Abstract
We investigated whether the greater degree of exercise-induced diaphragmatic fatigue previously reported in highly trained athletes in hypoxia (compared with normoxia) could have a contribution from limited respiratory muscle blood flow. Seven trained cyclists completed three constant load 5 min exercise tests at inspired O2 fractions (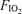 ) of 0.13, 0.21 and 1.00 in balanced order. Work rates were selected to produce the same tidal volume, breathing frequency and respiratory muscle load at each
) of 0.13, 0.21 and 1.00 in balanced order. Work rates were selected to produce the same tidal volume, breathing frequency and respiratory muscle load at each 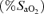 (63 ± 1, 78 ± 1 and 87 ± 1% of normoxic maximal work rate, respectively). Intercostals and quadriceps muscle blood flow (IMBF and QMBF, respectively) were measured by near-infrared spectroscopy over the left 7th intercostal space and the left vastus lateralis muscle, respectively, using indocyanine green dye. The mean pressure time product of the diaphragm and the work of breathing did not differ across the three exercise tests. After hypoxic exercise, twitch transdiaphragmatic pressure fell by 33.3 ± 4.8%, significantly (P < 0.05) more than after both normoxic (25.6 ± 3.5% reduction) and hyperoxic (26.6 ± 3.3% reduction) exercise, confirming greater fatigue in hypoxia. Despite lower leg power output in hypoxia, neither cardiac output nor QMBF (27.6 ± 1.2 l min−1 and 100.4 ± 8.7 ml (100 ml)−1 min−1, respectively) were significantly different compared with normoxia (28.4 ± 1.9 l min−1 and 94.4 ± 5.2 ml (100 ml)−1 min−1, respectively) and hyperoxia (27.8 ± 1.6 l min−1 and 95.1 ± 7.8 ml (100 ml)−1 min−1, respectively). Neither IMBF was different across hypoxia, normoxia and hyperoxia (53.6 ± 8.5, 49.9 ± 5.9 and 52.9 ± 5.9 ml (100 ml)−1 min−1, respectively). We conclude that when respiratory muscle energy requirement is not different between normoxia and hypoxia, diaphragmatic fatigue is greater in hypoxia as intercostal muscle blood flow is not increased (compared with normoxia) to compensate for the reduction in
(63 ± 1, 78 ± 1 and 87 ± 1% of normoxic maximal work rate, respectively). Intercostals and quadriceps muscle blood flow (IMBF and QMBF, respectively) were measured by near-infrared spectroscopy over the left 7th intercostal space and the left vastus lateralis muscle, respectively, using indocyanine green dye. The mean pressure time product of the diaphragm and the work of breathing did not differ across the three exercise tests. After hypoxic exercise, twitch transdiaphragmatic pressure fell by 33.3 ± 4.8%, significantly (P < 0.05) more than after both normoxic (25.6 ± 3.5% reduction) and hyperoxic (26.6 ± 3.3% reduction) exercise, confirming greater fatigue in hypoxia. Despite lower leg power output in hypoxia, neither cardiac output nor QMBF (27.6 ± 1.2 l min−1 and 100.4 ± 8.7 ml (100 ml)−1 min−1, respectively) were significantly different compared with normoxia (28.4 ± 1.9 l min−1 and 94.4 ± 5.2 ml (100 ml)−1 min−1, respectively) and hyperoxia (27.8 ± 1.6 l min−1 and 95.1 ± 7.8 ml (100 ml)−1 min−1, respectively). Neither IMBF was different across hypoxia, normoxia and hyperoxia (53.6 ± 8.5, 49.9 ± 5.9 and 52.9 ± 5.9 ml (100 ml)−1 min−1, respectively). We conclude that when respiratory muscle energy requirement is not different between normoxia and hypoxia, diaphragmatic fatigue is greater in hypoxia as intercostal muscle blood flow is not increased (compared with normoxia) to compensate for the reduction in 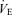 , thus further compromising O2 supply to the respiratory muscles.
, thus further compromising O2 supply to the respiratory muscles.
In healthy subjects of varying fitness, diaphragmatic fatigue is thought to be affected by the magnitude of diaphragmatic work, the degree of arterial hypoxaemia and the competition for blood flow between the diaphragm and the locomotor muscles (Babcock et al. 1996, 2002; Romer & Polkey, 2008). Accordingly, an imbalance of muscle force output versus blood flow or O2 transport availability to the diaphragm that favours fatigue appears to occur during high-intensity endurance exercise (Johnson et al. 1993) when the diaphragm must compete with locomotor muscles for its share of the available cardiac output (Harms et al. 1997, 1998, 2000) or when arterial O2 saturation drops below ∼90% (Babcock et al. 1995; Vogiatzis et al. 2006, 2007).
Our previous work (Vogiatzis et al. 2006, 2007) approached the question of whether, at near-maximal levels of exercise, arterial hypoxaemia per se or blood flow competition between the respiratory and locomotor muscles is more important in causing diaphragmatic fatigue in highly trained athletes. Isolating the role of hypoxaemia on diaphragmatic fatigue is difficult as hypoxaemia potentiates the hyperventilatory response to exercise, thus increasing the work of breathing and the degree of diaphragmatic fatigue (Babcock et al. 1995; Cibella et al. 1996; Gudjonsdottir et al. 2001). We therefore compared hypoxic and normoxic exercise at levels that produced the same respiratory work, which meant setting a lower leg work rate in hypoxia. We found greater fatigue in hypoxia (Vogiatzis et al. 2007). However, without any respiratory blood flow measurements we were unable to decide between hypoxemia per se or hypoxaemia combined with limited respiratory muscle blood flow as the principal reason for greater diaphragmatic fatigue.
Limited respiratory muscle blood flow could be caused by competition between the leg and respiratory muscles with the former ‘winning’ at the expense of the latter. However, based on the assumption that cardiac output would not be different between the two conditions (Stenberg et al. 1966), we noted that the lower leg work rate in hypoxia, probably requiring less leg blood flow than in normoxia, would if anything allow greater respiratory muscle blood flow in hypoxia, rather than less (compared with normoxia). The greater degree of diaphragmatic fatigue in hypoxia was therefore attributed to hypoxaemia, in spite of the potentially greater respiratory muscle blood flow availability. However, the possibility that in hypoxia, despite the lower work rate, leg blood flow could rise due to hypoxic sympathetic vasodilatation to similar or greater values as in normoxia (Koskolou et al. 1997; Calbet et al. 2003) to compensate for the reduced arterial O2 content and preserve O2 delivery, thus potentially competing with the diaphragm for the available blood flow, was not explored as blood flow to the leg and in particular the respiratory muscles, was not assessed.
Measuring blood flow to the respiratory muscles is difficult owing to their complex anatomical arrangement, the extensive vascular network and the large variation in muscular recruitment with varying degrees of ventilation. We recently succeeded in quantifying absolute changes in intercostal muscle blood flow at different levels of isocapnic hyperpnoea in healthy subjects (Guenette et al. 2008) by using near-infrared spectroscopy over the left 7th intercostal space and the light-absorbing tracer indocyanine green dye injected intravenously. Our data revealed that as ventilation rose, intercostal muscle blood flow was linearly correlated with the work of breathing and transdiaphragmatic pressure.
Accordingly, in the present study we repeated our previous measurements across a range of 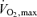 values with the addition of measurements of cardiac output, quadriceps and intercostal muscle blood flow. Again using work rates that were selected to equalize respiratory work across the
values with the addition of measurements of cardiac output, quadriceps and intercostal muscle blood flow. Again using work rates that were selected to equalize respiratory work across the 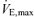 range, we investigated whether the greater degree of diaphragmatic fatigue previously documented in hypoxia (Vogiatzis et al. 2007) is mainly due to uncompensated hypoxaemia in spite of increased intercostal muscle blood flow, or rather due to hypoxaemia combined with similar or reduced intercostal muscle blood flow. We reasoned that if intercostal muscle blood flow in hypoxia was greater than in normoxia (due to lower work rate) the greater amount of fatigue would be due to arterial hypoxaemia per se as the greater intercostal muscle blood flow failed to counteract hypoxaemia. If intercostal muscle blood flow in hypoxia was, however, similar or lower than in normoxia, greater fatigue would be due to both hypoxaemia and insufficiently adjusted (to counteract hypoxaemia) respiratory muscle blood flow.
range, we investigated whether the greater degree of diaphragmatic fatigue previously documented in hypoxia (Vogiatzis et al. 2007) is mainly due to uncompensated hypoxaemia in spite of increased intercostal muscle blood flow, or rather due to hypoxaemia combined with similar or reduced intercostal muscle blood flow. We reasoned that if intercostal muscle blood flow in hypoxia was greater than in normoxia (due to lower work rate) the greater amount of fatigue would be due to arterial hypoxaemia per se as the greater intercostal muscle blood flow failed to counteract hypoxaemia. If intercostal muscle blood flow in hypoxia was, however, similar or lower than in normoxia, greater fatigue would be due to both hypoxaemia and insufficiently adjusted (to counteract hypoxaemia) respiratory muscle blood flow.
Methods
Subjects
Seven healthy competitive Greek male cyclists participated in the study which was approved by the authors’ University Ethics Committee and was conducted in accordance with the guidelines of the Declaration of Helsinki. Prior to participation in the study, all subjects (whose physical characteristics are given in Table 1) were informed of any risks and discomforts associated with the experiments and gave written, signed informed consent.
Table 1.
Pulmonary function and maximal exercise data of the study population
| Age (years) | 31 ± 5 |
| Height (cm) | 180 ± 2 |
| Weight (kg) | 73 ± 2 |
| FEV1 (l) | 4.67 ± 0.18 |
| FEV1 (% predicted) | 108 ± 3.67 |
| FVC (l) | 5.63 ± 0.17 |
| FVC (% predicted) | 109 ± 2.30 |
| WRmax (W) | 373 ± 10 |
 (ml kg−1 min−1) (ml kg−1 min−1) |
63.7 ± 4.2 |
| HRmax (beats min−1) | 182 ± 5 |
| RER at WRmax | 1.20 ± 0.02 |
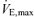 (l min−1) (l min−1) |
163 ± 9 |
| VTmax (l min−1) | 3.11 ± 0.14 |
| fmax (breaths min−1) | 53 ± 4 |
Values are means ±s.e.m. Exercise data depict the results of the incremental exercise test in room air. FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; WRmax, maximal work rate, 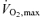 , maximal oxygen uptake, RER, respiratory exchange ratio,
, maximal oxygen uptake, RER, respiratory exchange ratio, 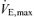 , maximal minute ventilation, VTmax, maximal tidal volume, fmax, maximal breathing frequency.
, maximal minute ventilation, VTmax, maximal tidal volume, fmax, maximal breathing frequency.
Experimental design
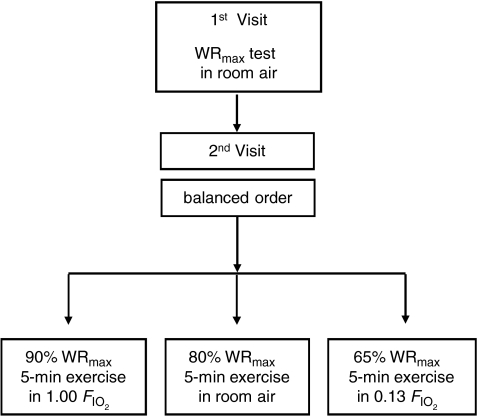
Experiments were conducted in two visits (Fig. 1) as previously described (Vogiatzis et al. 2008). In visit 1, subjects underwent an incremental exercise test to the limit of tolerance (WRmax). This test was carried out in room air. In visit 2 (Fig. 1), subjects completed three 5 min exercise tests, each separated by 90 min, at work rates corresponding to the following targeted intensities and 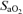 values: (i) 90% WRmax while breathing a high
values: (i) 90% WRmax while breathing a high 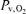 (1.00: hyperoxia); (ii) 80% WRmax in room air (normoxia); and (iii) 65% WRmax with
(1.00: hyperoxia); (ii) 80% WRmax in room air (normoxia); and (iii) 65% WRmax with 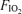 of 0.13 (hypoxia). Based on previous studies (Vogiatzis et al. 2006, 2007), work rate during the tests was slightly adjusted, if necessary, to keep both tidal volume and breathing frequency the same at each
of 0.13 (hypoxia). Based on previous studies (Vogiatzis et al. 2006, 2007), work rate during the tests was slightly adjusted, if necessary, to keep both tidal volume and breathing frequency the same at each  (Fig. 2). The order of application of each exercise level/
(Fig. 2). The order of application of each exercise level/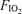 combination was balanced across subjects 1–6, each of these subjects having their own unique order. Subject 7 repeated the sequence order of subject 1. Room temperature during the testing days ranged between 24 and 28°C.
combination was balanced across subjects 1–6, each of these subjects having their own unique order. Subject 7 repeated the sequence order of subject 1. Room temperature during the testing days ranged between 24 and 28°C.
Figure 1. Experimental design.
Experiments were conducted in 2 visits. In visit 1, subjects underwent an incremental exercise test in room air. In visit 2, subjects completed in balanced ordering sequence three 5 min exercise tests, separated by 90 min, breathing the following fractions of inspired O2 gas mixtures (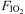 : hyperoxic (1.00), normoxic (0.21) and hypoxic (0.13).
: hyperoxic (1.00), normoxic (0.21) and hypoxic (0.13).
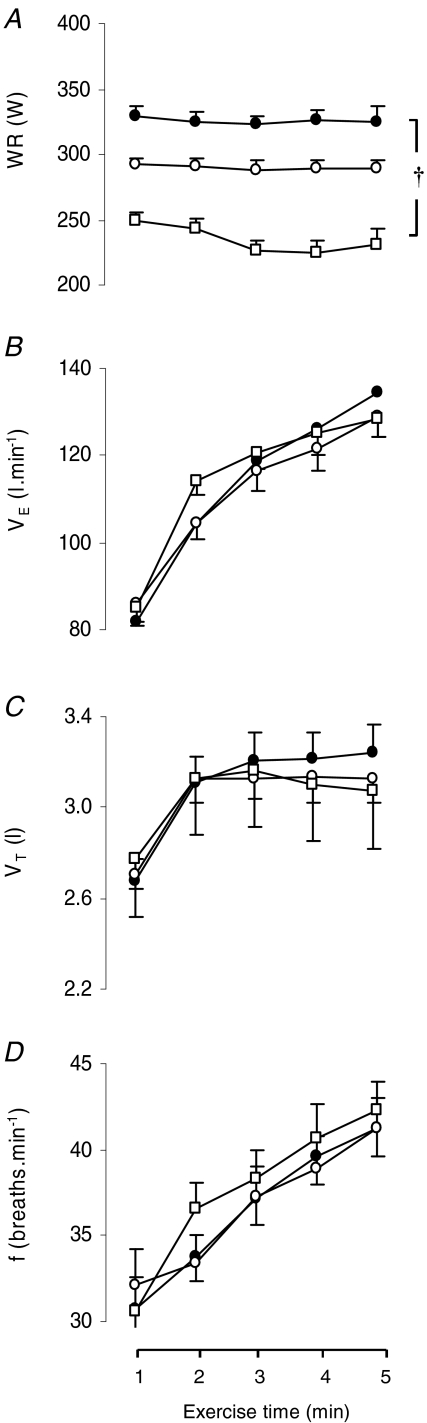
Figure 2. Exercise load and ventilatory responses during the three 5 min exercise tests.
Work rate (WR; A), minute ventilation (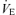 ; B), tidal volume (VT; C) and breathing frequency (f; D) throughout the hyperoxic (
; B), tidal volume (VT; C) and breathing frequency (f; D) throughout the hyperoxic (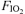 : 1.00 (•)), the normoxic (
: 1.00 (•)), the normoxic (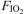 : 0.21 (○)) and the hypoxic exercise test (
: 0.21 (○)) and the hypoxic exercise test (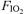 : 0.13 (□)). Values are means ±s.e.m. for 7 subjects. †Differences between the three conditions, P < 0.05. WR changes during the course of each test reflect minor real-time adjustments needed to maintain comparable tidal volume and breathing frequency at each
: 0.13 (□)). Values are means ±s.e.m. for 7 subjects. †Differences between the three conditions, P < 0.05. WR changes during the course of each test reflect minor real-time adjustments needed to maintain comparable tidal volume and breathing frequency at each 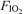 .
.
Incremental exercise tests
In visit 1, the incremental exercise tests were performed on an electromagnetically braked cycle ergometer (Ergoline 800; Sensor Medics, Anaheim, CA, USA) starting at 30 W and increasing by 30 W every minute, with the subjects maintaining a pedalling frequency of 70–90 r.p.m. Tests were preceded by a 3 min rest period, followed by 3 min of unloaded pedalling. The following pulmonary gas exchange and ventilatory variables were recorded breath by breath (Vmax 229; Sensor Medics): oxygen uptake (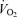 ), carbon dioxide elimination (
), carbon dioxide elimination (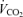 ), respiratory exchange ratio (RER), minute ventilation (
), respiratory exchange ratio (RER), minute ventilation (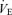 ), tidal volume (VT), and breathing frequency (f). Heart rate (HR) was determined using the R–R interval from a 12-lead on line electrocardiogram (Marquette Max; Marquette Hellige GmbH, Germany).
), tidal volume (VT), and breathing frequency (f). Heart rate (HR) was determined using the R–R interval from a 12-lead on line electrocardiogram (Marquette Max; Marquette Hellige GmbH, Germany).
Subject preparation (visit 2)
Subjects were prepared first with femoral arterial and venous catheters for blood flow measurements and blood sampling, and then with oesophageal and gastric balloons for assessment of diaphragm fatigue and work of breathing.
Using local anaesthesia (2% lidocaine) and sterile technique, identical catheters were introduced percutaneously into the right femoral artery and right femoral vein about 2 cm below the inguinal ligament, both orientated in the proximal direction. We used 20 cm long 16-gauge flexible catheters (CV-04301, Arrow International, Reading, PA, USA) placed over a 0.032 inch guide wire introduced through an 18-gauge needle. Catheters were secured in place by a 3-O skin suture and additionally by clear adhesive tape. The catheters were used to collect arterial and femoral venous blood samples and also to inject indocyanine green dye (ICG) and sample blood after each injection for blood flow measurements. They were kept patent throughout the experiment by periodic flushing with heparinized (1 unit ml−1) saline.
Gastric and oesophageal pressures were assessed by two commercially available thin-walled balloon catheters (Ackrad Laboratories, Inc., Crandford, NJ, USA) coupled to differential pressure transducers (MP-45, ±250 cmH2O; Validyne Corp., Northridge, CA, USA). The balloons were inserted by nasal intubation following the application of 2% lidocaine anaesthetic gel to the nose and with the assistance of continuous pressure monitoring. The two balloon tips were positioned in the middle third of the oesophagus and stomach, respectively.
Visit 2: 5 min exercise tests
Subjects initially warmed up by cycling at 50% of 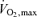 for 5 min in room air. Without stopping, they were switched to one of three
for 5 min in room air. Without stopping, they were switched to one of three 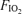 values (0.13, 0.21, 1.0) using a standard mouthpiece and two-way non-rebreathing valve system (model 2700, Hans Rudolph) and began 5 min of heavy exercise at the power output indicated above for the chosen
values (0.13, 0.21, 1.0) using a standard mouthpiece and two-way non-rebreathing valve system (model 2700, Hans Rudolph) and began 5 min of heavy exercise at the power output indicated above for the chosen 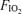 . During these tests, recording of pulmonary gas exchange and ventilatory variables was performed as mentioned above, whilst gas of the designated
. During these tests, recording of pulmonary gas exchange and ventilatory variables was performed as mentioned above, whilst gas of the designated 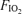 was inspired by the subjects from a Douglas bag connected to the two-way non-rebreathing valve by a piece of tubing. Femoral arterial and venous blood was taken during the third and fifth minutes of exercise, whereas swings in oesophageal pressure (Poes), gastric pressure (Pga) and transdiaphragmatic pressure (Pdi) were continuously monitored. Pdi was obtained from electronic subtraction of Poes from Pga. Flow was measured with a hot wire pneumotachograph (Vmax 229; Sensor Medics) near the mouthpiece, and tidal volume changes were obtained by integrating the flow signal. Gastric and oesophageal pressures and air flow rates were displayed on a computer screen, and digitized at 60 Hz using an analogue-to-digital converter connected to the same computer used for optoelectronic plethysmography (OEP system, BTS, Milan, Italy). End-inspiratory and expiratory chest wall volume regulation during exercise was determined by OEP as previously described (Vogiatzis et al. 2005). In brief, the movement of 89 retro-reflective markers placed front and back over the chest wall from clavicles to pubis was recorded. Each marker was tracked by six video cameras, three in front of the subject and three behind. Dedicated software recognizes in real time the markers on each camera, reconstructs their 3D co-ordinates by stereophotogrametry and calculates volume variations.
was inspired by the subjects from a Douglas bag connected to the two-way non-rebreathing valve by a piece of tubing. Femoral arterial and venous blood was taken during the third and fifth minutes of exercise, whereas swings in oesophageal pressure (Poes), gastric pressure (Pga) and transdiaphragmatic pressure (Pdi) were continuously monitored. Pdi was obtained from electronic subtraction of Poes from Pga. Flow was measured with a hot wire pneumotachograph (Vmax 229; Sensor Medics) near the mouthpiece, and tidal volume changes were obtained by integrating the flow signal. Gastric and oesophageal pressures and air flow rates were displayed on a computer screen, and digitized at 60 Hz using an analogue-to-digital converter connected to the same computer used for optoelectronic plethysmography (OEP system, BTS, Milan, Italy). End-inspiratory and expiratory chest wall volume regulation during exercise was determined by OEP as previously described (Vogiatzis et al. 2005). In brief, the movement of 89 retro-reflective markers placed front and back over the chest wall from clavicles to pubis was recorded. Each marker was tracked by six video cameras, three in front of the subject and three behind. Dedicated software recognizes in real time the markers on each camera, reconstructs their 3D co-ordinates by stereophotogrametry and calculates volume variations.
Pdi was averaged over 30 s breath samples in every minute of the exercise tests. Mean Pdi was measured by integration of Pdi during inspiration divided by the inspiratory time. The pressure–time product of the diaphragm (mean Pdi× inspiratory time × respiratory frequency) was then calculated and expressed in cmH2O s−1 min−1. The mechanical work of breathing (WOB) was determined by ensemble averaging several breaths to integrate the averaged esophageal pressure–tidal volume loop. The WOB was then multiplied by the breathing frequency to obtain the total amount of work done per minute by the respiratory system and expressed in J min−1 (Otis, 1964).
Assessment of diaphragmatic fatigue
To assess diaphragmatic fatigue, twitch transdiaphragmatic pressure (Pdi,tw) was measured before and at 10, 30 and 60 min following each of the three exercise tests. During recovery from each test, subjects breathed room air. Pdi,tw was recorded during stimulation of the phrenic nerves at the neck according to recommended techniques (Babcock et al. 2002). Bilateral supramaximal transcutaneous phrenic nerve single shocks (twitches) were performed with an electrical stimulator (Neuropack; Nichon-Kohden, Tokyo, Japan). The phrenic nerves were stimulated at the posterior border of the sternomastoid muscle at the level of the cricoid cartilage. Each nerve was separately stimulated by applying single square-wave electrical pulses (duration 100 μs). The stimulus voltage was progressively increased until there was no further increase in the amplitude of the ipsilateral peak-to-peak compound motor action potential of the diaphragm (Laghi et al. 1995). The intensity of the electrical stimulus was then increased by a further 20–50%. The position of stimulation of each phrenic nerve was marked, and the subsequent stimulations were performed at this point. Compound motor action potentials were recorded with surface electrodes placed at the seventh to eighth right intercostals space and the anterior axillary line. The signals were amplified, band-pass filtered (bandwidth 10 Hz to 1 kHz), displayed on the screen, and printed by the stimulator-electromyograph (Neuropack; Nichon-Kohden). Chest wall volumes were continuously monitored throughout the stimulation tests by optoelectronic plethysmography (Vogiatzis et al. 2005) to ensure similar thoraco-abdominal configuration during twitches (Chen et al. 2000). The electrical stimulations were performed with the subject seated on the bicycle ergometer, with a nose clip on and with the mouth closed. Subjects were instructed to breathe quietly, then to perform a gentle expiratory effort to functional residual capacity and to hold their breath while the stimulation took place. We relied on Poes as a measure of position in the respiratory cycle relative to functional residual capacity. Hence, Poes immediately before stimulation was always carefully evaluated to ensure constant lung volume. Once relaxation was achieved (as judged by levelling off of Pdi, Poes and Pga) the operator performed the stimulations. Criteria adopted for acceptance of Pdi,tw were those previously used (Laghi et al. 1995; Babcock et al. 2002). During each designated time point, 8–10 twitches were performed and the average value of the three measurements with highest Pdi,tw values was used for the analysis.
Cardiac output
Between the third and fifth minute of exercise, cardiac output was determined in duplicate by the dye dilution method (Dow, 1956), using known volumes of indocyanine green dye (ICG, range: 0.8–1.2 ml at 5 mg ml−1) injected into the right femoral vein followed by a 10 ml flush of isotonic saline. Blood was withdrawn from the femoral artery using an automated pump (Harvard Apparatus, USA) at 20 ml min−1 through a linear photodensitometer (Pulsion ICG, ViCare Medical, Denmark) connected to a cardiac output computer (Waters CO-10, Rochester, MN, USA) through a closed loop, sterile tubing system. The blood was re-infused into the femoral vein immediately upon completion of the measurements. The cardiac output computer was connected to a data acquisition system (DI-720, Dataq, OH, USA). Data were sampled at 100 Hz and stored on a computer for subsequent analysis. To remove the influence of dye recirculation, the downslopes of the dye concentration curves were linearly extrapolated using a semilogarithmic scale in the conventional manner (Dow, 1956). Cardiac output was calculated as the ratio of ICG mass injected to the mean arterial ICG concentration over the time interval of the curve and expressed as litres per minute. ICG calibration curves were obtained following each experiment by measuring the raw voltage deflection from three 20 ml blood samples containing various concentrations of ICG. Calibrations at each concentration were performed 2–3 times to ensure linearity and consistency.
Intercostals and quadriceps muscle blood flow by near-infrared spectroscopy (NIRS)
In order to measure intercostal and quadriceps muscle blood flow (IMBF and QMBF, respectively), two sets of NIRS optodes were placed on the skin over the left 7th intercostal space and over the vastus lateralis muscle 100–120 mm from the knee parallel to the major axis of the thigh and secured using double-sided adhesive tape. NIRS signals were collected continuously during exercise.
The optode separation distance was 4 cm, corresponding to a penetration depth of ∼2 cm. The left intercostal space was used in order to avoid potential blood flow contributions from the liver on the right side of the body. Respiratory muscles within view of the optode included primarily the internal and external intercostals. Optodes were connected to a NIRO 300 spectrophotometer (Hamamatsu Photonics KK, Hamamatsu, Japan) which was used to measure ICG concentration following the same 4–5 mg bolus injection of ICG in the right femoral vein as used above for cardiac output assessment. As previously described (Boushel et al. 2000; Kalliokoski et al. 2006), the ICG bolus circulates to the right heart and lungs and enters the arterial circulation where arterial blood is then withdrawn by a pump. The arterial ICG concentration is measured by photodensitometry, while in the tissue microcirculation ICG is detected transcutaneously by measuring light attenuation with NIRS at 775, 813, 850 and 913 nm wavelengths and analysed using an algorithm incorporating the Modified Beer–Lambert Law (van der Zee et al. 1992; Duncan et al. 1995; Boushel et al. 2000). Since the measured light attenuation in the tissue is influenced by ICG and oxy- and deoxyhemoglobin, the independent contribution of ICG to the light absorption signal was isolated using a matrix operation (MATLAB). The matrix operation incorporates path length-specific extinction coefficients for each of the light absorbing chromophores (haemoglobin + myoglobin and ICG) at each wavelength employed by the NIRS machine (Hamamatsu Photonics KK).
Blood analysis and calculations
Femoral arterial and venous tensions of O2 (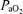 ) and CO2 (
) and CO2 (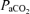 ), pH, haemoglobin concentration (Hb), whole-body lactate concentration and percentage arterial oxygen saturation
), pH, haemoglobin concentration (Hb), whole-body lactate concentration and percentage arterial oxygen saturation 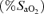 were measured from 2 ml blood samples using a blood gas electrode system combined with a co-oximeter (ABL 625, Radiometer, Copenhagen, Denmark) within 10 s of collection. Plasma HCO3− was determined as described by Siggaard-Andersen (1974). Arterial and venous O2 content (
were measured from 2 ml blood samples using a blood gas electrode system combined with a co-oximeter (ABL 625, Radiometer, Copenhagen, Denmark) within 10 s of collection. Plasma HCO3− was determined as described by Siggaard-Andersen (1974). Arterial and venous O2 content (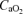 and
and 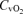 , respectively) were computed from the saturation (
, respectively) were computed from the saturation (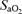 ) and haemoglobin (Hb) (i.e. (1.34[Hb]×
) and haemoglobin (Hb) (i.e. (1.34[Hb]×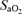 ) + (0.003 ×
) + (0.003 ×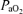 )). Venous samples were placed on ice until arterial samples had been analysed and were then immediately measured. No venous samples sat longer than 5 min in ice before measurements were made. The blood gas analyser was auto-calibrated every 4 h throughout the day and calibrating gases of known concentrations were run before each set of measurements.
)). Venous samples were placed on ice until arterial samples had been analysed and were then immediately measured. No venous samples sat longer than 5 min in ice before measurements were made. The blood gas analyser was auto-calibrated every 4 h throughout the day and calibrating gases of known concentrations were run before each set of measurements.
Arterio-venous O2 difference (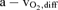 ) was calculated from the difference in femoral arterial and femoral venous O2 content. This difference was then divided by arterial O2 content to give leg O2 extraction. Systemic O2 delivery was computed as the product of cardiac output and
) was calculated from the difference in femoral arterial and femoral venous O2 content. This difference was then divided by arterial O2 content to give leg O2 extraction. Systemic O2 delivery was computed as the product of cardiac output and 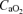 .
.
Statistical analysis
The minimum sample size was calculated based on 80% power and a two-sided 0.05 significance level. Sample size capable of detecting between-condition difference of 10% was estimated for the decrease in Pdi,tw using the standard deviation from our previous studies (Vogiatzis et al. 2006, 2007). The critical sample size was estimated to be seven subjects. All data are reported as mean ±s.e.m. because our primary interest is in mean group differences, not individual variation. Two-way ANOVA with repeated measures was used to identify statistically significant differences across different time points among the three 5 min tests for all respiratory and blood analysis variables. For cardiac output, IMBF and QMBF and O2 transport variables, one-way ANOVA with repeated measures was used to identify statistically significant differences across the mean values derived from averaging the data over the 3rd and 5th minute of each 5 min exercise tests. From previous work (Vogiatzis et al. 2006, 2007) showing that the greatest degree of fatigue occurs at 10 min post-exercise, we first examined whether the fall in Pdi,tw at 10 min of recovery was affected by 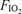 . Two-way ANOVA with repeated measures was used to identify statistically significant differences between values recorded at baseline and at the 10th minute of recovery following the three exercise tests. When one- or two-way ANOVA detected statistical significance, differences between the three conditions were identified with the LSD post hoc test. The level of significance for all analyses was set at P < 0.05.
. Two-way ANOVA with repeated measures was used to identify statistically significant differences between values recorded at baseline and at the 10th minute of recovery following the three exercise tests. When one- or two-way ANOVA detected statistical significance, differences between the three conditions were identified with the LSD post hoc test. The level of significance for all analyses was set at P < 0.05.
Results
Incremental and 5 min exercise tests
Table 1 shows the subjects’ responses to the incremental exercise tests in air. Relative to the maximal workload achieved in the incremental exercise tests in room air, the mean workload achieved in the three 5 min tests was 87 ± 1% in hyperoxia, 78 ± 1% in normoxia and 63 ± 1% in hypoxia. In absolute terms, these were power outputs of 325 ± 6 W in hyperoxia, 290 ± 5 W in normoxia and 235 ± 6 W in hypoxia. Across the three tests mean work rate was significantly (P = 0.0001) different (Fig. 2A).
The ventilatory response to the exercise tests is shown in Fig. 2B–D. 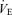 increased similarly with exercise in all tests. Peak
increased similarly with exercise in all tests. Peak 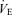 at the end of the three exercise tests ranged between 80 ± 5% to 84 ± 6% of the maximum value attained at the incremental test in room air (Fig. 2B). No significant differences were found in
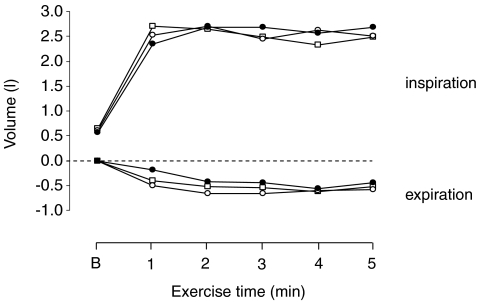
at the end of the three exercise tests ranged between 80 ± 5% to 84 ± 6% of the maximum value attained at the incremental test in room air (Fig. 2B). No significant differences were found in 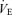 between the three tests. Neither tidal volume nor frequency differed across the three tests (Fig. 2C and D). The increase in tidal volume during exercise was achieved by an increase in end-inspiratory volume and a decrease in end-expiratory volume by approximately 2.5 l and 0.5 l, respectively (Fig. 3). End-expiratory volume remained reduced (by approximately 0.5 l) throughout the duration of all three exercise tests (Fig. 3).
between the three tests. Neither tidal volume nor frequency differed across the three tests (Fig. 2C and D). The increase in tidal volume during exercise was achieved by an increase in end-inspiratory volume and a decrease in end-expiratory volume by approximately 2.5 l and 0.5 l, respectively (Fig. 3). End-expiratory volume remained reduced (by approximately 0.5 l) throughout the duration of all three exercise tests (Fig. 3).
Figure 3. Chest wall volume regulation at baseline (B) and during exercise in hypoxia (□), normoxia (○) and hyperoxia (•).
End-inspiratory and end-expiratory chest wall volumes are expressed as the difference from the end-expiratory volume at baseline and at 1, 2, 3, 4 and 5 min of exercise. Error bars have been omitted for clarity.
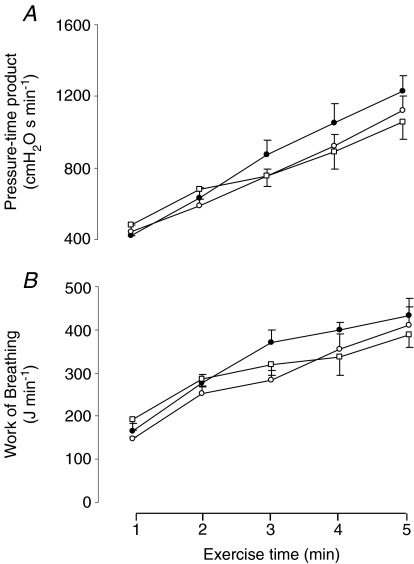
Diaphragmatic fatigue in recovery
Figure 4A shows the cumulative pressure–time product of the diaphragm, indicating total diaphragm effort over the 5 min, for each of the three exercise tests. Figure 4B shows the work of breathing itself. No significant differences were found for either variable across the three exercise tests.
Figure 4. Diaphragmatic work during exercise.
Changes in A: pressure–time product of the diaphragm and B: work of breathing throughout the hyperoxic (FIO2: 1.00 (•)), the normoxic (FIO2: 0.21 (○)) and the hypoxic exercise test (FIO2: 0.13 (□)). Values are means ±s.e.m. for 7 subjects.
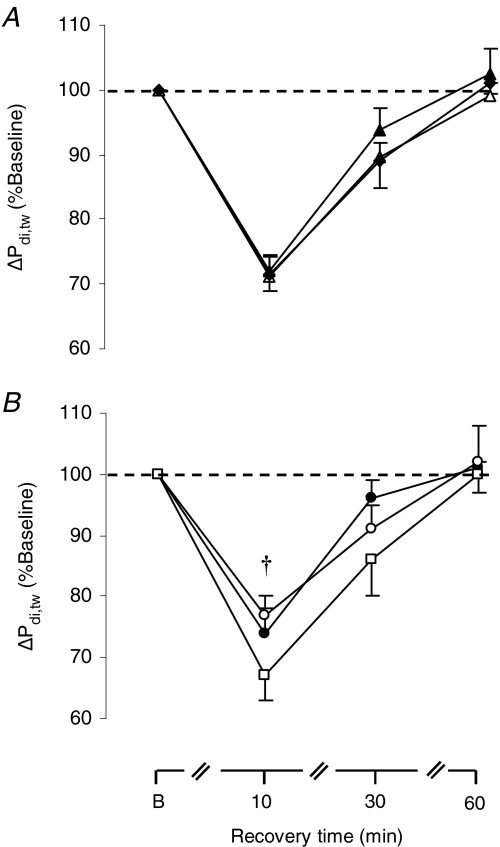
The time course of the percentage drop in Pdi,tw after the first, second and third exercise tests (irrespective of the order in which the three 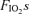 were administered) is shown in Fig. 5A, demonstrating lack of significant ordering effect. Note that 60 min after completion of each exercise test, mean Pdi,tw had fully recovered to the pre-testing levels (Fig. 5A). As a result, Pdi,tw at the baseline of each subsequent test was at similar resting levels as for the first test.
were administered) is shown in Fig. 5A, demonstrating lack of significant ordering effect. Note that 60 min after completion of each exercise test, mean Pdi,tw had fully recovered to the pre-testing levels (Fig. 5A). As a result, Pdi,tw at the baseline of each subsequent test was at similar resting levels as for the first test.
Figure 5. Response of twitch transdiaphragmatic pressure during recovery.
Percentage fall in twitch diaphragmatic pressure (ΔPdi,tw) during recovery after A: the 1st (▴), 2nd (▵) and the 3rd (♦) exercise test (irrespective of FIO2); B: the hyperoxic (FIO2:1.00 (•)), the normoxic (FIO2: 0.21 (□)) and the hypoxic exercise test (FIO2: 0.13 (○)). Values are means ±s.e.m. for 7 subjects. †Significant difference between the normoxic and the hypoxic test, P < 0.05.
As shown in Fig. 5B, we confirmed our prior observations that all exercise tests induced a significant (P = 0.001) fall in Pdi,tw that was greatest at 10 min into recovery (ranging from 25 ± 3 to 33 ± 5%). The degree of fall in Pdi,tw 10 min into recovery was significantly different (P = 0.047) across the three conditions (Fig. 5B). Post hoc analysis revealed significant differences in the percentage fall in Pdi,tw between the normoxic (by 25.5 ± 3.5%) and hypoxic run (by 33.3 ± 4.8%, P = 0.042), but not between normoxic and hyperoxic runs (by 26.6 ± 3.3%).
Haemodynamics
Haemodynamic data are shown in Table 2. Mean cardiac output, stroke volume and heart rate were each not significantly different across the three experimental conditions. In absolute values quadriceps muscle blood flow during exercise was not significantly different across the three exercise tests. Relative to work rate, however, quadriceps muscle blood flow in hypoxia was greater than in normoxia (Table 2). Mean intercostal muscle blood flow (measured in all three tests successfully in 5/7 subjects) was not different across the three exercise tests, whether examined as absolute values or per unit work of breathing (Table 2). Compared with normoxia, systemic O2 delivery was significantly lower in hypoxia and greater in hyperoxia (Table 2). Systemic 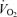 was significantly lower in hypoxia compared with normoxia, but not different between normoxia and hyperoxia (Table 2). Compared with normoxia, leg O2 extraction was significantly greater in hypoxia and lower in hyperoxia (Table 2).
was significantly lower in hypoxia compared with normoxia, but not different between normoxia and hyperoxia (Table 2). Compared with normoxia, leg O2 extraction was significantly greater in hypoxia and lower in hyperoxia (Table 2).
Table 2.
O2 transport and blood flow data during the 5 min exercise tests
| Variable | Hypoxia | Normoxia | Hyperoxia |
|---|---|---|---|
| WR (W) | 235 ± 6* | 290 ± 5 | 325 ± 6* |
| CO (l min−1) | 27.6 ± 1.2 | 28.4 ± 1.9 | 27.8 ± 1.6 |
| SV (l) | 0.166 ± 0.008 | 0.169 ± 0.019 | 0.166 ± 0.011 |
| HR (beats min−1) | 169 ± 8 | 175 ± 9 | 174 ± 9 |
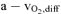 legs (ml l−1) legs (ml l−1) |
135.6 ± 9.2* | 170.5 ± 5.2 | 185.3 ± 10.1* |
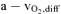 f systemic (ml l−1) f systemic (ml l−1) |
122.7 ± 14.5* | 151.2 ± 27.9 | 158.2 ± 25.5 |
| Syst O2 del (l min−1) | 4.15 ± 0.14* | 5.55 ± 0.32 | 6.07 ± 0.30* |
| Legs O2 ex (%) | 89.9 ± 1.0* | 86.9 ± 1.7 | 84.7 ± 2.2* |
Whole body 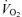 (l min−1) (l min−1) |
3.3 ± 0.1* | 4.2 ± 0.1 | 4.3 ± 0.1 |
| IMBF (ml (100 ml)−1 min−1) | 53.6 ± 8.5 | 49.9 ± 5.9 | 52.9 ± 5.9 |
| WOB (J min−1) | 353 ± 29 | 347 ± 34 | 401 ± 29 |
| IMBF/WOB (ml (100 ml)−1 J−1) | 0.151 ± 0.29 | 0.144 ± 0.17 | 0.132 ± 0.20 |
| QMBF (ml (100 ml)−1 min−1) | 100.4 ± 8.7 | 94.4 ± 5.2 | 95.1 ± 7.8 |
| QMBF/WR (ml (100 ml)−1 min−1 W−1) | 0.43 ± 0.9* | 0.33 ± 0.10 | 0.29 ± 0.8 |
WR, work rate; CO, cardiac output; SV, stroke volume; HR, heart rate; 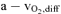 , arterio-venous difference; O2 del, O2 delivery; O2 ex (%), percentage O2 extraction;
, arterio-venous difference; O2 del, O2 delivery; O2 ex (%), percentage O2 extraction; 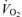 , oxygen uptake;. RMBF, respiratory muscle blood flow; WOB, work of breathing; IMBF/WOB, intercostal muscle blood flow as a function of the WOB calculated over the same period as IMBF; QMBF, quadriceps muscle blood flow assessed by NIRS; QMBF/WR quadriceps muscle blood flow as a function of the work rate. Asterisks denote significant differences (P < 0.05) compared with normoxia.
, oxygen uptake;. RMBF, respiratory muscle blood flow; WOB, work of breathing; IMBF/WOB, intercostal muscle blood flow as a function of the WOB calculated over the same period as IMBF; QMBF, quadriceps muscle blood flow assessed by NIRS; QMBF/WR quadriceps muscle blood flow as a function of the work rate. Asterisks denote significant differences (P < 0.05) compared with normoxia.
Blood analysis
Variables measured from blood samples are shown in Table 3. Throughout the exercise tests, 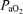 and
and 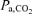 were both significantly (P < 0.005) higher in hyperoxia than in normoxia and hypoxia, whereas pH was significantly (P = 0.003) lower in hyperoxia compared with hypoxia. End-exercise arterial lactate in hypoxia (11.5 ± 2.6 mmol l−1) was not significantly different (P = 0.42) compared with that in normoxia (9.7 ± 1.6 mmol l−1) and in hyperoxia (10.6 ± 2.2 mmol l−1).
were both significantly (P < 0.005) higher in hyperoxia than in normoxia and hypoxia, whereas pH was significantly (P = 0.003) lower in hyperoxia compared with hypoxia. End-exercise arterial lactate in hypoxia (11.5 ± 2.6 mmol l−1) was not significantly different (P = 0.42) compared with that in normoxia (9.7 ± 1.6 mmol l−1) and in hyperoxia (10.6 ± 2.2 mmol l−1).
Table 3.
Femoral blood analysis variables during the three 5 min exercise tests
| Variable | Hypoxia | Normoxia | Hyperoxia |
|---|---|---|---|
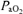 (mmHg) (mmHg) |
51.3 ± 9.5* | 89.2 ± 2.8 | 645.2 ± 11.2* |
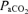 (mmHg) (mmHg) |
30.0 ± 0.5* | 35.1 ± 0.9 | 37.9 ± 1.1* |
| pHa | 7.34 ± 0.1* | 7.30 ± 0.1 | 7.26 ± 0.1* |
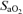 (%) (%) |
71.8 ± 2.3* | 95.0 ± 0.5 | 99.8 ± 0.1* |
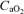 (ml l−1) (ml l−1) |
150.9 ± 4.3* | 196.6 ± 4.4 | 219.5 ± 6.2* |
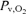 (mmHg) (mmHg) |
9.4 ± 1.1* | 15.2 ± 1.6 | 19.5 ± 2.1* |
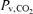 (mmHg) (mmHg) |
56.4 ± 2.7* | 68.9 ± 2.7 | 78.7 ± 2.7* |
| pHv | 7.20 ± 0.1 | 7.15 ± 0.1 | 7.10 ± 0.1 |
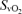 (%) (%) |
6.2 ± 0.7* | 11.4 ± 1.7 | 14.9 ± 2.3* |
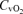 (ml l−1) (ml l−1) |
15.3 ± 1.8* | 26.1 ± 4.1 | 34.2 ± 6.1* |
| Arterial lactate (mmol l−1) | 11.5 ± 2.6 | 9.7 ± 1.6 | 10.6 ± 2.2 |
Values are means ±s.e.m. 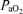 , arterial tension of O2;
, arterial tension of O2; 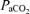 , arterial tension of CO2; pHa, arterial pH;
, arterial tension of CO2; pHa, arterial pH; 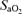 , arterial oxygen saturation;
, arterial oxygen saturation; 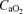 , arterial O2 content;
, arterial O2 content; 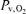 , venous tension of O2;
, venous tension of O2; 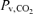 , venous tension of CO2; pHv, venous pH;
, venous tension of CO2; pHv, venous pH; 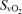 , venous oxygen saturation;
, venous oxygen saturation; 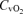 , venous O2 content. Asterisks denote significant differences (P < 0.05) compared with normoxia.
, venous O2 content. Asterisks denote significant differences (P < 0.05) compared with normoxia.
Discussion
We have confirmed our prior finding that compared with normoxia, hypoxia exaggerates the degree of diaphragmatic fatigue in highly trained endurance athletes during sustained heavy exercise at loads producing the same ventilation and work of breathing (Vogiatzis et al. 2007). In our prior study, greater diaphragmatic fatigue in hypoxia was speculatively attributed to hypoxaemia and not to limited respiratory muscle blood flow, reasoning that the lower exercise intensity in hypoxia would have allowed greater respiratory muscle blood flow to compensate for reduced arterial oxygenation. Such a presumption was based on the hypothesis that hypoxic exercise sustained at a lower work rate than in normoxia requires smaller leg blood flow, which would reduce the competition for blood flow between the leg and respiratory muscles. However, it is possible that hypoxic exercise might actually result in higher leg flow than at the same submaximal exercise intensity in normoxia (Calbet et al. 2003), which could then compromise respiratory muscle flow and contribute to diaphragm fatigue. Respiratory muscle blood flow could not be measured in our prior study, and accordingly, it was felt important to measure this to determine whether the greater diaphragm fatigue in hypoxia might be attributable, at least in part, to limited respiratory muscle flow. We found similar intercostal muscle blood flow values at each 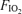 , suggesting that despite the lower work rate in hypoxia, respiratory muscle blood flow is not increased (compared with normoxia) to compensate for the reduction in
, suggesting that despite the lower work rate in hypoxia, respiratory muscle blood flow is not increased (compared with normoxia) to compensate for the reduction in 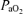 , thus further compromising O2 supply to the respiratory muscles.
, thus further compromising O2 supply to the respiratory muscles.
Effect of respiratory loading and metabolic acidosis on diaphragmatic fatigue
In hypoxia, twitch transdiaphragmatic pressure 10 min into recovery fell significantly more than after normoxic exercise, thus confirming greater fatigue in hypoxia (Vogiatzis et al. 2007). At this time, diaphragmatic pressures remain low and represent what is happening during exercise (Johnson et al. 1993, 1996). The workload sustained by the respiratory muscles is a critical determinant of the degree of diaphragmatic fatigue (Babcock et al. 2002). Thus, by design, neither the pressure–time product of the diaphragm and hence its energy requirement, nor the work of breathing measured from oesophageal pressure and volume changes breath by breath, were different across the three experimental conditions. Furthermore, arterial lactate was not significantly different between normoxia and hypoxia (Table 3) and therefore the significant difference found in diaphragmatic fatigue between these conditions is unlikely to be due to metabolic acidosis (Fregosi & Demspey, 1986). Hence, this study was focused on the contribution of intercostal muscle blood flow and its oxygen transport availability on the greater degree of diaphragmatic fatigue reported in hypoxia (Vogiatzis et al. 2007).
Respiratory muscle blood flow: principles, strengths and limitations
Using NIRS and ICG to measure intercostal muscle blood flow is a new technique we recently reported (Guenette et al. 2008). On the basis of tracer principles of mass conservation, the NIRS–ICG technique makes it possible to quantify blood flow because the rate of accumulation of ICG in a given tissue reflects perfusion in the specified tissue of interest. Thus, blood flow is determined as the ratio of ICG accumulated to the quantity of ICG introduced over a given period of time. The ability to measure intercostal muscle blood flow in humans has implications for understanding blood flow distribution patterns and circulatory regulation during exercise. Accordingly, the advantage of performing actual intercostal muscle blood flow measurements is that it provides an insight into the ‘blood flow competition’ theory, based on which it is thought that the respiratory muscles compete with the locomotor muscles for the available blood flow (Dempsey et al. 2006). A potential limitation of this technique when applied to measure intercostal muscle blood flow is the contribution of skin and subcutaneous tissue to the light-absorption signal and the small penetration depth (2 cm). This also means that blood flow measurements primarily reflect the internal and external intercostal muscles and only to a lesser extend the costal part of the diaphragm (Guenette et al. 2008). In the present study we compared intercostal muscle blood flow under the same NIR probe position across all conditions.
Cardiac output and quadriceps muscle blood flow
Cardiac output was recently reported by our group (Vogiatzis et al. 2008) as a factor in shunt calculations during exercise across the three conditions. The results of the present study are in line with those by Stenberg et al. (1966), Hartley et al. (1973) and Horstman et al. (1980) in that cardiac output in moderate hypoxia  is not different to that attained during normoxic exercise. In addition, quadriceps muscle blood flow was found not to differ between normoxia and hypoxia, despite the lower leg work rate in hypoxia. This is consistent with the finding that leg blood flow relative to work rate is increased in hypoxia (Table 2) to compensate for hypoxaemia (Koskolou et al. 1997; Calbet et al. 2003) and that at such high exercise intensities in hypoxia and normoxia (∼90% of respective
is not different to that attained during normoxic exercise. In addition, quadriceps muscle blood flow was found not to differ between normoxia and hypoxia, despite the lower leg work rate in hypoxia. This is consistent with the finding that leg blood flow relative to work rate is increased in hypoxia (Table 2) to compensate for hypoxaemia (Koskolou et al. 1997; Calbet et al. 2003) and that at such high exercise intensities in hypoxia and normoxia (∼90% of respective 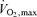 ) leg blood flow values are close to the maximal attainable (Knight et al. 1993). Accordingly, our previous hypothesis (Vogiatzis et al. 2007) that leg blood flow would be lower in hypoxia (due to the lower work rate), thus allowing greater blood flow availability to the respiratory muscles, is not confirmed by the results of this study.
) leg blood flow values are close to the maximal attainable (Knight et al. 1993). Accordingly, our previous hypothesis (Vogiatzis et al. 2007) that leg blood flow would be lower in hypoxia (due to the lower work rate), thus allowing greater blood flow availability to the respiratory muscles, is not confirmed by the results of this study.
There is accumulating evidence to implicate a significant role of fatiguing respiratory muscle work in the sympathetically mediated vasoconstriction of exercising limb muscle vasculature (Sheel et al. 2001, 2002; Romer & Polkey, 2008; Amann & Calbet, 2008). Accordingly, it has been assumed that the reduction in limb blood flow with fatiguing respiratory muscle work is directed towards the respiratory muscles (Dempsey et al. 2006). The results of the present study provide evidence that despite the greater degree of diaphragmatic fatigue in hypoxia compared with normoxia and hyperoxia, blood flow distribution to the intercostal and quadriceps muscles was not significantly different across the three conditions. It is known that sympathetically mediated vasoconstriction in the lower limbs is elicited by a metaboreflex originating in the diaphragm only when this reaches its threshold for activation during fatiguing contractions causing a reduction in diaphragm force output by 25–40% (Sheel et al. 2001, 2002). As in the present study the degree of reduction in diaphragmatic force output ranged between 25 and 33% across the three conditions, it is conceivable that activation of the diaphragm metaboreflex reached its threshold for activation in all exercise tests. Whether the effect of the diaphragm metaboreflex on limb blood flow was of the same magnitude in normoxia and hypoxia is uncertain as it is still unclear if this metaboreflex is sufficiently powerful to override the local vasodilator effects present in locomotor muscles during hypoxic exercise (Romer & Polkey, 2008).
Respiratory muscle blood flow in normoxia
It has been suggested that during maximal normoxic exercise, competition for blood flow between the leg and the respiratory muscles exists, such that respiratory muscle blood flow may increase at the expense of blood flow to working limb muscles (Harms et al. 1997, 1998). Intercostal muscle blood flow during normoxic exercise in the present study was 49.9 ± 5.9 ml (100 ml)−1 min−1 and mean minute ventilation was 128 ± 4 l min−1. This compares with 50.1 ± 12.5 ml (100 ml)−1 min−1 for intercostal muscle blood flow recently reported by our group (Guenette et al. 2008) in the same healthy subjects at rest during voluntary isocapnic hyperpnoea sustained at a mean minute ventilation of 123 ± 4 l min−1. Thus, at essentially the same ventilation and work of breathing (∼350 J min−1), intercostal muscle blood flow was the same at rest as during two-legged cycling. This occurred at ∼80% of maximal exercise capacity, the only intensity at which measurements were made in normoxia. At this intensity of exercise, the outcome suggests that ‘steal’ of blood flow away from the leg muscles to further supply the respiratory muscles is not occurring. This fits with the observation that during submaximal exercise respiratory muscles may not rank above limb muscles in terms of blood flow supply, most probably because the load placed on the respiratory muscles at submaximal exercise is insufficient to cause a profound change in blood flow distribution (Wetter et al. 1999). Whether such ‘steal’ would occur closer to maximal normoxic exercise remains to be studied by this technique.
Respiratory muscle blood flow in hypoxia and hyperoxia
Intercostal muscle blood flow during hypoxic and hyperoxic exercise (52.9 ± 5.9 and 53.6 ± 8.5 ml (100 ml)−1 min−1, respectively) was comparable to that recorded during normoxic exercise (Table 2) and to that recently reported by our group (Guenette et al. 2008) during resting isocapnic hyperpnoea at comparable levels of minute ventilation and work of breathing. Taken together, the tight relationship between intercostal muscle blood flow and work of breathing under conditions of varying cardiac output and leg exercise suggests that intercostal muscle blood flow during submaximal exercise is regulated mainly by respiratory muscle energy requirement and appears unaffected by other possible modifying factors such as arterial oxygenation or leg exercise intensity.
Expiratory flow limitation (EFL) reported during exercise in endurance athletes (Johnson et al. 1992) may influence respiratory muscle blood flow distribution (Robertson et al. 1977a). In the present study, however, we did not assess the incidence of EFL in our exercising subjects. Had EFL occurred in our subjects it is unlikely that this would have influenced differently respiratory muscle blood flow distribution across the three conditions as breathing frequency, tidal volume, minute ventilation (Fig. 2) operational lung volumes (Fig. 3) and most importantly work of breathing (Fig. 4) were not different.
Despite the finding that intercostal muscle blood flow was not different across normoxia, hypoxia and hyperoxia, arterial oxygen content was markedly different (Table 3). Consequently, reduced oxygen delivery in hypoxia compared with normoxia caused greater diaphragmatic fatigue, whereas increased oxygen delivery in hyperoxia compared with normoxia (Table 2) had no influence on the degree of diaphragmatic fatigue. The present study therefore confirms previous findings in normal healthy humans (Babcock et al. 1995) in that reduced oxygen supply to the respiratory muscles during high-intensity exercise (> 85%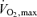 ) exacerbates the degree of diaphragmatic fatigue.
) exacerbates the degree of diaphragmatic fatigue.
Leg blood flow is a function of the absolute work performed (Rowell et al. 1986; Koskolou et al. 1997). Hence, in hypoxia, quadriceps muscle blood flow increased relative to work rate compared with normoxia (Table 2) most probably to compensate for hypoxaemia (Koskolou et al. 1997; Calbet et al. 2003). Intercostal muscle blood flow in hypoxia was, however, not different than that in normoxia despite the similar work of breathing (Table 2). Thus, lack of increase in intercostal muscle blood flow in hypoxia compared with normoxia (to compensate for the reduction in  ) is compatible with the suggestion that during high-intensity hypoxic exercise there may be very little or no room to increase respiratory muscle blood flow even in the face of superimposed hypoxia (Babcock et al. 1995). This would further compromise O2 supply to the respiratory muscles and exacerbate diaphragm fatigue.
) is compatible with the suggestion that during high-intensity hypoxic exercise there may be very little or no room to increase respiratory muscle blood flow even in the face of superimposed hypoxia (Babcock et al. 1995). This would further compromise O2 supply to the respiratory muscles and exacerbate diaphragm fatigue.
Potential limitations of the study
Intercostal muscle blood flow measured by the NIRS–ICG technique over the 7th intercostals space has been attributed to blood flow perfusing the external and internal intercostal muscles and to a lesser extend the costal diaphragm owing to the distance encompassed between the sampling point of NIRS on the skin and the diaphragmatic appositional area (Guenette et al. 2008). The amount of intercostal muscle blood flow recorded in the present study (∼50 ml (100 ml)−1 min−1) compares favourably to that reported by using radionuclide-labelled microspheres in dogs during moderately intense exercise (43 ml (100 g)−1 min−1) (Fixler et al. 1976) and at maximal work of resistive breathing (60 ml (100 g)−1 min−1) (Robertson et al. 1977b) as well as in rats during maximal exercise (68 ml (100 g)−1 min−1) (Poole et al. 2000), whilst it is lower than that reported in exercising ponies (132 ml (100 g)−1 min−1) (Manohar, 1986). Although in exercising ponies the fold increase in blood flow to the diaphragm and intercostal muscles from rest to maximal exercise was similar, diaphragm blood flow at maximal exercise was reported to be twice as high as that to intercostal muscles (261 versus 132 ml (100 g)−1 min−1) (Manohar, 1986). Whether in the present study the fold increase in blood flow measured over the left 7th intercostal space accurately reflects changes in blood flow to the diaphragm remains unknown.
In the study by Manohar (1986), blood flow in the major muscles involved in propulsion in the pony was not different from that in the diaphragm (∼240 and 261 ml (100 g)−1 min−1, respectively) whilst it was nearly double that recorded for intercostal muscles (132 ml (100 g)−1 min−1). This finding is in tandem with the results of the present study reporting twice as high blood flows to quadriceps compared with intercostal muscles in all exercise conditions (Table 2). Interestingly, in exercising ponies, vascular resistance in the muscles involved in propulsion (∼75 mm ml−1 min−1 g−1) was not different from that in the diaphragm (63 mm ml−1 min−1 g−1), but it was nearly half of that in the intercostal muscles (129 mm ml−1 min−1 g−1). Lower vasodilatory capacity of intercostal muscles compared with the muscles of propulsion has also been reported in exercising dogs (Fixler et al. 1976).
Whether the difference in relative blood flow to intercostal and quadriceps muscles in the present study is due to the different vasodilatory capacity of the two different muscle groups remains unknown. It is highly likely, however, that quadriceps and intercostal muscles may have different perfusion levels depending on the mass activated, force of contraction and flow capacity, defined by muscle characteristics such as fibre type and capillary density. This is the reason why the present study focuses on comparisons of blood flow under the same NIR probe position only within each muscle group across the three exercise conditions.
Studies in dogs undergoing unobstructed hyperventilation induced by CO2 re-breathing (Robertson et al. 1977c) have revealed blood flow heterogeneity in the respiratory muscles with the diaphragm receiving approximately 40% of total respiratory muscle blood flow and the intercostal and other inspiratory and expiratory muscles receiving the remaining 60%. Despite the linearity of intercostal muscle blood flow and cardiac output demonstrated by Guenette et al. (2008) during resting hyperventilation in the same subjects, the magnitude of intercostal blood flow cannot account for the full magnitude of the increase in total respiratory blood flow. Undoubtedly, the other respiratory muscles (i.e. diaphragm, parasternals, sternocleidomastoid, scalenus, abdominal and triangularis sterni) were also active to varying degrees during the exercise tests. These muscles will have different perfusion levels depending on the mass and force of contraction. As in the present study it was not possible to measure blood flow to all of the additional respiratory muscles, it remains difficult to estimate total respiratory muscle blood flow in absolute terms and express it as a fraction of cardiac output.
In conclusion, when the work of breathing is similar during heavy but submaximal normoxic and hypoxic exercise, diaphragm fatigue is confirmed to be greater during hypoxia. Intercostal muscle blood flow is not different between normoxia and hypoxia, suggesting that despite the lower leg work rate in hypoxia, respiratory muscle blood flow is not increased (compared with normoxia) to compensate for hypoxaemia, thus further compromising O2 supply to the respiratory muscles.
Acknowledgments
This work was supported by grants from the ‘A. Perotti’ visiting Professorship fund of the Thorax Foundation, the National Institutes of Health (NIH HL 84281), the Natural Sciences and Engineering Research Council (NSERC) of Canada and Fonds de la Recherche en Santé Québec (FRSQ). J.A.G. was supported by graduate scholarships from NSERC, the Michael Smith Foundation for Health Research and the Sir James Lougheed Award of Distinction. P.D.W. and H.E.W. were supported in part by NIH HL 84281.
References
- Amann M, Calbet JAL. Convective oxygen transport and fatigue. J Appl Physiol. 2008;104:861–870. doi: 10.1152/japplphysiol.01008.2007. [DOI] [PubMed] [Google Scholar]
- Babcock MA, Johnson BD, Pegelow DF, Suman OE, Griffin D, Dempsey JA. Hypoxic effects on exercise-induced diaphragmatic fatigue in normal healthy humans. J Appl Physiol. 1995;78:82–92. doi: 10.1152/jappl.1995.78.1.82. [DOI] [PubMed] [Google Scholar]
- Babcock MA, Pegelow DF, Harms CA, Dempsey JA. Effects of respiratory muscle unloading on exercise-induced diaphragmatic fatigue. J Appl Physiol. 2002;93:201–206. doi: 10.1152/japplphysiol.00612.2001. [DOI] [PubMed] [Google Scholar]
- Babcock MA, Pegelow DF, Johnson BD, Dempsey JA. Aerobic fitness effects on exercise-induced low-frequency diaphragm fatigue. J Appl Physiol. 1996;81:2156–2164. doi: 10.1152/jappl.1996.81.5.2156. [DOI] [PubMed] [Google Scholar]
- Boushel R, Langberg H, Olesen J, Nowak M, Simonsen L, Bulow J, Kjaer M. Regional blood flow during exercise in humans measured by near-infrared spectroscopy and indocyanine green. J Appl Physiol. 2000;89:1868–1878. doi: 10.1152/jappl.2000.89.5.1868. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Boushel R, Radegran G, Sondergaard H, Wagner PD, Saltin B. Determinants of maximal oxygen uptake in severe acute hypoxia. Am J Physiol Regul Integr Comp Physiol. 2003;287:R996–R999. doi: 10.1152/ajpregu.00155.2002. [DOI] [PubMed] [Google Scholar]
- Chen R, Kayser B, Macklem PT. Twitch transdiaphragmatic pressure depends critically on thoracoabdominal configuration. J Appl Physiol. 2000;88:54–60. doi: 10.1152/jappl.2000.88.1.54. [DOI] [PubMed] [Google Scholar]
- Cibella F, Cuttitta G, Kayser B, Narici M, Romano S, Saibene F. Respiratory mechanics during exhaustive submaximal exercise at high altitude in healthy humans. J Physiol. 1996;494:881–890. doi: 10.1113/jphysiol.1996.sp021540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA, Romer L, Rodman J, Miller J, Smith C. Consequences of exercise-induced respiratory muscle work. Respir Physiol Neurobiol. 2006;151:242–250. doi: 10.1016/j.resp.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Dow P. Estimations of cardiac output and central blood volume by dye dilution. Physiol Rev. 1956;36:77–102. doi: 10.1152/physrev.1956.36.1.77. [DOI] [PubMed] [Google Scholar]
- Duncan A, Meek JH, Clemence M, Elwell CE, Tyszczuk L, Cope M, Delpy DT. Optical pathlength measurements on adult head, calf and forearm and the head of the newborn infant using phase resolved optical spectroscopy. Phys Med Biol. 1995;40:295–304. doi: 10.1088/0031-9155/40/2/007. [DOI] [PubMed] [Google Scholar]
- Fixler DE, Atkins JM, Mitchell JH, Horwitz LD. Blood flow to respiratory, cardiac, and limb muscles in dogs during graded exercise. Am J Physiol. 1976;231:1515–1519. doi: 10.1152/ajplegacy.1976.231.5.1515. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Dempsey JA. Effects of exercise in normoxia and acute hypoxia on respiratory muscle metabolites. J Appl Physiol. 1986;60:1274–1283. doi: 10.1152/jappl.1986.60.4.1274. [DOI] [PubMed] [Google Scholar]
- Gudjonsdottir M, Appending L, Baderna P, Purro A, Patessio A, Vilianis G, et al. Diaphragm fatigue during exercise at high altitude: the role of hypoxia and workload. Eur Respir J. 2001;17:674–680. doi: 10.1183/09031936.01.17406740. [DOI] [PubMed] [Google Scholar]
- Guenette JA, Vogiatzis I, Zakynthinos S, Athanasopoulos D, Koskolou M, Golemati S, et al. Human respiratory muscle blood flow measured by near-infrared spectroscopy and indocyanine green. J Appl Physiol. 2008;104:1202–1210. doi: 10.1152/japplphysiol.01160.2007. [DOI] [PubMed] [Google Scholar]
- Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol. 1997;82:1573–1583. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- Harms CA, Thomas A, Wetter J, McClaran SR, Pegelow DF, Nickele GA, et al. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol. 1998;85:609–618. doi: 10.1152/jappl.1998.85.2.609. [DOI] [PubMed] [Google Scholar]
- Harms CA, Wetter TJ, Croix CM, Pegelow DF, Dempsey JA. Effects of respiratory muscle work on exercise performance. J Appl Physiol. 2000;89:131–138. doi: 10.1152/jappl.2000.89.1.131. [DOI] [PubMed] [Google Scholar]
- Hartley LH, Vogel JA, Landowne M. Central, femoral, and brachial circulation during exercise in hypoxia. J Appl Physiol. 1973;34:87–90. doi: 10.1152/jappl.1973.34.1.87. [DOI] [PubMed] [Google Scholar]
- Horstman D, Weiskopf R, Jackson RE. Work capacity during 3-wk sojourn at 4,300 m: effects of relative polycythemia. J Appl Physiol. 1980;49:311–318. doi: 10.1152/jappl.1980.49.2.311. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Aaron EA, Babcock MA, Dempsey JA. Respiratory muscle fatigue during exercise: implications for performance. Med Sci Sports Exerc. 1996;28:1129–1137. doi: 10.1097/00005768-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Babcock MA, Suman OE, Dempsey JA. Exercise-induced diaphragmatic fatigue in healthy humans. J Physiol. 1993;460:385–405. doi: 10.1113/jphysiol.1993.sp019477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, Saupe KW, Dempsey JA. Mechanical constraints on exercise hypernea in endurance athletes. J Appl Physiol. 1992;73:874–886. doi: 10.1152/jappl.1992.73.3.874. [DOI] [PubMed] [Google Scholar]
- Kalliokoski KK, Scheede-Bergdahl C, Kjaer M, Boushel R. Muscle perfusion and metabolic heterogeneity: insights from non-invasive imaging techniques. Exerc Sport Sci Rev. 2006;34:164–170. doi: 10.1249/01.jes.0000240018.07502.48. [DOI] [PubMed] [Google Scholar]
- Knight DR, Schaffartzik W, Poole DC, Hogan MC, Bebout DE, Wagner PD. Effects of hyperoxia on maximal leg O2 supply and utilization in men. J Appl Physiol. 1993;75:2586–2594. doi: 10.1152/jappl.1993.75.6.2586. [DOI] [PubMed] [Google Scholar]
- Koskolou MD, Calbet JA, Radegran G, Roach RC. Hypoxia and the cardiovascular response to dynamic knee-extensor exercise. Am J Physiol Heart Circ Physiol. 1997;272:H2655–H2663. doi: 10.1152/ajpheart.1997.272.6.H2655. [DOI] [PubMed] [Google Scholar]
- Laghi F, D'Alfonso N, Tobin MJ. Pattern of recovery from diaphragmatic fatigue over 24 hours. J Appl Physiol. 1995;79:539–546. doi: 10.1152/jappl.1995.79.2.539. [DOI] [PubMed] [Google Scholar]
- Manohar M. Blood flow to the respiratory and limb muscles and to abdominal organs during maximal exertion in ponies. J Physiol. 1986;377:25–35. doi: 10.1113/jphysiol.1986.sp016174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis AB. Handbook of Physiology. Washington, DC: American Physiological Society; 1964. The work of breathing; pp. 463–476. [Google Scholar]
- Poole DC, Sexton WL, Behnke BJ, Ferguson CS, Hageman KS, Musch TI. Respiratory muscle blood flow during physiological and chemical hyperpnea in the rat. J Appl Physiol. 2000;88:186–194. doi: 10.1152/jappl.2000.88.1.186. [DOI] [PubMed] [Google Scholar]
- Robertson CH, Jr, Eschenbacher WL, Johnson RL., Jr Respiratory muscle blood flow distribution during expiratory resistance. J Clin Invest. 1977c;60:43–480. doi: 10.1172/JCI108798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CH, Jr, Foster GH, Johnson RL., Jr The relationship of respiratory failure to the oxygen consumption of, lactate production by, and distribution of blood flow among respiratory muscles during increasing inspiratory resistance. J Clin Invest. 1977a;59:31–42. doi: 10.1172/JCI108619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CH, Jr, Pagel MA, Johnson RL., Jr The distribution of blood flow, oxygen consumption, and work output among respiratory muscles during unobstructed hyperventilation. J Clin Invest. 1977b;59:43–50. doi: 10.1172/JCI108620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer LM, Polkey MI. Exercise-induced respiratory muscle fatigue: implications for performance. J Appl Physiol. 2008;104:879–888. doi: 10.1152/japplphysiol.01157.2007. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Saltin B, Kiens B, Christensen NJ. Is peak quadriceps blood flow in humans higher during exercise with hypoxemia? Am J Physiol Heart Circ Physiol. 1986;251:H1038–H1044. doi: 10.1152/ajpheart.1986.251.5.H1038. [DOI] [PubMed] [Google Scholar]
- Sheel AW, Derchak PA, Morgan BJ, Pegelow DF, Jacques AJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex reduction in resting leg blood flow in humans. J Physiol. 2001;537:277–289. doi: 10.1111/j.1469-7793.2001.0277k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheel AW, Derchak PA, Pegelow DF, Dempsey JA. Threshold effects of respiratory muscle work on limb vascular resistane. Am J Physiol Heart Circ Physiol. 2002;282:H1732–H1738. doi: 10.1152/ajpheart.00798.2001. [DOI] [PubMed] [Google Scholar]
- Siggaard-Andersen O. The Acid-Base Status of Blood. Copenhagen, Denmark: Munksgaard; 1974. [Google Scholar]
- Stenberg J, Ekblom B, Messin R. Hemodynamic response to work at simulated altitude, 4,000 m. J Appl Physiol. 1966;21:1589–1594. doi: 10.1152/jappl.1966.21.5.1589. [DOI] [PubMed] [Google Scholar]
- Van Der Zee P, Cope M, Arridge SR, Essenpreis M, Potter LA, Edwards AD, et al. Experimentally measured optical pathlengths for the adult head, calf and forearm and the head of the newborn infant as a function of inter optode spacing. Adv Exp Med Biol. 1992;316:143–153. doi: 10.1007/978-1-4615-3404-4_17. [DOI] [PubMed] [Google Scholar]
- Vogiatzis I, Aliverti A, Golemati S, Georgiadou O, LoMauro A, Kosmas E, et al. Respiratory kinematics by optoelectronic plethysmography during exercise in men and women. Eur J Appl Physiol. 2005;93:581–587. doi: 10.1007/s00421-004-1249-4. [DOI] [PubMed] [Google Scholar]
- Vogiatzis I, Georgiadou O, Giannopoulou I, Koskolou M, Peraki E, Kostikas K, et al. Effects of exercise-induced arterial hypoxaemia and work rate on diaphragmatic fatigue in highly trained endurance athletes. J Physiol. 2006;572:539–549. doi: 10.1113/jphysiol.2005.102442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogiatzis I, Goergiadou O, Koskolou M, Athanasopoulos D, Kostikas K, Golemati S, et al. Effects of hypoxia on diaphragmatic fatigue in highly trained athletes. J Physiol. 2007;581:299–308. doi: 10.1113/jphysiol.2006.126136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogiatzis I, Zakynthinos S, Boushel R, Athanasopoulos D, Guenette JA, et al. The contribution of intrapulmonary shunts to the alveolar to arterial oxygen difference is very small. J Physiol. 2008;586:2381–2391. doi: 10.1113/jphysiol.2007.150128. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
Wetter TJ, Harms CA, Nelson WB, Pegelow DF, Dempsey JA. Influence of respiratory muscle work on
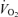 and leg blood flow during submaximal exercise. J Appl Physiol. 1999;87:643–651. doi: 10.1152/jappl.1999.87.2.643. [DOI] [PubMed] [Google Scholar]
and leg blood flow during submaximal exercise. J Appl Physiol. 1999;87:643–651. doi: 10.1152/jappl.1999.87.2.643. [DOI] [PubMed] [Google Scholar]