitates light-driven transducin translocation in rod photoreceptors. Evidence from the phosducin knockout mouse. J Biol Chem. 2004;279:19149–19156. doi: 10.1074/jbc.M311058200. [DOI] [PubMed] [Google Scholar]
- Strissel KJ, Lishko PV, Trieu LH, Kennedy MJ, Hurley JB, Arshavsky VY. Recoverin undergoes light-dependent intracellular translocation in rod photoreceptors. J Biol Chem. 2005;280:29250–29255. doi: 10.1074/jbc.M501789200. [DOI] [PubMed] [Google Scholar]
- Strissel KJ, Sokolov M, Trieu LH, Arshavsky VY. Arrestin translocation is induced at a critical threshold of visual signaling and is superstoichiometric to bleached rhodopsin. J Neurosci. 2006;26:1146–1153. doi: 10.1523/JNEUROSCI.4289-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Windh RT, Chen CA, Manning DR. N-Myristoylation and betagamma play roles beyond anchorage in the palmitoylation of the G protein alpha(o) subunit. J Biol Chem. 1999;274:37435–37442. doi: 10.1074/jbc.274.52.37435. [DOI] [PubMed] [Google Scholar]
- Wolfrum U, Giessl A, Pulvermuller A. Centrins, a novel group of Ca2+-binding proteins in vertebrate photoreceptor cells. Adv Exp Med Biol. 2002;514:155–178. doi: 10.1007/978-1-4615-0121-3_10. [DOI] [PubMed] [Google Scholar]