Abstract
Endothelial cells can induce contractions of the underlying vascular smooth muscle by generating vasoconstrictor prostanoids (endothelium-dependent contracting factor; EDCF). The endothelial COX-1 isoform of cyclooxygenase appears to play the dominant role in the phenomenon. Its activation requires an increase in intracellular Ca2+ concentration. The production of EDCF is inhibited acutely and chronically by nitric oxide (NO), and possibly by endothelium-dependent hyperpolarizing factor (EDHF). The main prostanoids involved in endothelium-dependent contractions appear to be endoperoxides (PGH2) and prostacyclin, which activate thromboxane-prostanoid (TP) receptors of the vascular smooth muscle cells. Oxygen-derived free radicals can facilitate the production and/or the action of EDCF. Endothelium-dependent contractions are exacerbated by ageing, obesity, hypertension and diabetes, and thus are likely to contribute to the endothelial dysfunction observed in older people and in essential hypertensive patients.
Besides playing an essential role in vasodilator responses by releasing endothelium-derived relaxing factor(s) (EDRF(s)) (Furchgott & Zawadzki, 1980), the endothelial cells of certain arteries and veins can also initiate contractions of the vascular smooth muscle that surrounds them (De Mey & Vanhoutte, 1982, 1983). Bioassay studies demonstrated that the transfer of diffusible factors is involved in such endothelium-dependent contractions (Rubanyi & Vanhoutte, 1985; Iqbal & Vanhoutte, 1988; Yang et al. 2003). Theoretically, endothelium-dependent contractions could be explained by either the withdrawal of endothelial inhibitory signals (prostacyclin, nitric oxide (NO), endothelium-derived hyperpolarizing factor (EDHF) or the production of vasoconstrictor substances. Over the years, it has become evident that prostanoids, derived from the endothelial cyclooxygenase, explain most endothelium-dependent contractions (see Vanhoutte et al. 2005). Obviously, endothelial cells can produce vasoconstrictor substances other than prostanoids in particular different peptides (Yanagisawa et al. 1988; Dhein et al. 1997; Saifeddine et al. 1998) or the non-peptidic dinucleotide uridine adenosine tetraphosphate (UP4A) (Jankowski et al. 2005). However, it is uncertain whether or not the instantaneous release of these non-prostanoid substances can lead to endothelium-dependent contractions. Thus, the present brief review will focus on cyclooxygenase-derived vasoconstrictor substances (EDCF) initiating endothelium-dependent contractions.
EDCF-mediated responses
Endothelium-dependent contractions to acetylcholine, and other vasoactive substances (e.g. arachidonic acid, ATP, the calcium ionophore A23187), have been reported in a variety of blood vessels from different species (see Furchgott & Vanhoutte, 1989; Lüscher & Vanhoutte, 1990; Vanhoutte et al. 2005).
The source of EDCF
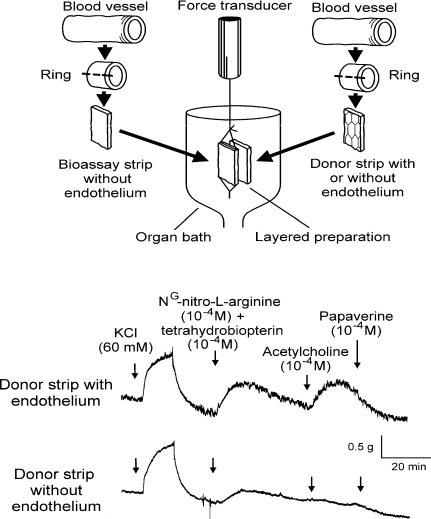
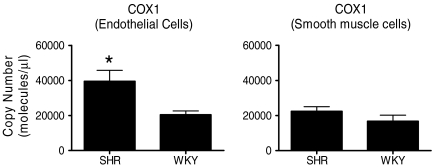
The endothelium-dependent contractions of canine veins to arachidonic acid were prevented by non-selective inhibitors of cyclooxygenase (e.g. indomethacin), as were those evoked by acetylcholine in the canine basilar artery or the aorta of the spontaneously hypertensive rat (SHR) (Miller & Vanhoutte, 1985; Lüscher & Vanhoutte, 1986; Katusic et al. 1988). This demonstrated the key role of the metabolism of arachidonic acid into prostanoids in the genesis of endothelium-dependent contractions (see Vanhoutte et al. 2005). Bioassay studies revealed that it is mainly the cyclooxygenase of the endothelial cells, rather than that of the vascular smooth muscle which is responsible (Fig. 1) (Yang et al. 2003). Studies in the SHR aorta using preferential and selective inhibitors of the two isoforms of the enzyme (cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2)) and molecular biology techniques (Fig. 2), as well as experiments in the aorta of genetically modified mice prompted the conclusion that the constitutive isoform, COX-1, plays the key role in the occurrence of endothelium-dependent contractions in those blood vessels (Ge et al. 1995; Traupe et al. 2002; Yang et al. 2003; Tang et al. 2005a; Gluais et al. 2006). However, in blood vessels where endothelial COX-2 is present, the prostanoids generated by this isoform can contribute to EDCF-mediated contractions (Camacho et al. 1998; Zerrouk et al. 1998; Garcia-Cohen et al. 2000; Álvarez et al. 2005; Blanco-Rivero et al. 2005; Hirao et al. 2008; Shi & Vanhoutte, 2008).
Figure 1.
Upper panel: a donor strip is stitched onto the bioassay tissue creating a ‘sandwich’-like layered preparation. Isometric tension is recorded from the bioassay strip and the donor tissue does not directly contribute to the recorded response. Lower panel: acetylcholine-induced contractions only occurred when the donor strip contained endothelium. The experiment was performed in the presence of nitro-l-arginine and tetrahydrobiopterin to optimize the EDCF-mediated response (reproduced from Vanhoutte et al. 2005, with permission).
Figure 2.
The mRNA expression of COX-1, measured by RT-PCR in freshly isolated endothelial cells, was significantly higher in 36-week-old SHR compared to 36-week-old WKY (n = 6). There was no difference in gene expression of COX-1 in smooth muscle cells between 36-week-old WKY and SHR (n = 8). Data are means ±s.e.m.*P < 0.05 (data from Tang & Vanhoutte, 2008b, reproduced with permission).
Pivotal role of TP receptors
Most cyclooxygenasedependent, endothelium-dependent contractions are abolished by TP-receptor antagonists (Tesfamariam et al. 1989; Auch-Schwelk et al. 1990; Kato et al. 1990; Mayhan, 1992; Yang et al. 2002, 2003; Zhou et al. 2005). Bioassay experiments demonstrate that the TP receptors involved are those located in the vascular smooth muscle cells (Yang et al. 2003). The contraction of the latter upon TP-receptor activation is due to the combination of an increased entry of Ca2+ resulting from the opening of both receptor-operated and voltage-gated Ca2+ channels and Rho-kinase-mediated sensitization of the myofilaments (Okon et al. 2002; Huang et al. 2004; O'Rourke et al. 2006).
Impact of ageing
Endothelium-dependent contractions become more prominent in arteries of older, compared to younger animals (Koga et al. 1989; Iwama et al. 1992; Abeywardena et al. 2002). This increased response is accompanied by an increased expression of COX-1 (Tang & Vanhoutte, 2008b). When COX-2 is induced by the ageing process, this isoform of the enzyme can contribute in part to endothelium-dependent contractions (Shi et al. 2008). The ability of prostacyclin to induce relaxation is lost in the aorta of 15-week-old and older WKY (Levy, 1980; Rapoport & Williams, 1996; Gluais et al. 2005). This inability is due to a dysfunction of the IP receptors, since the relaxation to isoproterenol (isoprenaline; a β-adrenoceptor agonist which also evokes cAMP-dependent dilatations) is maintained in those arteries (Rapoport & Williams, 1996).
Hallmark of vascular disease
Spontaneous hypertension. The endothelium-dependent relaxations to acetylcholine are blunted in the aorta of the SHR, and this is due to the concomitant release of EDCF rather than a reduced production of EDRF (Lockette et al. 1986; Lüscher & Vanhoutte, 1986; Lüscher et al. 1987b). The endothelium-dependent contractions to acetylcholine are more pronounced in the quiescent aorta of the adult SHR than in that of normotensive Wistar-Kyoto rats (WKY) (Lüscher & Vanhoutte, 1986) and this is accompanied by an increased expression/presence of COX-1 in the endothelial cells (Ge et al. 1995; Tang & Vanhoutte, 2008b). The overexpression of COX-1 is not observed in aortae of pre-hypertensive SHR, while the isoform is more prominent in arteries from ageing normotensive rats (Ge et al. 1999; Tang & Vanhoutte, 2008b). These findings then prompt the conclusion that the overexpression of COX-1 in arteries from adult hypertensive rats reflects a premature ageing of the endothelium rather than a genetic predisposition. The mRNA and protein expression of TP receptors do not differ between aortae of WKY and SHR (Tang & Vanhoutte, 2008b; Tang et al. 2008), indicating that alteration in their density is not a contributing factor in the augmented endothelium-dependent contractions observed in the aorta of the hypertensive rat. Despite the unaltered density of TP receptors, the aorta of the SHR is hyper-responsive to the vasoconstrictor effect of endoperoxides (Ge et al. 1995). This hyper-responsiveness is present early on in the hypertensive strain and is thus not a consequence of the chronic exposure of the vascular wall to the high arterial blood pressure (Ge et al. 1999).
Obesity and diabetes. Obesity potentiates the occurrence of EDCF-mediated responses in mouse arteries, possibly because of an up-regulation of the expression of TP receptors (Traupe et al. 2002; Gollasch, 2002). The endothelium-dependent relaxations to acetylcholine are blunted in a number of arteries from diabetic animals (see Tesfamariam, 1994; De Vriese et al. 2000). This is due in part to the concomitant release of EDCF and can be attributed to the exposure of the endothelial cells to high glucose, resulting in increased oxidative stress and overexpression of both COX-1 and COX-2 (Tesfamariam et al. 1990, 1991; Xu et al. 2006; Shi et al. 2007a,b, 2008; Michel et al. 2008b; Shi & Vanhoutte, 2008).
The nature of EDCF
When prostacyclin turns bad. Cyclooxygenase transforms arachidonic acid into endoperoxides which per se cause contraction of vascular smooth muscle. Indeed, endoperoxides are released during endothelium-dependent contractions of the SHR aorta and thus can be regarded as EDCF (Ito et al. 1991; Asano et al. 1994; Ge et al. 1995; Vanhoutte et al. 2005; Hirao et al. 2008). Endoperoxides are converted further into prostacyclin, thromboxane A2, prostaglandin D2, prostaglandin E2 and/or prostaglandin F2α by their respective synthases (Bos et al. 2004). Of those enzymes, the prostacyclin synthase gene is by far the most abundantly expressed in endothelial cells, and more so in the SHR than in the WKY endothelium (Tang & Vanhoutte, 2008b). The protein expression of the enzyme augments with age and by hypertension (Numaguchi et al. 1999). Acetylcholine causes a greater release of prostacyclin in the aorta of SHR than in that of the WKY (Gluais et al. 2005). The prostanoid no longer evokes relaxations in arteries from ageing or hypertensive rats, and induces larger contractions in the latter (Rapoport & Williams, 1996; Gluais et al. 2005). These are the main reasons to accept that, in the SHR aorta, endoperoxides and prostacyclin are the main mediators of the endothelium-dependent contractions evoked by acetylcholine (Ge et al. 1995; Blanco-Rivero et al. 2005; Gluais et al. 2005). In other blood vessels, or even in the SHR aorta exposed to other agonists (ADP, A23187, endothelin-1, nicotine), thromboxane A2 may contribute (Katusic et al. 1988; Shirahase et al. 1988; Auch-Schwelk & Vanhoutte, 1992; Taddei & Vanhoutte, 1993; Gluais et al. 2006, 2007). The contribution of prostaglandin E2 and prostaglandin F2α to endothelium-dependent contractions is marginal in most cases. However, when prostacyclin synthase is inhibited (pharmacologically or by peroxynitrite-dependent tyrosine nitration), or after photochemical endothelial injury, these two prostanoids can contribute to EDCF-mediated responses (Zou et al. 1999, 2002; Bachschmid et al. 2003; Gluais et al. 2005; Hirao et al. 2008).
Calcium, the trigger for release
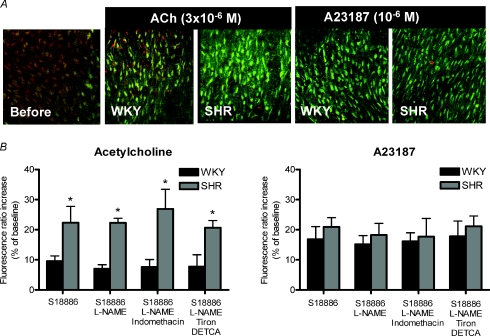
In certain vascular beds, a tonic release of EDCF may participate in the regulation of vasomotor tone (Iwatani et al. 2008). The release of EDCF can be triggered by vasoactive agonists acting at the cell membrane, such as acetylcholine (activating endothelial M3-muscarinic receptors (Boulanger et al. 1994) or ADP (activating purinoceptors; Koga et al. 1989; Mombouli & Vanhoutte, 1993). Endothelium-dependent contractions of basilar arteries also can be elicited by sudden stretch (Katusic et al. 1987), which raises the possibility of a role in autoregulation of the cerebral circulation. Endothelium-dependent contractions are reduced when the external Ca2+ concentration is lowered, and can be evoked by calcium ionophores (Katusic et al. 1988; Okon et al. 2002; Gluais et al. 2006; Shi et al. 2007a,b, 2008; Tang et al. 2007). They are accompanied by an increase in endothelial cytosolic Ca2+ concentration (Fig. 3) (Tang et al. (2007). The increase in intracellular endothelial Ca2+ concentration caused by acetylcholine is greater in the aorta of the SHR than in that of the WKY, which is in line with the absence of endothelium-dependent contraction in the latter (Tang et al. 2007). By contrast, the increase in Ca2+ concentration is comparable in endothelial cells of the two strains when exposed to A23187, which causes contractions in aortae of both SHR and WKY (Tang et al. 2007). These observations suggest that the increase in intracellular Ca2+ concentration is the initial trigger for endothelium-dependent contractions. The increased Ca2+ then presumably activates phospholipase A2 which makes arachidonic acid available for metabolism by the endothelial cyclooxygenase.
Figure 3.
A, representative merged images taken by confocal microscopy showing responses to infusion of acetylcholine (3 × 10−6m) or A23187 (10−6m) on cytosolic calcium of aortic endothelial cells from WKY and SHR. The addition of acetylcholine caused a rapid increase in intracellular calcium in aortic endothelial cells of both WKY and SHR (indicated by an increase in green fluorescence and a decrease of red fluorescence), which was greater in the latter. The calcium ionophore A23187 caused a comparable increase in intracellular calcium in preparations from WKY and SHR. B, the increase in fluorescence ratio in endothelial cells from WKY and SHR in response to acetylcholine (3 × 10−6m) (left) or A23187 (10−6m) (right) is expressed in percentages of the baseline values. Data are shown as means ±s.e.m.; n = 5. *P < 0.05 WKY versus SHR. Acetylcholine caused greater calcium increase in aortic endothelial cells of SHR than WKY, while A23187 caused a comparable response in the two strains. The increase of calcium was not affected by treatment with indomethacin, tiron plus diethyldithlocarbonate acid (DETCA; an inhibitor of superoxide dimutase) or NG-nitro-l-argine methylestes (L-NAME; an inhibitor of nitric oxide synthase) (reproduced from Tang et al. 2007, with permission). The experiment was performed in the presence of S18886, a TP-receptor antagonist, to prevent contraction of the smooth muscle.
Modulation by EDRF(s)
Many blood vessels exhibit a basal release of NO, which is augmented by increases in shear stress (Rubanyi et al. 1986). Hence, it is not surprising that if their smooth muscle possesses myogenic tone or is contracted by vasoconstrictor agents, a sudden reduction in the activity of endothelial NO synthase (NOS), for example by the administration of NOS inhibitors, results in endothelium-dependent contractions in vitro or vasoconstrictions in vivo (Rees et al. 1989). Thus, in the intact organism, inhibition of NOS (either by pharmacological agents or by gene deletion) causes an increase in arterial blood pressure (Rees et al. 1989; Huang et al. 1995), although part of the response is due to withdrawal of the inhibitory effect of NO on the release of angiotensin II and endothelin-1 (see Vanhoutte, 2000; Félétou et al. 2008) rather than to the absence of the direct inhibitory effect of the endothelial mediator on vascular smooth muscle cells. Likewise, the continuous presence of signals resulting in EDHF-mediated responses may contribute to vascular tone, and the genetic deletion of these signals may also result in an increase in arterial blood pressure (see Félétou & Vanhoutte, 2006a,b; Félétou & Vanhoutte, 2007). In addition, a reduction in the release of EDRF will facilitate or permit the occurrence of endothelium-dependent constrictor responses.
Reduction in NO production. Inhibitors of NOS cause a marked acute potentiation of EDCF-mediated responses of the rat aorta (Auch-Schwelk et al. 1992; Yang et al. 2002). Previous exposure to NO, whether released from the endothelium (by acetylcholine or the calcium ionophore A23187) or provided by NO donors, results in a prolonged inhibition of endothelium-dependent contractions (Tang et al. 2005b). Therefore, most experiments (at least in the authors’ laboratory) investigating EDCF-mediated responses are performed in the presence of an inhibitor of NOS, to optimize endothelium-dependent, cyclooxygenase-dependent contractions. In addition to unmasking EDCF-mediated responses, a reduction in endothelial NO production can sensitize the underlying vascular smooth muscle to hypoxia. When isolated arteries and veins are suddenly made hypoxic, this results in a distinct endothelium-dependent contraction (De Mey & Vanhoutte, 1982, 1983; Katusic & Vanhoutte, 1986; Iqbal & Vanhoutte, 1988; Gräser & Vanhoutte, 1991; Hoshino et al. 1994; Pearson et al. 1996). The hypoxia-induced endothelium-dependent contraction involves a diffusible factor (Rubanyi & Vanhoutte, 1985), which does not require the activity of cyclooxygenase. It is absent in preparations incubated with inhibitors of endothelial NOS but can be induced in preparations without endothelium by exogenous NO donors (Gräser & Vanhoutte, 1991; Pearson et al. 1996), which suggests the involvement of a critical concentration of NO. The hypoxic response of coronary arteries is potentiated in vitro and in vivo by previous ischaemia–reperfusion injury (Pearson et al. 1996) which makes the phenomenon highly relevant as a contributor to coronary vasospasm. However, the exact mechanism by which a reduction in NO production underlies this type of endothelium-dependent contraction remains elusive.
Reduction in EDHF-mediated responses. In the renal artery of WKY, inhibitors of EDHF-mediated responses potentiate the endothelium-dependent component of the contraction elicited by acetylcholine, suggesting that the absence of endothelium-dependent hyperpolarizations favours the production or the action of EDCF (Michel et al. 2008a). This is not seen in the renal artery of the SHR, presumably because the EDHF-mediated responses are already blunted in arteries of the hypertensive strain (Fujii et al. 1992; Hayakawa et al. 1995; Dohi et al. 1996; Hutri-Kahonen et al. 1997; Bussemaker et al. 2003; Michel et al. 2008a).
Modulation by oxygen-derived free radicals
To estimate the actual involvement of oxygen-derived free radicals (ROS) in cyclooxygenase-, endothelium-dependent contractions is beyond the scope of this focused review, as it appears variable depending on the species, the blood vessel and sometimes the laboratory involved. For example, superoxide dismutase (SOD), that does not permeate cells, abolishes endothelium-dependent contractions in the canine basilar artery (implying a pivotal role for superoxide anions as intercellular messengers; Katusic & Vanhoutte, 1989) and reduces them in layered ‘sandwich’ preparations (Yang et al. 2003) but not in intact rings (Auch-Schwelk et al. 1989) of SHR aorta. Tiron, a cell-permeable scavenger of superoxide anions, reduces endothelium-dependent contractions to acetylcholine in the SHR aorta in studies carried out in Paris (Yang et al. 2002) but not in Hong Kong (Tang & Vanhoutte, 2008a). In the same preparation, acetylcholine causes a burst of endothelial free radical production, which is larger in the endothelium of the SHR than in that of the WKY (Tang et al. 2007). Since the burst of ROS is prevented by indomethacin, cyclooxygenase appears to be the main source of free radicals under these conditions, and their production is a secondary event (Tang et al. 2007). However, once produced, the free radicals can amplify the EDCF-mediated response. They probably do so in part by activating/facilitating the production of vasoconstrictor prostanoids in the vascular smooth muscle cells (Auch-Schwelk et al. 1989; Yang et al. 2002, 2003; Álvarez et al. 2008), possibly reaching the latter through the shielded channels constituted by the myo-endothelial gap junctions (Tang & Vanhoutte, 2008a). Whether or not the ROS, liberated by the endothelial cyclooxygenase, can activate the enzyme through a positive feedback mechanism is still uncertain. In the case of diabetes, the production of ROS may play a more crucial role in triggering and amplifying EDCF-mediated responses (Shi et al. 2007b, 2008; Shi & Vanhoutte, 2008). Obviously, the scavenging action of superoxide anions on NO, by reducing the bioavailability of the latter (Rubanyi & Vanhoutte, 1986; Gryglewski et al. 1986; Auch-Schwelk et al. 1992; Cosentino et al. 1994; Tschudi et al. 1996; DeLano et al. 2006; Miyagawa et al. 2007; Macarthur et al. 2008) will also favour the occurrence of endothelium-dependent contractions.
Human relevance
The observations that indomethacin potentiates the relaxations to acetylcholine in isolated renal arteries of aged patients (Lüscher et al. 1987a) and the vasodilator response to the muscarinic agonist in the forearm of people with essential hypertension (Taddei et al. 1995, 1997a,b) suggest that endothelium-derived vasoconstrictor prostanoids also contribute to endothelial dysfunction in the human. This conclusion is supported by the finding that the TP-receptor inhibitor terutroban improves endothelial function in patients with coronary disease (Belhassen et al. 2003). To judge from the comparison of the effect of indomethacin in different age groups, the contribution of vasoconstrictor prostanoid augments with advancing age (Taddei et al. 1995, 1997b), as it does in animal blood vessels.
Conclusion
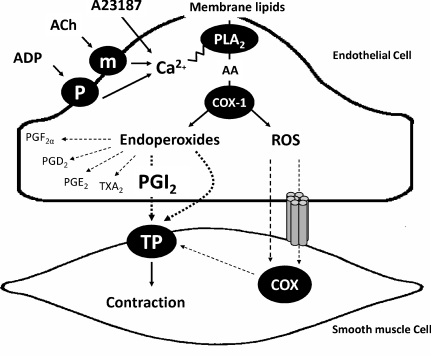
The sequence of events (Fig. 4) that leads to endothelium-dependent contractions first requires an increase in endothelial Ca2+ concentration, which activates endothelial COX-1, leading to the production of EDCF(s). The major prostanoids involved in EDCF-mediated contractions are endoperoxides, prostacyclin and, to a lesser extent, thromboxane A2. They activate TP receptors of the vascular smooth muscle cells which initiate the contractile process. Reactive oxygen species may stimulate cyclooxygenase both in the endothelium and in the vascular smooth muscle, with subsequent activation of the TP receptors by the produced prostanoids. Dysfunction in calcium handling is the leading causal factor for the exacerbated occurrence of endothelium-dependent contractions in the aorta of the SHR. An increased expression of endothelial COX-1, prostacyclin synthase, thromboxane synthase and enhanced TP receptor sensitivity are not prerequisites for but intensify the magnitude of endothelium-dependent contractions.
Figure 4.
The chain of events leading to the occurrence of endothelium-dependent contractions first involves an abnormal increase in intracellular calcium (which can be evoked by receptor-dependent agonists, such as acetylcholine or ADP, or mimicked with calcium-increasing agents, such as the calcium ionophore A23187) that presumably activates phospholipase A2 to release arachidonic acid. The endothelial COX-1 isoform metabolizes the fatty acid into endoperoxides which per se are EDCF or are transformed predominantly into prostacyclin that subsequently causes contraction by activating the TP receptors of the underlying vascular smooth muscle cells. Reactive oxygen species generated in the endothelium may reach the smooth muscle layer by passive diffusion or through myoendothelial gap junctions and serve to amplify TP receptor-mediated contractions by activating the cyclooxygenase of the vascular smooth muscle. AA, arachidonic acid; ACh, acetycholine; ADP, adenosine diphosphate; m, muscarinic receptors; P, purinergic receptors; PGD2, prostaglandin D2; PGE2, prostaglandin E2; PGF2α, prostaglandin F2α; PGI2, prostacyclin; PLA2, phospholipase A2; ROS, reactive oxygen species; TXA2, thromboxane A2.
References
- Abeywardena MY, Jablonskis LT, Head RJ. Age- and hypertension-induced changes in abnormal contractions in rat aorta. J Cardiovasc Pharmacol. 2002;40:930–937. doi: 10.1097/00005344-200212000-00015. [DOI] [PubMed] [Google Scholar]
- Álvarez Y, Briones AM, Balfagón G, Alonso MJ, Salaices M. Hypertension increases the participation of vasoconstrictor prostanoids from cyclooxygenase-2 in phenylephrine responses. J Hypertens. 2005;23:767–777. doi: 10.1097/01.hjh.0000163145.12707.63. [DOI] [PubMed] [Google Scholar]
- Álvarez Y, Briones AM, Hernanz R, Pérez-Girón JV, Alonso MJ, Salaixes M. Role of NADPH oxidase and iNOS in vasoconstrictor responses of vessels from hypertensive and normotensive rats. Br J Pharmacol. 2008;153:926–935. doi: 10.1038/sj.bjp.0707575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano H, Shimizu K, Muramatsu M, Iwama Y, Toki Y, Miyazaki Y, Okumura K, Hashimoto H, Ito T. Prostaglandin H2 as an endothelium-derived contracting factor modulates endothelin-1-induced contraction. J Hypertens. 1994;12:383–390. [PubMed] [Google Scholar]
- Auch-Schwelk W, Katusic ZS, Vanhoutte PM. Contractions to oxygen-derived free radicals are augmented in aorta of the spontaneously hypertensive rat. Hypertension. 1989;13:859–864. doi: 10.1161/01.hyp.13.6.859. [DOI] [PubMed] [Google Scholar]
- Auch-Schwelk W, Katusic ZS, Vanhoutte PM. Thromboxane A2 receptor antagonists inhibit endothelium-dependent contractions. Hypertension. 1990;15:699–703. doi: 10.1161/01.hyp.15.6.699. [DOI] [PubMed] [Google Scholar]
- Auch-Schwelk W, Katusic ZS, Vanhoutte PM. Nitric oxide inactivates endothelium-derived contracting factor in the rat aorta. Hypertension. 1992;19:442–445. doi: 10.1161/01.hyp.19.5.442. [DOI] [PubMed] [Google Scholar]
- Auch-Schwelk W, Vanhoutte PM. Contractions to endothelin in normotensive and spontaneously hypertensive rats: role of endothelium and prostaglandins. Blood Press. 1992;1:45–49. doi: 10.3109/08037059209065123. [DOI] [PubMed] [Google Scholar]
- Bachschmid M, Thurau S, Zou MH, Ullrich V. Endothelial cell activation by endotoxin involves superoxide/NO-mediated nitration of prostacyclin synthase and thromboxane receptor stimulation. FASEB J. 2003;17:914–916. doi: 10.1096/fj.02-0530fje. [DOI] [PubMed] [Google Scholar]
- Belhassen L, Pelle G, Dubois-Rande J, Adnot S. Improved endothelial function by the thromboxane A2 receptor antagonist S 18886 in patients with coronary artery disease treated with aspirin. J Am Coll Cardiol. 2003;41:1198–1204. doi: 10.1016/s0735-1097(03)00048-2. [DOI] [PubMed] [Google Scholar]
- Blanco-Rivero J, Cachofeiro V, Lahera V, Aras-Lopez R, Márquez-Rodas I, Salaices M, Xavier FE, Ferrer M, Balfagón G. Participation of prostacyclin in endothelial dysfunction induced by aldosterone in normotensive and hypertensive rats. Hypertension. 2005;46:107–112. doi: 10.1161/01.HYP.0000171479.36880.17. [DOI] [PubMed] [Google Scholar]
- Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH. Prostanoids and prostanoid receptors in signal transduction. Int J Biochem Cell Biol. 2004;36:1187–1205. doi: 10.1016/j.biocel.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Boulanger CM, Morrison KJ, Vanhoutte PM. Mediation by M3-muscarinic receptors of both endothelium-dependent contraction and relaxation to acetylcholine in the aorta of the spontaneously hypertensive rat. Br J Pharmacol. 1994;112:519–524. doi: 10.1111/j.1476-5381.1994.tb13104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussemaker E, Popp R, Fisslthaler B, Larson CM, Fleming I, Busse R, Brandes RP. Aged spontaneously hypertensive rats exhibit a selective loss of EDHF-mediated relaxation in the renal artery. Hypertension. 2003;42:562–568. doi: 10.1161/01.HYP.0000088852.28814.E2. [DOI] [PubMed] [Google Scholar]
- Camacho M, Lopez-Belmonte J, Vila L. Rate of vasoconstrictor prostanoids released by endothelial cells depends on cyclooxygenase-2 expression and prostaglandin I synthase activity. Circ Res. 1998;83:353–365. doi: 10.1161/01.res.83.4.353. [DOI] [PubMed] [Google Scholar]
- Cosentino F, Sill JC, Katusic ZS. Role of superoxide anions in the mediation of endothelium-dependent contractions. Hypertension. 1994;23:229–235. doi: 10.1161/01.hyp.23.2.229. [DOI] [PubMed] [Google Scholar]
- DeLano FA, Parks DA, Ruedi JM, Babior BM, Schmid-Schönbein GW. Microvascular display of xanthine oxidase and NADPH oxidase in the spontaneously hypertensive rat. Microcirculation. 2006;13:551–566. doi: 10.1080/10739680600885152. [DOI] [PubMed] [Google Scholar]
- De Mey JG, Vanhoutte PM. Heterogeneous behavior of the canine arterial and venous wall. Circ Res. 1982;51:439–447. doi: 10.1161/01.res.51.4.439. Importance of the endothelium. [DOI] [PubMed] [Google Scholar]
- De Mey JG, Vanhoutte PM. Anoxia and endothelium-dependent reactivity of the canine femoral artery. J Physiol. 1983;335:65–74. doi: 10.1113/jphysiol.1983.sp014519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhein S, Hartmann E, Salameh A, Klaus W. Characterization of a peptide endothelium-derived constricting factor EDCF. Pharmacol Res. 1997;35:43–50. doi: 10.1006/phrs.1996.0103. [DOI] [PubMed] [Google Scholar]
- Dohi Y, Kojima M, Sato K. Benidipine improves endothelial function in renal resistance arteries of hypertensive rats. Hypertension. 1996;28:58–63. doi: 10.1161/01.hyp.28.1.58. [DOI] [PubMed] [Google Scholar]
- Félétou M, Tang EHC, Vanhoutte PM. Nitric oxide the gatekeeper of endothelial vasomotor control. Front Biosci. 2008;13:4198–4217. doi: 10.2741/3000. [DOI] [PubMed] [Google Scholar]
- Félétou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006a;291:H985–H1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- Félétou M, Vanhoutte PM. EDHF. The Complete Story. Boca Raton: CRC Taylor & Francis; 2006b. [Google Scholar]
- Félétou M, Vanhoutte PM. Endothelium-dependent hyperpolarizations: past beliefs and present facts. Ann Med. 2007;39:495–516. doi: 10.1080/07853890701491000. [DOI] [PubMed] [Google Scholar]
- Fujii K, Tominaga M, Ohmori S, Kobayashi K, Koga T, Takata Y, Fujishima M. Decreased endothelium-dependent hyperpolarization to acetylcholine in smooth muscle of the mesenteric artery of spontaneously hypertensive rats. Circ Res. 1992;70:660–669. doi: 10.1161/01.res.70.4.660. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J. 1989;3:2007–2017. [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garcia-Cohen EC, Marin J, Diez-Picazo LD, Baena AB, Salaices M, Rodriguez-Martinez MA. Oxidative stress induced by tert-butyl hydroperoxide causes vasoconstriction in the aorta from hypertensive and aged rats: role of cyclooxygenase-2 isoform. J Pharmacol Exp Ther. 2000;293:75–81. [PubMed] [Google Scholar]
- Ge T, Hughes H, Junquero DC, Wu KK, Vanhoutte PM. Endothelium-dependent contractions are associated with both augmented expression of prostaglandin H synthase-1 and hypersensitivity to prostaglandin H2 in the SHR aorta. Circ Res. 1995;76:1003–1010. doi: 10.1161/01.res.76.6.1003. [DOI] [PubMed] [Google Scholar]
- Ge T, Vanhoutte PM, Boulanger C. Increased response to prostaglandin H2 precedes changes in PGH synthase-1 expression in the SHR aorta. Acta Pharmacol Sin. 1999;20:1087–1092. [PubMed] [Google Scholar]
- Gluais P, Lonchampt M, Morrow JD, Vanhoutte PM, Félétou M. Acetylcholine-induced endothelium-dependent contractions in the SHR aorta: the Janus face of prostacyclin. Br J Pharmacol. 2005;146:834–845. doi: 10.1038/sj.bjp.0706390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluais P, Paysant J, Badier-Commander C, Verbeuren T, Vanhoutte PM, Félétou M. In SHR aorta, calcium ionophore A-23187 releases prostacyclin and thromboxane A2 as endothelium-derived contracting factors. Am J Physiol Heart Circ Physiol. 2006;291:H2255–H2564. doi: 10.1152/ajpheart.01115.2005. [DOI] [PubMed] [Google Scholar]
- Gluais P, Vanhoutte PM, Félétou M. Mechanisms underlying ATP-induced endothelium-dependent contractions in the SHR aorta. Eur J Pharmacol. 2007;556:107–114. doi: 10.1016/j.ejphar.2006.10.050. [DOI] [PubMed] [Google Scholar]
- Gollasch M. Endothelium-derived contracting factor: a new way of looking at endothelial function in obesity. J Hypertens. 2002;20:2147–2149. doi: 10.1097/00004872-200211000-00011. [DOI] [PubMed] [Google Scholar]
- Gräser T, Vanhoutte PM. Hypoxic contraction of canine coronary arteries: role of endothelium and cGMP. Am J Physiol Heart Circ Physiol. 1991;261:H1769–H1777. doi: 10.1152/ajpheart.1991.261.6.H1769. [DOI] [PubMed] [Google Scholar]
- Gryglewski RJ, Palmer RMJ, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Hayakawa H, Hirata Y, Suzuki E, Kakoki M, Kikuchi K, Nagano T, Hirobe M, Omata M. Endothelium-derived relaxing factors in the kidney of spontaneously hypertensive rats. Life Sci. 1995;56:L401–L408. doi: 10.1016/0024-3205(95)00157-2. [DOI] [PubMed] [Google Scholar]
- Hirao A, Kondo K, Takeuchi K, Inui N, Umemura K. Cyclooxygenase-dependent vasoconstricting factor(s) in remodeled rat femoral arteries. Cardiovasc Res. 2008;79:161–168. doi: 10.1093/cvr/cvn111. [DOI] [PubMed] [Google Scholar]
- Hoshino Y, Morrison KJ, Vanhoutte PM. Mechanisms of hypoxic vasoconstriction in the canine isolated pulmonary artery: role of endothelium and sodium pump. Am J Physiol Lung Cell Mol Physiol. 1994;267:L120–L127. doi: 10.1152/ajplung.1994.267.2.L120. [DOI] [PubMed] [Google Scholar]
- Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- Huang JS, Ramamurthy SK, Lin X, Le Breton GC. Cell signalling through thromboxane A2 receptors. Cell Signal. 2004;16:521–533. doi: 10.1016/j.cellsig.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Hutri-Kahonen N, Kahonen M, Tolvanen JP, Wu X, Sallinen K, Porsti I. Ramipril therapy improves arterial dilation in experimental hypertension. Cardiovasc Res. 1997;33:188–195. doi: 10.1016/s0008-6363(96)00197-6. [DOI] [PubMed] [Google Scholar]
- Iqbal A, Vanhoutte PM. Flunarizine inhibits endothelium-dependent hypoxic facilitation in canine coronary arteries through an action on vascular smooth muscle. Br J Pharmacol. 1988;95:789–794. doi: 10.1111/j.1476-5381.1988.tb11706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Kato T, Iwama Y, Muramatsu M, Shimizu K, Asano H, Okumura K, Hashimoto H, Satake T. Prostaglandin H2 as an endothelium-derived contracting factor and its interaction with nitric oxide. J Hypertens. 1991;9:729–736. doi: 10.1097/00004872-199108000-00006. [DOI] [PubMed] [Google Scholar]
- Iwama Y, Kato T, Muramatsu M, Asano H, Shimizu K, Toki Y, Miyazaki Y, Okumura K, Hashimoto H, Ito T. Correlation with blood pressure of the acetylcholine-induced endothelium-derived contracting factor in the rat aorta. Hypertension. 1992;19:326–332. doi: 10.1161/01.hyp.19.4.326. [DOI] [PubMed] [Google Scholar]
- Iwatani Y, Kosugi K, Isobe-Oku S, Atagi S, Kitamura Y, Kawasaki H. Endothelium removal augments endothelium-independent vasodilatation in rat mesenteric vascular bed. Br J Pharmacol. 2008;154:32–40. doi: 10.1038/bjp.2008.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski V, Tolle M, Vanholder R, Schonfelder G, Van Der Giet M, Henning L, Schluter H, Paul M, Zidek W, Jankowski J. Uridine adenosine tetraphosphate: a novel endothelium-derived vasoconstrictive factor. Nat Med. 2005;11:223–227. doi: 10.1038/nm1188. [DOI] [PubMed] [Google Scholar]
- Kato T, Iwama Y, Okumura K, Hashimoto H, Ito T, Satake T. Prostaglandin H2 may be the endothelium-derived contracting factor released by acetylcholine in the aorta of the rat. Hypertension. 1990;15:475–481. doi: 10.1161/01.hyp.15.5.475. [DOI] [PubMed] [Google Scholar]
- Katusic ZS, Shepherd JT, Vanhoutte PM. Endothelium-dependent contraction to stretch in canine basilar arteries. Am J Physiol Heart Circ Physiol. 1987;252:H671–H673. doi: 10.1152/ajpheart.1987.252.3.H671. [DOI] [PubMed] [Google Scholar]
- Katusic ZS, Shepherd JT, Vanhoutte PM. Endothelium-dependent contractions to calcium ionophore A23187, arachidonic acid and acetylcholine in canine basilar arteries. Stroke. 1988;19:476–479. doi: 10.1161/01.str.19.4.476. [DOI] [PubMed] [Google Scholar]
- Katusic ZS, Vanhoutte PM. Anoxic contractions in isolated canine cerebral arteries: Contribution of endothelium-derived factors, metabolites of arachidonic acid, and calcium entry. J Cardiovasc Pharmacol. 1986;8:S97–S101. [PubMed] [Google Scholar]
- Katusic ZS, Vanhoutte PM. Superoxide anion is an endothelium-derived contracting factor. Am J Physiol Heart Circ Physiol. 1989;257:H33–H37. doi: 10.1152/ajpheart.1989.257.1.H33. [DOI] [PubMed] [Google Scholar]
- Koga T, Takata Y, Kobayashi K, Takishita S, Yamashita Y, Fujishima M. Age and hypertension promote endothelium-dependent contractions to acetylcholine in the aorta of the rat. Hypertension. 1989;14:542–548. doi: 10.1161/01.hyp.14.5.542. [DOI] [PubMed] [Google Scholar]
- Levy JV. Prostacyclin-induced contraction of isolated aortic strips from normal and spontaneously hypertensive rats (SHR) Prostaglandins. 1980;19:517–520. doi: 10.1016/s0090-6980(80)80002-5. [DOI] [PubMed] [Google Scholar]
- Lockette W, Otsuka Y, Carretero O. The loss of endothelium-dependent vascular relaxation in hypertension. Hypertension. 1986;8:II61–II66. doi: 10.1161/01.hyp.8.6_pt_2.ii61. [DOI] [PubMed] [Google Scholar]
- Lüscher TF, Cooke JP, Houston DS, Neves RJ, Vanhoutte PM. Endothelium-dependent relaxation in human arteries. Mayo Clin Proc. 1987a;62:601–606. doi: 10.1016/s0025-6196(12)62299-x. [DOI] [PubMed] [Google Scholar]
- Lüscher TF, Romero JC, Vanhoutte PM. Bioassay of endothelium-derived vasoactive substances in the aorta of normotensive and spontaneously hypertensive rats. J Hypertens. 1987b;4:S81–S83. [Google Scholar]
- Lüscher TF, Vanhoutte PM. Endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Hypertension. 1986;8:344–348. doi: 10.1161/01.hyp.8.4.344. [DOI] [PubMed] [Google Scholar]
- Lüscher TF, Vanhoutte PM. The Endothelium: Modulator of Cardiovascular Function. Boca Raton: CRC Press, Inc.; 1990. pp. 1–228. [Google Scholar]
- Macarthur H, Westfall TC, Wilken GH. Oxidative stress attenuates NO-induced modulation of sympathetic neurotransmission in the mesenteric arterial bed of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2008;294:H183–H189. doi: 10.1152/ajpheart.01040.2007. [DOI] [PubMed] [Google Scholar]
- Mayhan WG. Role of prostaglandin H2-thromboxane A2 in responses of cerebral arterioles during chronic hypertension. Am J Physiol Heart Circ Physiol. 1992;262:H539–H543. doi: 10.1152/ajpheart.1992.262.2.H539. [DOI] [PubMed] [Google Scholar]
- Michel FS, Man GS, Man RYK, Vanhoutte PM. Hypertension and the absence of EDHF-mediated responses favour endothelium-dependent contractions in renal arteries of the rat. Br J Pharmacol. 2008a;155:217–226. doi: 10.1038/bjp.2008.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel F, Simonet S, Vayssettes-Courchay C, Bertin F, Sansilvestri-Morel P, Bernhardt F, Paysant J, Silvestre JS, Levy BI, Félétou M, Verbeuren TJ. Altered TP receptor function in isolated, perfused kidneys of nondiabetic and diabetic ApoE-deficient mice. Am J Physiol Renal Physiol. 2008b;294:F120–F129. doi: 10.1152/ajprenal.00111.2007. [DOI] [PubMed] [Google Scholar]
- Miller VM, Vanhoutte PM. Endothelium-dependent contractions to arachidonic acid is mediated by products of cyclooxygenase. Am J Physiol Heart Circ Physiol. 1985;248:H432–H437. doi: 10.1152/ajpheart.1985.248.4.H432. [DOI] [PubMed] [Google Scholar]
- Miyagawa K, Ohashi M, Yamashita S, Kojima M, Sato K, Ueda R. Increased oxidative stress impairs endothelial modulation of contractions in arteries from spontaneously hypertensive rats. J Hypertens. 2007;25:415–421. doi: 10.1097/HJH.0b013e3280115b96. [DOI] [PubMed] [Google Scholar]
- Mombouli JV, Vanhoutte PM. Purinergic endothelium-dependent and -independent contractions in rat aorta. Hypertension. 1993;22:577–583. doi: 10.1161/01.hyp.22.4.577. [DOI] [PubMed] [Google Scholar]
- Numaguchi Y, Harada M, Osanai H, Hayashi K, Toki Y, Okumura K, Ito T, Hayakawa T. Altered gene expression of prostacyclin synthase and prostacyclin receptor in the thoracic aorta of spontaneously hypertensive rats. Cardiovasc Res. 1999;41:682–688. doi: 10.1016/s0008-6363(98)00239-9. [DOI] [PubMed] [Google Scholar]
- Okon EB, Golbabaie A, van Breemen C. In the presence of L-NAME SERCA blockade induces endothelium-dependent contraction of mouse aorta through activation of smooth muscle prostaglandin H2/thromboxane A2 receptors. Br J Pharmacol. 2002;137:545–553. doi: 10.1038/sj.bjp.0704884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke ST, Vanhoutte PM, Miller VM. Biology of blood vessels. In: Creager MA, Dzau V, Loscalso J, editors. Vascular Medicine, a Companion to Braunwald's Heart Disease. Philadelphia, PA, USA: Elsevier; 2006. pp. 71–100. [Google Scholar]
- Pearson PJ, Lin PJ, Schaff HV, Vanhoutte PM. Augmented endothelium-dependent constriction to hypoxia early and late following reperfusion of the canine coronary artery. Clin Exp Pharmacol Physiol. 1996;23:634–641. doi: 10.1111/j.1440-1681.1996.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Rapoport RM, Williams SP. Role of prostaglandins in acetylcholine-induced contraction of aorta from spontaneously hypertensive and Wistar-Kyoto rats. Hypertension. 1996;28:64–75. doi: 10.1161/01.hyp.28.1.64. [DOI] [PubMed] [Google Scholar]
- Rees DD, Palmer RMJ, Moncada S. The role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989;86:3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol Heart Circ Physiol. 1986;250:H1145–H1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Vanhoutte PM. Hypoxia releases a vasoconstrictor substance from the canine vascular endothelium. J Physiol. 1985;364:45–56. doi: 10.1113/jphysiol.1985.sp015728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi GM, Vanhoutte PM. Superoxide anions and hypoxia inactivate endothelium-derived relaxing factor(s) Am J Physiol Heart Circ Physiol. 1986;250:H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Saifeddine M, Roy SS, Al-Ani B, Triggle CR, Hollengerg MD. Endothelium-dependent contractile actions of proteinase-activated receptor-2-activating peptides in human umbilical vein: release of a contracting factor via a novel receptor. Br J Pharmacol. 1998;125:1445–1454. doi: 10.1038/sj.bjp.0702213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Félétou M, Ku DD, Man RYK, Vanhoutte PM. The calcium ionophore A23187 induces endothelium-dependent contractions in femoral arteries from rats with streptozotocin-induced diabetes. Br J Pharmacol. 2007a;150:624–632. doi: 10.1038/sj.bjp.0706999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Man RY, Vanhoutte PM. Two isoforms of cyclooxygenase contribute to augmented endothelium-dependent contractions in femoral arteries of 1-year-old rats. Acta Pharmacol Sin. 2008;29:185–192. doi: 10.1111/j.1745-7254.2008.00749.x. [DOI] [PubMed] [Google Scholar]
- Shi Y, So KF, Man RYK, Vanhoutte PM. Oxygen-derived free radicals mediate endothelium-dependent contractions in femoral arteries of rats with streptozotocin-induced diabetes. Br J Pharmacol. 2007b;152:1033–1041. doi: 10.1038/sj.bjp.0707439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Vanhoutte PM. Oxidative stress and COX cause hyper-responsiveness in vascular smooth muscle of the femoral artery from diabetic rats. Br J Pharmacol. 2008;154:639–651. doi: 10.1038/bjp.2008.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirahase H, Usui H, Kurahashi K, Fujiwara M, Fukui K. Endothelium-dependent contraction induced by nicotine in isolated canine basilar artery – possible involvement of a thromboxane A2 (TXA2) like substance. Life Sci. 1988;42:437–445. doi: 10.1016/0024-3205(88)90082-3. [DOI] [PubMed] [Google Scholar]
- Taddei S, Vanhoutte PM. Role of endothelium in endothelin-evoked contractions in the rat aorta. Hypertension. 1993;21:9–15. doi: 10.1161/01.hyp.21.1.9. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Cyclooxygenase inhibition restores nitric oxide activity in essential hypertension. Hypertension. 1997a;29:274–279. doi: 10.1161/01.hyp.29.1.274. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Fasolo CB, Sudano I, Salvetti A. Hypertension causes premature aging of endothelial function in humans. Hypertension. 1997b;29:736–743. doi: 10.1161/01.hyp.29.3.736. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- Tang EHC, Félétou M, Huang Y, Man RYK, Vanhoutte PM. Acetylcholine and sodium nitroprusside cause long-term inhibition of EDCF-mediated contraction. Am J Physiol Heart Circ Physiol. 2005b;289:H2434–H2440. doi: 10.1152/ajpheart.00568.2005. [DOI] [PubMed] [Google Scholar]
- Tang EH, Jensen BL, Skott O, Leung GP, Félétou M, Man RY, Vanhoutte PM. The role of prostaglandin E and thromboxane-prostanoid receptors in the response to prostaglandin E2 in the aorta of Wistar Kyoto rats and spontaneously hypertensive rats. Cardiovasc Res. 2008;78:130–138. doi: 10.1093/cvr/cvm112. [DOI] [PubMed] [Google Scholar]
- Tang EH, Ku BB, Tipoe GL, Félétou M, Man RY, Vanhoutte PM. Endothelium-dependent contractions occur in the aorta of wild-type and COX2–/– knockout but not COX1–/– knockout mice. J Cardiovasc Pharmacol. 2005a;46:761–765. doi: 10.1097/01.fjc.0000187174.67661.67. [DOI] [PubMed] [Google Scholar]
- Tang EH, Leung FP, Huang Y, Félétou M, So KF, Man RY, Vanhoutte PM. Calcium and reactive oxygen species increase in endothelial cells in response to releasers of endothelium-derived contracting factor. Br J Pharmacol. 2007;151:15–23. doi: 10.1038/sj.bjp.0707190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang EH, Vanhoutte PM. Gap junction inhibitors reduce endothelium-dependent contractions in the aorta of spontaneously hypertensive rats. J Pharmacol Exp Ther. 2008a;327:148–153. doi: 10.1124/jpet.108.140046. [DOI] [PubMed] [Google Scholar]
- Tang EH, Vanhoutte PM. Gene expression changes of prostanoid synthases in endothelial cells and prostanoid receptors in vascular smooth muscle cells caused by aging and hypertension. Physiol Genomics. 2008b;32:409–418. doi: 10.1152/physiolgenomics.00136.2007. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B. Free radicals in diabetic endothelial cell dysfunction. Free Rad Biol Med. 1994;16:383–391. doi: 10.1016/0891-5849(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B, Brown ML, Cohen RA. Elevated glucose impairs endothelium-dependent relaxation by activating protein kinase C. J Clin Invest. 1991;87:1643–1648. doi: 10.1172/JCI115179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfamariam B, Brown ML, Deykin D, Cohen RA. Elevated glucose promotes generation of endothelium-derived vasoconstrictor prostanoids in rabbit aorta. J Clin Invest. 1990;85:929–932. doi: 10.1172/JCI114521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfamariam B, Jakubowski JA, Cohen RA. Contraction of diabetic rabbit aorta caused by endothelium-derived PGH2-TXA2. Am J Physiol Heart Circ Physiol. 1989;257:H1327–H1333. doi: 10.1152/ajpheart.1989.257.5.H1327. [DOI] [PubMed] [Google Scholar]
- Traupe T, Lang M, Goettsch W, Münter K, Morawietz H, Vetter W, Barton M. Obesity increases prostanoidmediated vasoconstriction and vascular thromboxane receptor gene expression. J Hypertens. 2002;20:2239–2245. doi: 10.1097/00004872-200211000-00024. [DOI] [PubMed] [Google Scholar]
- Tschudi MR, Mesaros S, Lüscher TF, Malinski T. Direct in situ measurement of nitric oxide in mesenteric resistance arteries. Increased decomposition by superoxide in hypertension. Hypertension. 1996;27:32–35. doi: 10.1161/01.hyp.27.1.32. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM. Say NO to ET. J Auton Nerv Syst. 2000;81:271–277. doi: 10.1016/s0165-1838(00)00126-0. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Félétou M, Taddei S. Endothelium-dependent contractions in hypertension. Br J Pharmacol. 2005;144:449–458. doi: 10.1038/sj.bjp.0706042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Jiang B, Maitland KA, Bayat H, Gu J, Nadler JL, Corda S, Lavielle G, Verbeuren TJ, Zuccollo A, Cohen RA. The thromboxane receptor antagonist S18886 attenuates renal oxidant stress and proteinuria in diabetic apolipoprotein E-deficient mice. Diabetes. 2006;55:110–119. [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Mitsui Y, Kobayashi M, Watanabe TX, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yang D, Félétou M, Boulanger CM, Wu HF, Levens N, Zhang JN, Vanhoutte PM. Oxygen-derived free radicals mediate endothelium-dependent contractions to acetylcholine in aortas from spontaneously hypertensive rats. Br J Pharmacol. 2002;136:104–110. doi: 10.1038/sj.bjp.0704669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Félétou M, Levens N, Zhang JN, Vanhoutte PM. A diffusible substance(s) mediates endothelium-dependent contractions in the aorta of SHR. Hypertension. 2003;41:143–148. doi: 10.1161/01.hyp.0000047651.45322.16. [DOI] [PubMed] [Google Scholar]
- Zerrouk A, Auguet M, Chabrier PE. Augmented endothelium-dependent contraction to angiotensin II in the SHR aorta: role of an inducible cyclooxygenase metabolite. J Cardiovasc Pharmacol. 1998;31:525–533. doi: 10.1097/00005344-199804000-00009. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Varadharaj S, Zhao X, Parinandi N, Flavahan NA, Zweier JL. Acetylcholine causes endothelium-dependent contraction of mouse arteries. Am J Physiol Heart Circ Physiol. 2005;289:H1027–H1032. doi: 10.1152/ajpheart.00226.2005. [DOI] [PubMed] [Google Scholar]
- Zou MH, Leist M, Ullrich V. Selective nitration of prostacyclin synthase and defective vasorelaxation in atherosclerotic bovine coronary arteries. Am J Pathol. 1999;154:1359–1365. doi: 10.1016/S0002-9440(10)65390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou MH, Shi C, Cohen RA. High glucose via peroxynitrite causes tyrosine nitration and inactivation of prostacyclin synthase that is associated with thromboxane/prostaglandin H2 receptor-mediated apoptosis and adhesion molecule expression in cultured human aortic endothelial cells. Diabetes. 2002;51:198–203. doi: 10.2337/diabetes.51.1.198. [DOI] [PubMed] [Google Scholar]