Abstract
Most manual tasks demand a delicate control of the wrist. Sensory information for this control, e.g. about the position and movement velocity of the hand, is assumed to be primarily provided by muscle spindle afferents. It is known that human muscle spindles in relaxed muscles behave as stretch receptors but it is unclear how they discharge during ‘natural’ hand movements, since their discharges can also be affected by extrafusal contractions and fusimotor activity. We therefore let subjects perform a centre-out-centre key-pressing task on buttons laid out in a 3 × 3 pattern, a task that allowed unconstrained hand and finger movements and required precise control of the wrist. Microneurography recordings from muscle spindle afferents of the wrist extensor muscles were obtained along with wrist kinematics and electromyographic signals. The discharge rates of afferents were more phase advanced than expected on the length of the radial wrist extensor, which acted as an anti-gravity muscle in the key-pressing task. As such, both acceleration and velocity had significant impacts on the discharge rate of primary afferents, velocity on that of secondary afferents, and length had no impact on either afferent type. The response patterns were different for the two types of muscle spindle afferents from the predominantly eccentrically contracting ulnar wrist extensor: muscle length and velocity had significant impacts on the ensemble response of secondary afferents whereas the primary afferents showed highly variable responses. Accordingly, good predictions of the radial ulnar angular velocity were possible from spindle ensemble responses (R2= 0.85) whereas length could be predicted only for phases with lengthening of the ulnar wrist extensor. There are several possible explanations for the unexpectedly large phase advance of spindle afferents in the radial wrist extensor. Given the compliance of tendons, for instance, the phase relationship between the muscle fascicle length and the whole muscle length is conjectured to depend on the load. While additional phase advances are advantageous in motor control, it is concluded that if the central nervous system estimates length or velocity of a muscle from its muscle spindle discharges, this would require additional information about not only the concomitant extrafusal and fusimotor drive but also about the mechanical properties of the load on which the muscle acts.
Most manual tasks require a delicate control of the wrist. The sensory information for this control, such as the position and movement velocity of the hand, is assumed to be primarily provided by muscle spindle afferents. Human muscle spindles in relaxed muscles behave as stretch receptors (Edin & Vallbo, 1990a; Bergenheim, Ribot-Ciscar & Roll, 2000) but what their discharges represent in actively contracting muscles is less clear, since spindle discharges can also be affected by extrafusal contractions and fusimotor activity. During concentric contractions against an external load, for example, the discharges of human spindle afferents vary more with contraction strength than with muscle length (Burke et al. 1978). Both primary (type Ia) and secondary muscle spindle afferents (type II) in finger extensor muscles fail to represent finger joint positions during visually guided finger movements (Vallbo et al. 1981; Hulliger et al. 1982). Similarly, spindle afferents from actively contracting human wrist muscles show poor position sensitivity (Jones et al. 2001b). Thus, while muscle spindle afferents in passive muscles can encode joint kinematics, this is not necessarily true for actively contracting muscles.
It is conceivable that during active movements, spindle afferents from the less active antagonists provide the positional information required for adequate control (Jones et al. 2001b). Natural hand and finger movements are, however, characteristically associated with extensive co-contractions (Johansson & Westling, 1988; Maier & Hepp-Reymond, 1995). Since previous human studies have focused on spindle responses to isometric contractions or movements at single joints (Vallbo, 1971; Vallbo et al. 1981; Al-Falahe et al. 1990; Edin & Vallbo, 1990a,b; Bergenheim et al. 2000; Jones et al. 2001a; Cordo et al. 2002), little is known about muscle spindle activity during natural behaviours such as grasping, object manipulation and pointing movements. It remains to be determined to what extent spindle afferents represent naturally occurring hand positions and movements during everyday motor tasks.
We therefore developed a protocol in which participants used a single finger in a ‘centre-out-centre’ task to press sequences of buttons laid out in a 3 × 3 pattern, a task that was characterized by discrete, purposeful movements, involved object contact, and required precise control of the wrist. The discharge of individual muscle spindle afferents from the radial (RWE) and ulnar (UWE) wrist extensor muscles were recorded with microneurography along with electromyographic (EMG) signals from the muscles, and the kinematics of the wrist and the digits.
Methods
Ethical approval
Nineteen right-handed healthy volunteers (age 19–45, 9 females) participated in the experiments. All gave their informed consent prior to experimentation according to the Declaration of Helsinki and the experimental procedures were approved by the Ethics Committee of the Umeå University, Umeå, Sweden.
Behavioural task
The participants were seated with their right arm supported by a vacuum pillow around their forearm and a cushioned clamp just proximal to the wrist. In this position they could move their wrist and digits freely. The hand was placed in a semi-pronated position, so that radial deviations of the wrist coincided with vertical movements against gravity, that is, the RWE worked against gravity (see insets in Figs 2 and 3). For the key-pressing task, the participants were asked to press specific key sequences on a custom-built numeric key pad with nine buttons (diameter 5 mm, arranged 3 × 3) that required a normal force of 1.45 N (s.d.= 0.09) to be activated. The position of the key pad was adjusted so that the participants could reach the middle key (5) with the tip of digit III close to the mid-point of the movement range of the wrist in the ulnar–radial and flexion–extension planes.
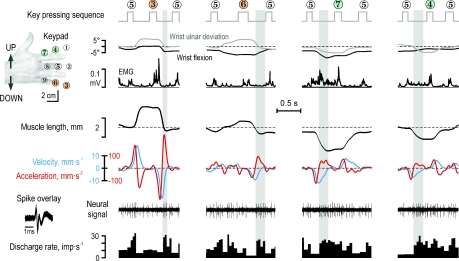
Figure 2. Responses of a typical type Ia muscle spindle afferent (65-02, cf., Fig. 1) from the radial wrist extensor (RWE) during four key-pressing sequences, two that required its parent muscle to first lengthen and then shorten (key 3 and 6) and two that required it to first shorten (keys 7 and 4).
Shown from top are the angular changes at the wrist, the EMG of the RWE, the length of the RWE muscle and its time derivatives (‘velocity’ and ‘acceleration’) and the neural activity. Unexpectedly, the afferent's discharge was characterized by bursting and pauses apparently related to the second derivate of the muscle length (‘acceleration’). In particular, note the discharge onset when acceleration reversed from negative to positive (grayed areas) and that this afferent discharged less when its parent muscle was at a longer length than when it was at a shorter length.
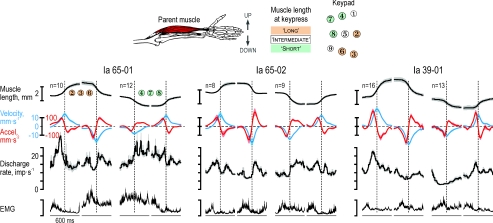
Figure 3. Averaged responses during key pressing in three single type Ia afferents from the radial wrist extensor muscle.
Data were aggregated in a 600 ms window centred on the zero crossing of the acceleration signal during muscle lengthening and shortening phases of the key-pressing task. Kinematic recordings, discharge rates of single type Ia afferents and EMG recordings were averaged separately across keys that required muscle elongation (keys 2, 3 and 6) and shortening (keys 4, 7, 8). Regression analyses showed that only acceleration had a significant impact on the discharge rates of afferent 65-01 and 65-02 (see Fig. 2) whereas that of afferent 39-01 was mostly related to both velocity and acceleration. Shaded areas around means represent ±s.e.m. Scale bars for EMG correspond to 0.1, 0.06 and 0.02 mV from left to right.
The participants were asked to use their middle finger to press series of key sequences at a comfortable pace of their own choice. Each key sequence comprised three keys: Key 5 was always the first and last key in a sequence and it was never the ‘target key’ of the sequence, i.e. the middle key (see inset of Fig. 2). The key sequences were presented in ‘blocks’ of eight separate sequences. For each block, the participants traversed the keyboard in a clock-wise manner, e.g. 5–2–5, 5–3–5, etc. Each sequence was presented on a VFD display 300 ms after pressing the terminal 5 key of the previous sequence, and the concurrent sequence remained on display until the terminal 5 key was pressed. The participants practiced the key-pressing task before microneurography begun. A buzzing sound indicated that an incorrect key had been pressed. Due to the simplicity and repetitive nature of the task all participants attained proficiency quickly, and for most participants, the practice session lasted less than 5 min.
Neurophysiological technique
Signals in single muscle spindle afferents were obtained by microneurographic recordings from the radial nerve in the upper arm (Vallbo & Hagbarth, 1968). The muscle from which an afferent originated was identified by palpation and by observing its responses to passive joint movements and active contractions. Muscle spindle afferents were discriminated from Golgi tendon organ afferents by their different responses to passive movements and isometric contraction (Edin & Vallbo, 1990a,b). Muscle spindle afferents were subdivided into type Ia and II depending on the variability of their interspike intervals during passive isometric conditions (Nordh et al. 1983), responses to passive muscle lengthening (Edin & Vallbo, 1990a), and their response to a sudden relaxation following a steady isometric contraction (Edin & Vallbo, 1990b).
EMG recordings
EMG was recorded from the four major forearm muscles involved in wrist movements and accessible by surface electrodes: the radial wrist extensor (RWE, m. extensor carpi radialis), the radial wrist flexor (RWF, m. flexor carpi radialis), the ulnar wrist flexor (UWF, m. flexor carpi ulnaris), and the UWE (m. extensor carpi ulnaris). The optimal recording site for each muscle was identified with help of a hand-held EMG recording probe during isometric contractions. Custom-built surface electrodes (diameter 2 mm; 12 mm apart) were coated with electrode jelly and attached to the skin at the optimal sites using double-sided adhesive tape. The activity in the long and short RWE (m. extensor carpi radialis brevis et longus) cannot be distinguished by surface electrodes although subtle differences can be demonstrated using intramuscular EMG recordings (Riek et al. 2000). However, because the experimental sessions lasted 4–5 h, intramuscular EMG recordings were not feasible.
Kinematics and muscle length estimates
Digit and wrist kinematics were recorded using the CyberGlove™ complemented in nine subjects with the Polhemus FasTrak™ motion tracking system (Skill Technologies Inc., AZ, USA) to record wrist joint movements. Muscle lengths were calculated from the recorded joint angles according to Pigeon et al. (1996). The estimate of muscle length used for the RWE was the average of those for the long and short RWE muscles. We let all participants hold their wrist straight in both the flexion–extension and the ulnar–radial planes to define a reference posture. All muscle lengths are reported relative to the length at this reference posture, e.g. −4 mm would represent a shortening and +4 mm an elongation compared to the whole muscle length when the wrist was held in the reference posture. For convenience, we refer to the first and second time derivatives of muscle length as ‘velocity’ and ‘acceleration’, respectively.
Since Fourier analyses of the kinematic data showed no significant frequency components above 10 Hz, the time derivatives were calculated in a moving window of ±40 ms resulting in a cut-off frequency of ∼10 Hz. The averaged total range of ulnar–radial deviation was 19 deg (subject range 11–32 deg) and 26 deg for flexion–extension angle (subject range 19–36 deg). For comparison, the wrist position had to be controlled within ±1.5 deg to successively press a key.
Depending on the key sequence, RWE and UWE would shorten or elongate significantly or change length very little when the participants moved from key 5 towards the target key of the sequence or vice versa. Across seven subjects, muscle lengths differed in a consistent manner when pressing specific keys: Fisher transformed averaged correlation coefficients between all pairs among the subjects was 0.99 and 0.91 for RWE and UWE, respectively. Grouping the keys into those associated with long, short and intermediate muscle lengths (see inset in Figs 3, and 4B and C), was found to be consistent with 96% of the measured muscle lengths across all subjects. We focused, of course, our analyses on sequences that resulted in appreciable length changes in the RWE and UWE. The keys associated with ‘long’ and ‘short’ muscle lengths of the RWE muscle were 2–3–6 and 4–7–8, respectively; the corresponding set of keys for the UWE muscle were 1–4–7 and 3–6–9.
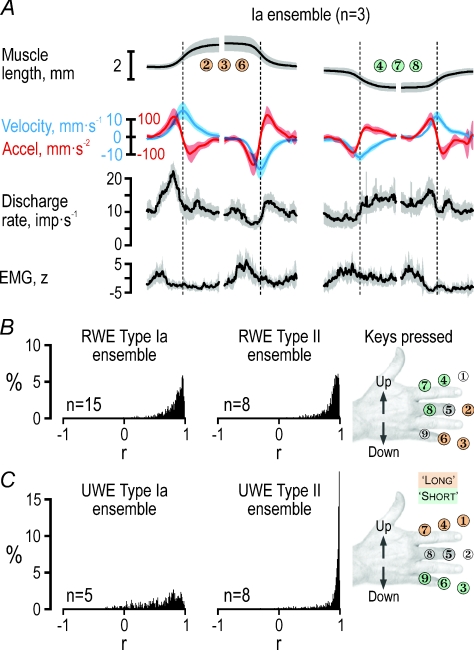
Figure 4. Creation of ensemble responses during key pressing.
A, averaged ensemble responses created by picking at random the recorded responses from each of the three type Ia afferents shown in Fig. 3. EMG was z-transformed before averaging. Shaded areas around means represent ±s.d. B, the same procedure was employed for creating ensemble responses for each key and phase (n = 1200) using all available afferents from the RWE muscle (n = 15). This time, however, the data were aggregated in a 400 ms time window starting 100 ms before the subject released a key to move to a key that required either elongation or shortening of the RWE (see inset). The histograms represent r values when each randomly generated average was correlated with the grand mean. The median r value obtained for the type Ia and type II ensemble were 0.87 and 0.90, respectively. C, the same as in B but pertaining to the UWE. The type Ia and type II median r values were 0.68 and 0.95, respectively. RWE, radial wrist extensor; UWE, ulnar wrist extensor.
Data sampling, processing and extraction
The data were digitally sampled using SC/ZOOM (Physiology Section, Department Integrative Medical Biology, Umeå University): the neural signal was sampled at 12.8 kHz, the root-mean-square processed EMG channels at 1600 Hz, the CyberGlove channels at 86 Hz and the Polhemus FasTrak at 30 Hz. Identification of single action potentials was made semi-automatically under visual control (Edin et al. 1988). Records with multi-unit discharges were discarded.
Population responses and cross-validation
We generated afferent population responses (see Figs 4, 5 and 6) by means of a random re-sampling procedure (so-called ‘bootstrapping’). For instance, to create the ensemble discharge rates of the three afferents and the respective EMG and kinematic variables in Fig. 4A, averaged responses synchronized at the time of acceleration reversal from specific key presses and phases were generated by picking at random recorded responses of the three afferents in Fig. 3. The ensemble responses pertaining to all available afferents from the RWE and UWE (Figs 4B and C, 5 and 6), were generated through a similar method but could not be synchronized using kinematics. Instead, the data for each discrete movement were extracted from a window of 400 ms, which included the 100 ms before the participants released a key and the following 300 ms when moving to another key. For each discrete movement (i.e. each key and phase), 100 ensemble responses were generated by random sampling the discharges of single afferents, for which at least two repetitions without key-pressing errors had been obtained. The number of afferents contributing to the ensemble responses therefore varied slightly depending on the key of interest. Of the 15 recorded Ia afferents from the RWE muscle, 12–14 contributed to the type Ia ensemble responses from the RWE muscle whereas 4–8 of the 8 type II afferents contributed to the type II ensemble responses from the UWE muscle.
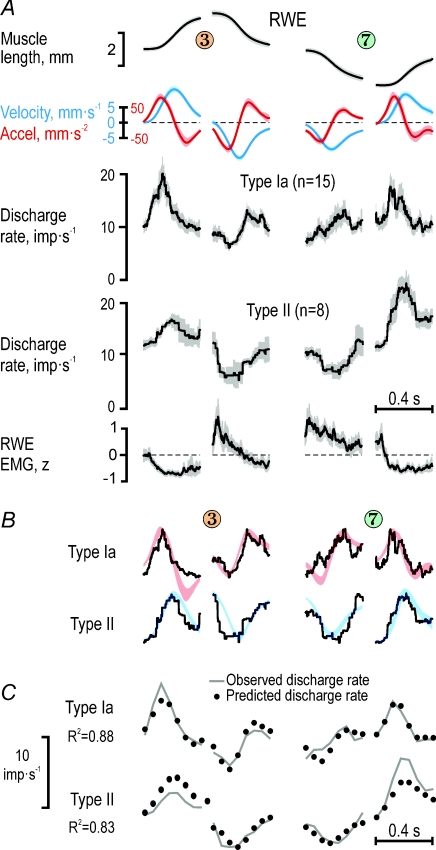
Figure 5. Ensemble responses of muscle spindle afferents from the radial wrist extensor.
A, averaged length, velocity, acceleration and EMG signals along with the corresponding ensemble discharges of type Ia afferents (n = 15) and type II afferents (n = 8) from the radial wrist extensor (RWE), for a ‘long’ key 3 and a ‘short’ key 7. EMG was z-transformed before averaging. Shaded areas around means represent ±s.d.B, qualitiative comparisons of ensemble discharge profiles and kinematic variables having the most significant impacts on the discharge rates. The kinematic variables were rescaled by eye to make a good fit to the discharge profiles using red for acceleration and blue for velocity (as in A). C, reconstructions of the observed ensemble discharge rates shown in A by regressions using data, each point representing the average of a 50 ms bin. Continuous lines represent the observed values and dots the values predicted from linear regressions using the kinematics signals and RWE EMG as independent predictors. For the key sequences shown, both acceleration and velocity affected significantly type Ia ensemble discharges from the RWE, and velocity alone sculpted the type II discharges from the same muscle.
Figure 6. Ensemble responses of muscle spindle afferents from the ulnar wrist extensor.
A, averaged length, velocity, acceleration and EMG signals along with the corresponding ensemble discharges of type Ia afferents (n = 5) and type II afferents (n = 8) from the UWE for a ‘long’ key 1 and a ‘short’ key 9. EMG was z-transformed before averaging. The type Ia afferents failed to generate a reliable ensemble response and were excluded from further analyses. Shaded areas around means represent ±s.d.B, qualitiative comparisons of ensemble discharge profiles and kinematic variables having statistically significant impacts on type II discharge rates. The kinematic variables were rescaled by eye to make a good fit to the discharge profiles, with blue signifying velocity and grey length (as in A). C, reconstructions of the observed type II ensemble discharge rates shown in A by regressions using data, each point representing the average of a 50 ms bin. Continuous lines represent the observed values and dots the values predicted from linear regressions using the kinematic signals and UWE EMG as independent predictors. For the key sequences shown, both length and velocity significantly affected type II ensemble discharges from the UWE.
The degree to which the ensemble responses were representative of the ‘true’ population responses can be readily assessed by the s.d.s shown in Figs 5 and 6. In addition, Fig. 4B and C shows the distribution of r values when each randomly generated response for each discrete movement towards a ‘long’, ‘short’ or 5 key (n = 100, n = 1200) was correlated with the grand mean. The median r value obtained for the RWE type Ia and type II responses was 0.87 and 0.90, respectively, with a range between the lower and upper quartile of 0.09 and 0.07, respectively. The median r value for type II from the UWE was 0.96 (range 0.06) whereas the corresponding value of the UWE type Ia was a mere 0.68 (range 0.24). Since the validity of an UWE type Ia ensemble response was questionable, the latter was not used when investigating the relationship between ensemble responses and observed kinematics. The kinematic data corresponding to the ensemble responses across all afferents were obtained by averaging across the participants from whom reliable kinematic data had been recorded (n = 9) whereas EMG measurements were gathered from all participants involved in the study (n = 19).
Statistical methods
All statistical tests were performed using STATISTICA® (StatSoft Inc., USA) choosing a significance level of P < 10−2 for all tests. The experimental protocol included symmetrical movements around the central 5 key of the keyboard, and therefore muscle lengths, velocities and accelerations should in theory all be pair-wise uncorrelated. Length and velocity were indeed uncorrelated for both muscles (r2 < 0.02) but length and acceleration were statistically significantly correlated but to such a small degree that it was ignored in the regression analyses (r2 < 0.20). The data used for regression analyses showed, however, a high degree of auto-correlation which if not taken into account would have led to inflated P values. The effective sampling rate was estimated to be ∼20 Hz (Dawdy & Matalas, 1964). Accordingly, all regressions were performed on data that were down-sampled to 20 Hz, i.e. each data point representing the average in a 50 ms window. For the regression analyses we assumed that length and its derivatives all must have a positive impact on the afferent's discharges, i.e. if all other factors were constant, the discharge would never decrease if the muscle length increased. In contrast, EMG could have a negative or positive impact depending on the balance between the associated mechanical unloading and fusimotor activation. The variables used in these non-linear regressions were standardized (z-transformed) in order to obtain normalized impact coefficients (β values; a value of unity implies that a change of 1 s.d. in the variable results in 1 s.d. change in the dependent variable).
Rationale for separate analyses of afferents from RWE and UWE
Various considerations motivated us to analyse afferent responses in the RWE and UWE muscles separately. First, the afferents obviously discharged differently in the two muscles, e.g. the discharge rates of type II afferents from UWE were much higher than those recorded from RWE (Figs 5A and 6A). Second, since the participants held their palm in the vertical plane (see insets in Figs 2 and 3), RWE in contrast to UWE counteracted gravity and was expected to be more or less continuously active. Third, visual analyses of the EMG patterns during the key-pressing task indicated that the RWE was primarily responsible for driving the wrist in concentric contractions, whereas the UWE contracted more when lengthening (eccentric contractions), and thus seemed to break the action of its antagonist. These observations were statistically confirmed. EMG averaged during phases characterized by short and long muscle lengths and either shortening and lengthening (see Fig. 4B and C) were entered in repeated measures ANOVAs, one for each muscle. The data were recorded across 19 participants and were z-transformed for each participant before analysis. For both muscles there were significant main effects of muscle length (long/short) and dynamical state (shortening/lengthening), and a significant interaction between the two (see Figs 5A and 6A). Specifically, for the RWE there was a main effect of length (short > long, F1,18= 16.5, P < 10−3), a main effect of the dynamic state (shortening > lengthening, F1,18= 37.4, P < 10−4), and a significant interaction effect between the two (F1,18= 23.4, P < 10−3) with the highest EMG values recorded when the muscle was shortening at a short length (F1,18= 54.2, P < 10−5). In contrast, the EMG recorded from the UWE muscle was higher at the long length (F1,18= 23.1, P < 10−3) and when lengthening rather than shortening (F1,18= 36.2, P < 10−4), and planned comparisons indicated that the UWE was most active when the muscle was lengthening at a long length (F1,18= 43.3, P < 10−5).
Results
Data were obtained from 36 sequentially recorded single muscle spindle afferents: 23 afferents from RWE and 13 from UWE during a total of 1231 key-pressing sequences (3693 key strokes). In what follows we will describe the impact of various kinematic variables on the discharges of primary (‘type Ia’) and secondary (‘type II’) afferents and how well wrist joint kinematics were represented by the ensemble discharges.
Single primary muscle afferents
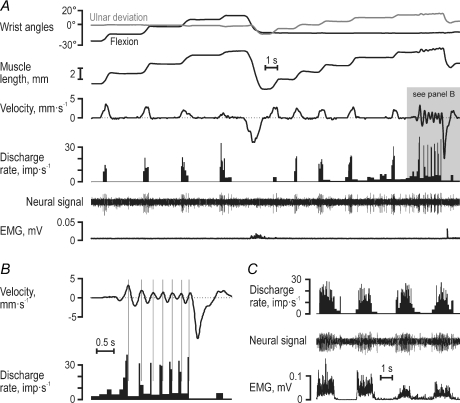
Figure 1 displays responses in a type Ia afferent (65-02) from the RWE muscle. When its parent muscle was relaxed, this afferent showed brisk responses to stretching, irregular firing rates, but no responses during muscle shortening (Fig. 1A and B), and discharged vigorously during isometric contractions (Fig. 1C). During the key-pressing task, however, its discharge pattern was not simply the approximate sum of its responses to muscle contraction and stretch (Fig. 2). Specifically, although the earlier stages of muscle shortening were associated with significant EMG and practically unchanged muscle length, the afferent discharged poorly. Instead, during shortening it displayed a burst of discharges that commenced around the time of negative peak velocities (shaded grey areas in Fig. 2). To assess quantitatively the impact of EMG, muscle length and its first and second derivatives (i.e. ‘velocity’ and ‘acceleration’) on this afferent's discharge rate, non-linear regression (see Methods) was performed across keys that initially required muscle lengthening and shortening (2–3–6 and 4–7–8, respectively; see inset of Fig. 3). Acceleration was the only variable with a significant impact on the discharge rate of this afferent (R2= 0.59; βAcc= 0.70, P < 10−6). Given the range of accelerations observed (117 mm s−2), acceleration accounted for a modulation of 12 impulses s−1 (compared to a total observed modulation of 14 impulses s−1). The discharge of type Ia afferent 65-01 was also predominantly dependent on acceleration (Fig. 3; R2= 0.56; βAcc= 0.68, P < 10−6; βEMG= 0.42, P < 10−2); these calculated impacts corresponded to a total estimated modulations of 12 and 5 impulses s−1 compared to a total observed modulation of 16 impulses s−1. Velocity had the dominant impact on afferent 39-01 (Fig. 3; R2= 0.79; βLen= 0.39, βVel= 0.66 and βAcc= 0.41; P < 10−4 for all β values), with the range of lengths, velocities and accelerations accounting for a modulation of 5, 13 and 8 impulses s−1, respectively, compared to a total modulation of 19 impulses s−1. During key pressing, therefore, the discharge of these type Ia afferents was particularly affected by the time derivatives of length rather than length itself.
Figure 1. Responses of a typical type Ia muscle spindle afferent from the radial wrist extensor.
A, during passive joint movements this Ia afferent (65–02) showed poor responses to static length but pronounced dynamic responses particularly evident during small rapid length changes (grey area) shown on an expanded time scale in B, and it discharged vigorously during isometric contractions (C).
Afferent ensemble responses
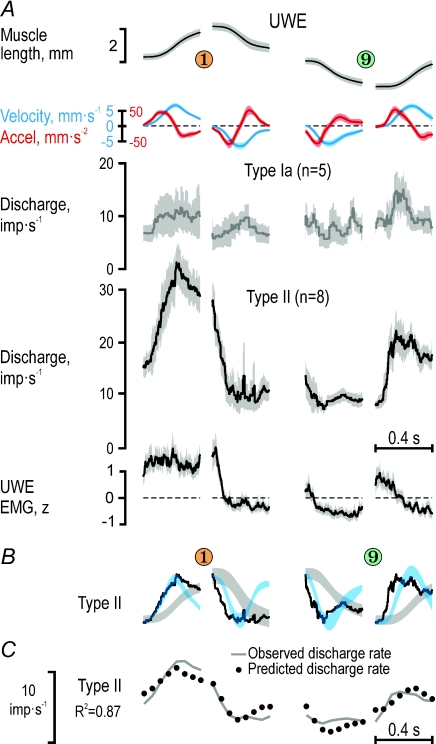
We also analysed responses of afferent populations (ensemble responses). Averaging responses across several neurones has been forwarded as a way by which the CNS deals with the effects of non-linearities and noise that are present at the single afferent level (Scott & Loeb, 1994; Faisal et al. 2008). The afferent recordings were obviously obtained neither simultaneously nor in the same participants. The participants’ behaviours were, however, sufficiently similar to allow construction of ensemble responses by aggregating responses recorded during similar phases of the task. These responses were generated for particular discrete movements during key pressing by randomly selecting empirically observed discharges by single afferents (see Methods). The results of this bootstrapping procedure involving the three single afferents in Fig. 3 are shown in Fig. 4A. A regression analysis using muscle length, velocity, acceleration and parent EMG as independent predictors indicated that only velocity and acceleration had a significant impact on the three-unit ensemble discharge rate, with acceleration having the largest effect (R2= 0.67; βVel= 0.43 and βAcc= 0.69; P < 10−3 for both β values). When the same bootstrapping procedure was applied to all afferents from RWE and UWE (Fig. 4B and C), it was concluded that reliable ensemble responses were obtained for RWE type Ia (n = 15) and type II (n = 8) and UWE type II (n = 8). Although UWE type Ia afferents (n = 5) discharged more when their parent muscle was lengthening than when it was shortening, their responses were otherwise too variable, and their ensemble discharge was therefore excluded from further analyses. Qualitatively, the discharge profile of RWE type Ia seemed sculpted primarily by acceleration and type II from both RWE and UWE by velocity (Figs 5B and 6B). These relationships were quantitatively assessed by means of non-linear regression analyses.
When length, velocity, acceleration and parent muscle EMG were included as independent predictors of RWE type Ia discharge rates across the four dynamic phases of key pressing sequences 5–3–5 and 5–7–5 (i.e. the sequences with the strongest RWE activation; Fig. 5A), high R2 values were obtained with acceleration and velocity both having significant impacts (R2= 0.88; βAcc= 0.54, P < 10−6; βVel= 0.31, P < 10−4; Fig. 5C) Moreover, the R2 values increased further when only ‘ramp-and-hold’ sequences were included in the regression (e.g. when subjects moved in sequence 5–3–5: R2= 0.92, βAcc= 0.56, P < 10−3; βVel= 0.39, P < 10−3). When all phases during which the participants moved to and from the ‘short’ and ‘long’ keys were used in the regressions, again only acceleration and velocity had significant impacts, with that of acceleration being the largest (R2= 0.62, βAcc= 0.50, P < 10−6; βVel= 0.38, P < 10−6) just as for the small ensemble of three RWE type Ia afferents across all short and long keys, i.e. across 12 dynamic phases (see Fig. 4A).
The same independent predictors were used to reconstruct the discharge rates of the type II ensemble (n = 8) from the RWE. For the sequences shown in Fig. 5A, only velocity was found to have a significant impact (R2= 0.83; βVel= 0.67, P < 10−6; Fig. 5C). When ‘ramp-and-hold’ sequences were used (i.e. only movements in sequence 5–3–5), the R2 values again increased markedly (R2= 0.96; βVel= 0.53, P < 10−6). In addition, only velocity was found to exert a significant impact on type II discharges when regressing across all 12 discrete movements (R2= 0.72; βVel= 0.75, P < 10−6).
The discharge rates of the type II ensemble from the UWE (n = 8) were higher than those from the RWE and were clearly modulated in parallel with the kinematic signals (Fig. 6). Both velocity and length had significant impacts (R2= 0.87; βLen= 0.34, P < 10−3; βVel= 0.72, P < 10−6; Fig. 6C). When using all phases during which the participants moved to and from the ‘short’ and ‘long’ keys (12 discrete movements), both length and velocity had a strong impact on type II discharge whereas that of acceleration was small (R2= 0.82; βLen= 0.53, P < 10−6; βVel= 0.83, P < 10−6; βAcc= 0.15, P < 10−2).
Afferent encoding of wrist joint angle, velocity and acceleration
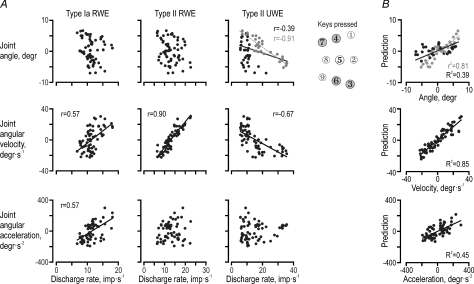
To analyse the ability of the spindle afferent populations from RWE and UWE to individually and jointly encode wrist joint kinematics, we focused on the dynamic phases of key sequences associated with substantial length changes in both muscles, i.e. keys 3, 6, 4 and 7 (see insets in Figs 4B and C, and 7). The correlation matrix relating the discharge of the three afferent populations to wrist kinematics was congruent with previous analyses: only the type Ia ensemble responses from the RWE displayed a significant relationship to angular acceleration (r = 0.57, P < 10−6), and only the type II discharge from the UWE was related to the wrist joint angle (r =−0.39, P < 10−6), but with a good representation of the joint angle limited to phases of UWE lengthening (r =−0.91, P < 10−4; grey dots in Fig. 7A). Moreover, all three ensemble responses were related to angular velocity, with the type II ensemble from the RWE displaying the closest relationship (r = 0.90, P < 10−6). The latter r value was higher than those of both type Ia from the RWE and type II from the UWE (P < 10−3). Finally, the type II discharges from the lengthening UWE were significantly more strongly correlated with the angle (r =−0.91) than with angular velocity (r =−0.67; during only UWE lengthening: r =−0.38; P < 10−2).
Figure 7. Encoding of wrist joint kinematics by muscle spindle afferents.
A, the instantaneous ensemble discharge plotted against the ulnar–radial joint angle, angular velocity and acceleration for eight discrete movements: the phases towards and from the four keys (3, 4, 6, 7) that required ‘short’ or ‘long’ muscle lengths during key pressing in radial (RWE) and ulnar wrist extensor (UWE; see Fig. 4B). Regression lines are shown for statistically significant correlations only. Joint angle was well correlated with type II discharges only during phases of UWE lengthening (grey circles). B, reconstructions across all eight movements of ulnar–radial angle, angular velocity and acceleration from single linear regressions including the ensemble discharges of type Ia and type II from the RWE and type II from the UWE as independent predictors. The models predicted velocity well but the joint angle only during phases with UWE lengthening (grey circles).
Multiple linear regressions were performed with the three afferent populations as predictors of the wrist kinematics (Fig. 7B). Across all movements, the afferents reconstructed the angle poorly with only the two type II populations having a significant impact (R2= 0.39; βII-RWE= 0.64, P < 10−4; βII-UWE=−0.63, P < 10−3). In fact, fair predictions were only possible during periods of UWE lengthening (r2= 0.81, P < 10−6; grey dots in Fig. 7B). Although type Ia and II from both the wrist extensor muscles were significantly correlated with velocity, only the type II population from the RWE was found to have a significant effect when reconstructing angular velocity (R2= 0.85; βII-RWE= 0.75, P < 10−6). As expected, only the type Ia population contributed significantly to the reconstruction of angular acceleration, which was nevertheless quite poor (R2= 0.45; βIa-RWE= 0.77, P < 10−6).
Responses during passive and active sinusoidal movements
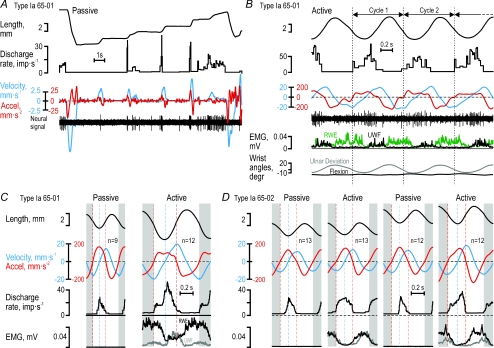
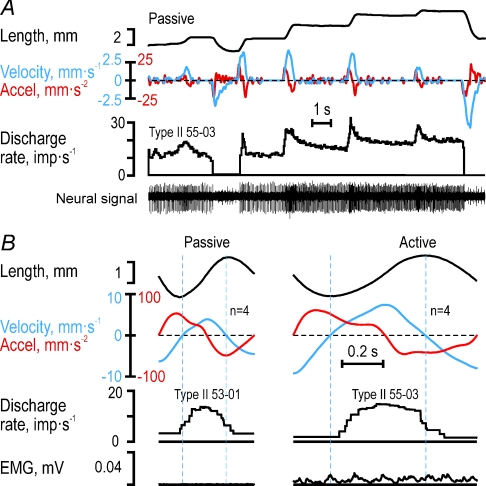
To establish if the observations above were phenomena specific to the key-pressing task, we also studied the responses of four muscle spindle afferents to passive and active sinusoidal wrist movements. During passive ramp-and-hold stretching of type Ia afferent 65-01 (Fig. 8A, see Fig. 3), its discharge began at the onset of stretching but lasted only as long as acceleration was positive, with a poor static response and an obvious lack of responses during passive muscle shortening. The same response pattern was evident when ulnar–radial sinusoidal movements were induced by the experimenter while the participant was relaxed (Fig. 8C), i.e. the afferent discharge began at the onset of positive velocity but ceased at the onset of negative acceleration. In contrast, when sinusoidal movements were actively produced by the participant, the afferent's discharge commenced when acceleration turned positive although the muscle length was decreasing (Fig. 8B and C). While the discharge increased further when velocity also turned positive, the afferent's discharge ceased once acceleration turned negative. The discharge of this afferent obviously corresponded to positive acceleration during active movements. Similarly, type Ia afferent 65-02 discharged when acceleration was positive during active, continuous cyclical movements (Fig. 8D; see Figs 1 and 2), and only when both velocity and acceleration were positive during passive sinusoidal movements. Finally, type II afferent 55-03 responded both to velocity and length during passive ramp-and-hold stretching (Fig. 9A), while, just as type II 55-01, responded only when velocity was positive during passive and active sinusoidal movements (Fig. 9B). In conclusion, the dependence of the afferents’ discharges on length, velocity and acceleration as revealed by the detailed analyses of the key-pressing task was qualitatively the same as those observed during active sinusoidal wrist movements.
Figure 8. Type Ia responses to passive and active sinusoidal movements.
A, responses of type Ia afferent 65-01 to passive length changes of its parent muscle, the radial wrist extensor (RWE), and, B, its discharge during active sinusoidal movements. Vertical lines delimit individual cycles. C, cycle averages for type Ia 65-01 during passive and active sinusoidal movements. D, cycle averages for type Ia 65-02 (see Fig. 1 and 2) during passive and active sinusoidal movements at two different mean lengths. During active wrist movements, RWE type Ia afferents discharged during phases of positive acceleration irrespective of the sign of the velocity whereas they discharged only when both acceleration and velocity were positive during passive wrist movements. Note the lack of any obvious relationship between the afferents’ discharges and the length of their parent muscle. The blue and red dashed lines mark the zero crossing of velocity and acceleration, respectively. The first and last 90 deg of the averaged cycles (grey areas) were concatenated to the end and the beginning of the full cycle to simplify comparison.
Figure 9. Type II responses to passive and active sinusoidal movements.
A, example of type II 55-03 afferent to passive length changes of their parent muscle, the ulnar wrist extensor (UWE). B, cycle averages (see Fig. 8B and C) of recordings of type II afferents 55-01 and 55-03 both from UWE during passive and active sinusoidal wrist movements. In both conditions, type II afferents – in contrast to type Ia afferents – discharged while the velocity signal was positive even if the acceleration was negative. Dashed blue lines indicate zero crossings of velocity. Again note lack of apparent ‘length encoding’ during both passive and active conditions.
Discussion
We have presented the discharge behaviour of human muscle spindle afferents from the wrist extensor muscles when the hand was engaged in a key-pressing task. The task was conducted at subject-preferred speeds and the hand was unrestrained. We show that during this task, the discharges of both type Ia and type II afferents from the RWE were more phase advanced on muscle length than expected. As such, both kinds of afferents failed to encode the length of their parent muscles: the discharge rate of type Ia afferents was correlated with both acceleration and velocity, and type II afferents were correlated with velocity. In contrast, both muscle length and velocity affected the responses of the type II ensemble from the UWE muscle, whereas the type Ia afferents of the UWE showed no obvious relationship to muscle length.
Before addressing the above issues further, we need to comment on some pertinent biological and technical aspects of the study. Firstly, discharge rates of human muscle spindle afferents are known to be low compared to other species. It has been proposed that this is simply a consequence of the low muscle stretch velocities typical of human microneurography studies (Prochazka & Gorassini, 1998a). While this may be true, we put no constraints on the subjects’ behaviours in our study, and therefore the spindle discharge rates observed in the afferents must be considered ‘physiological’. Low discharge rates raise questions concerning the ability of muscle afferents to deliver useful information during short-lasting movements. Nevertheless, there is “…a kaleidoscope of functions, in which proprioceptive feedback has been supposed to be involved” (Windhorst, 2007). For example, lack of proprioceptive input has been linked to increased variability of rapid single-joint movements (Forget & Lamarre, 1987) and severe deficits in the coordination of multi-joint movements (Sainburg et al. 1993). It has been shown that the coordination of discrete movement sequences of durations 210 ms and longer requires proprioceptive information concerning velocity and position (Cordo et al. 1994). The effects of vibrating the biceps branchii tendon during forearm movements (Cordo et al. 1995) and the RWE and UWE tendons during wrist movement (Verschueren et al. 1998) strongly implicate muscle spindles in their control. Hence, despite presumed ‘low’ discharge rates in single afferents, muscle spindles apparently provide important information for the execution of even short-lasting movements. In fact, the RWE and UWE each are supplied by some 150–200 primary afferents and perhaps an even higher number of secondary afferents (Voss, 1959; Matthews, 1972), implying that at least 1000 action potentials were elicited in primary afferents from the RWE muscle during the 400 ms when moving from key 5 to key 3 (Fig. 5A), whereas more than 1500 impulses were generated in secondary afferents from the UWE when the subjects moved to key 1 (Fig. 6A). Encoding movement parameters by muscle receptors most probably relies on the combination of populations of afferents from several muscles, as proposed to be the case in behavioural studies (Verschueren et al. 1998, 1999).
Secondly, the muscle spindle afferents represented wrist angle poorly across all movement directions in the key-pressing task. It has been shown that populations of spindle afferents in the passive human wrist extensor muscles can represent joint angles within the whole physiological range even if individual spindle afferents represent only limited segments of the movement range (∼15 deg; Cordo et al. 2002). The precision required to successfully complete the key-pressing task corresponded to a mere fraction of the movement amplitudes displayed by our participants. Hence, even if the muscle spindle afferents also of active wrist extensor muscles are able to encode the length of their parent muscles, their sensitivity nevertheless appears to be insufficient for effectively monitoring wrist positions in our task.
Thirdly, we used simple linear and non-linear equations to describe the determinants of afferent discharge and the ability of afferents to encode kinematic variables. More complex equations might have captured even more of the variances observed. It should be noted, however, that when more complex models have been used to predict muscle spindle responses in behaving animals on apparently far less complex data sets, the reported fits were not dramatically better than those obtained in this study (see, for instance, Prochazka & Gorassini, 1998a,b).
Finally, we find no reason to doubt that the muscle length, velocity and acceleration signals derived from the angular measurements during the key-pressing task were essentially correct, in particular since the quantitative analyses of the key-pressing task were qualitatively supported by the results from the active sinusoidal movements.
Three unexpected findings
We report three unexpected findings. First, the type II afferent population in the RWE encoded angular velocity better than the type Ia population from the same muscle. Second, the muscle spindles in the RWE muscle were not significantly affected by muscle length. Lastly, type Ia afferents can encode muscle acceleration as well as velocity. All three findings above contradict the consensus according to which primary afferents encode muscle length and velocity and type II afferents encode muscle length (e.g. Prochazka, 1996). Moreover, they imply that during the key-pressing task, the discharge rates of both primary and secondary afferents in the RWE muscle were significantly more phase advanced on length than expected from previous studies. In the physiological range of frequencies (< 10 Hz) and amplitudes, the discharge rates of cat spindle afferents show a phase advance on the length of the parent muscles significantly above 0 deg but below 90 deg and this is unaffected by concurrent fusimotor actions (Hulliger et al. 1977a); accordingly, the spindles are said to encode both length and velocity. The discharge rates of primary and secondary muscle spindles in our recordings were roughly 90 deg more phase advanced than expected from these results. Yet we have no reasons to believe that the encoding properties of human muscle spindles are fundamentally different from those in the cat; in fact, apart from lower discharge rates, sinusoidal stretching of human muscle spindle afferents in passive muscles has yielded results similar to those observed in cat (Kakuda, 2000).
If both primary and secondary muscle spindles were ∼90 deg more phase advanced on muscle length than expected, it is understandable that secondary afferents from the RWE apparently ‘encode’ velocity better than primaries. Furthermore, since the primary afferents from the same muscle were significantly correlated to both velocity and acceleration they obviously could not be well correlated to both of these orthogonal signals.
The lack of a substantial length response in RWE afferents is in line with previous recordings of muscle spindle afferents in actively contracting muscles (Vallbo et al. 1981; Hulliger et al. 1982; Jones et al. 2001b). In the study that most closely resembles ours (Jones et al. 2001b), recordings from RWE and UWE spindle afferents were obtained while participants made discrete hand movements in different directions. That study also found poor length sensitivities in RWE spindle afferents in contrast to UWE. We can only speculate on the reasons for these findings. Information about the static and dynamic wrist joint position can, however, be provided by stretch-sensitive skin mechanoreceptors as shown in neurophysiological (Edin & Abbs, 1991; Edin, 1992, 2001, 2004; Aimonetti et al. 2007) and behavioural studies (Edin & Johansson, 1995; Collins & Prochazka, 1996; Collins et al. 2005).
Finally, several models of spindle afferents take into account muscle length and velocity but not acceleration, and yet the simulated responses match well experimentally obtained muscle spindle responses (Hasan, 1983; Prochazka & Gorassini, 1998a; Mileusnic et al. 2006). These models have not, however, been demonstrated to work well for muscle spindles residing in muscles actively working against loads (Prochazka & Gorassini, 1998b). There is indirect evidence from behavioural studies that acceleration signals, or rather, signals much more phase advanced on muscle length than what is traditionally expected from muscle spindle afferents, play a role in motor control. The responses of cats to sudden loss of balance were, for instance, recently studied using optimal control theory (Lockhart & Ting, 2007). It was possible to account for the experimentally observed muscle activation patterns by taking into account the position, velocity and acceleration of the cats’ centre of mass. The estimated feedback gains were compared in normal cats and cats intoxicated with pyridoxine which destroys large-diameter afferents (Stapley et al. 2002). Interestingly, the main difference was a significantly reduced acceleration feedback gain in the intoxicated cats. Moreover, in a study of the reflexes evoked by postural and vestibular disturbances, Fitzpatrick et al. (1996) found unexpectedly large phase advances which they suggested might be attributed to central nervous processes. Our study indicates that some of this ‘additional’ phase advance may in fact be attributed to phase advances in the muscle afferent discharges.
Possible reasons for the phase advanced responses
In the key-pressing task, we have observed spindle discharges during varying shortening and lengthening velocities and contraction levels of their parent muscles, conditions not previously explored in humans. It therefore seems rational to primarily consider factors related to muscular activity. Moreover, since both type Ia and II from RWE were unexpectedly phase advanced on muscle length – i.e. that the discharge rates of type Ia was correlated with both acceleration and velocity rather than velocity and length and that of type II to velocity rather than length – a parsimonious explanation would identify mechanisms common to both kinds of afferents. We have therefore primarily considered the state of the parent muscle itself and the fusimotor control. A simple explanation that does not give credit to all complexities involved, is that the RWE muscle were acting against a viscous load constituted by its antagonists, the skin and connective tissues. Given that the mechanical properties of both contracting fascicles and tendons are dominated by stiffness, their lengths will be proportional to the tendon force. However, if the muscle is acting on a viscous load, the force will be 90 deg phase advanced on the length, and hence both type Ia and II discharges should appear phase advanced. That the muscle fascicle compartment ‘measured’ by the spindle afferents is not always in phase with the whole tendon–muscle complex has been known for many years (Hoffer et al. 1989; Griffiths, 1991; Fukunaga et al. 1997; Kawakami et al. 2002). Indeed, even slight contractions alter the relationship between fascicle lengths – i.e. the muscle compartment ‘measured’ by the spindle afferents – and the length of the whole tendon–muscle complex (Herbert et al. 2002). Following muscle contraction, muscle fascicle stretch can be powered by tendon recoil (Roberts et al. 1997), which might explain muscle spindle discharges being phase advanced on muscle length and joint angles in the shortening RWE (Figs 2–5).
Fusimotor activity as such seems not to provide a viable explanation. It changes the afferents’ relative sensitivity to length and velocity but not the phase relationship of either type Ia or type II afferents (Matthews & Stein, 1969; Hulliger et al. 1977a; Cussons et al. 1977; Baumann & Hulliger, 1991).
Both type Ia and II afferents show an ‘initial burst’ when their parent muscles are stretched after some period of rest. While this has been forwarded as an ‘acceleration response’ (Schäfer, 1967), it cannot explain acceleration responses during muscle shortening (see Fig. 5A). Yet, the underlying mechanism of the initial burst, i.e. the formation of intrafusal cross-bridges, may perhaps play a role. Intrafusal cross-bridges form spontaneously but can also be invoked by repeated stretching (Proske, 1975; Edin & Vallbo, 1988), are usually eliminated by ongoing fusimotor stimulation (Jansen & Matthews, 1962), but interestingly, are enhanced by fusimotor stimulation followed by rest (Brown et al. 1969). Indeed, this mechanism has been suggested to play a role in spindle afferents during chewing in monkeys when the jaw muscles are contracting to shorten, relax and then are lengthened by the antagonists (Goodwin & Luschei, 1975). The apparent responsiveness to acceleration displayed by type Ia afferents in our study may thus be the result of stretching spindles that have just previously been exposed to fusimotor activity.
Results from animal experiments
Our experimental protocol essentially de-correlated parent muscle length, velocity and acceleration and this enabled us to identify their contribution to the variance of the afferents’ discharge rate. The same can be achieved by sinusoidal stimuli but to our knowledge this technique has never been applied during natural behaviours and not while the muscles have been acting on anything but stiff servo loads (for examples see Crowe & Matthews, 1964; Matthews & Stein, 1969; Poppele & Bowman, 1970; Goodwin et al. 1975; Hulliger et al. 1977a,b; Greer & Stein, 1990; Baumann & Hulliger, 1991; Kakuda, 2000).
Several studies have reported recordings of spindle afferents during natural movements in animals, such as locomotion, stepping and paw shaking. The primary goal of these studies was to elucidate fusimotor control and less attention was paid to the issue of spindle encoding of kinematics. Accordingly, and unfortunately, we have found no detailed analyses of the phase relationship of muscle spindle afferents during such movements. Yet these studies seem to qualitatively agree with several findings in the key-pressing task. For instance, Bessou et al. (1990) claimed that the proprioceptive role of muscle spindles of the hip flexor muscles during cat locomotion are ‘cancelled’ during most of the step cycle because they increase their discharge during active shortening of their parent muscles. Similarly, Loeb et al. (1985) reported that spindles in both knee extensor and biarticular knee–hip muscles discharged vigorously during active muscle shortening. In general, the discharge patterns across primary afferents appeared to have little relationship to the length of their parent muscles (their Fig. 7). Finally, models including muscle length and velocities have been applied to muscle spindle afferent recordings from cats during normal stepping (Prochazka & Gorassini, 1998a). These models performed well in hamstring muscles but not in the triceps surae and the authors explained this by the effects of tendon compliances (Prochazka & Gorassini, 1998b; see Hoffer et al. 1989).
Conclusions
One obvious conclusion of the present study is that muscle spindle discharges in active muscles do not provide a simple representation of muscle length or velocity of muscle length changes. Estimating the length of a muscle from its muscle spindle discharges seems to require additional information about not only the concomitant fusimotor drive but also – if our proposed explanation for the unexpected additional phase advances is correct – independent information about the mechanical properties of the load. Nevertheless, the additional phase advance observed in spindle responses has advantages from a control perspective (Fitzpatrick et al. 1996; Lockhart & Ting, 2007). Finally, during the key-pressing task that appears simple to any healthy human but in fact requires precise and complex control of the muscles acting on the wrist, the responses of the spindle afferents could not be surmised from their responses to passively imposed muscle length changes or to isometric contractions. This is not surprising given the interplay between the intricate biomechanical properties of tendinous tissues and contracting muscle fascicles (Hoffer et al. 1989; Griffiths, 1991; Fukunaga et al. 1997; Kawakami et al. 2002), the local actions of muscle contractions (Windhorst & Meyer-Lohmann, 1977), co-activity in synergists and antagonists, and the fusimotor drive. The consequences of this interplay will only be identified in empirical investigations of natural movements.
Acknowledgments
This work was supported by grants from the Swedish Medical Research Council (projects 08667 and 2005-6994) and the 6th Framework Program of EU (SENSOPAC, IST-001917). We thank Anders Bäckström and Göran Westling for technical support, and Professors G. E. Loeb and P. B. C. Matthews for valuable discussions about our results.
References
- Aimonetti JM, Hospod V, Roll JP, Ribot-Ciscar E. Cutaneous afferents provide a neuronal population vector that encodes the orientation of human ankle movements. J Physiol. 2007;580:649–658. doi: 10.1113/jphysiol.2006.123075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Falahe NA, Nagaoka M, Vallbo ÅB. Lack of fusimotor modulation in a motor adaptation task in man. Acta Physiol Scand. 1990;140:23–30. doi: 10.1111/j.1748-1716.1990.tb08972.x. [DOI] [PubMed] [Google Scholar]
- Baumann TK, Hulliger M. The dependence of the response of cat spindle Ia afferents to sinusoidal stretch on the velocity of concomitant movement. J Physiol. 1991;439:325–350. doi: 10.1113/jphysiol.1991.sp018669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergenheim M, Ribot-Ciscar E, Roll JP. Proprioceptive population coding of two-dimensional limb movements in humans. I. Muscle spindle feedback during spatially oriented movements. Exp Brain Res. 2000;134:301–310. doi: 10.1007/s002210000471. [DOI] [PubMed] [Google Scholar]
- Bessou P, Joffroy M, Montoya R, Pagès B. Evidence of the co-activation of ?-motoneurons and static α-motoneurons of the sartorius medialis muscle during locomotion in the thalamic cat. Exp Brain Res. 1990;82:191–198. doi: 10.1007/BF00230851. [DOI] [PubMed] [Google Scholar]
- Brown MC, Goodwin GM, Matthews PBC. After-effects of fusimotor stimulation on the response of muscle spindle primary afferent endings. J Physiol. 1969;205:677–694. doi: 10.1113/jphysiol.1969.sp008990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Hagbarth KE, Löfstedt L. Muscle spindle responses in man to changes in load during accurate position maintenance. J Physiol. 1978;276:159–164. doi: 10.1113/jphysiol.1978.sp012225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Prochazka A. Movement illusions evoked by ensemble cutaneous input from the dorsum of the human hand. J Physiol. 1996;496:857–871. doi: 10.1113/jphysiol.1996.sp021733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Refshauge KM, Todd G, Gandevia SC. Cutaneous receptors contribute to kinesthesia at the index finger, elbow, and knee. J Neurophysiol. 2005;94:1699–1706. doi: 10.1152/jn.00191.2005. [DOI] [PubMed] [Google Scholar]
- Cordo P, Carlton L, Bevan L, Carlton M, Kerr GK. Proprioceptive coordination of movement sequences: role of velocity and position information. J Neurophysiol. 1994;71:1848–1861. doi: 10.1152/jn.1994.71.5.1848. [DOI] [PubMed] [Google Scholar]
- Cordo P, Gurfinkel VS, Bevan L, Kerr GK. Proprioceptive consequences of tendon vibration during movement. J Neurophysiol. 1995;74:1675–1688. doi: 10.1152/jn.1995.74.4.1675. [DOI] [PubMed] [Google Scholar]
- Cordo PJ, Flores-Vieira C, Verschueren SM, Inglis JT, Gurfinkel V. Position sensitivity of human muscle spindles: single afferent and population representations. J Neurophysiol. 2002;87:1186–1195. doi: 10.1152/jn.00393.2001. [DOI] [PubMed] [Google Scholar]
- Crowe A, Matthews PBC. Further studies of static and dynamic fusimotor fibres. J Physiol. 1964;174:132–151. doi: 10.1113/jphysiol.1964.sp007477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cussons PD, Hulliger M, Matthews PB. Effects of fusimotor stimulation on the response of the secondary ending of the muscle spindle to sinusoidal stretching. J Physiol. 1977;270:835–850. doi: 10.1113/jphysiol.1977.sp011984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawdy DR, Matalas NC. Statistical and probability analysis of hydrologic data, part III: Analysis of variance, covariance and time series. In: Chow VT, editor. Handbook of Applied Hydrology, a Compendium of Water-Resources Technology. New York: McGraw-Hill Co.; 1964. pp. 68–90. [Google Scholar]
- Edin BB. Quantitative analysis of static strain sensitivity in human mechanoreceptors from hairy skin. J Neurophysiol. 1992;67:1105–1113. doi: 10.1152/jn.1992.67.5.1105. [DOI] [PubMed] [Google Scholar]
- Edin BB. Cutaneous afferents provide information about knee joint movements in humans. J Physiol. 2001;531:289–297. doi: 10.1111/j.1469-7793.2001.0289j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin BB. Quantitative analyses of dynamic strain sensitivity in human skin mechanoreceptors. J Neurophysiol. 2004;92:3233–3243. doi: 10.1152/jn.00628.2004. [DOI] [PubMed] [Google Scholar]
- Edin BB, Abbs JH. Finger movement responses of cutaneous mechanoreceptors in the dorsal skin of the human hand. J Neurophysiol. 1991;65:657–670. doi: 10.1152/jn.1991.65.3.657. [DOI] [PubMed] [Google Scholar]
- Edin BB, Bäckström PA, Bäckström LO. Single unit retrieval in microneurography: a microprocessor-based device controlled by an operator. J Neurosci Methods. 1988;24:137–144. doi: 10.1016/0165-0270(88)90057-x. [DOI] [PubMed] [Google Scholar]
- Edin BB, Johansson N. Skin strain patterns provide kinaesthetic information to the human central nervous system. J Physiol. 1995;487:243–251. doi: 10.1113/jphysiol.1995.sp020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin BB, Vallbo ÅB. Stretch sensitization in human muscle spindles. J Physiol. 1988;400:101–111. doi: 10.1113/jphysiol.1988.sp017113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin BB, Vallbo ÅB. Dynamic response of human muscle spindle afferent to stretch. J Neurophysiol. 1990a;63:1297–1306. doi: 10.1152/jn.1990.63.6.1297. [DOI] [PubMed] [Google Scholar]
- Edin BB, Vallbo ÅB. Muscle afferent responses to isometric contractions and relaxations in humans. J Neurophysiol. 1990b;63:1307–1313. doi: 10.1152/jn.1990.63.6.1307. [DOI] [PubMed] [Google Scholar]
- Faisal AA, Selen LP, Wolpert DM. Noise in the nervous system. Nat Rev Neurosci. 2008;9:292–303. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick R, Burke D, Gandevia SC. Loop gain of reflexes controlling human standing measured with the use of postural and vestibular disturbances. J Neurophysiol. 1996;76:3994–4008. doi: 10.1152/jn.1996.76.6.3994. [DOI] [PubMed] [Google Scholar]
- Forget R, Lamarre Y. Rapid elbow flexion in the absence of proprioceptive and cutaneous feedback. Hum Neurobiol. 1987;6:27–37. [PubMed] [Google Scholar]
- Fukunaga T, Kawakami Y, Kuno S, Funato K, Fukashiro S. Muscle architecture and function in humans. J Biomech. 1997;30:457–463. doi: 10.1016/s0021-9290(96)00171-6. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, Hulliger M, Matthews PBC. The effect of fusimotor stimulation during small amplitude stretching on the frequency-response of the primary ending of the mammalian muscle spindle. J Physiol. 1975;253:175–206. doi: 10.1113/jphysiol.1975.sp011186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, Luschei ES. Discharge of spindle afferents from jaw-closing muscles during chewing in alert monkeys. J Neurophysiol. 1975;38:560–571. doi: 10.1152/jn.1975.38.3.560. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Stein RB. Fusimotor control of muscle spindle sensitivity during respiration in the cat. J Physiol. 1990;422:245–264. doi: 10.1113/jphysiol.1990.sp017982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RI. Shortening of muscle fibres during stretch of the active cat medial gastrocnemius muscle: the role of tendon compliance. J Physiol. 1991;436:219–236. doi: 10.1113/jphysiol.1991.sp018547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan Z. A model of spindle afferent response to muscle stretch. J Neurophysiol. 1983;49:989–1006. doi: 10.1152/jn.1983.49.4.989. [DOI] [PubMed] [Google Scholar]
- Herbert RD, Moseley AM, Butler JE, Gandevia SC. Change in length of relaxed muscle fascicles and tendons with knee and ankle movement in humans. J Physiol. 2002;539:637–645. doi: 10.1113/jphysiol.2001.012756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffer JA, Caputi AA, Pose IE, Griffiths RI. Roles of muscle activity and load on the relationship between muscle spindle length and whole muscle length in the freely walking cat. Prog Brain Res. 1989;80:75–85. doi: 10.1016/s0079-6123(08)62201-3. discussion p. 57. [DOI] [PubMed] [Google Scholar]
- Hulliger M, Matthews PBC, Noth J. Static and dynamic fusimotor action on the response of Ia fibres to low frequency sinusoidal stretching of widely ranging amplitudes. J Physiol. 1977a;267:811–836. doi: 10.1113/jphysiol.1977.sp011839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulliger M, Matthews PBC, Noth J. Effects of combining static and dynamic fusimotor stimulation on the response of the muscle spindle primary ending to sinusoidal stretching. J Physiol. 1977b;267:839–856. doi: 10.1113/jphysiol.1977.sp011840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulliger M, Nordh E, Vallbo ÅB. The absence of position response in spindle afferent units from human finger muscles during accurate position holding. J Physiol. 1982;322:167–179. doi: 10.1113/jphysiol.1982.sp014030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen JKS, Matthews PBC. The central control of the dynamic response of muscle spindel receptors. J Physiol. 1962;161:357–378. doi: 10.1113/jphysiol.1962.sp006892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Coordinated isometric muscle commands adequately and erroneously programmed for the weight during lifting task with precision grip. Exp Brain Res. 1988;71:59–71. doi: 10.1007/BF00247522. [DOI] [PubMed] [Google Scholar]
- Jones KE, Wessberg J, Vallbo A. Proprioceptive feedback is reduced during adaptation to a visuomotor transformation: preliminary findings. Neuroreport. 2001a;12:4029–4033. doi: 10.1097/00001756-200112210-00035. [DOI] [PubMed] [Google Scholar]
- Jones KE, Wessberg J, Vallbo AB. Directional tuning of human forearm muscle afferents during voluntary wrist movements. J Physiol. 2001b;536:635–647. doi: 10.1111/j.1469-7793.2001.0635c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuda N. Response of human muscle spindle afferents to sinusoidal stretching with a wide range of amplitudes. J Physiol. 2000;527:397–404. doi: 10.1111/j.1469-7793.2000.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y, Muraoka T, Ito S, Kanehisa H, Fukunaga T. In vivo muscle fibre behaviour during counter-movement exercise in humans reveals a significant role for tendon elasticity. J Physiol. 2002;540:635–646. doi: 10.1113/jphysiol.2001.013459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart DB, Ting LH. Optimal sensorimotor transformations for balance. Nat Neurosci. 2007;10:1329–1336. doi: 10.1038/nn1986. [DOI] [PubMed] [Google Scholar]
- Loeb GE, Hoffer JA, Pratt CA. Activity of spindle afferents from cat anterior thigh muscles. I. Identification and patterns during normal locomotion. J Neurophysiol. 1985;54:549–564. doi: 10.1152/jn.1985.54.3.549. [DOI] [PubMed] [Google Scholar]
- Maier MA, Hepp-Reymond MC. EMG activation patterns during force production in precision grip. II. Muscular synergies in the spatial and temporal domain. Exp Brain Res. 1995;103:123–136. doi: 10.1007/BF00241970. [DOI] [PubMed] [Google Scholar]
- Matthews PBC. Mammalian Muscle Receptors and Their Central Actions. London: Edward Arnold Publishers Ltd; 1972. The structure of the receptors; pp. 1–66. [Google Scholar]
- Matthews PBC, Stein RB. The sensitivity of muscle spindle afferents to small sinusoidal changes of length. J Physiol. 1969;200:723–743. doi: 10.1113/jphysiol.1969.sp008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileusnic MP, Brown IE, Lan N, Loeb GE. Mathematical models of proprioceptors. I. Control and transduction in the muscle spindle. J Neurophysiol. 2006;96:1772–1788. doi: 10.1152/jn.00868.2005. [DOI] [PubMed] [Google Scholar]
- Nordh E, Hulliger M, Vallbo AB. The variability of inter-spike intervals of human spindle afferents in relaxed muscles. Brain Res. 1983;271:89–99. doi: 10.1016/0006-8993(83)91367-7. [DOI] [PubMed] [Google Scholar]
- Pigeon P, Yahia L, Feldman AG. Moment arms and lengths of human upper limb muscles as functions of joint angles. J Biomech. 1996;29:1365–1370. doi: 10.1016/0021-9290(96)00031-0. [DOI] [PubMed] [Google Scholar]
- Poppele RE, Bowman RJ. Quantitative description of linear behavior of mammalian muscle spindles. J Neurophysiol. 1970;33:59–72. doi: 10.1152/jn.1970.33.1.59. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Proprioceptive feedback and movement regulation. In: Rowell L, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: American Physiological Society; 1996. pp. 89–127. [Google Scholar]
- Prochazka A, Gorassini M. Models of ensemble firing of muscle spindle afferents recorded during normal locomotion in cats. J Physiol. 1998a;507:277–291. doi: 10.1111/j.1469-7793.1998.277bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A, Gorassini M. Ensemble firing of muscle afferents recorded during normal locomotion in cats. J Physiol. 1998b;507:293–304. doi: 10.1111/j.1469-7793.1998.293bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U. Stretch-evoked potentiation of responses of muscle spindles in the cat. Brain Res. 1975;88:378–383. doi: 10.1016/0006-8993(75)90403-5. [DOI] [PubMed] [Google Scholar]
- Riek S, Carson RG, Wright A. A new technique for the selective recording of extensor carpi radialis longus and brevis EMG. J Electromyogr Kinesiol. 2000;10:249–253. doi: 10.1016/s1050-6411(00)00017-1. [DOI] [PubMed] [Google Scholar]
- Roberts TJ, Marsh RL, Weyand PG, Taylor CR. Muscular force in running turkeys: the economy of minimizing work. Science. 1997;275:1113–1115. doi: 10.1126/science.275.5303.1113. [DOI] [PubMed] [Google Scholar]
- Sainburg RL, Poizner H, Ghez C. Loss of proprioception produces deficits in interjoint coordination. J Neurophysiol. 1993;70:2136–2147. doi: 10.1152/jn.1993.70.5.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer SS. The acceleration response of a primary muscle-spindle ending to ramp stretch of the extrafusal muscle. Experientia. 1967;23:1026–1027. doi: 10.1007/BF02136428. [DOI] [PubMed] [Google Scholar]
- Scott SH, Loeb GE. The computation of position sense from spindles in mono- and multiarticular muscles. J Neurosci. 1994;14:7529–7540. doi: 10.1523/JNEUROSCI.14-12-07529.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapley PJ, Ting LH, Hulliger M, Macpherson JM. Automatic postural responses are delayed by pyridoxine-induced somatosensory loss. J Neurosci. 2002;22:5803–5807. doi: 10.1523/JNEUROSCI.22-14-05803.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo ÅB. Muscle spindle response at the onset of isometric voluntary contractions in man. Time difference between fusimotor and skeletomotor effects. J Physiol. 1971;218:405–431. doi: 10.1113/jphysiol.1971.sp009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo ÅB, Hagbarth K-E. Activity from skin mechanoreceptors recorded percutaneously in awake human subjects. Exp Neurol. 1968;21:270–289. doi: 10.1016/0014-4886(68)90041-1. [DOI] [PubMed] [Google Scholar]
- Vallbo ÅB, Hulliger M, Nordh E. Do spindle afferents monitor joint position in man? A study with active position holding. Brain Res. 1981;204:209–213. doi: 10.1016/0006-8993(81)90666-1. [DOI] [PubMed] [Google Scholar]
- Verschueren SM, Cordo PJ, Swinnen SP. Representation of wrist joint kinematics by the ensemble of muscle spindles from synergistic muscles. J Neurophysiol. 1998;79:2265–2276. doi: 10.1152/jn.1998.79.5.2265. [DOI] [PubMed] [Google Scholar]
- Verschueren SM, Swinnen SP, Cordo PJ, Dounskaia NV. Proprioceptive control of multijoint movement: unimanual circle drawing. Exp Brain Res. 1999;127:171–181. doi: 10.1007/s002210050787. [DOI] [PubMed] [Google Scholar]
- Voss H. Weitere Untersuchungen Über die absolute und relative Zahl der Muskelspindeln in verschiedenen Muskelgruppen (Hüft, Oberschenkel- und Unterarmmuskeln) des Menschen. Anat Anz. 1959;107:190–197. [Google Scholar]
- Windhorst U. Muscle proprioceptive feedback and spinal networks. Brain Res Bull. 2007;73:155–202. doi: 10.1016/j.brainresbull.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Windhorst U, Meyer-Lohmann J. The influence of extrafusal muscle activity on discharge patterns of primary muscle spindle endings. Pflugers Arch. 1977;372:131–138. doi: 10.1007/BF00585326. [DOI] [PubMed] [Google Scholar]