Abstract
Sepsis causes muscle atrophy and insulin resistance, but the underlying mechanisms are unclear. Therefore, the present study examined the effects of lipopolysaccharide (LPS)-induced endotoxaemia on the expression of Akt, Forkhead Box O (FOXO) and its downstream targets, to identify any associations between changes in FOXO-dependent processes influencing muscle atrophy and insulin resistance during sepsis. Chronically instrumented male Sprague–Dawley rats received a continuous intravenous infusion of LPS (15 μg kg−1 h−1) or saline for 24 h at 0.4 ml h−1. Animals were terminally anaesthetized and the extensor digitorum longus muscles from both hindlimbs were removed and snap-frozen. Measurements were made of mRNA and protein expression of selected signalling molecules associated with pathways regulating protein synthesis and degradation and carbohydrate metabolism. LPS infusion induced increases in muscle tumour necrosis factor-α (8.9-fold, P < 0.001) and interleukin-6 (8.4-fold, P < 0.01), paralleled by reduced insulin receptor substrate-1 mRNA expression (−0.7-fold, P < 0.01), and decreased Akt1 protein and cytosolic FOXO1 and FOXO3 phosphorylation. These changes were accompanied by significant increases in muscle atrophy F-box mRNA (5.5-fold, P < 0.001) and protein (2-fold, P < 0.05) expression, and pyruvate dehydrogenase kinase 4 mRNA (15-fold, P < 0.001) and protein (1.6-fold, P < 0.05) expression. There was a 29% reduction in the muscle protein: DNA ratio, a 56% reduction in pyruvate dehydrogenase complex (PDC) activity (P < 0.05), and increased glycogen degradation and lactate accumulation. The findings of this study suggest a potential role for Akt/FOXO in the simultaneous impairment of carbohydrate oxidation, at the level of PDC, and up-regulation of muscle protein degradation, in LPS-induced endotoxaemia.
Sepsis is a complex and potentially fatal condition arising from an uncontrolled, systemic inflammatory response to an infection (Angus et al. 2001), and circulating cytokines are thought to play a major role (Cohen, 2002). Two of the metabolic characteristics of sepsis are muscle insulin resistance (Lang et al. 1990) and severe muscle wasting (Hasselgren & Fischer, 2001; Lang et al. 2007).
Various animal models have indicated that increased protein degradation via the ATP-dependent ubiquitin-proteasome pathway (UPP) is primarily responsible for the regulation of skeletal muscle protein degradation in wasting conditions (Tawa et al. 1997; Lecker et al. 2004). Ubiquitination is achieved by the action of several enzymes: ubiquitin-activating (E1) enzyme, ubiquitin-conjugating (E2) enzymes and ubiquitin ligases (E3). Two muscle-specific ubiquitin ligases, muscle atrophy F-box (MAFbx) and muscle RING finger 1 (MuRF1), have emerged as key regulators of skeletal muscle proteolysis under catabolic conditions (Bodine et al. 2001).
Evidence suggests that impaired signalling via the insulin-like growth factor-1 (IGF-1)/phosphatidylinositol-3 kinase (PI3K)/Akt1 pathway may be involved in the suppression of protein synthesis and induction of protein degradation in catabolic conditions (see Glass, 2005). IGF-1, which can induce skeletal muscle hypertrophy via Akt1, has been shown to block the upregulation of certain mediators of skeletal muscle protein degradation in C2C12 myotubes, namely ubiquitin ligases MAFbx and MuRF1 (Stitt et al. 2004). Specifically, IGF-1-mediated inhibition of proteolysis may, partly, reflect Akt1-mediated inhibition (phosphorylation) of Forkhead box O (FOXO) transcription factors (Stitt et al. 2004). The FOXO family of transcription factors have been implicated in initiating protein degradation during muscle atrophy (Sandri et al. 2004), and in an active (dephosphorylated) state, participate in the transcriptional activation of specific target genes, including MAFbx and MuRF1 (Stitt et al. 2004). Therefore, in catabolic states, where Akt signalling is impaired, muscle atrophy may arise through increased activity of FOXO and activation of MAFbx and MuRF1 transcription.
Muscle atrophy has been shown to occur concomitantly with insulin resistance in numerous catabolic states, including sepsis (Hasselgren et al. 1987), but to our knowledge, to date no-one has investigated them concurrently in sepsis to determine any commonality in molecular changes that may explain these physiological responses. Dysregulation of Akt1 signalling has been reported in vivo in the catabolic, insulin resistant state (Wang et al. 2006), and FOXO transcription factors have also been implicated in muscle insulin resistance. Indeed, an important role for FOXO transcription factors in the aetiology of muscle insulin resistance may be through inhibition of muscle carbohydrate oxidation, via increased pyruvate dehydrogenase kinase 4 (PDK4) transcription (Furuyama et al. 2003), as a result of impaired insulin stimulation of PI3K and Akt1 (Kim et al. 2006). PDK4 specifically phosphorylates and therefore inactivates the pyruvate dehydrogenase complex (PDC), the rate limiting step in carbohydrate oxidation. Thus, increased PDK4 expression through FOXO transcriptional activity in catabolic states may lead to the development of a muscle insulin resistant state.
Evidence therefore points towards a single signalling pathway (via Akt1 and the FOXO family members) being involved in the simultaneous regulation of muscle protein synthesis and degradation (see Hoffman & Nader, 2004), and muscle insulin resistance (Furuyama et al. 2003). Elevated cytokines, specifically TNF-α, may contribute to insulin resistance (Hotamisligil et al. 1996; Plomgaard et al. 2005) and TNF-α has been shown to mediate Akt1 degradation in vitro (Medina et al. 2005), thus impairing Akt1-dependent signalling. Therefore, we hypothesized that the simultaneous induction of muscle atrophy and development of insulin resistance during sepsis, a condition associated with increased circulating cytokines (Cohen, 2002), may be associated with a reduction in Akt1 activity, and therefore FOXO activation, and up-regulation of FOXO gene targets MAFbx, MuRF1 and PDK4. A suppression of Akt1 activity may also lead to a reduction in muscle protein synthesis (Hoffman & Nader, 2004), which may also promote muscle atrophy in sepsis, but this was not the focus of the present study. The aim of this study was to measure the effects of lipopolysaccharide (LPS)-induced endotoxaemia on the expression of Akt, FOXO and its downstream targets, to examine the potential dual role of FOXO in both muscle protein loss and impairment of carbohydrate oxidation in vivo during sepsis. To investigate this, conscious, chronically instrumented rats were infused intravenously with a non-lethal dose of bacterial LPS, as a clinically relevant model of endotoxaemia that results in a systemic inflammatory response syndrome and represents the early stages of clinical sepsis (Waller et al. 1995).
Methods
Experimental design and tissue collection
Male Sprague–Dawley rats (380–480 g; Charles River, Sandwich, UK) were anaesthetized with fentanyl citrate (Janssen-Cilag, High Wycombe, UK) and medetomidine (Domitor; Pfizer, Sandwich, UK; 300 μg kg−1 of each intraperitoneally (i.p.)) and a catheter was implanted in the jugular vein. Whilst under anaesthesia, rats were fitted with a harness connected to a counter-balanced spring to carry the catheter, which allowed relatively free movement within the cage. Anaesthesia was reversed and analgesia provided with atipamezole (Antisedan; Pfizer; 1 mg kg−1 subcutaneously (s.c.)) and buprenorphine (Vetergesic; Alstoe Animal Health, York, UK; 0.02 mg kg−1s.c.). Animals were allowed open access to food and water and left to recover for 24 h in individual housing, with continuous sterilized saline infusion (0.4 ml h−1 intravenously (i.v.)) via a fluid-filled swivel.
Prepared rats were divided into two groups; one group (n = 8) was subjected to continuous 24 h intravenous infusion of LPS (E. coli, serotype 0127: B8, Sigma-Aldrich, Poole, UK) dissolved in saline (15 μg kg−1 h−1). A saline-control group (n = 8) received an equal volume of saline (0.4 ml h−1) for 24 h. After 24 h, animals were terminally anaesthetized with thiobutabarbital sodium ((Inactin), Sigma-Aldrich, St Louis, MO, USA; 80 mg kg−1i.v.), the extensor digitorum longus (EDL) muscle from both hindlimbs was removed, and samples were immediately snap-frozen in liquid nitrogen. The fast-twitch EDL muscle was chosen due to its higher susceptibility to sepsis than slow-twitch muscle (Tiao et al. 1997). All procedures were approved by the University of Nottingham Ethical Review Committee and were performed under Home Office Project Licence authority.
Muscle analyses
Protein and DNA measurements
A portion of frozen muscle was freeze-dried and powdered, and alkaline soluble protein and DNA were extracted from approximately 3 mg of the powdered muscle, using perchloric acid (PCA), with the protein finally isolated by the addition of KOH. Muscle alkaline soluble protein and DNA were quantified according to the method described by Forsberg et al. (1991).
Real-time PCR measurements
Total RNA was isolated from frozen wet EDL muscle (20–30 mg) using Tri Reagent (Sigma-Aldrich, Poole, UK), according to the manufacturer's protocol. Following extraction, total RNA was quantified spectrophotometrically at 260 nm, and RNA purity was determined as the ratio of readings at 260/280 nm. First-strand cDNA synthesis and real-time PCR protocols were carried out according to the methods of Constantin et al. (2007). Taqman primer/probe sets were obtained from ABI (Foster City, CA, USA): TNF-α, IL-6, IRS-1, Akt1, FOXO1, peroxisome proliferator activated receptor γ (PPAR γ) coactivator 1α (PGC-1α), MAFbx, MuRF1, PDK4, PDK-2 and pyruvate dehydrogenase phosphatase 1 (PDP1). Due to its stability, i.e. not being affected by the treatment, the housekeeping gene hydroxymethylbilane synthase (HMBS) was used as an internal control.
Relative quantification of gene expression between LPS-treated and control groups was calculated using the 2−ΔΔCt method. ΔΔCt was calculated using the difference in ΔCt of LPS-treated samples and the mean ΔCt of controls, following normalization of Ct values for the target gene to the Ct values of HMBS. The saline control group was given a value of 1, and fold changes in mRNA expression for the LPS-treated group were calculated, relative to the control group.
Protein extraction and Western blotting measurements
Cytosolic and nuclear proteins were extracted from approximately 30 mg frozen wet tissue using a modification of the method of Blough et al. (1999). Extracted proteins in both fractions were quantified using the Bradford assay (Bio-Rad, UK) and Western blotting was carried out, as previously described (Constantin et al. 2007). The antibodies used were purchased from Cell Signaling Technology (Danvers, MA, USA), except for PDK4 and MAFbx, which were gifts from AstraZeneca (Alderley Park, UK) and Pfizer Inc. (Groton, CT, USA), respectively. Bands were quantified by densitometry using GeneTools software (Syngene, Frederick, MD, USA). Values were adjusted by subtracting the background and normalized to an actin protein control for cytosolic proteins and lamin (New England BioLabs, Hitchin, UK) for nuclear proteins.
Muscle metabolite measurements
Freeze-dried and powdered EDL muscle samples (1–2 mg) were alkaline extracted and used for muscle glycogen determination according to Harris et al. (1974). Additionally, 5–10 mg of muscle powder was extracted with 0.5 mol l−1 PCA containing 1 mmol l−1 EDTA, then neutralized with 2.1 mol l−1 KHCO3. Muscle extracts were analysed for glucose-6-phosphate and lactate concentrations, using a modification of the spectrophotometric methods of Harris et al. (1974).
Muscle PDC activity measurements
A portion of frozen muscle (5–10 mg) was used to measure PDC in its dephosphorylated (active) form (PDCa), according to the method of Constantin-Teodosiu et al. (1991). Briefly, muscles were homogenized in a buffer containing NaF and dichloroacetate (DCA), and the activity of PDC in its dephosphorylated (active) form was measured as a rate of acetyl-CoA formation (mmol min−1 (kg wet muscle)−1) at 37°C.
Statistical analysis
All data are presented as means ±s.e.m. and comparisons between LPS treatment and control groups were performed using Student's unpaired t test, with the exception of real-time PCR results, which were analysed by one-way analysis of variance (ANOVA). Differences were considered statistically significant when P < 0.05.
Results
Protein: DNA ratio
Total alkaline-soluble protein and DNA from EDL muscle of saline and LPS-treated rats was quantified in order to calculate the protein: DNA ratio, and thereby obtain a sensitive index of muscle protein mass (Gamrin et al. 1996). Twenty-four hours of LPS infusion resulted in a significant, 29% reduction in the protein: DNA ratio compared to control (123.0 ± 12.8 versus 87.0 ± 6.3; P < 0.05).
Muscle mRNA expression
Selected mRNA transcripts were examined in EDL muscle, encoding cytokine proteins and proteins thought to be involved in muscle fuel metabolism, insulin signalling and protein degradation pathways. Figure 1 shows the fold-changes in mRNA expression in LPS-treated animals compared to control. Twenty-four hours of LPS infusion induced marked increases in mRNA expression of TNF-α and IL-6 relative to control (8.9-fold, P < 0.001 and 8.4-fold, P < 0.001, respectively). LPS administration also elicited significant increases in Akt1 (2-fold, P < 0.001 versus control), FOXO1 (2.7-fold, P < 0.001 versus control), MAFbx (5.5-fold, P < 0.001 versus control) and MuRF1 (21-fold, P < 0.001 versus control) mRNA expression. Additionally, PDK4 transcript levels were markedly increased compared to control (15-fold, P < 0.001 versus control; Fig. 2). Finally, there was also evidence of decreased expression of PDP1 (0.5-fold change, P < 0.001; Fig. 2), PGC-1α (0.7-fold change, P < 0.01; Fig. 1) and IRS-1 (0.7-fold change, P < 0.01; Fig. 1) compared to control.
Figure 1. mRNA expression of selected genes in rat extensor digitorum longus muscle, following 24 h of lipopolysaccharide (LPS) infusion.
Relative mRNA expression of saline controls was set at 1. Values are means and vertical bars represent s.e.m. Significantly different from corresponding control: **P < 0.01; ***P < 0.001.
Figure 2. mRNA expression of pyruvate dehydrogenase kinase 4 (PDK4) and pyruvate dehydrogenase phosphatase 1 (PDP1) in rat extensor digitorum longus muscle, following 24 h of lipopolysaccharide (LPS) infusion.
Relative expression of saline controls was set at 1. Values are means and vertical bars represent s.e.m. Significantly different from control: ***P < 0.001.
Muscle protein expression
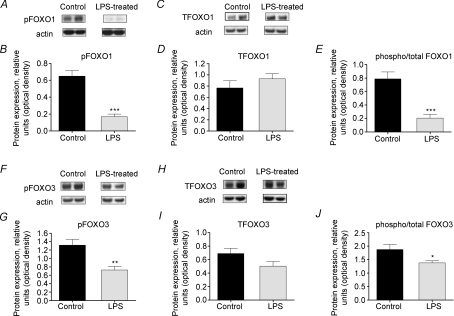
LPS infusion resulted in a 3.9-fold decrease in phosphorylated FOXO1 (serine256) within the cytosol (P < 0.001 versus control; Fig. 3A and B), but no change in total FOXO1 protein (Fig. 3C and D). Cytosolic levels of phosphorylated FOXO3 (serine253) were also reduced in muscle from LPS-treated rats (1.7-fold; P < 0.05 versus control; Fig. 3F and G), with no significant change in total FOXO3 levels (Fig. 3H and I). Hence, there was a significant reduction in the phosphorylated/total FOXO1 and FOXO3 ratio within the cytosol in muscle from LPS-treated rats (both P < 0.001 versus control; Fig. 3E and J). FOXO3 protein levels were also analysed within the nuclear fraction; similar to cytosolic protein content, phosphorylated FOXO3 levels were significantly reduced by ∼27% in muscle from LPS-treated rats (P < 0.05 versus control; Fig. 4A and B). Overall, a significant reduction in the phosphorylated/total FOXO3 ratio within the nuclear fraction of LPS-treated samples was observed in comparison to saline controls (P < 0.05; Fig. 4E).
Figure 3. Cytosolic Forkhead box O (FOXO) 1 and 3 protein expression in rat extensor digitorum longus (EDL) muscle.
A, representative Western blot of phosphorylated FOXO1 protein. B, density of FOXO1 protein bands, measured by Western blotting, in EDL muscle of rats administered lipopolysaccharide (LPS) or saline for 24 h. C, representative blot of total FOXO1 protein. D, density of FOXO1 protein bands, measured by Western blotting, in EDL muscle from rats administered LPS or saline for 24 h. E, ratio of phosphorylated to total FOXO1 protein levels in EDL muscle of LPS-treated and control rats. F, representative blot of phosphorylated FOXO3 protein. G, density of FOXO3 protein bands, measured by Western blotting, in EDL muscle of rats administered LPS or saline for 24 h. H, representative Western blot of total FOXO3 protein. I, density of FOXO3 protein bands, measured by Western blotting, in EDL muscle from rats administered LPS or saline for 24 h. J, ratio of phosphorylated to total FOXO3 protein levels in EDL muscle of LPS-treated and control rats. Significantly different from control: *P < 0.05; ***P < 0.001.
Figure 4. Nuclear Forkhead box O (FOXO) 3 protein expression in rat extensor digitorum longus (EDL) muscle.
A, representative Western blot of phosphorylated FOXO3 protein. B, density of FOXO3 protein bands, measured by Western blotting, in EDL muscle of rats administered lipopolysaccharide (LPS) or saline for 24 h. C, representative blot of total FOXO3 protein. D, density of FOXO3 protein bands, measured by Western blotting, in EDL muscle from rats administered LPS or saline for 24 h. E, ratio of phosphorylated to total FOXO3 protein levels in EDL muscle of LPS-treated and control rats. Significantly different from control: *P < 0.05.
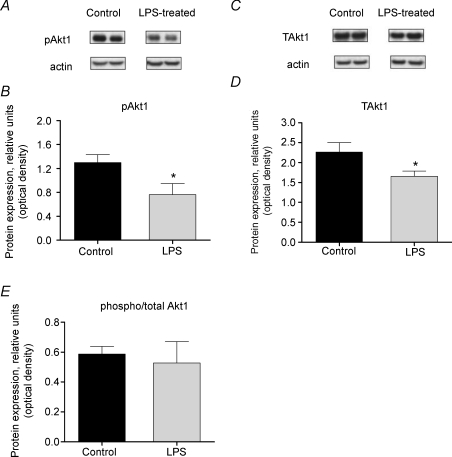
The level of cytosolic phosphorylated Akt1 (serine473) was found to be 1.7-fold lower than control with LPS administration (P < 0.05 versus control; Fig. 5A and B). There was also a 1.4-fold reduction in the levels of total Akt1 (P < 0.05; Fig. 5C and D). Hence there was no change in the phosphorylated/total Akt1 ratio (Fig. 5E).
Figure 5. Cytosolic Akt1 protein expression in rat extensor digitorum longus (EDL) muscle.
A, representative Western blot of phosphorylated Akt1 protein. B, density of Akt1 protein bands, measured by Western blotting, in EDL muscle of rats administered lipopolysaccharide (LPS) or saline for 24 h. C, representative blot of total Akt1 protein. D, density of Akt1 protein bands, measured by Western blotting, in EDL muscle from rats administered LPS or saline for 24 h. E, ratio of phosphorylated to total Akt1 protein levels in EDL muscle of LPS-treated and control rats. Significantly different from control: *P < 0.05.
Figure 6A illustrates a typical blot of MAFbx protein, identified as a band at around 50 kDa. The average level of MAFbx protein expression increased 2-fold with LPS administration compared to the control group (P < 0.05; Fig. 6B). Analysis of phosphorylated IRS-1 protein (serine612) revealed no differences in protein levels between the two groups (data not shown). A 1.6-fold increase in PDK4 protein expression with LPS administration was observed relative to the control group (P < 0.05 versus control; Fig. 7A and B).
Figure 6. Muscle atrophy F-box (MAFbx) protein expression in rat extensor digitorum longus (EDL) muscle.
A, representative Western blot of MAFbx protein. B, density of MAFbx protein bands, measured by Western blotting, in EDL muscle of rats administered lipopolysaccharide or saline for 24 h. Significantly different from control: *P < 0.05.
Figure 7. Pyruvate dehydrogenase kinase 4 (PDK4) protein expression and pyruvate dehydrogenase complex (PDC) activity in rat extensor digitorum longus (EDL) muscle.
A, representative blot of PDK4 protein. B, density of PDK4 protein bands, measured by Western blotting, in EDL muscle of rats administered with lipopolysaccharide (LPS) or saline for 24 h. C, muscle PDC activity following 24 h LPS or saline infusion. Significantly different from control: *P < 0.05. Values represent means +s.e.m.
Muscle PDC activity and metabolites
Muscle PDCa (active form) activity was reduced by 56% in the LPS-treated group compared to control (P < 0.05; Fig. 7C). Muscle glycogen content was ∼16% lower in EDL muscle following 24 h LPS administration, compared to the control group (172.0 ± 8.8 mmol kg−1versus 145.0 ± 9.3 mmol kg−1, respectively; P < 0.05), and muscle lactate values were 131% higher in the LPS-treated group (29.8 ± 5.9 mmol kg−1versus 12.9 ± 0.7 mmol kg−1 in control group; P < 0.05), but there was no difference in glucose-6-phosphate levels (1.65 ± 0.49 mmol kg−1 for control group, 2.38 ± 0.97 mmol kg−1 for LPS-treated group).
Discussion
Muscle atrophy and insulin resistance are frequently observed in numerous catabolic disease states, including sepsis (Hasselgren & Fischer, 2001; Marik & Raghavan, 2004), but the mechanisms underlying these processes are poorly understood. The results of this study suggest that 24 h low dose LPS infusion elicited atrophy of fast-twitch EDL muscle, which was likely to have been at least partly due to increased UPP-mediated proteolysis, as indicated by the marked up-regulation of ubiquitin ligases MAFbx and MuRF1 mRNA transcription, and MAFbx protein expression. These events coincided with the impairment of muscle carbohydrate metabolism, as indicated by decreased pyruvate oxidation and increased lactate accumulation, which is consistent with the observed PDK4-mediated inhibition of PDC activity and inefficient muscle glycogen utilization, and hence greater glycogen degradation. These changes were accompanied by reduced phosphorylation (inhibition) of Akt1 protein, and reduced phosphorylation (activation) of FOXO 1 and 3, suggesting a potential dual role for FOXO in inducing UPP-mediated muscle atrophy and altering muscle fuel use, especially in highly glycolytic fibres, during sepsis. Overall, the present data suggest that a common signalling pathway exists (see Fig. 8 for schematic diagram), involving Akt1 and FOXO, that may simultaneously trigger the induction of muscle protein degradation and impairment of carbohydrate oxidation, at the level of PDC, during LPS-induced endotoxaemia. A likely factor for triggering dysregulation of Akt1, and thereby FOXO signalling, is TNF-α (Medina et al. 2005).
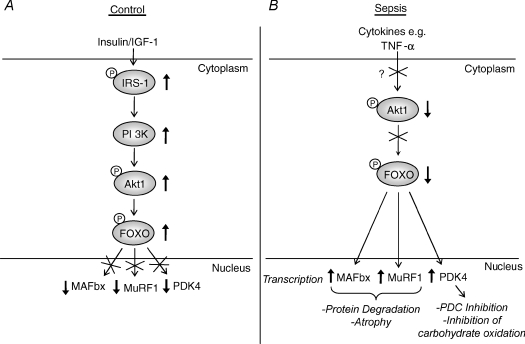
Figure 8. A single signalling pathway potentially links muscle protein degradation and regulation of carbohydrate metabolism during lipopolysaccharide (LPS)-induced endotoxaemia.
A, in a non-catabolic state, stimulation with growth factors, such as insulin-like growth factor-1 (IGF-1), and hormones, such as insulin, leads to the activation of the phosphatidylinositol-3 kinase (PI3K)/Akt signalling cascade. Phosphorylation of Forkhead box O (FOXO) 1 or 3 by Akt1 leads to their exclusion from the nucleus, and a reduction in the expression of key genes involved in protein degradation, namely muscle atrophy F-box (MAFbx) and muscle RING finger 1 (MuRF1), and regulation of carbohydrate oxidation (pyruvate dehydrogenase kinase 4 (PDK4)). B, during LPS-induced endotoxaemia, Akt1 is in a predominantly dephosphorylated/inactive state, possibly due to elevated cytokine levels, such as tumour necrosis factor-α (TNF-α). This enables FOXO factors to up-regulate MAFbx and MuRF1 expression, promoting ubiquitin-proteasome pathway (UPP)-mediated protein degradation, as well as PDK4, causing inhibition of the pyruvate dehydrogenase complex (PDC), down-regulation of carbohydrate oxidation, and potentially inducing insulin resistance.
Sepsis is a complex and potentially fatal condition that is known to induce rapid and marked muscle atrophy (Hasselgren & Fischer, 2001). Various models of endotoxaemia, including caecal ligation and puncture (Tiao et al. 1996) and endotoxin administration (Dehoux et al. 2003), have shown that muscle proteolysis induced by sepsis is primarily associated with increased UPP-dependent protein degradation. In this study, continuous LPS administration caused the fast-twitch EDL muscle to undergo atrophy, as illustrated by a 29% reduction in the protein: DNA ratio, which was accompanied by the transcriptional up-regulation of the muscle-specific E3 ubiquitin ligases MAFbx and MuRF1 (Fig. 1), and increased MAFbx protein levels (Fig. 6). MAFbx and MuRF1 are thought to be an important feature of the muscle atrophy programme in numerous animal models of muscle wasting (Bodine et al. 2001), and rapid increases in MAFbx and MuRF1 expression have been observed in a model of sepsis (Dehoux et al. 2003). Hence, the findings of this study are in agreement with previous observations of up-regulation of MAFbx and MuRF1 in endotoxaemia (Dehoux et al. 2003), and their central role in the initiation and regulation of muscle protein degradation via the UPP during atrophy (Bodine et al. 2001). Nevertheless, this study provides novel insight relating to the potential role of Akt/FOXO signalling in the simultaneous induction of muscle atrophy and development of muscle insulin resistance during sepsis, as will be presented in further detail below. This study has not explored the specific proteins that are being degraded during LPS-induced endotoxaemia. However, one potential protein target of MuRF1 is myosin heavy chain (Clarke et al. 2007), and therefore the loss of muscle mass could potentially arise directly from a loss of myofibrillar protein. Substrates of MAFbx, however, do not appear to include myofibrillar proteins, but include the transcription factor MyoD (Tintignac et al. 2005), and eukaryotic initiation factor 3 subunit 5 (eIF3-f) (Lagirand-Cantaloube et al. 2008), both of which are linked to anabolic signalling pathways in muscle. It is possible, therefore, that MAFbx may indirectly target myofibrillar proteins through instigating an overall reduction in muscle protein synthesis.
An important novel observation of the present study was that levels of phosphorylated FOXO protein were reduced in both the cytosol and nucleus of muscles from LPS-treated rats. Although there was no detectable change in total FOXO levels in the cytosol or nucleus, reduced levels of phosphorylated FOXO protein indicate that transcriptionally active FOXO was likely to have been primarily responsible for the increased expression of MAFbx and MuRF1. Consistent with reduced levels of phosphorylated FOXO 1 and 3, a reduction in phosphorylated Akt1, thereby inactivating it, was observed in muscles of LPS-treated animals (Fig. 5). Therefore, it is possible that LPS administration induced a suppression of Akt1 activity, thus altering Akt1/FOXO signalling, and potentially inducing atrophy. Furthermore, it could be predicted that since Akt1 activity was suppressed, protein synthesis was also inhibited, as previous evidence has suggested a coupling of MAFbx and MuRF1 up-regulation via Akt/FOXO signalling and a suppression of muscle protein synthesis in catabolic states (Hoffman & Nader, 2004). The observed reduction in total Akt1 (Fig. 5D), along with increased Akt1 mRNA expression, suggests that the reduced levels of phosphorylated (active) Akt1 may have resulted from degradation of the Akt1 protein. Since total Akt1 protein levels were decreased, and both TNF-α and IL-6 mRNA expression increased with LPS treatment, reduced activity of Akt1 most likely occurred through degradation of Akt1 via a TNF-α-mediated mechanism (Medina et al. 2005). Although only mRNA expression of TNF-α was measured and this does not necessarily correlate with increased protein levels, given the proposed role of cytokines in inducing muscle wasting (Cai et al. 2004), it is likely that increased TNF-α gene expression following LPS treatment was partially responsible for triggering the observed muscle atrophy, through altering Akt1/FOXO signalling (Fig. 8). However, it has recently been shown that calpain activation may also be important in the activation of UPP-dependent protein degradation through inhibition of Akt signalling (Smith & Dodd, 2007); therefore it is possible that calpain activation may have been at least partly responsible for the reduction in Akt1 activity in this study. Also, although not investigated in this study, it cannot be ruled out that glucocorticoids may have an important role in the induction of muscle atrophy by LPS infusion, as circulating glucocorticoids have been shown to be involved in regulating muscle proteolysis in sepsis (Tiao et al. 1996) via the UPP.
As well as inducing rapid muscle atrophy, dysregulation of intracellular insulin signalling is likely to have occurred as a result of reduced Akt1 activity. Other aspects of the insulin signalling pathway were affected, including significantly reduced IRS-1 gene expression in muscle of LPS-treated animals (Fig. 1), which could potentially down-regulate intracellular insulin signalling upstream of Akt1. However, analysis revealed no difference in the amount of phosphorylated IRS-1 protein (serine612; data not shown), suggesting there was no effect of LPS infusion on regulation of IRS-1 protein, since serine phosphorylation of IRS-1 is associated with inhibition of insulin signalling (Hotamisligil et al. 1996), although a different phosphorylation site may have been affected on the IRS-1 protein. Indeed, TNF-α has been shown to inhibit IRS-1 (serine307) and induce insulin resistance (Hotamisligil et al. 1996). With its important role in glucose metabolism and normal insulin signalling in skeletal muscle (Ueki et al. 1998), reduced Akt signalling by LPS is an indication of disturbed carbohydrate metabolism and potentially the induction of a muscle insulin resistant state. Future work using accepted in vivo measurements of insulin resistance will provide further evidence for the existence of a muscle insulin resistant state in this model of endotoxaemia.
Other compelling evidence that LPS infusion elicited an impairment of muscle carbohydrate metabolism include the observed inhibition of PDC activity (Fig. 7C), which was probably mediated by the simultaneous up-regulation of PDK4 mRNA (Fig. 2), and PDK4 protein expression (Fig. 7B), and the down-regulation of the PDC phosphatase (PDP1) mRNA (Fig. 2).
PDC is the rate-limiting step in carbohydrate oxidation, and its activity is regulated by the concerted action of one of four kinases (PDK 1–4) and one of two phosphatases (PDP 1–2) (Bowker-Kinley et al. 1998; Huang et al. 1998). PDK2 and PDK4, which are the most prevalent isoforms in skeletal muscle, inhibit the activity of PDC by phosphorylation of the complex, thereby preventing the conversion of carbohydrate-derived pyruvate into acetyl-CoA. On the other hand, muscle PDC phosphatases such as PDP1 act against the actions of PDK2 and PDK4, by dephosphorylating the complex and thereby activating PDC. However, in the present study, LPS administration resulted in down-regulation of carbohydrate oxidation at the level of PDC inhibition apparently by both increasing muscle PDK4 protein expression and reducing PDP1 mRNA transcription. These findings provide, for the first time, compelling evidence that a dual inhibition of PDC activity exists in LPS-induced endotoxaemia. Protein measurements of PDP1 need to be carried out in future work to provide further support for the role of reduced PDP1 in the observed inhibition of PDC.
Findings in the present study of PDC inhibition are in agreement with a recent study using a similar model of LPS infusion (albeit at a 10-fold higher dose) over 24 h that elicited a 65% reduction in PDC activity (Alamdari et al. 2008). In both studies, the inhibition of PDC activity appeared to be due to a cytokine-mediated increase in PDK4 transcription, and this was probably responsible for the accumulation of muscle lactate observed after 24 h LPS infusion. Vary and colleagues have also demonstrated the involvement of increased muscle PDK activity in the sepsis-induced inhibition of PDC and development of hyperlactataemia (Vary, 1996; Vary & Hazen, 1999). Thus, observations in the present study of a marked elevation of muscle lactate accumulation and reduced glycogen content in the LPS-administered group are more than likely attributable to an inhibition of PDC activity. Increased muscle glycogen breakdown, in conjunction with greater lactate accumulation suggests that LPS administration resulted in inefficient muscle glycogen utilization, and impaired pyruvate oxidation following PDC inhibition.
Since levels of dephosphorylated (active) FOXO1 and 3, which are transcription factors for PDK4 (Furuyama et al. 2003), were increased in the LPS group, it is likely that they might have also been involved in PDK4 up-regulation and PDC inhibition. The present results suggest that in the septic state, inhibition of PDC, due to FOXO-mediated up-regulation of PDK4, could be an important factor in the cascade of events that lead to the impairment of carbohydrate oxidation and to the development of insulin resistance. Indeed, up-regulation of muscle PDK4 has been reported in insulin-resistant and -deficient states, through dysregulation of a PI3K/Akt-mediated pathway (Kwon et al. 2004), and it has recently been shown that PDK4 deficiency slightly improves glucose tolerance and insulin sensitivity in PDK4 knockout mice on a high-fat diet (Jeoung & Harris, 2008). As elevated TNF-α has been implicated in inducing insulin resistance (Plomgaard et al. 2005), it most probably had a role in the induction of a muscle insulin resistant state in this model of endotoxaemia.
A noticeable reduction in PGC-1α gene expression was also observed in muscles of LPS-treated rats (Fig. 1). Reduced PGC-1α has been observed in other insulin resistant states (Handschin & Spiegelman, 2006), and is associated with reduced oxidative phosphorylation. The actions of PGC-1α appear to be diverse (Handschin & Spiegelman, 2006); however, with a possible role of PGC-1α in promoting skeletal muscle glucose uptake and involvement in fibre-type switching towards more oxidative fibres (Lin et al. 2002), reduced expression in this model of endotoxaemia may have had an effect on muscle fuel metabolism that is entirely consistent with the other observations made pertaining to carbohydrate oxidation.
The findings presented in this study are in line with previous results obtained from studies involving septic patients. For example, increased proteasome activity (Klaude et al. 2007) and protein and mRNA expression of a variety of components of the ubiquitin-proteasome pathway (Mansoor et al. 1996; Helliwell et al. 1998; Biolo et al. 2000; Winkelman, 2007) have been demonstrated in muscles from septic patients, which supports the proposed role of MAFbx and MuRF1 in muscle protein degradation in this model of endotoxaemia. Furthermore, the observed reduction in muscle PDC activity, and increased muscle lactate accumulation and glycogen degradation may be relevant to the clinical situation, with evidence of reduced mitochondrial content and function in patients with sepsis-induced multiple organ failure (Fredriksson et al. 2006). Additionally, the various findings of our study pointing towards the development of a muscle insulin resistant state during LPS infusion are consistent with findings from patient based studies (Chambrier et al. 2000; Marik & Raghavan, 2004). Hence, some findings in this model of endotoxaemia appear to be highly relevant to the clinical situation in sepsis patients. Nevertheless, it should be recognized that critically ill patients present with a host of different clinical complications, each potentially able to modify the catabolic and metabolic response of muscle.
The results from this study demonstrate that up-regulation of MAFbx and MuRF1, and atrophy of the EDL muscle induced by continuous LPS infusion occurred simultaneously with altered regulation of carbohydrate metabolism, which was accompanied by appropriate changes in Akt and FOXO, to suggest that a common, FOXO-dependent, pathway may be important to both the regulation of muscle fuel metabolism and protein turnover in vivo in sepsis (Fig. 8). Although these data may be regarded as confirmatory to some extent, this is the first study that has shown a concomitant induction of muscle atrophy and inhibition of carbohydrate oxidation in vivo during sepsis, paralleled by changes in Akt and FOXO phosphorylation status that suggest they may have a role, since FOXO gene targets MAFbx and MuRF1 have been implicated in muscle atrophy (Bodine et al. 2001), and FOXO target PDK4 is important for PDC inhibition (Bowker-Kinley et al. 1998). Pharmacological and/or nutritional strategies aimed at suppressing atrophy or reducing the transcriptional activity of FOXO transcription factors could potentially facilitate recovery of muscle mass and improve insulin sensitivity during sepsis, by restoring LPS-mediated dysregulation of Akt/FOXO signalling.
Acknowledgments
We would like to thank Julie March and Philip Kemp for their excellent technical assistance. This study was funded by the Biotechnology and Biological Sciences Research Council (BBSRC).
References
- Alamdari N, Constantin-Teodosiu D, Murton AJ, Gardiner SM, Bennett T, Layfield R, Greenhaff PL. Temporal changes in the involvement of pyruvate dehydrogenase complex in muscle lactate accumulation during lipopolysaccharide infusion in rats. J Physiol. 2008;586:1767–1775. doi: 10.1113/jphysiol.2007.149625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Biolo G, Bosutti A, Iscra F, Toigo G, Gullo A, Guarnieri G. Contribution of the ubiquitin-proteasome pathway to overall muscle proteolysis in hypercatabolic patients. Metabolism. 2000;49:689–691. doi: 10.1053/meta.2000.6236. [DOI] [PubMed] [Google Scholar]
- Blough E, Dineen B, Esser K. Extraction of nuclear proteins from striated muscle tissue. Biotechniques. 1999;26:202–204. 206. doi: 10.2144/99262bm05. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J. 1998;329:191–196. doi: 10.1042/bj3290191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Chambrier C, Laville M, Rhzioual Berrada K, Odeon M, Bouletreau P, Beylot M. Insulin sensitivity of glucose and fat metabolism in severe sepsis. Clin Sci (Lond) 2000;99:321–328. [PubMed] [Google Scholar]
- Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, Rakhilin SV, Stitt TN, Patterson C, Latres E, Glass DJ. The E3 ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. 2007;6:376–385. doi: 10.1016/j.cmet.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- Constantin D, Constantin-Teodosiu D, Layfield R, Tsintzas K, Bennett AJ, Greenhaff PL. PPARδ agonism induces a change in fuel metabolism and activation of an atrophy programme, but does not impair mitochondrial function in rat skeletal muscle. J Physiol. 2007;583:381–390. doi: 10.1113/jphysiol.2007.135459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin-Teodosiu D, Cederblad G, Hultman E. A sensitive radioisotopic assay of pyruvate dehydrogenase complex in human muscle tissue. Anal Biochem. 1991;198:347–351. doi: 10.1016/0003-2697(91)90437-x. [DOI] [PubMed] [Google Scholar]
- Dehoux MJ, van Beneden RP, Fernandez-Celemin L, Lause PL, Thissen JP. Induction of MafBx and Murf ubiquitin ligase mRNAs in rat skeletal muscle after LPS injection. FEBS Lett. 2003;544:214–217. doi: 10.1016/s0014-5793(03)00505-2. [DOI] [PubMed] [Google Scholar]
- Forsberg AM, Nilsson E, Werneman J, Bergstrom J, Hultman E. Muscle composition in relation to age and sex. Clin Sci (Lond) 1991;81:249–256. doi: 10.1042/cs0810249. [DOI] [PubMed] [Google Scholar]
- Fredriksson K, Hammarqvist F, Strigard K, Hultenby K, Ljungqvist O, Wernerman J, Rooyackers O. Derangements in mitochondrial metabolism in intercostal and leg muscle of critically ill patients with sepsis-induced multiple organ failure. Am J Physiol Endocrinol Metab. 2006;291:E1044–E1050. doi: 10.1152/ajpendo.00218.2006. [DOI] [PubMed] [Google Scholar]
- Furuyama T, Kitayama K, Yamashita H, Mori N. Forkhead transcription factor FOXO1 (FKHR)-dependent induction of PDK4 gene expression in skeletal muscle during energy deprivation. Biochem J. 2003;375:365–371. doi: 10.1042/BJ20030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamrin L, Essen P, Forsberg AM, Hultman E, Wernerman J. A descriptive study of skeletal muscle metabolism in critically ill patients: free amino acids, energy-rich phosphates, protein, nucleic acids, fat, water, and electrolytes. Crit Care Med. 1996;24:575–583. doi: 10.1097/00003246-199604000-00005. [DOI] [PubMed] [Google Scholar]
- Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005;37:1974–1984. doi: 10.1016/j.biocel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor γ coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- Harris RC, Hultman E, Nordesjo LO. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Laboratory Invest. 1974;33:109–120. [PubMed] [Google Scholar]
- Hasselgren PO, Fischer JE. Muscle cachexia: current concepts of intracellular mechanisms and molecular regulation. Ann Surg. 2001;233:9–17. doi: 10.1097/00000658-200101000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselgren PO, Warner BW, James JH, Takehara H, Fischer JE. Effect of insulin on amino acid uptake and protein turnover in skeletal muscle from septic rats. Arch Surg. 1987;122:228–233. doi: 10.1001/archsurg.1987.01400140110015. Evidence for insulin resistance of protein breakdown. [DOI] [PubMed] [Google Scholar]
- Helliwell TR, Wilkinson A, Griffiths RD, McClelland P, Palmer TE, Bone JM. Muscle fibre atrophy in critically ill patients is associated with the loss of myosin filaments and the presence of lysosomal enzymes and ubiquitin. Neuropathol Appl Neurobiol. 1998;24:507–517. doi: 10.1046/j.1365-2990.1998.00144.x. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Nader GA. Balancing muscle hypertrophy and atrophy. Nat Med. 2004;10:584–585. doi: 10.1038/nm0604-584. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- Huang B, Gudi R, Wu P, Harris RA, Hamilton J, Popov KM. Isoenzymes of pyruvate dehydrogenase phosphatase. J Biol Chem. 1998;273:17680–17688. doi: 10.1074/jbc.273.28.17680. DNA-derived amino acid sequences, expression, and regulation. [DOI] [PubMed] [Google Scholar]
- Jeoung NH, Harris RA. Pyruvate dehydrogenase kinase 4 (PDK4) deficiency lowers blood glucose and improves glucose tolerance in diet-induced obese mice. Am J Physiol Endocrinol Metab. 2008;295:E46–54. doi: 10.1152/ajpendo.00536.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YI, Lee FN, Choi WS, Lee S, Youn JH. Insulin regulation of skeletal muscle PDK4 mRNA expression is impaired in acute insulin-resistant states. Diabetes. 2006;55:2311–2317. doi: 10.2337/db05-1606. [DOI] [PubMed] [Google Scholar]
- Klaude M, Fredriksson K, Tjader I, Hammarqvist F, Ahlman B, Rooyackers O, Wernerman J. Proteasome proteolytic activity in skeletal muscle is increased in patients with sepsis. Clin Sci (Lond) 2007;112:499–506. doi: 10.1042/CS20060265. [DOI] [PubMed] [Google Scholar]
- Kwon HS, Huang B, Unterman TG, Harris RA. Protein kinase B-α inhibits human pyruvate dehydrogenase kinase-4 gene induction by dexamethasone through inactivation of FOXO transcription factors. Diabetes. 2004;53:899–910. doi: 10.2337/diabetes.53.4.899. [DOI] [PubMed] [Google Scholar]
- Lagirand-Cantaloube J, Offner N, Csibi A, Leibovitch MP, Batonnet-Pichon S, Tintignac LA, Segura CT, Leibovitch SA. The initiation factor eIF3-f is a major target for atrogin1/MAFbx function in skeletal muscle atrophy. EMBO J. 2008;27:1266–1276. doi: 10.1038/emboj.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CH, Dobrescu C, Meszaros K. Insulin-mediated glucose uptake by individual tissues during sepsis. Metabolism. 1990;39:1096–1107. doi: 10.1016/0026-0495(90)90172-9. [DOI] [PubMed] [Google Scholar]
- Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293:E453–E459. doi: 10.1152/ajpendo.00204.2007. [DOI] [PubMed] [Google Scholar]
- Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18:39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Mansoor O, Beaufrere B, Boirie Y, Ralliere C, Taillandier D, Aurousseau E, Schoeffler P, Arnal M, Attaix D. Increased mRNA levels for components of the lysosomal, Ca2+-activated, and ATP-ubiquitin-dependent proteolytic pathways in skeletal muscle from head trauma patients. Proc Natl Acad Sci U S A. 1996;93:2714–2718. doi: 10.1073/pnas.93.7.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marik PE, Raghavan M. Stress-hyperglycemia, insulin and immunomodulation in sepsis. Intensive Care Med. 2004;30:748–756. doi: 10.1007/s00134-004-2167-y. [DOI] [PubMed] [Google Scholar]
- Medina EA, Afsari RR, Ravid T, Castillo SS, Erickson KL, Goldkorn T. Tumor necrosis factor-α decreases Akt protein levels in 3T3-L1 adipocytes via the caspasedependent ubiquitination of Akt. Endocrinology. 2005;146:2726–2735. doi: 10.1210/en.2004-1074. [DOI] [PubMed] [Google Scholar]
- Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor necrosis factor-α induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes. 2005;54:2939–2945. doi: 10.2337/diabetes.54.10.2939. [DOI] [PubMed] [Google Scholar]
- Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith IJ, Dodd SL. Calpain activation causes a proteasome-dependent increase in protein degradation and inhibits the Akt signalling pathway in rat diaphragm muscle. Exp Physiol. 2007;92:561–573. doi: 10.1113/expphysiol.2006.035790. [DOI] [PubMed] [Google Scholar]
- Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- Tawa NE, Jr, Odessey R, Goldberg AL. Inhibitors of the proteasome reduce the accelerated proteolysis in atrophying rat skeletal muscles. J Clin Invest. 1997;100:197–203. doi: 10.1172/JCI119513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiao G, Fagan J, Roegner V, Lieberman M, Wang JJ, Fischer JE, Hasselgren PO. Energy-ubiquitin-dependent muscle proteolysis during sepsis in rats is regulated by glucocorticoids. J Clin Invest. 1996;97:339–348. doi: 10.1172/JCI118421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiao G, Lieberman M, Fischer JE, Hasselgren PO. Intracellular regulation of protein degradation during sepsis is different in fast- and slow-twitch muscle. Am J Physiol Regul Integr Comp Physiol. 1997;272:R849–R856. doi: 10.1152/ajpregu.1997.272.3.R849. [DOI] [PubMed] [Google Scholar]
- Tintignac LA, Lagirand J, Batonnet S, Sirri V, Leibovitch MP, Leibovitch SA. Degradation of MyoD mediated by the SCF (MAFbx) ubiquitin ligase. J Biol Chem. 2005;280:2847–2856. doi: 10.1074/jbc.M411346200. [DOI] [PubMed] [Google Scholar]
- Ueki K, Yamamoto-Honda R, Kaburagi Y, Yamauchi T, Tobe K, Burgering BM, Coffer PJ, Komuro I, Akanuma Y, Yazaki Y, Kadowaki T. Potential role of protein kinase B in insulin-induced glucose transport, glycogen synthesis, and protein synthesis. J Biol Chem. 1998;273:5315–5322. doi: 10.1074/jbc.273.9.5315. [DOI] [PubMed] [Google Scholar]
- Vary TC. Sepsis-induced alterations in pyruvate dehydrogenase complex activity in rat skeletal muscle: effects on plasma lactate. Shock. 1996;6:89–94. doi: 10.1097/00024382-199608000-00002. [DOI] [PubMed] [Google Scholar]
- Vary TC, Hazen S. Sepsis alters pyruvate dehydrogenase kinase activity in skeletal muscle. Mol Cell Biochem. 1999;198:113–118. doi: 10.1023/a:1006993910781. [DOI] [PubMed] [Google Scholar]
- Waller J, Gardiner SM, Jose J, Bennett T. Lack of effect of TNF antibodies on the cardiovascular sequelae of lipopolysaccharide infusion in conscious rats. Br J Pharmacol. 1995;116:2487–2495. doi: 10.1111/j.1476-5381.1995.tb15100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hu Z, Hu J, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: Activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology. 2006;147:4160–4168. doi: 10.1210/en.2006-0251. [DOI] [PubMed] [Google Scholar]
- Winkelman C. Inactivity and inflammation in the critically ill patient. Crit Care Clin. 2007;23:21–34. doi: 10.1016/j.ccc.2006.11.002. [DOI] [PubMed] [Google Scholar]