Abstract
Interferon tau (IFNT), the maternal recognition of pregnancy signal in sheep and other ruminants, is secreted by the conceptus and regulates the expression of a number of genes in a cell-specific manner within the uterus. The response of different endometrial cell types to IFNT appears to be specified by IFN regulatory factors (IRFs). IRF2, a potent repressor of gene transcription, is expressed only by luminal (LE) and superficial glandular epithelia (sGE), whereas IRF1 and IRF9, activators of gene transcription, are expressed only in GE and stromal cells of the uterus during early pregnancy. In the present study, IRF6 was found to be expressed in LE/sGE and middle GE of the ovine uterine endometrium as well as conceptus trophectoderm. IRF family members can regulate transcription via IFN-stimulated response elements (ISREs). Transient transfection analyses found that IRF6 enhanced basal activity of ISRE-containing promoters, but did not enhance IFNT stimulation of ISRE-containing promoters in variety of different cell types. Further, IRF6 did not cooperate with IRF1 or reduce IRF2 repression of ISRE-containing promoter activity. These results establish that IRF6 is a transcriptional activator that is preferentially expressed in the endometrial epithelia and conceptus trophectoderm. IRF6 is hypothesized to play critical roles in endometrial gene expression as well as in conceptus trophectoderm growth and differentiation.
1. Introduction
Biochemical signaling between the conceptus (embryo and associated extraembryonic membranes) and the mother must occur prior to and following conceptus attachment to the uterine luminal epithelium (LE) to prevent the mother from returning to cyclicity and to establish an altered physiological milieu favorable for implantation, placentation, and survival and development of the conceptus. In sheep, mononuclear trophectoderm cells of the conceptus secrete copious amounts of interferon tau (IFNT), a Type I IFN, from Day 11 to Days 21 to 25 of pregnancy (Bazer et al., 1992). In sheep, IFNT silences estrogen receptor alpha (ESR1) gene transcription in endometrial LE and superficial glandular epithelia (sGE) (Fleming et al., 2001), which inhibits ESR1 induction of oxytocin receptor gene transcription, thereby preventing oxytocin-induced release of luteolytic pulses of prostaglandin F2 alpha (PGF) and maintaining production of progesterone by the corpus luteum. Progesterone is essential for establishment and maintenance of pregnancy. In addition, IFNT stimulates a number of IFN-stimulated genes (ISGs) in a cell-specific manner within the uterus (Spencer et al., 2008b, Spencer et al., 2007). These ISGs are hypothesized to regulate endometrial receptivity to implantation and placentation, as well as survival, growth and development of the conceptus.
The classical pathway of Type I IFN action is via activation of the Janus kinase/signal transducers and activators of transcription (JAK-STAT) signaling pathway (Stark et al., 1998). Using cell lines deficient in various components of this pathway, IFNT was found to activate the JAK-STAT pathway similar to other Type I IFNs (Stewart et al., 2001a). STAT1, STAT2 and IFN regulatory factor 9 (IRF9), which comprise the IFN-stimulated gene factor 3 (ISGF3) transcription factor complex that transactivates ISG expression via IFN-stimulated response elements (ISREs) in their promoter regions, are up-regulated by IFNT only in GE and stromal cells of the ovine uterus during early pregnancy and are down-regulated or absent in LE/sGE of the endometrium between Days 11 and 19 of pregnancy (Choi et al., 2001). The lack of STAT1, STAT2, IRF9 and other classical ISGs, such as ISG15 and beta-2 microglobulin (B2M), in LE/sGE and cell-specific effects of IFNT on uterine gene expression is hypothesized to involve IFN regulatory factors (IRFs) (Choi et al., 2001, Choi et al., 2003, Spencer et al., 2008a, Spencer et al., 2007).
The IRFs are key regulators of IFN induction during antiviral responses (Stark et al., 1998, Mamane et al., 1999). The nine mammalian members of the IRF gene family share a conserved 115 amino acid N-terminal DNA binding domain (DBD) that bind ISREs and closely related IRF elements in the promoter region of target genes (Mamane et al., 1999). Most IRFs contain a transcriptional activation domain, although IRF2 contains a potent transcriptional repression domain. The transcriptional regulatory functions of most IRFs, with the exception of IRF6, have been determined (Mamane et al., 1999, Cheng et al., 2006, Takaoka et al., 2005). In the ovine uterus, the IRF2 repressor is expressed exclusively by endometrial LE/sGE during the estrous cycle and expression increases during early pregnancy (Choi et al., 2001). Available results support the hypothesis that increased expression of IRF2 in endometrial LE/sGE during early pregnancy represses transcription of many classical ISGs, such as STAT1, STAT2, IRF9, ISG15 and B2M (see (Choi et al., 2001, Spencer et al., 2008a, Spencer et al., 2007). Thus, the induction and stimulation of many classical ISGs by conceptus IFNT is restricted to endometrial stromal cells and GE, which are IRF2-negative although IFN receptors are abundant on LE/sGE (Rosenfeld et al., 2002). In addition, IRF2 is involved in IFNT inhibition of ESR1 transcription in LE/sGE (Fleming et al., 2006, Fleming et al., 2001).
A comprehensive search for all IRF family members in the ovine uterine endometrium found expression of only IRF1, IRF2, IRF6 and IRF9 (Choi et al., 2001). IRF6 is a member of the IRF gene family based on the presence of the N-terminal DBD characteristic of IRFs and has much greater sequence homology with IRF5 in both the DBD and the C-terminal domain than with the other paralogs (Mamane et al., 1999, Nguyen et al., 1997). The relative lack of information on the transcriptional function and target genes regulated by IRF6 is somewhat surprising in view of its strong genetic association with cleft lip and/or palate, one of the most common birth defects of humans (Cobourne, 2004). Mutation of IRF6 has been strongly associated with human cranofacial developmental defects in van der Woude and popliteal pterygium syndromes as well as in nonsyndromic cleft lip and palate and tooth agenesis worldwide (Cobourne, 2004, Blanton et al., 2005, Zucchero et al., 2004, Kondo et al., 2002, Gorlin et al., 1968). Besides the better known craniofacial defects, popliteal pterygium syndrome also includes genitourinary abnormalities and digital and limb anomalies (Gorlin et al., 1968). IRF6 is expressed in epithelial cells of several tissue types in mouse, chicken, zebrafish and Xenopus laevis embryos (Ben et al., 2005, Knight et al., 2006, Washbourne and Cox, 2006, Hatada et al., 1997). Irf6 is found in a variety of adult mouse tissues, including eyes, liver, lung, skin, spleen, placenta and testes (Kondo et al., 2002). In contrast to knockout mice for the other IRFs which have compromised immune responses, Irf6 null mice have morphological defects that include severely abnormal skin, limb and craniofacial development resulting in perinatal lethality, consistent with the defects seen to varying extents in humans with IRF6 mutations (Ingraham et al., 2006, Richardson et al., 2006). Thus, Irf6 is a primary regulator of keratinocyte proliferation and differentiation during embroygenesis in mice (Ingraham et al., 2006, Richardson et al., 2006).
These studies determined the temporal and spatial patterns of IRF6 expression in the ovine uterus during early pregnancy and whether IRF6 functions as an activator or repressor of ISRE-regulated promoter activity and effects of IFNT. The results indicate that IRF6 is exclusively expressed in endometrial epithelia of the ovine uterus and in conceptus trophectoderm. Further, in vitro transfection assays found that IRF6 is a transcriptional activator, but it does not enhance IFNT actions on ISRE-containing promoters.
2. Materials and Methods
2.1. Cells and reagents
Human 2fTGH (parental), U3A (STAT1-deficient 2fTGH) fibrosarcoma cells (Pelligrini), immortalized ovine endometrial luminal epithelial cells (LE) (Johnson et al., 1999) and bovine endometrial cells (BEND) (Staggs et al., 1998) were maintained in DMEM-F12 medium (Sigma-Aldrich Corp., St. Louis, MO) supplemented with penicillin/streptomycin sulfate/amphotericin B (PSA) solution (Invitrogen, Carlsbad, CA) and fetal bovine serum (Hyclone, Logan, UT) (5% FBS for 2fTGH and U3A and 10% FBS for LE and BENDs). Ovine mononuclear trophectoderm cells (oTr1) (Farmer et al., 2008) were maintained in DMEM-F12 plus PSA, 1 mM pyruvate, 2 mM L-glutamine, non-essential amino acids, 700 nM insulin and 10% FBS (complete trophoblast medium). Recombinant ovine IFNT (IFNT) (108 antiviral units/mg) was prepared and assayed as described previously (Van Heeke et al., 1996). Restriction endonucleases, T4 DNA ligase, cell culture lysis reagent and luciferase substrate were purchased from Promega (Madison, WI). Ex Taq™ polymerase (Takara Bio USA, Madison, WI) was used. Plasmid DNAs were purified by the alkaline lysis method with kits from Qiagen (Qiagen, Valencia, CA). An affinity purified rabbit polyclonal antibody against IRF6 was purchased from Active Motif (Carlsbad, CA).
2.2. Cloning of ovine IRF6 and luciferase constructs
An ovine endometrial IRF6 DNA was cloned as described previously for ovine IRF1 and IRF2 (Choi et al., 2001). Briefly, an ovine IRF6 partial cDNA was amplified by reverse transcription-polymerase chain reaction (RT-PCR) of total RNA isolated from Day 15 pregnant ovine endometrium with primers designed from the human orthologue (forward: 5′-CCA GAA CG TGG ATC AGC-3′ and reverse: 5′-GAC CTC CAG GAT CAG TCC-3′). The PCR reaction was performed using PCR Optimized Buffer F (Invitrogen, Carlsbad, CA) as follows: 1) 95 °C for 2 min; 2) 1.5 min at 95 °C, 2 min at 42 °C, 1 min at 72 °C for 4 cycles; 3) 30 sec at 95 °C, 30 sec at 53 °C, 1 min at 72 °C for 30 cycles; and 4) 72 °C for 7 min. The partial ovine endometrial IRF6 cDNA was cloned into pCRII plasmid vector using a T/A cloning Kit (Invitrogen), verified by sequencing, and used to screen a Lambda Zap II cDNA library made from Day 15 pregnant ovine endometria (Stratagene, LaJolla, CA) using methods described previously (Choi et al., 2001). A full-length ovine endometrial IRF6 cDNA (GenBank AF228446) was subcloned into pEF1-Myc/His LacZ mammalian expression vector (Invitrogen) by standard methods.
The sequence of PRE2-TATA-LUC (a gift from Dr. Ming-jer Tsai, Baylor College of Medicine, Houston, TX) was determined by standard methods. Oligonucleotides corresponding to the sense (5′-CTA GCCAGCTGA GAG GGT ATA TAA TGG ATC TGC GAT CTA AGT AAG CTT GGC ATT CCG GTA CTG TTG GTA AAA-3′) and antisense (5′-GAT CTT TTA CCA ACA GTA CCG GAA TGC CAA GCT TAC TTA GAT CGC AGA TCC ATT ATA TAC CCT CTC AGC TGG-3′) strands of the core promoter were synthesized (Integrated DNA Technologies, Inc., Coralville, IA) and annealed. The double-stranded oligonucleotide was cloned into the luciferase vector pGL3-Basic (Promega, Madison, WI) that had been digested with Bgl II and NheI to create pTATA-LUC. Five copies of an ISRE was inserted by ligating a double-stranded oligonucleotide (sense strand 5′-CGA TAT CCT GCA GGA AAC TGA AAC TGA AAC TGA AAC TGA AAC TGA AAC TGA AAC TGA AAC TGA AAC TGA AAC TG-3′; antisense 5′-CTA GCA GTT TCA GTT TCA GTT TCA GTT TCA GTT TCA GTT TCA GTT TCA GTT TCA GTT TCA GTT TCC TGC AGG ATA TCG AGC T-3′) into pTATA-LUC that had been digested with SacI and NheI to create 5xISRE-TATA-LUC. The reporter construct containing the IFNT-responsive bovine ISG17 promoter (ISG17-LUC) (Perry et al., 1999) was kindly provided by Dr. Thomas R. Hansen (Colorado State University, Fort Collins).
2.3. Collection of Ovine Uterine Tissues
Crossbred Suffolk ewes (Ovis aries) were observed daily for estrus in the presence of vasectomized rams and used in the experiments after they exhibited at least two estrous cycles of normal duration (16–18 days). All experimental and surgical procedures were in compliance with the Guide for the Care and Use of Agriculture Animals in Teaching and Research and approved by the Institutional Animal Care and Use Committee of Texas A&M University.
At estrus (Day 0), ewes were mated to either an intact or a vasectomized ram and then hysterectomized (n = 5 ewes/day) on either Day 11, 13 or 15 of the estrous cycle, Days 11, 13, 15, 17 or 19 of pregnancy, Days 10, 12, 14 or 16 of the estrous cycle or Days 10, 12, 14, 16, 18 or 20 of pregnancy. Pregnancy was confirmed on Days 10 to 16 post-mating by the presence of a morphologically normal conceptus(es) in the uterus. At hysterectomy, several sections (~0.5 cm) from the mid-portion of each uterine horn ipsilateral to the corpus luteum (CL) were fixed in fresh 4% paraformaldehyde in PBS (pH 7.2). After 24 h, fixed tissues were changed to 70% ethanol for 24 h and then dehydrated and embedded in Paraplast-Plus (Oxford Labware, St. Louis, MO). In monovulatory pregnant ewes, uterine tissue samples were marked as either contralateral or ipsilateral to the ovary bearing the CL. No tissues from the contralateral uterine horn were used for this study. The uterine lumen was flushed with saline on Days 10 to 16 of estrous cycle or Days 10 to 16 of pregnancy. The presence of a morphologically normal conceptus was evidence for ewes being pregnant. It was not possible to obtain uterine flushings on either Day 18 or Day 20 of pregnancy because the conceptus is firmly adhered to the endometrial luminal epithelium (LE) and basal lamina on those days of pregnancy.
2.4. In Situ Hybridization Analyses
Location of IRF6 mRNA in sections (5 μm) of ovine uteri from Days 11, 13, and 15 of the estrous cycle and Days 11, 13, 15, 17 and 19 of early pregnancy was determined by radioactive in situ hybridization analysis as described previously (Choi et al., 2001). Briefly, deparaffinized, rehydrated and deproteinated uterine tissue sections were hybridized with radiolabeled antisense or sense cRNA probes generated from linearized ovine IRF6 partial cDNA using in vitro transcription with [α-35S]-UTP. After hybridization, washing and ribonuclease A digestion, slides were dipped in NTB-2 liquid photographic emulsion (Kodak, Rochester, NY), and exposed at 4°C for two weeks. Slides were developed in Kodak D-19 developer, counterstained with Gill’s hematoxylin (Fisher Scientific, Fairlawn, NJ), and then dehydrated through a graded series of alcohol to xylene. Coverslips were then affixed with Permount (Fisher). Images of representative fields were recorded under brightfield and darkfield illumination using a Nikon Eclipse 1000 photomicroscope (Nikon Instruments Inc., Lewisville, TX) fitted with a Nikon DXM1200 digital camera.
2.5. Immunohistochemical Analyses
Immunocytochemical localization of IRF6 protein in ovine uteri was performed as described previously (Song et al., 2005) in sections from Days 10, 12, 14 and 16 of the estrous cycle and Days 10, 12, 14, 16, 18 and 20 of pregnancy with affinity purified rabbit anti-human IRF6 polyclonal antibody at 0.5 μg per ml final dilution. Antigen retrieval was performed by using a boiling citrate buffer, and negative controls included substitution of purified rabbit IgG for the primary antibody at the same final concentration. Images of representative fields were recorded using a Nikon Eclipse 1000 photomicroscope (Nikon Instruments Inc., Lewisville, TX) fitted with a Nikon DXM1200 digital camera.
2.6. Transient transfections and luciferase assays
Transient transfections of 2fTGH and U3A cells were conducted as described previously (Fleming et al., 2006). The oTr1 cells were seeded to about 90% confluency before transfection of each well with 1 μg luciferase construct and 1 μg expression plasmid or pEF1-Myc/His/LacZ vector lacking an insert or containing full-length ovine IRF1, ovine IRF2 or ovine IRF6 cDNAs in the presence of ExGen 500 in vitro transfection reagent (Fermentas, Inc., Glen Burnie, MD) according to the manufacturer’s instructions. Cells were maintained in complete trophoblast medium containing 10% FBS during transfection and an overnight incubation (approximately 14–16 h). The oTr1 cells then were cultured in serum-free, antibiotic supplemented DMEM-F12 medium and treated with IFNT (104 antiviral units/ml) in the same medium for 24 h before collecting cell lysates. BEND cells were cultured similarly to oTr1 cells except that antibiotic supplemented DMEM-F12 plus 10% FBS was used during the transfection and the overnight recovery period. The ovine LE cells were seeded to 67% confluency and transferred to serum-free antibiotic containing DMEM-F12 before transfection with DNAs and ExGen 500 in vitro transfection reagent as described above. In order to maintain good cell condition throughout the transfection and to avoid cytopathic effects resulting from overgrowth and crowding of this rapidly proliferating immortalized cell line, the overnight recovery period was omitted and IFNT treatments were added to LE cells in serum-free medium approximately 2 to 4 h after addition of the DNA complexes. The LE cells continued to proliferate under these conditions and typically achieved 100% confluency by the time cells were lysed 24 h after the addition of DNA complexes to the monolayers. Cell lysates were collected and luciferase assays were conducted as described previously (Fleming et al., 2006). In experiments with all cell types, the amount of reporter plasmid per well and the total amount of DNA per well was kept constant. The pEF1-Myc/His/LacZ empty vector was used, as necessary, to maintain constant amounts of DNA when expression plasmids were used at varying ratios. Each DNA and treatment combination was tested in four replicate wells in each experiment, and all experiments were repeated independently a minimum of three times.
2.7. Statistical analyses
The effect of the IRFs or IFNT on activities of reporter constructs in transient transfection assays were analyzed by least squares ANOVA using the General Linear Models procedure of the Statistical Analysis System (Cary, NC). A P value of 0.05 or less was considered statistically significant effect of treatment.
3. Results
3.1. IRF6 expression in the ovine uterus and conceptus
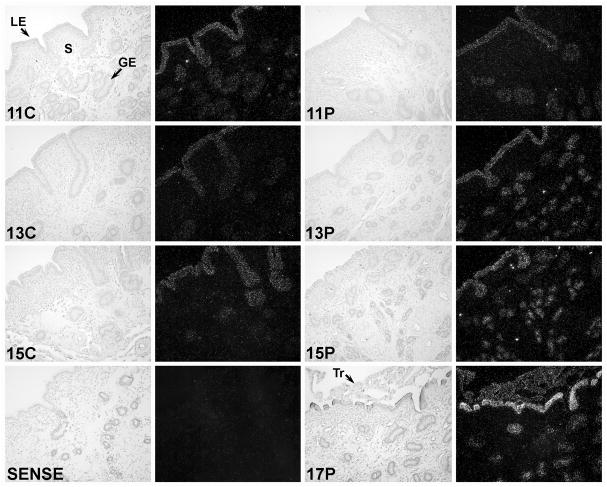
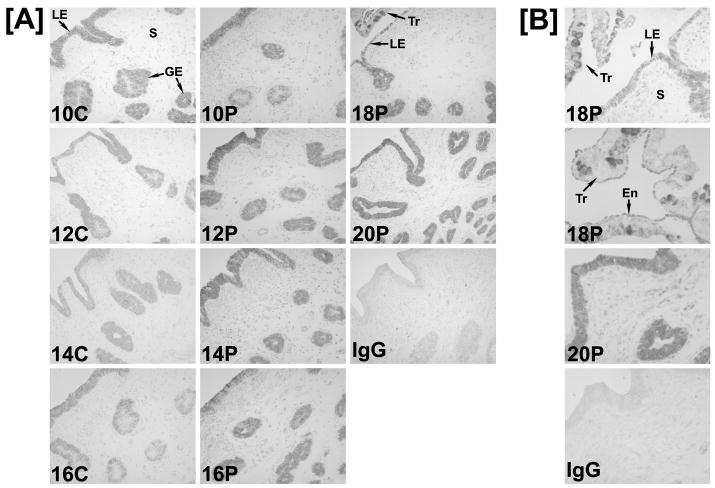
An ovine IRF6 cDNA (GenBank Accession AF228446) of 1404 nucleotides was isolated as described from a Lambda Zap II cDNA library of Day 15 pregnant ovine endometrium. The ovine endometrial IRF6 cDNA had high sequence identity with IRF6 cDNAs of human (93%) and other mammalian species. In situ hybridization analyses detected IRF6 mRNA in the LE/sGE and GE of endometria from cyclic and early pregnant ewes. The abundance of IRF6 mRNA in the LE/sGE, and to some extent in GE, increased between Days 11 and 17 of pregnancy, but not between Days 11 and 15 of the estrous cycle, and IRF6 mRNA was detected in the conceptus trophectoderm on Day 17 of pregnancy (Fig. 1). Immunohistochemical analyses of IRF6 protein in cyclic and pregnant ewes indicated that IRF6 protein was similar between Days 10 and 16 of the estrous cycle, increased in abundance between Days 10 and 20 of pregnancy in LE/sGE and GE, and was detected in trophectoderm and extra-embryonic endoderm of conceptuses (Fig. 2). IRF6 protein increased in LE/sGE and, to some extent, in GE in pregnant ewes. In addition, IRF6 protein was detected in the trophectoderm of the conceptus as was particularly abundant in the nuclei of trophoblast giant binucleate cells. The IRF6 protein was present in both the cytoplasm and nucleus of LE/sGE, GE and trophectoderm cells.
Fig. 1. In situ hybridization analysis of IRF6 mRNA in the ovine uterus.
Cross-sections of the uterine wall from cyclic (C) and pregnant (P) ewes were hybridized with radiolabeled antisense or sense ovine IRF6 cRNAs. IRF6 mRNA was detected in the uterine LE/sGE and GE as well as trophectoderm of the conceptus on Day 17 of pregnancy. Note the apparent increase in IRF6 mRNA in LE/sGE during pregnancy. Legend: GE, glandular epithelium; LE, luminal epithelium; S, stroma; Tr, trophectoderm. Scale bar represents 10 μm.
Fig. 2. Immunohistochemical localization of IRF6 protein in uteri from cyclic and pregnant ewes.
In the IgG control, normal rabbit IgG was substituted for rabbit IgG specific for human IRF6. Sections were not counterstained. Note the immunoreactive IRF6 protein in uterine LE/sGE and GE, as well as conceptus trophectoderm. IRF6 protein increased in uterine LE/sGE and GE during pregnancy. Legend: LE, luminal epithelium; GE, glandular epithelium; S, stroma; Tr, trophectoderm; En, endoderm. Scale bar represents 10 μm in [A] and 100 μm in [B].
3.2. Biological activity of IRF6 in human 2fTGH and U3A cells
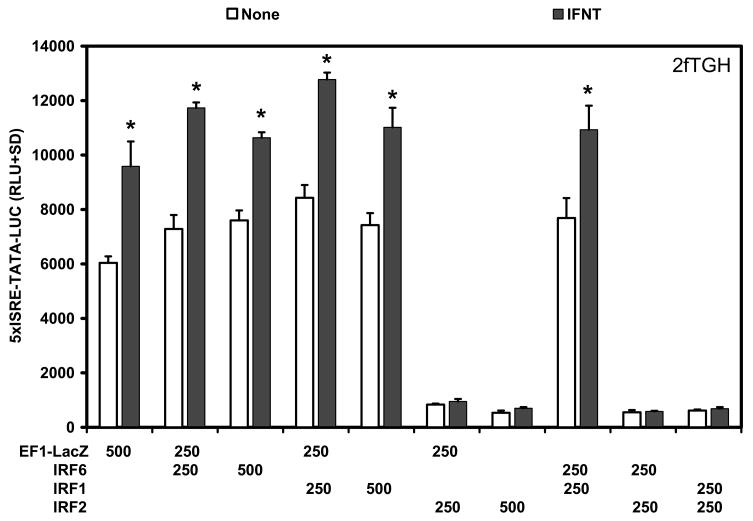
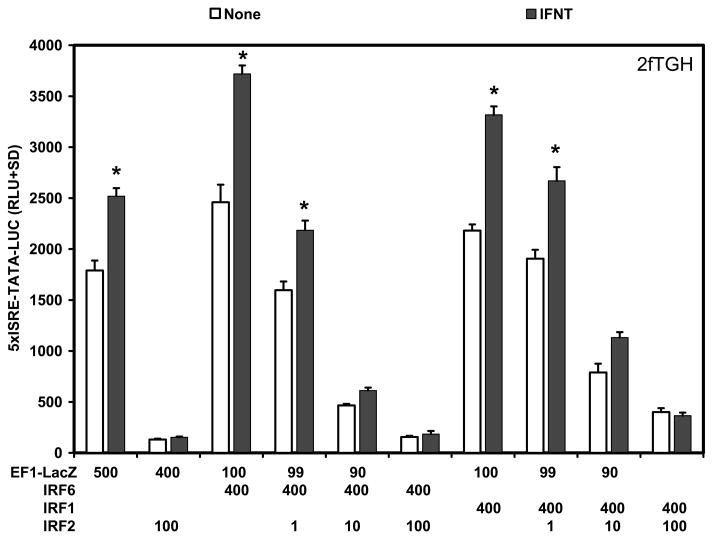
In the early pregnant ovine uterus, the transcriptional activators IRF1 and IRF9 are expressed in stromal cells and GE, whereas the transcriptional repressor IRF2 is expressed in LE/sGE that lack detectable STAT1, STAT2, IRF9 and IRF1 (Choi et al., 2001). Transient transfection analyses were conducted in human 2fTGH and U3A STAT1 null cells to determine if ovine endometrial IRF6 was an activator or repressor of transcription and if it was involved in responses of cells to IFNT. In human 2fTGH cells, IFNT treatment alone increased (P<0.05) activity of the 5xISRE-TATA-LUC reporter about 1.5-fold. Co-transfection with IRF6 or IRF1 stimulated (P<0.05) basal activity of the reporter only slightly (1.3-fold) compared to the empty vector (EF1-LacZ), but co-transfection with both IRF6 and IRF1 did not have (P>0.10) additive effects on 5xISRE-TATA-LUC activity (Fig. 3). Further, IRF6 and IRF1 alone or together failed (P>0.10) to increase IFNT stimulation of 5xISRE reporter activity in 2fTGH cells. In contrast, co-transfection of ovine IRF2 repressed (P<0.01) both basal and IFNT-stimulated reporter activity.
Fig. 3. Effect of IRFs and IFNT on 5xISRE-TATA-LUC reporter activity in 2fTGH cells.
Cells were co-transfected with indicated constructs (amount indicated in ng/well) and treated with nothing or IFNT (104 antiviral units) for 24 h. Transfection assays and luciferase activities were determined as described in Materials and Methods. The data are presented as mean relative light units (RLU) with standard deviation (SD). Four replicate determinations for each treatment group were conducted in each of three independent experiments. The asterisk (*) denotes a significant effect of treatment with IFNT on reporter activity (P<0.05).
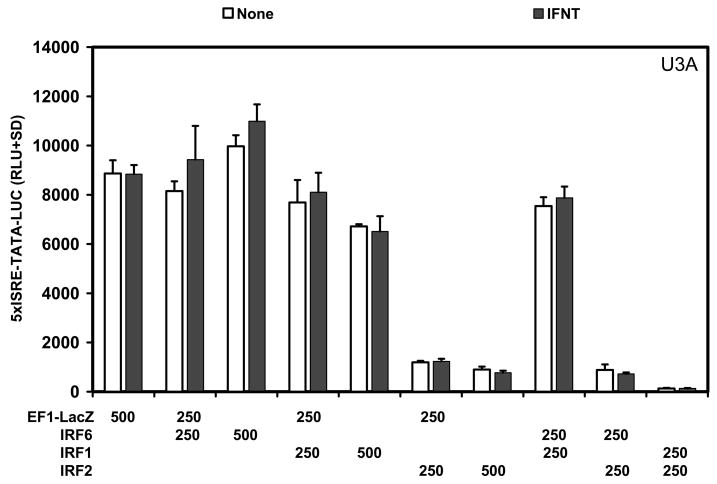
In U3A STAT1 null cells (Fig. 4), IFNT did not (P>0.10) stimulate 5xISRE-TATA-LUC reporter activity due to the lack of STAT1 and the inability to form the ISGF3 transcription factor complex (Choi et al., 2001, Stewart et al., 2002). Transfection of U3A cells with either IRF6 or IRF1 did not (P>0.10) affect basal activity of the reporter, whereas IRF2 alone or when co-transfected with either IRF6 or IRF1 repressed (P<0.01) basal activity of the reporter alone.
Fig. 4. Effect of IRFs and IFNT on 5xISRE-TATA-LUC reporter activity in U3A STAT1 null cells.
Cells were co-transfected with indicated constructs (amount indicated in ng/well) and treated with nothing or IFNT (104 antiviral units) for 24 h. Transfection assays and luciferase activities were determined as described in Materials and Methods. The data are presented as mean relative light units (RLU) with SD. Four replicate determinations for each treatment group were conducted in each of three independent experiments. Note the lack of IFNT stimulation of reporter activity (P>0.10) in the transfected U3A cells.
Next, 2fTGH cells were transfected with a constant amount of IRF6 or IRF1 and increasing amounts of the IRF2 repressor (Fig. 5). In the absence of IRF2, IRF6 and IRF1 stimulated (P<0.05) basal activity of the reporter by 1.5- and 1.3-fold, respectively, but neither potentiated IFNT stimulation of 5xISRE-TATA-LUC reporter activity. IRF2 decreased (P<0.01) both basal and IFNT-stimulated reporter activity in a dose-dependent manner in cells co-transfected with either IRF6 or IRF1. Ratios of IRF2 to IRF6 (or IRF1) as low 1:40 (10 ng IRF2 DNA/well) had pronounced effects on reporter activity, although on a fold-change basis IFN still stimulated reporter activity to approximately the same extent as in cells transfected with lower amounts of IRF2 DNA. Higher relative amounts of IRF2 compared to IRF6 or IRF1 (1:4, or 100 ng IRF2 DNA/well) further reduced (P<0.01) reporter activity and completely abolished IFNT stimulation.
Fig. 5. Effect of IRFs and IFNT on 5xISRE-TATA-LUC reporter activity in 2fTGH cells.
Cells were co-transfected with indicated constructs (amount indicated in ng/well) and treated with nothing or IFNT (104 antiviral units) for 24 h. Transfection assays and luciferase activities were determined as described in Materials and Methods. The data are presented as mean relative light units (RLU) with SD. Four replicate determinations for each treatment group were conducted in each of three independent experiments. The asterisk (*) denotes a significant effect of treatment with IFNT on reporter activity (P<0.05).
3.3. Biological activity of IRF6 in ruminant endometrial cells
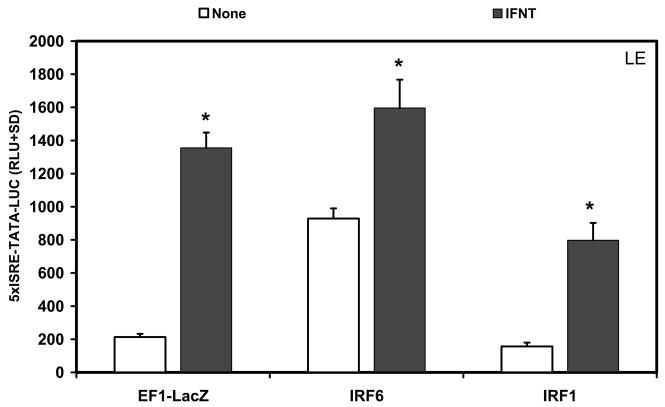
The effect of IRF6 on 5xISRE-TATA-LUC reporter activity was conducted next in an immortalized ovine LE cell line (Johnson et al., 1999). In untreated LE cells, IRF6 stimulated (P<0.05) basal activity of the 5xISRE-TATA-LUC reporter by almost 4-fold compared to the empty vector control, whereas IRF1 had no effect (P>0.10) on reporter activity (Fig. 6). IFNT stimulated (P<0.01) reporter activity about 7-fold in cells transfected with the empty vector, 4-fold in cells transfected with IRF1, and 1.7-fold in cells transfected with IRF6.
Fig. 6. Effect of IRFs and IFNT on 5xISRE-TATA-LUC reporter activity in immortalized ovine endometrial luminal epithelia (LE) cells.
Cells were co-transfected with indicated constructs (amount indicated in ng/well) and treated with nothing or IFNT (104 antiviral units) for 24 h. Transfection assays and luciferase activities were determined as described in Materials and Methods. The data are presented as mean relative light units (RLU) with SD. Four replicate determinations for each treatment group were conducted in each of three independent experiments. The asterisk (*) denotes a significant effect of treatment with IFNT on reporter activity (P<0.05).
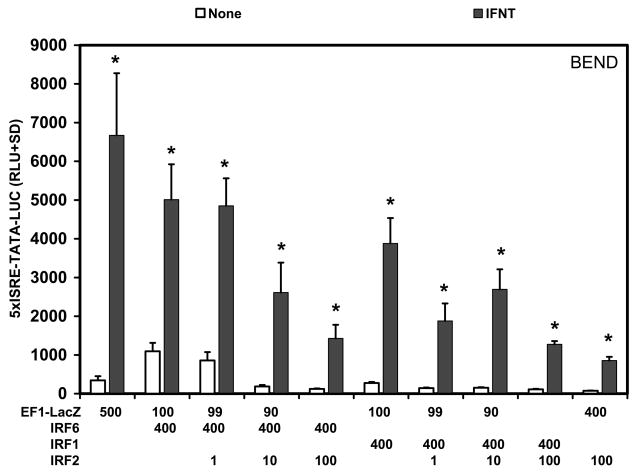
In a bovine endometrial cell line (BEND), IRF6 stimulated (P<0.05) basal activity of the 5xISRE-TATA-LUC reporter approximately 3-fold in cells transfected with the empty vector (Fig. 7). Transfection of IRF1 did not (P>0.10) increase basal activity of the reporter. IFNT induced (P<0.01) a 19.3-fold increase in 5xISRE-TATA-LUC reporter activity in cells transfected with the empty vector, but neither IRF1 nor IRF6 enhanced (P>0.10) IFNT stimulation of reporter activity. On a fold-change basis, IFNT stimulated (P<0.01) reporter activity 19-fold and 14-fold for empty vector and IRF1-transfected cells, respectively, and 4.6-fold for IRF6-transfected cells, reflecting the higher basal reporter activity in the IRF6-transfected cells. Transfection of ovine IRF2 decreased (P<0.01) basal reporter activities in a dose-dependent manner regardless of whether IRF6 or IRF1 was co-transfected into the cells.
Fig. 7. Effect of IRFs and IFNT on 5xISRE-TATA-LUC reporter activity in a bovine endometrial cell line (BEND).
Cells were co-transfected with indicated constructs (amount indicated in ng/well) and treated with nothing or IFNT (104 antiviral units) for 24 h. Transfection assays and luciferase activities were determined as described in Materials and Methods. The data are presented as mean relative light units (RLU) with SD. Four replicate determinations for each treatment group were conducted in each of three independent experiments. The asterisk (*) denotes a significant effect of treatment with IFNT on reporter activity (P<0.05).
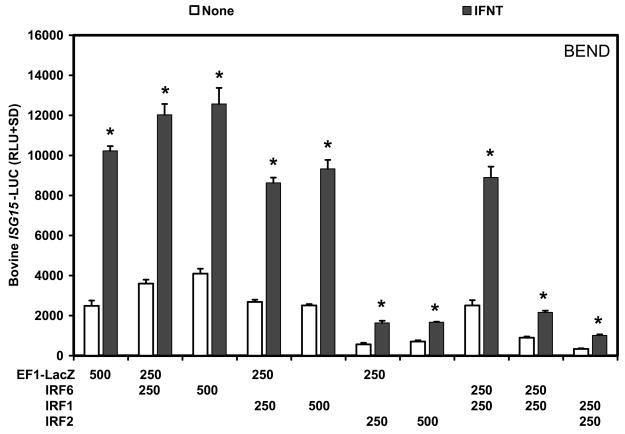
A similar experiment was conducted in BEND cells using the promoter region of the bovine ISG15 gene that contains four ISREs (Perry et al., 1999). IRF6, but not IRF1, stimulated (P<0.05) basal activity of the bovine ISG15-LUC reporter approximately 1.5-fold (Fig. 8). Treatment with IFNT induced a 4-fold increase (P<0.05) in reporter activity in cells transfected with the empty vector, but neither IRF6 nor IRF1 enhanced (P>0.10) the response of the reporter to IFNT. Co-transfection of IRF2 decreased (P<0.01) both basal and IFNT-stimulated reporter activity in BEND cells transfected with the empty vector, IRF6 or IRF1.
Fig. 8. Effect of IRFs and IFNT on 5xISRE-TATA-LUC reporter activity in a bovine endometrial cell line (BEND).
Cells were co-transfected with indicated constructs (amount indicated in ng/well) and treated with nothing or IFNT (104 antiviral units) for 24 h. Transfection assays and luciferase activities were determined as described in Materials and Methods. The data are presented as mean relative light units (RLU) with SD. Four replicate determinations for each treatment group were conducted in each of three independent experiments. The asterisk (*) denotes a significant effect of treatment with IFNT on reporter activity (P<0.05).
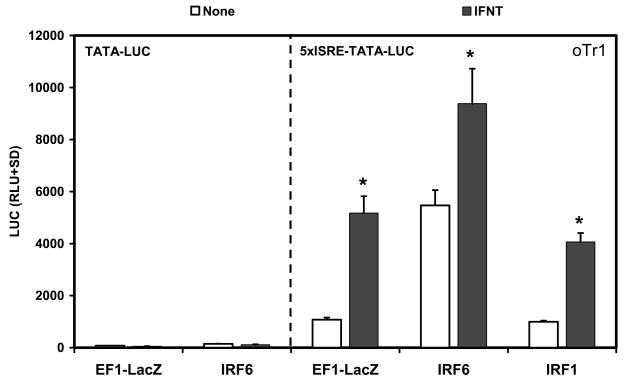
3.4. Biological activity of IRF6 in ovine trophectoderm cells
Since IRF6 is expressed in trophectoderm cells of the ovine conceptus, IRF6 activity in the oTr1 trophectoderm cell line was determined. Transfection of IRF6, but not IRF1, increased (P<0.05) basal activity of the 5xISRE-TATA-LUC reporter 5-fold compared to the empty vector (Fig. 9). IFNT increased (P<0.05) activity of the reporter by 4.8-fold in cells transfected with the empty vector and 4.1-fold in IRF1-transfected cells. In IRF6 transfected cells, IFNT stimulated (P<0.05) reporter activity only 1.7-fold. Both basal and IFNT-stimulated luciferase activity depended on the presence of the concatamerized ISRE in front of TATA in the luciferase reporter (Fig. 9).
Fig. 9. Effect of IRFs and IFNT on 5xISRE-TATA-LUC and TAT-LUC reporter activity in ovine trophectoderm (oTr1) cells.
Cells were co-transfected with indicated constructs (amount indicated in ng/well) and treated with nothing or IFNT (104 antiviral units) for 24 h. Transfection assays and luciferase activities were determined as described in Materials and Methods. The data are presented as mean relative light units (RLU) with SD. Four replicate determinations for each treatment group were conducted in each of three independent experiments. The asterisk (*) denotes a significant effect of treatment with IFNT on reporter activity (P<0.05).
4. Discussion
In the present study, IRF6 mRNA was present only the in endometrial epithelia and conceptus trophectoderm during early pregnancy in sheep. The expression of IRF6 in uterine epithelia is consistent with its expression in epithelia of tissues in other species (Washbourne and Cox, 2006, Hatada et al., 1997, Ben et al., 2005, Knight et al., 2006, Bailey et al., 2005). IRF6 levels in human mammary epithelial cells are highest in normal tissues, but diminished in mammary carcinomas (Bailey et al., 2005). The abundance of IRF6 was inversely correlated with the invasiveness of several breast cancer cell lines or tumor grade, which suggested that IRF6 declines as epithelial-mesenchymal transition progresses and cancer cells acquire more mesenchymal characteristics (Bailey et al., 2005). Alternatively, these observations may reflect higher rate of proliferation of breast cancer cells versus their fully differentiated normal counterparts since IRF6 was higher in quiescent cells than in dividing cells and decreased mitotic activity was associated with decreasing levels of IRF6 (Bailey et al., 2008). Similar to findings from studies of sheep placentae, Irf6 is expressed in placentae of mice (Kondo et al., 2002). The role of Irf6 in placental differentiation is not known, because homozygous Irf6 null mice die shortly after birth (Richardson et al., 2006). Thus, IRF6 likely has a biological role in both ovine uterine endometrial epithelia and conceptus trophectoderm.
IFN plays a critical role in the induction and activation of several IRFs (Mamane et al., 1999, Nguyen et al., 1997, Stark et al., 1998, Platanias, 2005), but IRF6 expression is expressed in endometrial epithelia of ewes during the estrous cycle independent of IFNs. However, IRF6 mRNA does increase in abundance in LE/GE between Days 11 and 17 of pregnancy in concert with onset of conceptus elongation and production of IFNT that is maximal between Days 14 and 16 of pregnancy (Ashworth and Bazer, 1989). These correlative results suggest that IFNT regulates IRF6 and perhaps IRF2 expression in LE/sGE, because IRF2 mRNA and protein abundance also increase in uterine LE/sGE of early pregnant, but not cyclic ewes (Choi et al., 2001). Treatment of human MCF-10A mammary cancer cells with the classic IFN inducer poly dI-C resulted in a rapid phosphorylation and translocation of activated IRF6 to the nucleus and later increased IRF6 levels in the cytosol (Bailey and Hendrix, 2008). Future studies are required to dissect out the mechanism underlying pregnancy-specific increases in IRF6 and IRF2 expression in endometrial LE/sGE.
With the exception of human U3A cells, IRF6 is a transcriptional activator of ISRE-containing promoter reporter constructs in transfected cells. The ability of IRF6 to activate the ISRE-containing reporter constructs depended on the presence of an ISRE upstream of TATA in the TATA-based constructs. Constructs that lacked ISREs were not activated by either IRF6 or IRF1 in the absence or presence of IFNT. The dependence on the presence of an upstream ISRE suggests that the DBD of IRF6 binds to ISRE and increases transcription via an intrinsic activation domain similar to most other IRFs, except IRF2. A high percentage of missense and nonsense mutations in IRF6 in individuals with van der Woude’s or popliteal pterygium syndromes occur in the DBD region, underscoring the importance of the interaction of IRF6 with an ISRE or ISRE-like element in target genes (Kondo et al., 2002). Compared to the TATA-LUC reporter, the 5xISRE-TATA-LUC reporter had significantly greater basal activity in the absence of IFNT regardless of whether IRF6 or IRF1 was co-transfected. These findings suggest that an endogenous factor(s) interacts with the ISREs to increase basal levels of transcription. Several IRFs are likely to be expressed in cells used for transfection analyses. Indeed, IRF5, but not IRF1, IRF3 or IRF7, stimulated basal activities of a reporter in 2fTGH cells (Barnes et al., 2001). Moreover, the C-terminal regions of IRFs contain domains termed IRF-association domains (IADs) required for interaction of IRFs with other IRFs or other transcription factors (Mamane et al., 1999, Taniguchi et al., 2001). Transactivation or repression is effected by these higher order complexes, and complexes of an IRF such IRF8 with different interacting partners can determine whether the complex functions as an activator or repressor (Levi et al., 2002). For instance, IRF2 is normally a strong transcriptional repressor of many genes, but a PU.1-IRF2-IRF8 complex activates the neurofibromin 1 gene in myeloid cells, emphasizing the importance of heterocomplexes in regulatory activity of genes (Mamane et al., 1999, Nguyen et al., 1997, Huang et al., 2007). Indeed, a high proportion of the IRF6 variants associated with van de Woude syndrome occur in the C-terminal protein interaction domain, suggesting that the IRF6 IAD is important to its function in gene regulation (Kondo et al., 2002). Although IRF6 is co-expressed with IRF1 in ovine endometrial LE/sGE (Choi et al., 2001), co-transfection of IRF6 and IRF1 did not enhance transactivation of ISRE-containing reporter constructs in any cell type tested. Conversely, titrations of IRF2 suggest that very low amounts of IRF2 are sufficient to repress both the 5xISRE-TATA-LUC and ISG17-LUC reporter activities in vitro, and that neither IRF6 nor IRF1 is sufficient to prevent IRF2-mediated repression. The DBD of IRF1 and IRF2 have a high degree of sequence similarity (76%) and function through the same cis element (Nguyen et al., 1997, Harada et al., 1989). The DBD of IRF6 has limited similarity to those of IRF1 and IRF2 (36% and 40%, respectively) and is most similar to the DBD of IRF5 (63%) (Nguyen et al., 1997). Repression of basal reporter activity by IRF2 suggests that it binds ISREs in the absence of IFNT and displaces IRF6 and IRF1 in co-transfected cells.
In addition to regulating gene expression via classical ISREs found in many IFN-stimulated genes, comparisons of IADs with C-terminal domains of SMAD2 and SMAD4 predicted some conserved structural features in the SMADs and seven IRFs, including IRF6, but not IRF1 or IRF2 (Levi et al., 2002,Eroshkin and Mushegian, 1999, Qin et al., 2003). The C-terminal protein-binding domain of IRFs is sometimes termed SMIR (SMAD and IRF) (Eroshkin and Mushegian, 1999). The conserved predicted folding patterns suggest that SMADs and some IRFs may interact similarly with other proteins. X-ray crystallographic analyses confirmed that the C-terminus of IRF3 and the Mad homology domain 2 (MH2 domain) of SMAD 2 or SMAD3 have considerable similarities in structural and surface electrostatic potential (Qin et al., 2003, Takahasi et al., 2003). Although SMADs can bind DNA directly, the receptor-activated SMADs can physically interact with several sequence-specific transcription factors that are regulated by other signaling pathways as well as co-activators and co-repressors to form complexes with different transcriptional activities (Derynck and Zhang, 2003). This combinatorial approach affects the specificity of gene regulation by SMAD complexes and provides a mechanism for cross-talk among different signaling pathways (Zhang and Derynck, 1999). Thus, IRF6 interactions with other transcription factors may be important for its biological roles in endometrial epithelial cell functions as well as conceptus trophectoderm growth and differentiation. Indeed, IRF6 is involved in the development of multiple tissues and organs in the embryo (Knight et al., 2006, Richardson et al., 2006, Vieira et al., 2007, Xu et al., 2006).
In the present study, IRF6 did not affect 5xISRE reporter activity in U3A cells, a STAT1 null derivative of 2fTGH cells, used as a model for studies of gene regulation in LE/sGE of early pregnancy in sheep (Stewart et al., 2001b) which also lack STAT1 (Choi et al., 2001). During early pregnancy, IRF6 and IRF2 are both expressed in endometrial LE/sGE and increase in abundance in LE (Choi et al., 2001). The increase in IRF2 expression in uterine LE/sGE of pregnancy is hypothesized to repress expression of most classical ISGs that contains multiple ISREs and are induced by Type I IFNs (Choi et al., 2001, Spencer et al., 2007, Spencer et al., 2004, Spencer et al., 2008a). Indeed, the LE/sGE of pregnancy lack detectable STAT1, STAT2, IRF9 and IRF1 (Choi et al., 2001), as well as ISG15, B2M and many other ISGs (Spencer et al., 2007, Spencer et al., 2004, Spencer et al., 2008a). Results of the present study support that hypothesis, because IRF2 dose-dependently inhibited IRF6 effects on basal activity of the ISRE-containing reporter constructs in all cells tested. Therefore, it is unlikely that IRF6 transactivates ISRE-containing genes in uterine LE/sGE, whereas IRF6 may regulate ISRE-containing genes in uterine GE and trophectoderm that are IRF2-negative. However, IRF6 may regulate expression of specific genes in endometrial LE/sGE as well as GE and trophectoderm through interactions with SMADs and other transcription factors.
In summary, IRF6 is co-expressed with IRF1 and IRF9, as well as IRF2 in ovine uterine GE and LE/sGE, respectively, and IRF6 is also expressed in trophectoderm of the conceptus. Moreover, IRF6 is a transactivator of ISRE-containing promoters that acts independent of IFNT. Based on differences in both their DBD and IADs, IRF6 and IRF1 and/or IRF2 likely regulate different, but possibly overlapping, expression of subsets of genes, depending on sequences of the cis elements in the promoters of the target genes and the different interacting proteins that may lead to the formation of different transcriptional regulatory heterocomplexes. Of particular interest are potential effects of IRF6 proliferation and differentiated functions of trophectoderm cells, as well as interactions between conceptus trophectoderm and extra-embryonic mesoderm during formation of the choriovitelline (yolk sac) and then chorioallantoic placentae during pregnancy in sheep (see (Kondo et al., 2002, Bailey et al., 2008, Bailey et al., 2005)). There is also the need to explore interactions between IRF6 and TGFB (Xu et al., 2006, Knight et al., 2006). For example, TGFB1 signaling involving SMADs can induce WNT-signaling by inhibiting DKK1 (Kane et al., 2008) and has been implicated in peri-implantation conceptus development in sheep (Dore et al., 1996, Dore et al., 1995). Future investigations should focus on determining the biological functions of IRF6 and target genes regulated by IRF6 in ovine uterine epithelia and conceptus trophectoderm during pregnancy.
Acknowledgments
We thank Dr. Ming-jer Tsai (Baylor College of Medicine) for the PRE2-TATA-LUC construct and Dr. Thomas R. Hansen (Colorado State University) for the ISG17-LUC construct. Financial support for these studies was obtained from National Institutes of Health R01 Grant HD32534.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ASHWORTH CJ, BAZER F. Interrelationships of Proteins Secreted by the Ovine Conceptus and Endometrium during the Periattachment Period. Animal Reproduction Science. 1989;20:117–130. [Google Scholar]
- BAILEY CM, ABBOTT DE, MARGARYAN NV, KHALKHALI-ELLIS Z, HENDRIX MJ. Interferon regulatory factor 6 promotes cell cycle arrest and is regulated by the proteasome in a cell cycle-dependent manner. Mol Cell Biol. 2008;28:2235–43. doi: 10.1128/MCB.01866-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAILEY CM, HENDRIX MJ. IRF6 in development and disease: a mediator of quiescence and differentiation. Cell Cycle. 2008;7:1925–30. doi: 10.4161/cc.7.13.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAILEY CM, KHALKHALI-ELLIS Z, KONDO S, MARGARYAN NV, SEFTOR RE, WHEATON WW, AMIR S, PINS MR, SCHUTTE BC, HENDRIX MJ. Mammary serine protease inhibitor (Maspin) binds directly to interferon regulatory factor 6: identification of a novel serpin partnership. J Biol Chem. 2005;280:34210–7. doi: 10.1074/jbc.M503523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNES BJ, MOORE PA, PITHA PM. Virus-specific activation of a novel interferon regulatory factor, IRF-5, results in the induction of distinct interferon alpha genes. J Biol Chem. 2001;276:23382–90. doi: 10.1074/jbc.M101216200. [DOI] [PubMed] [Google Scholar]
- BAZER FW, MIRANDO MA, OTT TL, HARNEY JP, DUBOIS DH, SCHALUE TK, PONTZER CH, HOSTETLER C, JOHNSON HM, OGLE T. Roles of ovine trophoblast protein-1 and oestradiol/prolactin in the establishment of pregnancy in sheep and pigs. Reprod Fertil Dev. 1992;4:335–40. doi: 10.1071/rd9920335. [DOI] [PubMed] [Google Scholar]
- BEN J, JABS EW, CHONG SS. Genomic, cDNA and embryonic expression analysis of zebrafish IRF6, the gene mutated in the human oral clefting disorders Van der Woude and popliteal pterygium syndromes. Gene Expr Patterns. 2005;5:629–38. doi: 10.1016/j.modgep.2005.03.002. [DOI] [PubMed] [Google Scholar]
- BLANTON SH, CORTEZ A, STAL S, MULLIKEN JB, FINNELL RH, HECHT JT. Variation in IRF6 contributes to nonsyndromic cleft lip and palate. Am J Med Genet A. 2005;137A:259–62. doi: 10.1002/ajmg.a.30887. [DOI] [PubMed] [Google Scholar]
- CHENG TF, BRZOSTEK S, ANDO O, VAN SCOY S, KUMAR KP, REICH NC. Differential activation of IFN regulatory factor (IRF)-3 and IRF-5 transcription factors during viral infection. J Immunol. 2006;176:7462–70. doi: 10.4049/jimmunol.176.12.7462. [DOI] [PubMed] [Google Scholar]
- CHOI Y, JOHNSON GA, BURGHARDT RC, BERGHMAN LR, JOYCE MM, TAYLOR KM, STEWART MD, BAZER FW, SPENCER TE. Interferon regulatory factor-two restricts expression of interferon- stimulated genes to the endometrial stroma and glandular epithelium of the ovine uterus. Biol Reprod. 2001;65:1038–49. doi: 10.1095/biolreprod65.4.1038. [DOI] [PubMed] [Google Scholar]
- CHOI Y, JOHNSON GA, SPENCER TE, BAZER FW. Pregnancy and interferon tau regulate MHC class I and beta-2-microglobulin expression in the ovine uterus. Biol Reprod. 2003;68:1703–1710. doi: 10.1095/biolreprod.102.012708. [DOI] [PubMed] [Google Scholar]
- COBOURNE M. The complex genetics of cleft lip and palate. European Journal of Orthodontics. 2004;26:7–16. doi: 10.1093/ejo/26.1.7. [DOI] [PubMed] [Google Scholar]
- DERYNCK R, ZHANG YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- DORE JJ, WILKINSON JE, JR, GODKIN JD. Early gestational expression of transforming growth factor beta isoforms by the ovine placenta. Biol Reprod. 1995;53:143–52. doi: 10.1095/biolreprod53.1.143. [DOI] [PubMed] [Google Scholar]
- DORE JJ, WILKINSON JE, JR, GODKIN JD. Ovine endometrial expression of transforming growth factor beta isoforms during the peri-implantation period. Biol Reprod. 1996;54:1080–7. doi: 10.1095/biolreprod54.5.1080. [DOI] [PubMed] [Google Scholar]
- EROSHKIN A, MUSHEGIAN A. Conserved transactivation domain shared by interferon regulatory factors and Smad morphogens. J Mol Med. 1999;77:403–5. doi: 10.1007/s001090050369. [DOI] [PubMed] [Google Scholar]
- FARMER JL, BURGHARDT RC, JOUSAN FD, HANSEN PJ, BAZER FW, SPENCER TE. Galectin 15 (LGALS15) functions in trophectoderm migration and attachment. FASEB J. 2008;22:548–60. doi: 10.1096/fj.07-9308com. [DOI] [PubMed] [Google Scholar]
- FLEMING JA, CHOI Y, JOHNSON GA, SPENCER TE, BAZER FW. Cloning of the ovine estrogen receptor-alpha promoter and functional regulation by ovine interferon-tau. Endocrinology. 2001;142:2879–87. doi: 10.1210/endo.142.7.8245. [DOI] [PubMed] [Google Scholar]
- FLEMING JG, SPENCER TE, SAFE SH, BAZER FW. Estrogen regulates transcription of the ovine oxytocin receptor gene through GC-rich SP1 promoter elements. Endocrinology. 2006;147:899–911. doi: 10.1210/en.2005-1120. [DOI] [PubMed] [Google Scholar]
- GORLIN RJ, SEDANO HO, CERVENKA J. Popliteal pterygium syndrome. A syndrome comprising cleft lip-palate, popliteal and intercrural pterygia, digital and genital anomalies. Pediatrics. 1968;41:503–9. [PubMed] [Google Scholar]
- HARADA H, FUJITA T, MIYAMOTO M, KIMURA Y, MARUYAMA M, FURIA A, MIYATA T, TANIGUCHI T. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell. 1989;58:729–39. doi: 10.1016/0092-8674(89)90107-4. [DOI] [PubMed] [Google Scholar]
- HATADA S, KINOSHITA M, TAKAHASHI S, NISHIHARA R, SAKUMOTO H, FUKUI A, NODA M, ASASHIMA M. An interferon regulatory factor-related gene (xIRF-6) is expressed in the posterior mesoderm during the early development of Xenopus laevis. Gene. 1997;203:183–8. doi: 10.1016/s0378-1119(97)00512-x. [DOI] [PubMed] [Google Scholar]
- HUANG W, HORVATH E, EKLUND EA. PU.1, interferon regulatory factor (IRF) 2, and the interferon consensus sequence-binding protein (ICSBP/IRF8) cooperate to activate NF1 transcription in differentiating myeloid cells. J Biol Chem. 2007;282:6629–43. doi: 10.1074/jbc.M607760200. [DOI] [PubMed] [Google Scholar]
- INGRAHAM CR, KINOSHITA A, KONDO S, YANG B, SAJAN S, TROUT KJ, MALIK MI, DUNNWALD M, GOUDY SL, LOVETT M, MURRAY JC, SCHUTTE BC. Abnormal skin, limb and craniofacial morphogenesis in mice deficient for interferon regulatory factor 6 (Irf6) Nat Genet. 2006;38:1335–40. doi: 10.1083/ng1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON GA, BURGHARDT RC, NEWTON GR, BAZER FW, SPENCER TE. Development and characterization of immortalized ovine endometrial cell lines. Biol Reprod. 1999;61:1324–30. doi: 10.1095/biolreprod61.5.1324. [DOI] [PubMed] [Google Scholar]
- KANE N, JONES M, BROSENS JJ, SAUNDERS PT, KELLY RW, CRITCHLEY HO. Transforming growth factor-beta1 attenuates expression of both the progesterone receptor and Dickkopf in differentiated human endometrial stromal cells. Mol Endocrinol. 2008;22:716–28. doi: 10.1210/me.2007-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNIGHT AS, SCHUTTE BC, JIANG R, DIXON MJ. Developmental expression analysis of the mouse and chick orthologues of IRF6: the gene mutated in Van der Woude syndrome. Dev Dyn. 2006;235:1441–7. doi: 10.1002/dvdy.20598. [DOI] [PubMed] [Google Scholar]
- KONDO S, SCHUTTE BC, RICHARDSON RJ, BJORK BC, KNIGHT AS, WATANABE Y, HOWARD E, DE LIMA RL, DAACK-HIRSCH S, SANDER A, MCDONALD-MCGINN DM, ZACKAI EH, LAMMER EJ, AYLSWORTH AS, ARDINGER HH, LIDRAL AC, POBER BR, MORENO L, ARCOS-BURGOS M, VALENCIA C, HOUDAYER C, BAHUAU M, MORETTI-FERREIRA D, RICHIERI-COSTA A, DIXON MJ, MURRAY JC. Mutations in IRF6 cause Van der Woude and popliteal pterygium syndromes. Nat Genet. 2002;32:285–9. doi: 10.1038/ng985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVI BZ, HASHMUELI S, GLEIT-KIELMANOWICZ M, AZRIEL A, MERARO D. ICSBP/IRF-8 transactivation: a tale of protein-protein interaction. J Interferon Cytokine Res. 2002;22:153–60. doi: 10.1089/107999002753452764. [DOI] [PubMed] [Google Scholar]
- MAMANE Y, HEYLBROECK C, GENIN P, ALGARTE M, SERVANT MJ, LEPAGE C, DELUCA C, KWON H, LIN R, HISCOTT J. Interferon regulatory factors: the next generation. Gene. 1999;237:1–14. doi: 10.1016/s0378-1119(99)00262-0. [DOI] [PubMed] [Google Scholar]
- NGUYEN H, HISCOTT J, PITHA PM. The growing family of interferon regulatory factors. Cytokine Growth Factor Rev. 1997;8:293–312. doi: 10.1016/s1359-6101(97)00019-1. [DOI] [PubMed] [Google Scholar]
- PERRY DJ, AUSTIN KJ, HANSEN TR. Cloning of interferon-stimulated gene 17: the promoter and nuclear proteins that regulate transcription. Mol Endocrinol. 1999;13:1197–206. doi: 10.1210/mend.13.7.0294. [DOI] [PubMed] [Google Scholar]
- PLATANIAS LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nature Reviews Immunology. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- QIN BY, LIU C, LAM SS, SRINATH H, DELSTON R, CORREIA JJ, DERYNCK R, LIN K. Crystal structure of IRF-3 reveals mechanism of autoinhibition and virus-induced phosphoactivation. Nat Struct Biol. 2003;10:913–21. doi: 10.1038/nsb1002. [DOI] [PubMed] [Google Scholar]
- RICHARDSON RJ, DIXON J, MALHOTRA S, HARDMAN MJ, KNOWLES L, BOOT-HANDFORD RP, SHORE P, WHITMARSH A, DIXON MJ. Irf6 is a key determinant of the keratinocyte proliferation-differentiation switch. Nat Genet. 2006;38:1329–34. doi: 10.1038/ng1894. [DOI] [PubMed] [Google Scholar]
- ROSENFELD CS, HAN CS, ALEXENKO AP, SPENCER TE, ROBERTS RM. Expression of interferon receptor subunits, IFNAR1 and IFNAR2, in the ovine uterus. Biology of Reproduction. 2002;67:847–53. doi: 10.1095/biolreprod.102.004267. [DOI] [PubMed] [Google Scholar]
- SONG G, SPENCER TE, BAZER FW. Cathepsins in the ovine uterus: regulation by pregnancy, progesterone, and interferon tau. Endocrinology. 2005;146:4825–33. doi: 10.1210/en.2005-0768. [DOI] [PubMed] [Google Scholar]
- SPENCER TE, JOHNSON GA, BAZER FW, BURGHARDT RC. Fetal-maternal interactions during the establishment of pregnancy in ruminants. Soc Reprod Fertil Suppl. 2007;64:379–96. doi: 10.5661/rdr-vi-379. [DOI] [PubMed] [Google Scholar]
- SPENCER TE, JOHNSON GA, BURGHARDT RC, BAZER FW. Progesterone and placental hormone actions on the uterus: insights from domestic animals. Biol Reprod. 2004;71:2–10. doi: 10.1095/biolreprod.103.024133. [DOI] [PubMed] [Google Scholar]
- SPENCER TE, SANDRA O, WOLF E. Genes involved in conceptus-endometrial interactions in ruminants: insights from reductionism and thoughts on holistic approaches. Reproduction. 2008a;135:165–79. doi: 10.1530/REP-07-0327. [DOI] [PubMed] [Google Scholar]
- SPENCER TE, SANDRA O, WOLF E. Genes involved in conceptus-endometrial interactions in ruminants: insights from reductionism and thoughts on holistic approaches: Focus on Mammalian Embryogenomics. Reproduction. 2008b;135:165–179. doi: 10.1530/REP-07-0327. [DOI] [PubMed] [Google Scholar]
- STAGGS KL, AUSTIN KJ, JOHNSON GA, TEIXEIRA MG, TALBOTT CT, DOOLEY VA, HANSEN TR. Complex induction of bovine uterine proteins by interferon-tau. Biol Reprod. 1998;59:293–7. doi: 10.1095/biolreprod59.2.293. [DOI] [PubMed] [Google Scholar]
- STARK GR, KERR IM, WILLIAMS BR, SILVERMAN RH, SCHREIBER RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–64. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- STEWART DM, JOHNSON GA, VYHLIDAL CA, BURGHARDT RC, SAFE SH, YU-LEE LY, BAZER FW, SPENCER TE. Interferon-tau activates multiple signal transducer and activator of transcription proteins and has complex effects on interferon-responsive gene transcription in ovine endometrial epithelial cells. Endocrinology. 2001a;142:98–107. doi: 10.1210/endo.142.1.7891. [DOI] [PubMed] [Google Scholar]
- STEWART MD, CHOI Y, JOHNSON GA, YU-LEE LY, BAZER FW, SPENCER TE. Roles of Stat1, Stat2, and interferon regulatory factor-9 (IRF-9) in interferon tau regulation of IRF-1. Biol Reprod. 2002;66:393–400. doi: 10.1095/biolreprod66.2.393. [DOI] [PubMed] [Google Scholar]
- STEWART MD, JOHNSON GA, BAZER FW, SPENCER TE. Interferon-tau (IFNtau) regulation of IFN-stimulated gene expression in cell lines lacking specific IFN-signaling components. Endocrinology. 2001b;142:1786–94. doi: 10.1210/endo.142.5.8138. [DOI] [PubMed] [Google Scholar]
- TAKAHASI K, SUZUKI NN, HORIUCHI M, MORI M, SUHARA W, OKABE Y, FUKUHARA Y, TERASAWA H, AKIRA S, FUJITA T, INAGAKI F. X-ray crystal structure of IRF-3 and its functional implications. Nat Struct Biol. 2003;10:922–7. doi: 10.1038/nsb1001. [DOI] [PubMed] [Google Scholar]
- TAKAOKA A, YANAI H, KONDO S, DUNCAN G, NEGISHI H, MIZUTANI T, KANO S, HONDA K, OHBA Y, MAK TW, TANIGUCHI T. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434:243–9. doi: 10.1038/nature03308. [DOI] [PubMed] [Google Scholar]
- TANIGUCHI T, OGASAWARA K, TAKAOKA A, TANAKA N. IRF family of transcription factors as regulators of host defense. Annu Rev Immunol. 2001;19:623–55. doi: 10.1146/annurev.immunol.19.1.623. [DOI] [PubMed] [Google Scholar]
- VAN HEEKE G, OTT TL, STRAUSS A, AMMATURO D, BAZER FW. High yield expression and secretion of the ovine pregnancy recognition hormone interferon-tau by Pichia pastoris. J Interferon Cytokine Res. 1996;16:119–26. doi: 10.1089/jir.1996.16.119. [DOI] [PubMed] [Google Scholar]
- VIEIRA AR, MODESTO A, MEIRA R, BARBOSA AR, LIDRAL AC, MURRAY JC. Interferon regulatory factor 6 (IRF6) and fibroblast growth factor receptor 1 (FGFR1) contribute to human tooth agenesis. Am J Med Genet A. 2007;143:538–45. doi: 10.1002/ajmg.a.31620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WASHBOURNE BJ, COX TC. Expression profiles of cIRF6, cLHX6 and cLHX7 in the facial primordia suggest specific roles during primary palatogenesis. BMC Dev Biol. 2006;6:18. doi: 10.1186/1471-213X-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU X, HAN J, ITO Y, BRINGAS P, URATA MM, JR, CHAI Y. Cell autonomous requirement for Tgfbr2 in the disappearance of medial edge epithelium during palatal fusion. Dev Biol. 2006;297:238–48. doi: 10.1016/j.ydbio.2006.05.014. [DOI] [PubMed] [Google Scholar]
- ZHANG Y, DERYNCK R. Regulation of Smad signalling by protein associations and signalling crosstalk. Trends Cell Biol. 1999;9:274–9. doi: 10.1016/s0962-8924(99)01579-2. [DOI] [PubMed] [Google Scholar]
- ZUCCHERO TM, COOPER ME, MAHER BS, DAACK-HIRSCH S, NEPOMUCENO B, RIBEIRO L, CAPRAU D, CHRISTENSEN K, SUZUKI Y, MACHIDA J, NATSUME N, YOSHIURA K, VIEIRA AR, ORIOLI IM, CASTILLA EE, MORENO L, ARCOS-BURGOS M, LIDRAL AC, FIELD LL, LIU YE, RAY A, GOLDSTEIN TH, SCHULTZ RE, SHI M, JOHNSON MK, KONDO S, SCHUTTE BC, MARAZITA ML, MURRAY JC. Interferon regulatory factor 6 (IRF6) gene variants and the risk of isolated cleft lip or palate. N Engl J Med. 2004;351:769–80. doi: 10.1056/NEJMoa032909. [DOI] [PubMed] [Google Scholar]