Abstract
Angiotensin II (ANGII) plays a central role in the enhanced sodium reabsorption in early type 1 diabetes in man and in streptozotocin-induced (STZ) diabetic rats. This study investigates the effect of untreated STZ-diabetes leading to diabetic nephropathy in combination with ANGII treatment, on the abundance and localization of the renal Na+,K+-ATPase (NKA), a major contributor of renal sodium handling. After 7 weeks of STZ-diabetes (i.v. 65 mg kg−1) a subgroup of control (C) and diabetic (D7) Wistar rats were treated with ANGII (s.c. minipump 33 μg kg−1 h−1 for 24 h; CA and D7A). We measured renal function and mRNA expression, protein level, Serin23 phosphorylation, subcellular distribution, and enzyme activity of NKA α-1 subunit in the kidney cortex. Diabetes increased serum creatinine and urea nitrogen levels (C versus D7), as did ANGII (C versus CA, D7 versus D7A). Both diabetes (C versus D7) and ANGII increased NKA α-1 protein level and enzyme activity (C versus CA, D7 versus D7A). Furthermore, the combination led to an additive increase (D7 versus D7A, CA versus D7A). NKA α-1 Ser23 phosphorylation was higher both in D7 and ANGII-treated rats in the non-cytoskeletal fraction, while no signal was detected in the cytoskeletal fraction. Control kidneys showed NKA α-1 immunopositivity on the basolateral membrane of proximal tubular cells, while both D7 and ANGII broadened NKA immunopositivity towards the cytoplasm. Our study demonstrates that diabetes mellitus (DM) increases the mRNA expression, protein level, Ser23 phosphorylation and enzyme activity of renal NKA, which is further elevated by ANGII. Despite an increase in total NKA quantity in diabetic nephropathy, the redistribution to the cystosol suggests the Na+ pump is no longer functional. ANGII also caused translocation from the basolateral membrane, thus in diabetic states where ANGII level is acutely elevated, the loss of NKA will be exacerbated. This provides another mechanism by which ANGII blockade is likely to be protective.
Diabetes mellitus is a metabolic disorder characterized by hyperglycaemia and long-term microvascular complications in various organs, including the kidney. Diabetic nephropathy occurs in 20–40% of patients with DM and is the leading cause of end-stage renal disease (American Diabetes Association, 2005). In type 1 DM in men and in streptozotocin (STZ)-induced diabetic rats an early sign of diabetic nephropathy is glomerular hyperfiltration, which may be due to the resetting of tubular glomerular feedback and increased Na+ reabsorption (Vallon et al. 1999). There are conflicting data on the relationship between type 1 DM and the renin–angiotensin–aldosterone system (RAS) (Campbell et al. 1999; Vallon et al. 1995; Zimpelmann et al. 2000; Blüher et al. 2001; Zhang et al. 2006). However, there is no doubt that acute hyperglycaemia plays a causal role in the short term RAS activation of patients with type 1 DM (Miller, 1999). It is also obvious that both systemic and glomerular hypertension caused by increased angiotensin II (ANGII) level accelerate the progression of diabetic nephropathy. It has been well established that in the proximal tubules, the main site for renal water and salt regulation, ANGII plays a critical role in the regulation of renal Na+ and water homeostasis by increasing Na+ reabsorption (Burns et al. 1993). In the renal proximal tubules Na+ is actively reabsorbed primarily by the apical Na+–H+ exchanger and the Na+–HCO3− cotransporter and extruded through the basolateral sodium pump Na+,K+-ATPase (NKA). Previous experiments demonstrated the involvement of ANGII in the regulation of subcellular abundance of Na+–H+ exchanger (Beutler et al. 2003) and Na+–HCO3− cotransporter (Leong et al. 2006). It has been also established that a combination of high glucose and ANGII is involved in diabetic nephropathy by regulating the Na+–glucose cotransporter activity (Lee et al. 2007; Vidotti et al. 2008). However, there have been no literary data about the combined effect of ANGII and DM on the subcellular localization of NKA, which is the key element of Na+ transport and the driving force of the other secondary Na+ transporters. The transporting enzyme NKA consists of a heterotetramer of two α and two β glycoprotein subunits. The α subunits are responsible mainly for the catalytic activity (Sweadner, 1989). Under physiological circumstances NKA actively secretes Na+ into the interstitium on the basolateral membrane of renal proximal tubular cells (Yingst et al. 2004). This physiological localization is essential for efficient enzyme function and Na+ reabsorption (Woroniecki et al. 2003).
The catalytic α subunit of NKA is phosphorylated by protein kinase (PK) C in the N-terminal at Ser11, Ser18 and Ser23 (Logvinenko et al. 1996), as well as by PKA in the C-terminal at Ser943 (Feschenko & Sweadner, 1995). Reversible covalent modification by phosphorylation takes part in the regulation of NKA trafficking between subcellular compartments (Chibalin et al. 1999; Efendiev et al. 2003). Furthermore, phosphorylation is generally associated with altered enzyme activity (Bertuccio et al. 2007; Lal et al. 2000), which may be in connection with altered subcellular NKA distribution. The influence of DM and ANGII on the expression and function of NKA has been studied separately in rodents, but their common effect is still not known.
In diabetic experimental models, there is controversial data about NKA with time-, dose- and tissue-specific changes. In the kidney, STZ-induced DM resulted in increased enzyme activity (Ng et al. 1993), while others found a biphasic time-dependent effect (Tsimarato et al. 2001). DM-induced alterations of renal NKA activity are only partially restored by insulin therapy (Vér et al. 1995), indicating that other factors, including ANGII, may also influence the enzyme function (Féraille & Doucet, 2001). There are also conflicting data about the effect of ANGII on NKA abundance and activity. While some showed increased NKA activity with ANGII treatment (Shah & Hussain, 2006), other studies revealed no or even opposite effects (Hakam et al. 2006; Siddiqui & Hussain, 2007). In most studies, ANGII has been administered chronically with a subpressor dose (5–15 μg kg−1 h−1), while short-term effects and pressor doses of ANGII in the kidney are poorly defined. To our knowledge, there are only scarce data in rat kidney that used short-term ANGII in a pressor dose of 30 μg kg−1 h−1 (Wilcox & Welch, 1990). Based on the study of Gonzalez-Villalobos et al. (2008) this dose would result in an approximately 1.5-fold increase in serum ANGII level. Therefore, we postulate that 24 h infusion of 33 μg kg−1 h−1 ANGII – chosen in our experiment – would also correspond to an approximately 1.5 times higher ANGII level reported in several studies both in DM patients and STZ-diabetic rat models (men: Hollenberg et al. 2004; rats: Zhang et al. 2006; Kobayashi et al. 2006; Xu et al. 2007). In several disease states (e.g. diabetic ketoacidosis, hyperosmolar non-ketotic hyperglycaemia, acute cardiac failure, or acute exacerbation of chronic heart failure) RAS activation, as a result of acute elevation of ANGII levels, superimposes to diabetes and diabetic nephropathy. Therefore, the goal of our study was to differentiate the effect of untreated STZ-diabetes leading to diabetic nephropathy, in combination with short-term ANGII treatment, on the abundance and localization of the renal NKA, a major contributor of renal Na+ handling.
Methods
Experimental protocol
Experiments were performed on 4-week-old male Wistar rats (weighing 100 ± 30 g). Rats were housed at a constant temperature (20°C), humidity, 12 h light–dark cycle, and were allowed free access to standard chow and water. All experimental protocols were in accordance with the guidelines of the Committee on the Care and Use of Laboratory Animals of the Council on Animal Care at the Semmelweis University of Budapest, Hungary (TUKEB 99/94).
STZ-induced diabetes, ANGII treatment and experimental groups
Rats were rendered diabetic with STZ (65 mg kg−1i.v., Sigma Chemical Co.) dissolved in 0.1 m citrate buffer (pH 4.5). Only STZ-treated animals with plasma glucose concentrations above 15 mmol l−1 were considered diabetic and included in the study. All experiments were performed 7 weeks after DM induction.
Seven weeks after the induction of DM (defined as day 0), an osmotic minipump (containing ANGII, pumping rate: 24 h, 33 μg kg−1 h−1 was implanted subcutaneously under pentobarbital anaesthesia (40 mg kg−1 pentobarbital sodium, Abbott Laboratories). After 24 h of ANGII treatment the animals were re-anaesthetized, blood and post mortem urinary samples were collected; the kidneys were removed and immediately snap-frozen for further investigations. Sham-operated, vehicle-only treated rats served as controls. The experimental protocol consisted of four groups: (1) control (C); (2) control treated with ANGII (CA); (3) STZ-induced 7 week-diabetic rats (D7); (4) STZ-induced 7 week-diabetic rats treated with ANGII (D7A) (n = 6, in each group) (Ruzicska et al. 2004).
Measurement of general and laboratory (metabolic and renal) parameters
Body and kidney weight were measured to calculate kidney/body weight ratio. Serum and urinary sodium, potassium levels, and renal parameters (blood urea nitrogen (BUN) and creatinine) were photometrically determined with commercially available kits (Boehringer-Mannheim Diagnostic Systems) on a Hitachi-917 automated spectrophotometer. Non-fasting serum glucose concentration was measured using a reagent kit from Boehringer-Mannheim. Serum fructosamine concentration was measured using a kit from Roche Diagnostics Ltd.
Fractional sodium excretion was calculated as follows:
Measurement of mean arterial blood pressure
Mean arterial blood pressure (MAP) was monitored with TL11M2-C50-PXT radiotelemetry transmitters (Data Sciences International) implanted in the peritoneal cavity of conscious, freely moving rats. Under sterile conditions, in anaesthetized rats (pentobarbital sodium, 40 mg kg−1, i.p. Abbott Laboratories) a catheter was introduced into the abdominal aorta and the body of the transmitter was sutured to the abdominal wall. After surgery the animals were treated with a single dose of antibiotic (1 mg kg−1i.m., Tardomyocel, Bayer AG).
A post-operative period of at least 7 days was allowed for the animals to recover completely. RLA1000 receivers placed under each animal's cage detected radio signals emitted by the transmitters. The data were collected, and evaluated using Dataquest IV Software (Data Sciences International). The computer was set to sample the parameters for 10 s every other minute. Both parameters were averaged for 30 min periods using the ‘Sort Utility’ of the Dataquest IV System. The upper and lower limits of the evaluating routine were set to exclude biologically improbable values.
RT-PCR reaction
All reagents, enzymes and isolation kits were purchased from Qiagen GmBH. Total RNA extraction, first-strand cDNA synthesis and PCR reaction for NKA α-1 and glyceraldeyde-3-phosphate dehydrogenase (GAPDH) were performed as previously described (Fekete et al. 2004).
Tissue homogenization and Western blot analysis
Protein determinations were performed in triplicate by Bradford analysis using bovine serum albumin as a standard. All reagents for PAGE and Western blot were purchased from Sigma Chemical Co.
Tissue homogenization and cellular protein fractionation
A sample of kidney cortex (100 mg) was homogenized in chilled extraction buffer (60 mm Hepes, 1 mm EDTA, 1 mm EGTA, 100 mm NaCl, 100 mm NaF, 0.5 mm PMSF, 0.75 mg l−1 leupeptin and 0.1 mm DTT) using a Potter-Elvehjem homogenizer. The total renal cortical tissue homogenate (TOT) was centrifuged at 680 g for 5 min at 4°C and stored at −80°C for further analysis. Triton X-100 extraction fractionates the cellular pool of NKA into an insoluble pellet (cytoskeletal-associated fraction) and a soluble supernatant by simple differential centrifugation. This method has been used in several studies as a reproducible marker for NKA subcellular distribution (Bidmonn et al. 2000; Aufricht et al. 2002; Fekete et al. 2004). Therefore, cold Triton X-100 (0.1%, 4°C) was added to the extraction buffer for cellular protein fractionation: another 100 mg of renal cortical tissue was homogenized in this chilled buffer and then centrifuged at 35000 g for 15 min at 4°C to separate the Triton-soluble fraction (detergent soluble (DS), non-cytoskeletal) from the Triton-insoluble pellet (detergent resistant (DR), cytoskeletal). DR was resuspended in extraction buffer and both fractions (DR and DS) were saved at −80°C.
Western blot analysis
NKA Western blot analysis was performed as previously described, with monoclonal antibodies to NKA α-1 (Upstate Biotechnology) diluted to 1: 750 (Fekete et al. 2004). The phosphorylation of NKA was verified by detecting the changes in the immunoreactivity to a phosphorylation state-specific Ser23 antibody (Santa Cruz Biotechnology) that had been subjected to SDS-PAGE and transferred to nitrocellulose membranes. Blots were developed with enhanced chemiluminescence Western blotting detection (AP-Biotech). Computerized densitometry of the specific bands was analysed with Gel-Pro Analyser 3.1 software. The values were normalized to β-actin (Sigma Chemical Co.) as an internal standard and expressed as relative optical density.
NKA enzyme activity
NKA activity was assayed in TOT renal cortical tissue homogenate by measuring the strophantidine sensitive 3-O-methylfluoresceinphosphatase activity (Vér et al. 1995,1997). Briefly, the activity was determined in the presence of 19.5 mmol l−1 3-O-methylfluorescein-phosphate, 4 mmol l−1 MgCl2, 1 mmol l−1 EDTA, 80 mmol l−1 Tris-HCl (pH 7.6), 10 mmol l−1 KCl and 100 μg TOT homogenate, and pre-incubated with 0.1% Na-deoxycholate (pH 7.4) for 30 min at 24°C. Inhibition percentages were calculated by comparing the activities in the presence of 5 mmol l−1 strophanthidine. NKA activity represents the difference between the activity with or without strophantidine in units of μmol fluorescein (mg protein)−1 h−1.
Fluorescent immunohistochemistry
Kidney sections were immediately snap-frozen in 30% sucrose for immunohistochemical analysis. They were embedded in Shandon cryomatrix (ThermoElectron Co.), cut into 5–10 μm thick slices with a cryostat, and stored at −80°C. Slides were washed in phosphate-buffered saline (PBS) for 10 min and were incubated for 2 h at room temperature with the NKA α-1 antibody (diluted to 1: 100). After repeated washing, slides were incubated with the appropriate secondary antibody (Alexa Fluor 488, Invitrogen) for 30 min at room temperature. DNA was stained with Hoechst 33342 (Sigma) for 10 min at room temperature. Slides were then rinsed in PBS and covered with Vectashield fluorescent mounting medium (Vector Laboratories). Appropriate controls were performed by omitting the primary antibodies to ensure the same specificity with Western blot methods and to avoid auto-fluorescence. Confocal images were taken on a Zeiss Axiovert LSM510.
Statistical analysis
Data were analysed on STATISTICA.6 software (StatSoft Inc.). Data are presented as means ±s.d., and were tested for normal distribution with a Kolmogorov–Smirnov test. Multiple comparisons and possible interactions were evaluated by two-way ANOVA followed by Scheffe correction as a post hoc test. For non-parametrical data the Kruskal–Wallis ANOVA on ranks was used. Criterion for significance was P < 0.05 in all experiments.
Results
Effect of STZ-diabetes and ANGII treatment on metabolic and renal parameters
General, metabolic and renal parameters are summarized in Table 1. Seven weeks after the induction of diabetes, D7 rats had lower body weight versus controls (P < 0.05 C versus D7; CA versus D7A, respectively.). Non-fasting blood glucose and fructosamine levels were elevated in D7 animals compared to controls (P < 0.05 C versus D7; CA versus D7A, respectively), while ANGII treatment had no further effect on these metabolic parameters. Kidney weight to body weight ratio, serum creatinine and BUN were higher in diabetic versus control rats (P < 0.05 C versus D7; CA versus D7A, respectively) suggesting the development of diabetic nephropathy. Both STZ-diabetes and ANGII treatment increased fractional sodium excretion values compared to controls (P < 0.05 C versus D7; C versus CA, respectively). Elevated MAP was observed after ANGII treatment in control rats (C versus CA, P < 0.05), while D7 animals showed trends of MAP increase.
Table 1.
General and laboratory parameters in samples of control (C), ANGII-treated control (CA), 7 week streptozotocin-diabetic (D7), and ANGII-treated D7 (D7A) rats
| C | CA | D7 | D7A | |
|---|---|---|---|---|
| Body weight (g) | 390 ± 37 | 393 ± 42 | 280 ± 32+ | 273 ± 38++ |
| Kidney weight (g) | 1.9 ± 0.4 | 1.8 ± 0.4 | 2.0 ± 0.4 | 2 ± 0.4 |
| Kidney/body weight | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.7 ± 0.3+ | 0.7 ± 0.4++ |
| Non-fasting blood glucose (mm l−1) | 8.6 ± 1.4 | 8.2 ± 1.7 | 30.9 ± 2.5+ | 27.9 ± 3.2++ |
| Fructosamine (μm l−1) | 137 ± 4 | 143 ± 4 | 252 ± 8+ | 265 ± 32++ |
| Serum sodium (mm l−1) | 149 ± 7 | 142 ± 4 | 145 ± 10 | 143 ± 8 |
| Serum potassium (mm l−1) | 4.7 ± 0.1 | 4.9 ± 0.2 | 4.9 ± 0.7 | 5.5 ± 0.6++ |
| Serum creatinine (μm l−1) | 68 ± 5 | 65 ± 2 | 81 ± 10+ | 97 ± 5++ |
| Blood urea nitrogen (mm l−1) | 4.7 ± 0.3 | 7.5 ± 1.3* | 10 ± 1.9+** | 21 ± 3.0++ |
| Urinary sodium (mm l−1) | 69 ± 11 | 55 ± 27 | 35 ± 8+** | 13 ± 1++ |
| Urinary potassium (mm l−1) | 38 ± 17 | 35 ± 30 | 16 ± 7 | 22 ± 3 |
| Urinary creatinine (μm l−1) | 1156 ± 231 | 573 ± 300* | 285 ± 173+** | 175 ± 96++ |
| Urinary urea nitrogen (mm l−1) | 83 ± 44 | 95 ± 63 | 69 ± 22** | 181 ± 26++ |
| Fractional sodium excretion (%) | 2.8 ± 0.6 | 4.5 ± 0.3* | 8.1 ± 0.3+ | 6.3 ± 0.3++ |
| Mean arterial pressure (mmHg) | 98.7 ± 1.7 | 125.2 ± 2.4* | 100.7 ± 1.6 | 109.1 ± 5.3 |
n = 6 per group.
P < 0.05, C vs CA;
P < 0.05, D7 vs D7A;
P < 0.05, C vs D7;
P < 0.05, CA vs D7A.
Effect of STZ-diabetes and ANGII treatment on the mRNA expression, protein level of α-1 subunit and NKA enzyme activity in renal cortical tissue homogenates
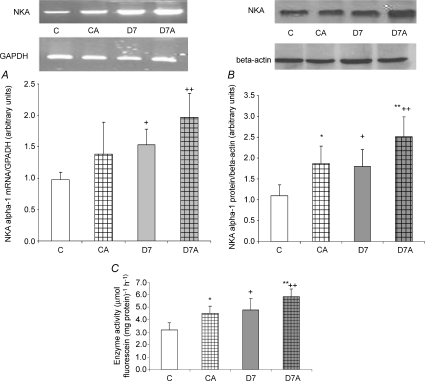
NKA α-1 subunit mRNA expression (Fig. 1A) and protein level (Fig. 1B) increased in D7 rats (P < 0.05, C versus D7, CA versus D7A, respectively). ANGII administration further elevated protein levels (P < 0.05, C versus CA, D7 versus D7A, respectively). In accordance with these, enzyme activity (Fig. 1C) was also increased in STZ-diabetic and ANGII-treated rats (P < 0.0001, C versus D7; P < 0.001, C versus CA; P < 0.01, D7 versus D7A, respectively).
Figure 1. mRNA expression, protein level of Na+,K+-ATPase (NKA) α-1 subunit and enzyme activity in cortical tissue homogenates of angiotensin II(ANGII)-treated and streptozotocin diabetic kidneys.
mRNA expression (A), protein level (B) and enzyme activity (C) were determined in kidney samples of control (C), ANGII-treated control (CA), 7 week diabetic (D7) and ANGII-treated D7 (D7A), rats (n = 6 per group). Top panel: representative examples of RT-PCR and Western blot analysis. Results are given as a ratio of intensity of NKA α-1 subunit and GAPDH mRNA, as a ratio of optical density of NKA α-1 subunit and β-actin and the difference between the activity with or without strophantidine in units of μmol fluorescein (mg protein)−1 h−1, respectively. mRNA and protein α-1 subunit: *P < 0.05, C vs D7; **P < 0.05, CA vs D7A. Enzyme activity: *P < 0.01, C vs CA; **P < 0.05, D7 vs D7A; +P < 0.0001, C vs D7.
Effect of STZ-diabetes and ANGII treatment on the subcellular distribution of NKA α-1 subunit
To determine whether STZ-diabetes and ANGII treatment influenced subcellular distribution of NKA, Triton X-100 extraction was used to divide TOT renal cortical tissue homogenate into Triton-soluble (DS) and Triton-insoluble (DR) fractions.
α-1 subunit in DS
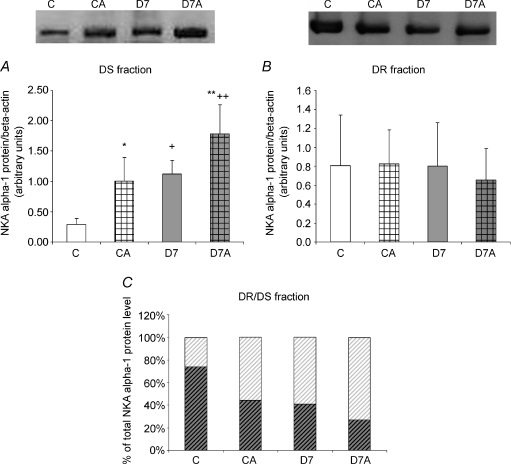
Similarly to TOT homogenates, in the cytosol-representing DS fraction, protein level of NKA α-1 subunit was higher in D7 rats (P < 0.002, C versus D7; P < 0.05, CA versus D7A, respectively; Fig. 2A), which was further increased by ANGII treatment both in control and diabetic animals (P < 0.01, C versus CA; D7 versus D7A, respectively).
Figure 2. Protein levels of Na+,K+-ATPase (NKA) α-1 in (A) detergent-soluble (DS) and (B) detergent-insoluble (DR) fraction of angiotensin II (ANGII)-treated and streptozotocin-diabetic kidneys and (C) DR/DS ratio.
Protein level of NKA α-1 subunit was measured in kidney samples of control (C), ANGII-treated control (CA), 7 week diabetic (D7) and ANGII-treated D7 (D7A) rats (n = 6 per group). Top panel: representative examples of Western blot analysis. Results are given as a ratio of optical density of NKA α-1 subunit and β-actin protein level. NKA α-1 protein level ratio of DR (dark hatching)/DS (light hatching) fraction is expressed in the percentage of TOT. *P < 0.05, C vs CA; **P < 0.05, D7 vs D7A; +P < 0.05, C vs D7; ++P < 0.05, CA vs D7A.
α-1 subunit in DR
In the DR fraction no significant changes were observed in any of the groups (Fig. 2B).
α-1 subunit protein level in the DS and DR fractions expressed as the percentage of TOT homogenates
Although the absolute amount of NKA α-1 protein levels were similar in the DR fraction of all groups, its relative value expressed as the percentage of TOT was lower both in diabetic and ANGII-treated animals (Fig. 2C). In parallel, with these the relative fraction of DS increased demonstrating a translocation between these subcellular compartments.
Effect of STZ-diabetes and ANGII treatment on Ser phosphorylation of Na+,K+-ATPase α-1 subunit
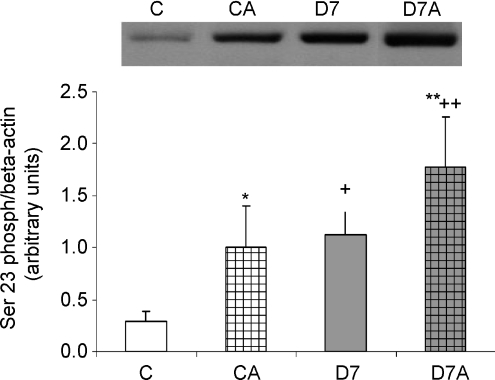
Ser23 phosphorylation was only detectable in the DS fraction. Ser23 phosphorylation of NKA α-1 increased in diabetes (P < 0.05, C versus D7, CA versus D7A, respectively, Fig. 3). ANGII treatment increased Ser23 phosphorylation both in control and D7 rats (P < 0.05, C versus CA, D7 versus D7A, respectively). There were no changes in the state of Ser943 phosphorylation in any of the groups or fractions (data not shown).
Figure 3. Serine23 phosphorylation of Na+,K+-ATPase (NKA) α-1 in angiotensin II (ANGII)-treated streptozotocin-diabetic kidneys.
Serin23 phosphorylation was determined in the detergent-soluble fraction of kidney samples of control (C), ANGII-treated control (CA), 7 week diabetic (D7), ANGII-treated D7 (D7A) rats (n = 6 per group). Results are given as a ratio of optical density of Ser23phospho-NKA α-1 and β-actin protein level. No signal was detected in the detergent-insoluble fraction in any of the groups. *P < 0.05, C vs CA; **P < 0.05, D7 vs D7A; +P < 0.05, C vs D7; ++P < 0.05, CA vs D7A.
Fluorescent immunohistochemistry
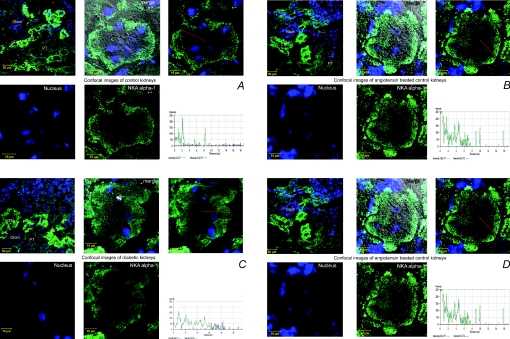
Figure 4A–C shows representative pictures of superficial cortex marked by the presence of glomeruli (GL). Control kidney NKA α-1 stained heavily in cortical thick ascending limbs (cTAL). Weaker staining was seen mainly in the basolateral surface of intact proximal tubular epithelial cells (PT) (Fig. 4A). NKA staining and distribution were not affected by ANGII administration in slightly stained proximal and heavily stained distal convoluted tubules compared to controls; however, the basal membrane was slightly widened (Fig. 4B). In D7 kidneys, broadened staining into the cytosol and towards the apical surface was seen in the markedly sustained proximal tubular cells (Fig. 4C). D7A kidneys showed a patchy distribution of stain. Some of the tubular cells showed marked NKA immunopositivity along the whole width of severely damaged proximal tubular cells, while there were other cells without any staining (Fig. 4D).
Figure 4. Confocalimages of angiotensin II (ANGII)-treated streptozotocin-diabetic kidney sections labelled with Na+,K+-ATPase (NKA) α-1 antibody.
Representative examples of NKA α-1 (green) localization in kidney sections of (A) control (C), (B) ANGII-treated control (CA), (C) 7 week diabetic (D7) and (D) ANGII-treated D7 (D7A) rats. Nuclei are stained blue. Fluorescence signal intensities of NKA α-1 (green) generated from a scanned horizontal line shown as red arrow in the merged image are shown on the bottom right of each panel. Glom, glomerulus; cTAL, cortical thick ascending limb; PT, proximal tubule. Scale bars: top left images for all panels, 50μm, all other images, 10 μm.
Discussion
The pathogenesis of diabetic nephropathy and end-stage renal failure is clearly multifactorial. Both hyperglycaemia and hypertension are major contributing factors in the progression of kidney disease, which are often simultaneously present in diabetic patients. Moreover, the impact of these two factors may be different during the development and progression of diabetic nephropathy and there are several clinical situations such as diabetic ketoacidosis, hyperosmolar non-ketotic hyperglycaemia, acute cardiac failure, etc., when acute RAS activation, as a result of acute elevation of ANGII, superimposes to DM and diabetic nephropathy.
The STZ-treated rat is a widely used animal model of type 1 DM. It is associated with severe hyperglycaemia and carbohydrate disorder that we also observed in the present study. In line with previous findings (Vallon et al. 1999; Marwaha et al. 2004; Hakam et al. 2006), diabetic rats were shown to have higher serum creatinine and BUN levels, and increased kidney/body weight ratio, in addition to elevated urinary creatinine, urea nitrogen levels, and increased fractioned sodium excretion. All these results strongly suggest the presence of structural and functional kidney damage. ANGII treatment resulted in hypertension, reflected by higher MAP in CA animals and also deterioration of renal function.
The first important finding is that STZ-diabetes along with ANGII treatment led to a ‘superimposed’ nephropathy with a supposed diabetic and RAS-activated factor. These animals had severely damaged kidney function (serum creatinine and BUN levels increased in parallel with elevated serum potassium and fractional sodium excretion). MAP also tended to be higher in D7A animals, though not significantly, which can be explained also by severe diabetic exsiccosis, as reflected by a high BUN. However, it is also well known that DM leads to cardiac, vascular and renal malfunction, which in turn alters MAP, heart rate and cardiovascular homeostasis as a whole. In the present study the lack of MAP increase in D7A rats might be also explained by malfunctioning pressor responses, as it was also seen in several other experiments using animal models of type 1 DM (Yu & McNeill, 1992; Fitzgerald & Brands, 2000; Ishikawa et al. 2004). This phenomenon can be detected as early as 3 weeks (Nagareddy et al. 2005), or as late as 7 weeks after the induction of DM, as it was seen in our experiment. However, the main aim of our experiments was not to differentiate the direct and indirect effects of ANGII on MAP. This would need further studies, considering the fact that elevated MAP has also direct effect on kidney function, maybe through up-regulated AT1 receptors, especially in diabetes (Sechi et al. 1994; Yoo et al. 2007).
Since the majority of filtered sodium is being reabsorbed by NKA in the proximal tubule, alterations of NKA may play a key role in the development of impaired renal Na+ handling. Previous findings on intestinal (Wild et al. 1999) and kidney tissues (Scherzer & Popovtzer, 2002) have reported an enhanced NKA α-1 mRNA and protein expression in linear correlation with enzyme activity in STZ-diabetic animals; however, they have not investigated the effect of ANGII. Another study demonstrated that elevated ANGII level increased NKA mRNA expression in hyperinsulinaemic diabetic rat aortas. Furthermore, ANGII administration made this effect even more pronounced (Kobayashi et al. 2006); however, protein level and enzyme activity have not been measured.
Our study is unique in that we investigated both the effect of untreated STZ-diabetes leading to diabetic nephropathy in combination with acute RAS activation referred by ANGII administration on renal NKA. We found that STZ-diabetes and ANGII increased NKA α-1 subunit mRNA expression and protein level, as well as the enzyme activity in renal cortex. Moreover, ANGII treatment had an additive effect on NKA in STZ-diabetic rats. One can hypothesize that the increased NKA expression and enzyme activity in the diabetic state and/or ANGII-related RAS activation may involve alterations in transcriptional and post-transcriptional events, and may represent an adaptive response to increased filtered and delivered load of Na+ to the tubular epithelial cells.
Previously, a transcriptional Na+ response mechanism has been characterized, defining a positive Na+ response regulatory region in the NKA α-1 genes (Azuma et al. 1993). In vascular smooth muscles, ANGII-stimulated α-1 gene transcription is mediated through phosphatidylinositol-3 kinase and MAPK signalling (Isenovic et al. 2004). Therefore, it is also conceivable that similar mechanisms might be responsible for the ANGII effect in the kidney as well; however, the molecular basis should be further investigated.
In agreement with several earlier reports in the diabetic kidney (Wald & Popovtzer, 1984; Khadouri et al. 1987; Marwaha et al. 2004), we found higher NKA α-1 protein level and enzyme activity in the renal cortical tissue homogenate of STZ-diabetic rats and also after ANGII treatment. Since the absolute protein amount of cytoskeleton-representing fraction did not change significantly, one can postulate that the elevated NKA α-1 protein level observed in the total renal cortical tissue homogenate is rather a result of the higher level in the cytosol-representing soluble fraction. Our results demonstrate that both DM and ANGII promote NKA α-1 subunit translocation from the cytoskeletal fraction into the cytosol, as reflected by the DR/DS ratio. However, it is also conceivable that the newly synthesized NKA α-1 subunits have not yet reached their physiological destination on the basolateral surface of proximal tubular cells and this might be a reason for their presence in the cytosol. All these data suggest that even though the total expression and enzyme activity can be increased under experimental circumstances, the membrane-associated pump does not necessarily follow these changes.
Besides the protein amount, phosphorylation takes a major part in the regulation of NKA enzyme activity and subcellular distribution. Previous studies demonstrated that in the proximal tubule ANGII action is mediated by PKC (Rangel et al. 2001; Rangel et al. 2002). The Ser23 phosphorylation site is within a PKC motif in the NH2 terminus of α-1 and it is not phosphorylated by any other known kinases (Feschenko & Sweadner, 1995). Recent data indicate that phosphorylation of the NKA α1 subunit at Ser18 does not alter NKA activity per se, but provides the signal for the removal of NKA from the plasma membrane and endocytosis into intracellular compartments (Chibalin et al. 1999; Lecuona et al. 2006). In accordance with these data, we found a marked increment in Ser23 phosphorylation of the cytosolic α-1 subunit in both ANGII-treated and STZ-diabetic rats, indicating that the translocated NKA protein is also phosphorylated. On the contrary, no signal has been detected in the cytoskeleton-associated detergent resistant fraction.
It is conceivable that PKC in addition to directly phosphorylating NKA also phosphorylates other proteins that interact with NKA, thus altering its activity. Since the modulation of NKA by PKC is complex, the exact role of Ser23 still remains elusive (Andersson et al. 2004).
We confirmed the altered subcellular distribution of NKA also by confocal microscopy. In control kidneys, NKA was localized on the basolateral surface of most tubules. ANGII-treated control kidneys showed increased staining in the cytoplasm which coincided with the lack of distinct basolateral membrane in-folding. In DM, parallel with typical histological picture of diabetic nephropathy, NKA translocation was even more pronounced. ANGII treatment in DM rats led to superimposed kidney damage with markedly increased staining and altered subcellular distribution. On the basis of our results, it can be hypothesized that DM, which also changes systemic and locally produced ANGII level, provokes trafficking of NKA from the basolateral membrane into the cytoplasm of proximal tubular cells. Literary data report that ANGII stimulates rapid subcellular trafficking of other Na+ channel in rat kidney further support our hypothesis (Sandberg et al. 2007); however, the question still remains as to how the translocation of NKA is controlled.
Caveolins might have a relevant role in intracellular trafficking, especially in DM: caveolin-1 and caveolae may play a dual role in the regulation of glucose homeostasis, both through a direct interaction between caveolin-1 and the insulin receptor, and as an agent for GLUT4-mediated glucose uptake (Cohen et al. 2003). Several other transporters and receptors, including angiotensin receptor 1, and NKA can be found associated to caveolae (Cohen et al. 2003; Wyse et al. 2003; Liu et al. 2002). Caveolin-dependent internalization of NKA might modulate NKA activity by regulating subcellular localization and providing a microdomain to protein–lipid or protein–protein interaction (Liu et al. 2002; Komers et al. 2006) Previous studies showed a reduced caveolin-1 protein level both in ANGII-treated and diabetic animals (Kobayashi et al. 2001). Based on all these observations one can also postulate that the reduced caveolin-1 level in diabetic animals might lead to altered integration of transmembrane proteins and result in impaired subcellular trafficking and activity.
In summary, our study demonstrates that: (i) DM increases the mRNA expression, protein level and enzyme activity of renal NKA, and is further elevated by ANGII treatment; (ii) Ser23 phosphorylation of the NKA α-1 subunit is higher in both DM and ANGII-treated animals, which might result in enzyme translocation and internalization from the basolateral membrane of proximal tubular cells; (iii) ANGII potentates the effect of DM on renal NKA, which underlines the relevance of RAS blockade in diabetes therapy. This beneficial effect might be in connection with the ‘conservation’ of the physiological localization of NKA on the basolateral membrane of renal proximal tubules.
Owing to improving glycaemic control and stricter follow-up of diabetic patients, such high glucose levels – seen in our study – are fortunately not that common nowadays. However, it still occurs in patients of untreated or uncontrolled type 1 DM, especially in adolescents. Furthermore, due to non-compliance or dietary mistakes, also old, obese, diabetic patients with hypertension and atherosclerosis often have acute hyperglycaemia, combined with acute RAS activation caused by, for example, myocardial ischaemia (angina, infarction or arrhythmias). Our experimental model provides data supporting another mechanism by which ANGII blockade is likely to be further protective in clinical situations of acute RAS activation in diabetic patients.
Acknowledgments
This work was supported by grants from The Hungarian Scientific Research Fund (OTKA) F048842-F68638, ETT 291-225/2006 and the Semmelweis Foundation. A.F. is a recipient of a Magyary, and A.F. and A.J.S. of a Bolyai scholarship. We are grateful to Professor Istvan Wittmann for his kind help by establishing the immunfluroescence staining, to Tunde Visnyei and Attila Cselenyak for technical assistance, and to N. Moningka and G. F. Chen for the careful reading of this manuscript.
References
- American Diabetes Association. Standards of medical care in diabetes (Position Statement) Diabetes Care. 2005;28:S4–S36. [PubMed] [Google Scholar]
- Andersson RM, Aizman O, Aperia A, Brismar H. Modulation of Na+,K+-ATPase activity is of importance for RVD. Acta Physiol Scand. 2004;180:329–334. doi: 10.1111/j.1365-201X.2003.01256.x. [DOI] [PubMed] [Google Scholar]
- Aufricht C, Bidmon B, Ruffingshofer D, Regele H, Herkner K, Siegel NJ, Kashgarian M, Van Why S. Ischemic conditioning prevents Na,K-ATPase dissociation from the cytoskeletal cellular fraction after repeat renal ischemia in rats. Pediatr Res. 2002;51:722–727. doi: 10.1203/00006450-200206000-00010. [DOI] [PubMed] [Google Scholar]
- Azuma KK, Hensley CB, Tang MJ, McDonough AA. Thyroid hormone specifically regulates skeletal muscle Na+-K+-ATPase alpha 2- and beta 2-isoforms. Am J Physiol Cell Physiol. 1993;265:C680–C687. doi: 10.1152/ajpcell.1993.265.3.C680. [DOI] [PubMed] [Google Scholar]
- Bertuccio CA, Arrizurieta EE, Ibarra FR, Martín RS. Mechanisms of PKC-dependent Na+ K+ ATPase phosphorylation in the rat kidney with chronic renal failure. Ren Fail. 2007;29:13–22. doi: 10.1080/08860220601038496. [DOI] [PubMed] [Google Scholar]
- Beutler KT, Masilamani S, Turban S, Nielsen J, Brooks HL, Ageloff S, Fenton RA, Packer RK, Knepper MA. Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension. 2003;41:1143–1150. doi: 10.1161/01.HYP.0000066129.12106.E2. [DOI] [PubMed] [Google Scholar]
- Bidmonn B, Endemann M, Mueller T, Arbeiter K, Herkner K, Aufricht C. HSP-70 repairs tubule cell structure after renal ischemia. Kidney Int. 2000;58:2400–2407. doi: 10.1046/j.1523-1755.2000.00423.x. [DOI] [PubMed] [Google Scholar]
- Blüher M, Kratzsch J, Paschke R. Plasma levels of tumor necrosis factor-alpha, angiotensin II, growth hormone, and IGF-I are not elevated in insulin-resistant obese individuals with impaired glucose tolerance. Diabetes Care. 2001;24:328–334. doi: 10.2337/diacare.24.2.328. [DOI] [PubMed] [Google Scholar]
- Burns KD, Homma T, Harris RC. The intrarenal renin-angiotensin system. Semin Nephrol. 1993;13:13–30. [PubMed] [Google Scholar]
- Campbell DJ, Kelly DJ, Wilkinson-Berka JL, Cooper ME, Skinner SL. Increased bradykinin and ‘normal’ angiotensin peptide levels in diabetic Sprague-Dawley and transgenic (mRen-2)27 rats. Kidney Int. 1999;56:211–221. doi: 10.1046/j.1523-1755.1999.00519.x. [DOI] [PubMed] [Google Scholar]
- Chibalin AV, Ogimoto G, Pedemonte CH, Pressley TA, Katz AI, Féraille E, Berggren PO, Bertorello AM. Dopamine-induced endocytosis of Na+,K+-ATPase is initiated by phosphorylation of Ser-18 in the rat alpha subunit and is responsible for the decreased activity in epithelial cells. J Biol Chem. 1999;274:1920–1927. doi: 10.1074/jbc.274.4.1920. [DOI] [PubMed] [Google Scholar]
- Cohen AW, Combs TP, Scherer PE, Lisanti MP. Role of caveolin and caveolae in insulin signaling and diabetes. Am J Physiol Endocrinol Metab. 2003;285:E1151–E1160. doi: 10.1152/ajpendo.00324.2003. [DOI] [PubMed] [Google Scholar]
- Efendiev R, Budu CE, Cinelli AR, Bertorello AM, Pedemonte CH. Intracellular Na+ regulates dopamine and angiotensin II receptors availability at the plasma membrane and their cellular responses in renal epithelia. J Biol Chem. 2003;78:28719–28726. doi: 10.1074/jbc.M303741200. [DOI] [PubMed] [Google Scholar]
- Fekete A, Vannay A, Ver A, Vasarhelyi B, Muller V, Ouyang N, Reusz G, Tulassay T, Szabo AJ. Sex differences in the alterations of Na+,K+-ATPase following ischaemia-reperfusion injury in the rat kidney. J Physiol. 2004;555:471–480. doi: 10.1113/jphysiol.2003.054825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Féraille E, Doucet A. Sodium-potassium-adenosinetriphosphatase-dependent sodium transport in the kidney: hormonal control. Physiol Rev. 2001;81:345–418. doi: 10.1152/physrev.2001.81.1.345. [DOI] [PubMed] [Google Scholar]
- Feschenko MS, Sweadner KJ. Structural basis for species-specific differences in the phosphorylation of Na,K-ATPase by protein kinase C. J Biol Chem. 1995;270:14072–14077. doi: 10.1074/jbc.270.23.14072. [DOI] [PubMed] [Google Scholar]
- Fitzgerald SM, Brands MW. Nitric oxide may be required to prevent hypertension at the onset of diabetes. Am J Physiol Endocrinol Metab. 2000;279:E762–E768. doi: 10.1152/ajpendo.2000.279.4.E762. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Villalobos RA, Seth DM, Satou R, Horton H, Ohashi N, Miyata K, Katsurada A, Tran DV, Kobori H, Navar LG. Intrarenal angiotensin II and angiotensinogen augmentation in chronic angiotensin II-infused mice. Am J Physiol Renal Physiol. 2008;295:F772–F779. doi: 10.1152/ajprenal.00019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakam AC, Siddiqui AH, Hussain T. Renal angiotensin II AT2 receptors promote natriuresis in streptozotocin-induced diabetic rats. Am J Physiol Renal Physiol. 2006;290:F503–F508. doi: 10.1152/ajprenal.00092.2005. [DOI] [PubMed] [Google Scholar]
- Hollenberg NK, Stevanovic R, Agarwal A, Lansang MC, Price DA, Laffel LM, Williams GH, Fisher ND. Plasma aldosterone concentration in the patient with diabetes mellitus. Kidney Int. 2004;65:1435–1439. doi: 10.1111/j.1523-1755.2004.00524.x. [DOI] [PubMed] [Google Scholar]
- Isenovic ER, Meng Y, Jamali N, Milivojevic N, Sowers JR. Ang II attenuates IGF-1 stimulated Na+,K+-ATPase activity via PI3K/Akt pathway in vascular smooth muscle cells. Int J Mol Med. 2004;13:915–922. [PubMed] [Google Scholar]
- Ishikawa T, Kohno F, Kawase R, Yamamoto Y, Nakayama K. Contribution of nitric oxide produced by inducible nitric oxide synthase tovascular responses of mesenteric arterioles in streptozotocin-diabetic rats. Br J Pharmacol. 2004;141:269–276. doi: 10.1038/sj.bjp.0705611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadouri C, Barlet-Bas C, Doucet A. Mechanism of increased tubular Na-K-ATPase during streptozotocin-induced diabetes. Pflugers Arch. 1987;409:296–301. doi: 10.1007/BF00583479. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Hayashi Y, Taguchi K, Matsumoto T, Kamata K. ANG II enhances contractile responses via PI3-kinase p110 pathway in aortas from diabetic rats with systemic hyperinsulinemia. Am J Physiol Heart Circ Physiol. 2006;291:H846–H853. doi: 10.1152/ajpheart.01349.2005. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Mori Y, Nakano S, Tsubokou Y, Kobayashi T, Shirataki H, Matsuoka H. TCV116 stimulates eNOS and caveolin-1 expression and improves coronary microvascular remodeling in normotensive and angiotensin II-induced hypertensive rats. Atherosclerosis. 2001;158:359–368. doi: 10.1016/s0021-9150(01)00458-0. [DOI] [PubMed] [Google Scholar]
- Komers R, Schutzer WE, Reed JF, Lindsley JN, Oyama TT, Buck DC, Mader SL, Anderson S. Altered endothelial nitric oxide synthase targeting and conformation and caveolin-1 expression in the diabetic kidney. Diabetes. 2006;55:1651–1659. doi: 10.2337/db05-1595. [DOI] [PubMed] [Google Scholar]
- Lal MA, Körner A, Matsuo Y, Zelenin S, Cheng SXJ, Jaremko G, DiBona GF, Eklof AC, Aperia A. Combined antioxidant and COMT inhibitor treatment reverses renal abnormalities in diabetic rats. Diabetes. 2000;49:1381–1389. doi: 10.2337/diabetes.49.8.1381. [DOI] [PubMed] [Google Scholar]
- Lecuona E, Dada LA, Sun H, Butti ML, Zhou G, Chew TL, Sznajder JI. Na,K-ATPase α1-subunit dephosphorylation by protein phosphatase 2A is necessary for its recruitment to the plasma membrane. FASEB J. 2006;20:2618–2620. doi: 10.1096/fj.06-6503fje. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Lee YJ, Han HJ. Regulatory mechanisms of N+/glucose cotransporters in renal proximal tubule cells. Kidney Int. 2007;106:S27–S35. doi: 10.1038/sj.ki.5002383. [DOI] [PubMed] [Google Scholar]
- Leong PK, Devillez A, Sandberg MB, Yang LE, Yip DK, Klein JB, McDonough AA. Effects of ACE inhibition on proximal tubule sodium transport. Am J Physiol Renal Physiol. 2006;290:F854–F863. doi: 10.1152/ajprenal.00353.2005. [DOI] [PubMed] [Google Scholar]
- Liu P, Rudick M, Anderson RG. Multiple functions of caveolin-1. J Biol Chem. 2002;277:41295–41298. doi: 10.1074/jbc.R200020200. [DOI] [PubMed] [Google Scholar]
- Logvinenko NS, Dulubova I, Fedosova N, Larsson SH, Nairn AC, Esmann M, Greengard P, Aperia A. Phosphorylation by protein kinase C of serine-23 of the α1 subunit of rat Na+,K+-ATPase affects its conformational equilibrium. Proc Nat Acad Sci USA. 1996;93:9132–9137. doi: 10.1073/pnas.93.17.9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwaha A, Banday A, Lokhandwala M. Reduced renal dopamine D1 receptor function in streptozotocin-induced diabetic rats. Am J Physiol Renal Physiol. 2004;286:F451–F457. doi: 10.1152/ajprenal.00227.2003. [DOI] [PubMed] [Google Scholar]
- Miller JA. Impact of hyperglycemia on the renin angiotensin system in early human type 1 diabetes mellitus. J Am Soc Nephrol. 1999;10:1778–1785. doi: 10.1681/ASN.V1081778. [DOI] [PubMed] [Google Scholar]
- Nagareddy PR, Xia Z, McNeill JH, MacLeod KM. Increased expression of iNOS is associated with endothelial dysfunction and impaired pressor responsiveness in streptozotocin-induced diabetes. Am J Physiol Heart Circ Physiol. 2005;289:H2144–H2152. doi: 10.1152/ajpheart.00591.2005. [DOI] [PubMed] [Google Scholar]
- Ng YC, Tolerico PH, Book CB. Alterations in levels of Na+-K+-ATPase isoforms in heart, skeletal muscle, and kidney of diabetic rats. Am J Physiol Endocrinol Metab. 1993;265:E243–E251. doi: 10.1152/ajpendo.1993.265.2.E243. [DOI] [PubMed] [Google Scholar]
- Rangel LB, Caruso-Neves C, Lara LS, Lopes AG. Angiotensin II stimulates renal proximal tubule Na+-ATPase activity through the activation of protein kinase C. Biochim Biophys Acta. 2002;1564:310–316. doi: 10.1016/s0005-2736(02)00472-8. [DOI] [PubMed] [Google Scholar]
- Rangel LB, Malaquias AT, Lara LS, Silva IV, De Souza AM, Lopes AG, Caruso-Neves C. Protein kinase C-induced phosphorylation modulates the Na+-ATPase activity from proximal tubules. Biochim Biophys Acta. 2001;1512:90–97. doi: 10.1016/s0005-2736(01)00305-4. [DOI] [PubMed] [Google Scholar]
- Ruzicska E, Foldes G, Lako-Futo Z, Sarman B, Wellmann J, Szenasi G, Tulassay Zs Ruskoaho H, Toth M, Somogyi A. Cardiac gene expression of natriuretic substances is altered in streptozotocin-induced diabetes during angiotensin II-induced pressure overload. J Hypertens. 2004;22:1191–1200. doi: 10.1097/00004872-200406000-00021. [DOI] [PubMed] [Google Scholar]
- Sandberg MB, Riquier ADM, Pihakaski-Maunsbach K, McDonough AA, Maunsbach AB. ANG II provokes acute trafficking of distal tubule Na+-Cl cotransporter to apical membrane. Am J Physiol Renal Physiol. 2007;293:F662–F669. doi: 10.1152/ajprenal.00064.2007. [DOI] [PubMed] [Google Scholar]
- Scherzer P, Popovtzer MM. Segmental localization of mRNAs encoding Na+-K+-ATPase α1- and β1-subunits in diabetic rat kidneys using RT-PCR. Am J Physiol Renal Physiol. 2002;282:F492–F500. doi: 10.1152/ajprenal.00053.2001. [DOI] [PubMed] [Google Scholar]
- Sechi LA, Griffin CA, Schambelan M. The cardiac renin-angiotensin system in STZ-induced diabetes. Diabetes. 1994;43:1180–1184. doi: 10.2337/diab.43.10.1180. [DOI] [PubMed] [Google Scholar]
- Shah S, Hussain T. Enhanced angiotensin II-induced activation of Na+,K+-ATPase in the proximal tubules of obese Zucker rats. Clin Exp Hypertens. 2006;28:29–40. doi: 10.1080/10641960500386650. [DOI] [PubMed] [Google Scholar]
- Siddiqui A, Hussain T. Impaired angiotensin II AT1 receptor function and enhanced Na,K-ATPase affinity for sodium in proximal tubule of streptozotocin-treated diabetic rats. Clin Exp Hypertens. 2007;29:435–444. doi: 10.1080/10641960701615659. [DOI] [PubMed] [Google Scholar]
- Sweadner KJ. Isozymes of the Na+/K+-ATPase. Biochim Biophys Acta. 1989;988:185–220. doi: 10.1016/0304-4157(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Tsimarato M, Coste TC, Djemli-Shipkolye A, Daniel L, Shipkolye F, Vague P, Raccah D. Evidence of time-dependent changes in renal medullary Na,K-ATPase activity and expression in diabetic rats. Cell Mol Biol. 2001;47:239–245. [PubMed] [Google Scholar]
- Vallon V, Richter K, Blantz RC, Thomson S, Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol. 1999;10:2569–2576. doi: 10.1681/ASN.V10122569. [DOI] [PubMed] [Google Scholar]
- Vallon V, Wead LM, Blantz RC. Renal hemodynamics and plasma and kidney angiotensin II in established diabetes mellitus in rats: effect of sodium and salt restriction. J Am Soc Nephrol. 1995;5:1761–1767. doi: 10.1681/ASN.V5101761. [DOI] [PubMed] [Google Scholar]
- Vér A, Szántó I, Bányász T, Csermely P, Végh E, Somogyi J. Changes in the expression of of Na+/K+-ATPase isoenzymes in left ventricle of diabetic rat hearts: effect of insulin treatment. Diabetologia. 1997;40:1255–1262. doi: 10.1007/s001250050818. [DOI] [PubMed] [Google Scholar]
- Vér A, Szántó I, Csermely P, Kalff K, Végh E, Bányász T, Marcsek Z, Kovács T, Somogyi J. Effect of streptozotocin-induced diabetes on kidney Na+/K+-ATPase. Acta Physiol Hung. 1995;83:323–332. [PubMed] [Google Scholar]
- Vidotti DB, Arnoni CP, Maquigussa E, Boim MA. Effect of long-term type 1 diabetes on renal sodium and water transporters in rats. Am J Nephrol. 2008;28:107–114. doi: 10.1159/000109967. [DOI] [PubMed] [Google Scholar]
- Wald H, Popovtzer MM. The effect of streptozotocin-induced diabetes mellitus on urinary excretion of sodium and renal Na+-K+-ATPase activity. Pflugers Arch. 1984;401:97–100. doi: 10.1007/BF00581539. [DOI] [PubMed] [Google Scholar]
- Wilcox CS, Welch WJ. Angiotensin II and thromboxane in the regulation of blood pressure and renal function. Kidney Int Suppl. 1990;30:S81–S83. [PubMed] [Google Scholar]
- Wild GE, Thompson JA, Searles L, Turner R, Hasan J, Thomson AB. Small intestinal Na+,K+-adenosine triphosphatase activity and gene expression in experimental diabetes mellitus. Dig Dis Sci. 1999;44:407–414. doi: 10.1023/a:1026631207219. [DOI] [PubMed] [Google Scholar]
- Woroniecki R, Ferdinand JR, Morrow JS, Devarajan P. Dissociation of spectrin-ankyrin complex as a basis for loss of Na-K-ATPase polarity after ischemia. Am J Physiol Renal Physiol. 2003;284:F358–F364. doi: 10.1152/ajprenal.00100.2002. [DOI] [PubMed] [Google Scholar]
- Wyse BD, Prior IA, Qian H, Morrow IC, Nixon S, Muncke C, Kurzchalia TV, Thomas WG, Parton RG, Hancock JF. Caveolin interacts with the angiotensin II type 1 receptor during exocytic transport but not at the plasma membrane. J Biol Chem. 2003;278:23738–23746. doi: 10.1074/jbc.M212892200. [DOI] [PubMed] [Google Scholar]
- Xu X, Ha CH, Wong C, Wang W, Hausser A, Pfizenmaier K, Olson EN, McKinsey TA, Jin ZG. Angiotensin II stimulates protein kinase d-dependent histone deacetylase 5 phosphorylation and nuclear export leading to vascular smooth muscle cell hypertrophy. Arterioscler Thromb Vasc Biol. 2007;27:2355–2362. doi: 10.1161/ATVBAHA.107.151704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yingst DR, Massey KJ, Rossi NF, Mohanty MJ, Mattingly RR. Angiotensin II directly stimulates activity and alters the phosphorylation of Na-K-ATPase in rat proximal tubule with a rapid time course. Am J Physiol Renal Physiol. 2004;287:F713–F721. doi: 10.1152/ajprenal.00065.2004. [DOI] [PubMed] [Google Scholar]
- Yoo TH, Li JJ, Kim JJ, Jung DS, Kwak SJ, Ryu DR, Choi HY, Kim JS, Kim HJ, Han SH, Lee JE, Han DS, Kang SW. Activation of the renin-angiotensin system within podocytes in diabetes. Kidney Int. 2007;71:1019–1027. doi: 10.1038/sj.ki.5002195. [DOI] [PubMed] [Google Scholar]
- Yu Z, McNeill JH. Blood pressure and heart rate response to vasoactive agents in conscious diabetic rats. Can J Physiol Pharmacol. 1992;70:1542–1548. doi: 10.1139/y92-221. [DOI] [PubMed] [Google Scholar]
- Zhang YW, Xie D, Chen YX, Zhang HY, Xia ZX. Protective effect of Gui Qi mixture on the progression of diabetic nephropathy in rats. Exp Clin Endocrinol Diabetes. 2006;114:563–568. doi: 10.1055/s-2006-948307. [DOI] [PubMed] [Google Scholar]
- Zimpelmann J, Kumar D, Levine DZ, Wehbi G, Imig JD, Navar LG, Burns KD. Early diabetes mellitus stimulates proximal tubule renin mRNA expression in the rat. Kidney Int. 2000;58:2320–2330. doi: 10.1046/j.1523-1755.2000.00416.x. [DOI] [PubMed] [Google Scholar]