Abstract
The magnocellular oxytocin and vasopressin neurones of the hypothalamus are now understood in exceptional detail. Extensive quantitative details from many independent sources are available describing the electrical activity of the neurones in diverse circumstances, the subcellular localization of vesicles, and rates of hormone secretion from nerve endings into the blood and from dendrites into the brain. These data enable the relationship of electrical (spike) activity to vesicle exocytosis to be inferred with some precision. Such calculations lead to the conclusion that exocytosis of peptide-containing vesicles is a relatively rare event even in this vesicle-dense system. At any given release site in the neurohypophysis, it seems that several hundred spikes are needed on average to release a single vesicle. Release from compartments within the brain seems also to be very rare, making it implausible that peptides can act in a temporally precise, anatomically specific manner. However, very large amounts of peptide are released by these infrequent events, consistent with their likely role as neurohormonal messengers.
Neurones encode information as trains of action potentials (spikes), and information passes between them when these spikes trigger the release of neurotransmitters at synapses. Neurones use many different molecules to communicate with each other, acting in many different ways via specific receptors. Amongst these molecules are more than a hundred different peptides, expressed in different subpopulations of neurons, and many of these peptides are known for the distinctive effects on specific physiological functions that follow central administration of peptide agonists or antagonists. Given the complexity, and the spatial and temporal precision of information processing in the brain, how is it conceivable that a peptide, when administered with no anatomical or temporal sophistication, can evoke functionally coherent consequences? Here we ask, are neuropeptides really like classical neurotransmitters at all?
Classical neurotransmitters are released from axon terminals by Ca2+-dependent exocytosis (Burgoyne & Morgan, 2003); they are packaged in small synaptic vesicles which are preferentially localized at synapses, although recent evidence indicates that extrasynaptic vesicular release can also occur from the somato/dendritic regions of neurones (Cheramy et al. 1981; Huang & Neher, 1996; Zilberter et al. 2005). Peptides are also released by Ca2+-dependent exocytosis, but they are packaged in large dense-core vesicles which generally are not localized to synapses; some are found at synapses, but these vesicles tend to be distributed in soma, dendrites and in axonal varicosities as well as at nerve endings. As the dendrites of a typical neurone contain more than 80% of the total cell volume, for at least some neurons this compartment is the major site of peptide vesicle stores.
The dendrites of the magnocellular neurones of the hypothalamic supraoptic (SON) and paraventricular nuclei (PVN) are the source of very large amounts of oxytocin and vasopressin released within the brain (Ludwig & Pittman, 2003; Ludwig & Leng, 2006). Although many neurones produce peptides as well as a conventional neurotransmitter, and although these are often described as being ‘co-released’, release of peptide-containing vesicles is regulated semi-independently of release of small synaptic vesicles. Here we consider stimulus–secretion coupling of peptide release from magnocellular neurones, and compare it with what is known for conventional transmitters.
A typical synapse contains several thousand small synaptic vesicles, but at any one time, only a few are docked at the membrane, ready to be released when a spike invades the presynaptic terminal. In hippocampal neurones, how many are released can be measured by recording the excitatory postsynaptic potentials (EPSPs) that arise when the synapse is stimulated. It is unusual for more than one vesicle to be released by a single spike, but often not even one is released; typically, the probability that a spike will release one vesicle (PREL) is between 0.1 and 0.3 at any given synapse. However, when spikes occur in a cluster, PREL increases progressively towards 1. This facilitation of release, which is due to augmentation of Ca2+ entry into the nerve ending, means that clusters of spikes are disproportionately important. The converse to facilitation is fatigue, reflecting depletion of the readily releasable pool of vesicles, and the combination of facilitation and fatigue means that brief bursts of spikes are most efficient for transmitter release (Dobrunz & Stevens, 1997; Dobrunz, 2002).
Secretion from the nerve endings in the neural lobe
Neuropeptide secretion is also subject to facilitation and fatigue (Dutton & Dyball, 1979; Ingram et al. 1982; Bicknell et al. 1984; Shaw et al. 1984; Cazalis et al. 1985), but there are large quantitative discrepancies between peptide release and neurotransmitter release.
The hypothalamo-neurohypophysial system (HNS) comprises several thousand magnocellular neurones that project to the pituitary neural lobe (estimates vary from ∼9000 according to Rhodes et al. 1981, to ∼18 000 according to Bandaranayake, 1971); half make mainly vasopressin and half oxytocin, and many of them are aggregated into the SON and the PVN. The SON is particularly homogeneous, containing more vasopressin cells than oxytocin cells (Swaab et al. 1975; Rhodes et al. 1981), but no significant numbers of any other neuronal subtype. Vasopressin and oxytocin are secreted into the circulation from the pituitary nerve terminals, and plasma concentrations have been measured in many circumstances in which neuronal electrical activity has also been recorded (e.g. Leng et al. 1999). From these, we can calculate how much hormone is secreted for a given spike activity, and as the ultrastructure of the HNS is well described (Nordmann & Morris, 1984), we can also estimate PREL.
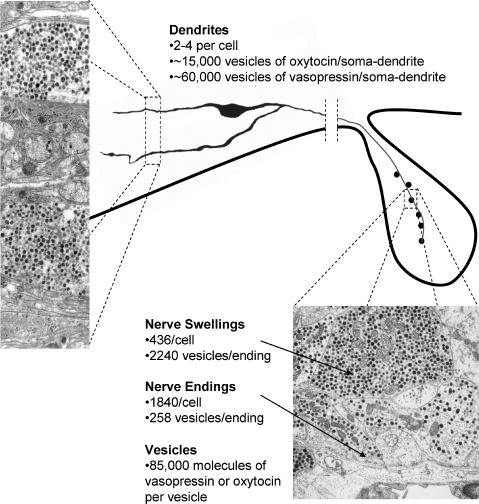
Taking 18 000 as the total number of magnocellular neurones, then from morphometric studies of the neural lobe, it appears that each magnocellular neuron has one long axon that, gives rise to about 2000 nerve terminals and swellings, all packed with vesicles that contain oxytocin or vasopressin (Fig. 1). Altogether, the neural lobe contains ∼1.48 × 1010 vesicles that contain vasopressin, and as many that contain oxytocin, each containing ∼85 000 molecules of hormone (see Morris, 1976a,b; Nordmann, 1977; Nordmann & Morris, 1984). Vasopressin and oxytocin have a molecular weight of ∼1000, implying a neural lobe content of ∼2 μg for each peptide (using Avogadro's number, 6.022 × 1023, for the number of molecules in 1000 g of peptide). Direct measurements of content are usually lower than this, but vary between 1 and 2.5 μg of vasopressin, depending on age, strain, and physiological status. However, some of the largest axonal swellings (the Herring bodies) contain aged granules which are undergoing degradation and have a lower hormone content (Krsulovic et al. 2005), and this may explain the small discrepancy.
Figure 1. Quantitative features of vesicle distribution in magnocellular neurones.
See Bandaranayake (1971), Morris (1976a,b), Nordmann (1977), Rhodes et al. (1981), Nordmann & Morris (1984) and Morris & Pow (1988). The electron micrograph shows a section through dendrites in the supraoptic nucleus. The electron micrograph of the posterior pituitary was kindly provided by Professor John Morris (Oxford).
In conscious rats, basal plasma vasopressin concentrations are ∼1 pg ml−1; for a distribution volume of 60 ml (assuming that extracellular fluid (ECF) volume is 20% of body weight in a 300 g rat), the circulating content is thus ∼60 pg. Vasopressin is cleared with a half-life of ∼2 min (Czaczkes & Kleeman, 1964), so this implies an average secretion of 0.35 pg s−1, or ∼2500 vesicles s−1. A similar conclusion was drawn by Morris (1976a); from considerations of daily turnover, he estimated that about 10 000 vesicles s−1 (comprising both oxytocin and vasopressin vesicles) were secreted in basal conditions.
Under urethane anaesthesia, vasopressin cells fire at ∼4 spikes s−1 and vasopressin concentrations are ∼85 pg ml−1 (in the study of Leng et al. 1994); assuming that there are 9000 vasopressin cells, ∼23 vesicles are secreted from each cell each second, or ∼5 vesicles spike−1. In that study, stimulating the neural lobe at a mean of 10 Hz raised the circulating vasopressin concentration to 213 pg ml−1, again ∼5 vesicles spike−1. Thus, as each cell has about 2000 release sites, the PREL at each site is just ∼0.0025. This calculation is independent of the actual number of vasopressin cells, depending only on the number of release sites as estimated by morphometric analyses.
Similar estimates come from calculations of electrically evoked secretion from the neural lobe in vitro. The optimal frequency for evoking vasopressin secretion is ∼13 Hz, and in response to 3 min of such stimulation Bicknell & Leng (1981), observed that the maximum secretion was ∼10 ng of each peptide. Again assuming that there are 9000 vasopressin cells and 9000 oxytocin cells, then this is 1.7 vesicles cell−1 spike−1. However, secretion fatigues during 3 min of stimulation, and with shorter stimulations, maximal secretion rates were ∼6.5 pg pulse−1 for oxytocin (at 52 Hz) and ∼5 pg pulse−1 for vasopressin (at 13 Hz), corresponding to ∼5 vesicles cell−1 spike−1 for oxytocin and ∼4 vesicles cell−1 spike−1 for vasopressin.
The physiological secretion rate for neurohypophysial hormones is maximal at reflex milk ejection, when a pulse of oxytocin is triggered by a brief, intense burst of spikes (Lincoln & Wakerley, 1974). A typical burst comprises ∼75 spikes, and involves a near-simultaneous activation of all magnocellular oxytocin cells (Belin & Moos, 1986). Comparing the accompanying intramammary pressure response with the response to i.v. injections of oxytocin indicates that each burst releases ∼0.5 ng oxytocin. This is equivalent to 3.5 × 106 vesicles, ∼5 vesicles cell−1 spike−1 (again for 9000 cells). These examples converge on a conclusion that, in response to each spike, a magnocellular neurone secretes at most ∼5 vesicles in all from its 2000 endings. Thus, although these endings contain far more large vesicles than seen at any peptidergic nerve endings in the brain, it typically requires about 400 spikes to release one vesicle at any given ending.
However, some capacitance measurements in isolated ‘single terminals’ (probably axonal swellings, given their size) have suggested a much higher rate of exocytosis. Interpretation of these measurements is complex; capacitance changes reflect exocytosis of small as well as large vesicles, and many terminals contain small vesicles of unknown function as well as peptide-containing vesicles (Lindau et al. 1992). Depolarization can also produce large capacitance changes unrelated to exocytosis, reflecting gating charge movements associated with voltage-dependent conductances (Matthews, 1998; Barg et al. 2002). Nevertheless, even allowing for some of these factors, Giovannucci & Stuenkel (1997) suggested that the ‘terminals’ contained an immediately releasable pool of about 19 vesicles that is released by a single 5 ms depolarization. This is about 0.8% of the mean content of swellings; if such a rate were sustained, 80% of the neural lobe content would be depleted by 2 s of stimulation at 50 Hz – a stimulus known to release only ∼1 ng of oxytocin (< 0.1% of gland content) in vivo and less in vitro. A similar discrepancy arises in capacitance measurements in the SON (de Kock et al. 2003; Soldo et al. 2004). de Kock et al. (2003) reported that a single 2 ms depolarization could apparently release ∼40 ± 8 vesicles from the somatodendritic compartment of oxytocin cells. At this rate, a basal discharge rate of only 1 spike s−1 would deplete the entire somatodendritic content in 5 min, and as much oxytocin would be released into the brain every second as is secreted from the pituitary into the periphery during an entire milk-ejection burst.
It has been noted by others that capacitance measurements often indicate rates of exocytosis much higher than expected from direct measurements of secretion. For example, Barg et al. (2002) measured glucose-induced second-phase insulin secretion from pancreatic β-cells, and calculated the rate of exocytosis as 5 vesicles min−1 (2.5% of total content h−1). However, they noted that the maximum observed rate of capacitance increase in β-cells is equivalent to 600 vesicles s−1 (5% of content), a discrepancy of about four orders of magnitude. This very high rate is only for a brief period (< 0.5 s), but estimates of exocytosis rate in the steady state, although much lower at < 15 granules s−1, still exceed direct measurements by over 100-fold.
Capacitance measurements only report changes in the cell surface area and there might be delays between the fusion of the granules with the plasma membrane, the establishment of the fusion pore and cargo release. Thus capacitance increases might be reporting not exocytosis itself but a preparatory phase, which may be reversible. If these measurements reflect brief transient membrane fusion events, is it possible that peptide release may occur by a ‘kiss-and-run’ mechanism (He & Wu, 2007)? For the vesicles that contain oxytocin and vasopressin, the answer is probably no; the contents of these are so densely packed that they allow little scope for free diffusion. Morris & Pow (1988) compared the peptide released from the SON in response to high K+ depolarization with the number of exocytoses visualized using the tannic acid method to capture exocytotic cores for electron microscopy, and concluded that the visualized exocytoses accounted for all the measured release.
Release of oxytocin and vasopressin within the brain
Oxytocin and vasopressin secreted from the pituitary does not enter the brain in significant amounts (Ermisch et al. 1993), but both are also released within the brain by centrally projecting parvocellular neurones, and also from the soma and dendrites of magnocellular neurones (Pow & Morris, 1989; Landgraf & Neumann, 2004; Ludwig & Leng, 2006). What is released centrally is degraded within brain tissue by aminopeptidases, and what survives this arrives in the cerebrospinal fluid (CSF), from where it is cleared into the circulation by bulk flow. In the rat, basal CSF concentrations of oxytocin are ∼50 pg ml−1; as oxytocin in CSF has a half-life of ∼20 min (Mens et al. 1983), to maintain this concentration in a CSF volume of ∼400 ml, about 0.7 pg must reach the CSF every minute (∼80 vesicles s−1). Only some of the oxytocin released reaches the CSF, but we can get closer to the true release rate by measuring neurophysin, a large peptide fragment of the oxytocin precursor. Neurophysin is co-packaged and co-released with oxytocin in equimolar amounts, but is not significantly degraded by enzymatic activity in the brain; it is cleared from the CSF by bulk flow with a half-life of ∼40 min (Jones & Robinson, 1982). CSF thus contains much more neurophysin than oxytocin and vasopressin; indeed, for every molecule of oxytocin that reaches the CSF, ∼50 molecules of the associated neurophysin arrive there (Jones & Robinson, 1982). If neurophysin is ultimately all cleared via the CSF, this will reflect the true basal level of central oxytocin release, giving an estimated central oxytocin release of ∼4000 vesicles s−1.
There may be as many parvocellular as magnocellular oxytocin neurones, although there have been no detailed quantitative analyses. The parvocellular neurones project densely to the nucleus tractus solitarii (the brain stem contains ∼3 ng oxytocin; Möhring et al. 1983) and the spinal cord, but generally sparsely to other sites, and individually the neurones contain less oxytocin than do the magnocellular neurones. If the central basal release rate of oxytocin is ∼4000 vesicles s−1, and if say 9000 parvocellular neurones were the sole source, then each releases one vesicle every 2 s or so; if each has 2000 nerve endings, this would be only one release event per ending every 4000 s or so. Clearly there are uncertainties in this estimate; 2000 is a conservative estimate for the number of nerve endings, but 9000 may be an overestimate of the number of parvocellular oxytocin cells. However, even if the total number of endings is overestimated by a factor of 10, which seems very unlikely, then this still suggests only one release event per ending every 400 s or so. Remembering that the parvocellular oxytocin neurones also release a classical neurotransmitter from their nerve endings, oxytocin release from these oxytocin cells may be rare compared to conventional neurotransmitter release.
The axonal terminal plexus of parvocellular neurones in the nucleus tractus solitarii lies close to the floor of the fourth ventricle, and oxytocin released there may reach the CSF readily; however, these are not the only source of oxytocin in the brain. The dendrites of magnocellular neurones contain a large store of vesicles that can be released either by agents that mobilize intracellular calcium, or, after appropriate priming, by spike activity (Ludwig et al. 2002; Ludwig & Leng, 2006). The SON contains only magnocellular neurones, so release from this nucleus reflects release from dendrites. Each SON contains ∼2 ng of oxytocin, giving an estimated 15 000 vesicles cell−1 for 1000 oxytocin cells, consistent with ultrastructural analyses (Morris, 1976a,b; Morris & Pow, 1991) (the vasopressin cells contain many more vesicles than this in their dendrites). In vitro, high potassium depolarization can release ∼50 pg of oxytocin from each SON over a 5 min period (Ludwig et al. 2002). This is only about 1 vesicle cell−1 s−1, but the total is a very large amount of oxytocin, and indeed, dendritic oxytocin release can make a major contribution even to CSF concentrations. Coombes et al. (1991) measured CSF concentrations of oxytocin in response to morphine withdrawal in morphine-dependent rats. This stimulus increases the electrical discharge rate of magnocellular oxytocin neurons by 3–4 spikes s−1, and even after complete lesion of the paraventricular nucleus to exclude any contribution from parvocellular neurons, it raised plasma CSF concentrations of oxytocin to > 1500 pg ml−1.
Estimating the rate of dispersal of peptide released within the brain
To estimate the radius of effectiveness of oxytocin released from dendrites, we need to estimate the secretion rate, the rate of spread through brain extracellular fluid, and the degradation rate. Höistad et al. (2005) asked how soon a peptide reaches the CSF after central release. After microinjection of 0.1 nmol of radiolabelled β-endorphin into the striatum, 2 mm away from the lateral ventricle, CSF concentrations in the cisterna magna rose within 15 min, and the concentration at 30 min (∼400 nm) indicates that virtually all of the injected β-endorphin had by then arrived in the CSF. Thus there is rapid dispersal of peptide within brain tissue in vivo; this does not reflect diffusion alone, but bulk flow of ECF combined with convection currents influenced by pressure gradients established by capillary blood flow.
Oxytocin and vasopressin disappear with a half-life of 2–3 min in plasma, and ∼20 min in CSF (Jones & Pickering, 1972; Mens et al. 1983; Fitzsimmons et al. 1992). The only direct measure of degradation rate in vivo suggested that the half-life in brain is ∼1 min (Stark et al. 1989), but there may be regional variations. Despite the uncertainties, we can begin to estimate the likely radius of effect of oxytocin released from the dendrites of magnocellular neurones. First, we might estimate that the basal oxytocin release from the dendrites is only ∼0.1 vesicle cell−1 s−1 (which would account for half of all oxytocin released in the brain), rising to a maximum of 1 vesicle cell−1 s−1. This basal rate, from 9000 magnocellular neurones, would give a secretion of 0.13 pg s−1. Many of the oxytocin cells are in isolated cells or small accessory cell groups between the PVN and the SON (Rhodes et al. 1981). The volume of the anterior hypothalamus, from the lamina terminalis of the third ventricle to the neural stalk, from the ventral surface of the brain to the fornix, and up to 3 mm lateral of the midline, is approximately that of a tissue block of 5 mm × 6 mm × 3 mm, i.e. ∼90 μl. Of this, extracellular space comprises about 11% of tissue volume, or ∼10 μl. Thus we might consider the consequences of 0.13 pg s−1 of oxytocin secreted into 10 μl, from which it is rapidly cleared. Even with a half-life of just 10 s, then within a minute, enough oxytocin would be present to achieve a mean basal concentration of ∼260 pg ml−1 throughout the anterior hypothalamus.
These concentrations are consistent with those commonly inferred from microdialysis experiments. Microdialysate samples from the SON commonly collect 1–4 pg oxytocin over 30 min (Neumann et al. 1993a,b) at concentrations of 10–100 pg ml−1, and as the recovery rate of these probes is ∼2% for peptides of this size, this suggests that the oxytocin concentration in the ECF of the SON is ∼1000 pg ml−1. This seems consistent with secretion of 0.1–0.4 vesicles cell−1 s−1 from 1000 oxytocin cells for 30 min, which would release 25–100 pg over 30 min.
Brief high frequency trains of action potentials may, however, cause higher immediate release rates. Kombian et al. (1997) showed that when SON neurones were depolarized to trigger about 50 spikes over 1 s, EPSC frequency was depressed for ∼5 min. This effect could be blocked by an oxytocin antagonist, and mimicked by exogenous oxytocin or by blocking aminopeptidase activity, indicating that it reflected the presynaptic effects of activity-evoked oxytocin release. Oxytocin binds to its receptors with high affinity (IC50 at 0.28 nm, Kimura et al. 1997) and is effective at concentrations of 1–10 pg ml−1; the contents of a single vesicle diluted in 1 nl will give a concentration in the nanomolar range, so the effects observed by Kombian et al. (1997) may reflect the release of just one vesicle from the stimulated cell.
The gradient of oxytocin concentrations within the hypothalamus remains unclear; we do not know how oxytocin spreads after release, exactly where it is taken or how quickly, or how local concentrations are affected by degradation. At present, the best indications come by comparing oxytocin concentrations measured by microdialysis in various brain areas after stimuli that induce release from the SON. One place to test this is the septum, which is 4–5 mm distant from the SON and which has a high density of oxytocin receptors but few endogenous oxytocin fibres. Here, oxytocin concentrations are raised consistently by stimuli that evoke large oxytocin release from the SON, although the concentrations are about 10-fold lower than in the SON itself (Engelmann et al. 2000).
Conclusions
These arguments suggest that, in the neural lobe, exocytosis of a large dense-core vesicle is a surprisingly rare event; at any given nerve terminal, it may take about 400 spikes to release a single vesicle. As these endings contain far more vesicles than are found at any synapse, synaptic release of peptides generally in the CNS seems likely to occur with a much lower probability of release. Release of oxytocin within the brain from the dendrites of magnocellular neurones is also infrequent, likely to occur at rates of only about 1 vesicle per cell every few seconds. This seems incompatible with the notion of peptides being effective and faithful mediators of information flow at short time scales and with spatial precision. However, the total amount released by these infrequent events is considerable and oxytocin and vasopressin are effective at very low concentrations. Accordingly, when rates of peptide exocytosis are increased in a population of neurones, the outflow is likely to provide a potent wave of peptide release, spreading through the brain in a way that depends on the direction of bulk ECF flow and local aminopeptidase activity, and acting at distant receptors.
We have focused on the magnocellular oxytocin and vasopressin. Just how generally these conclusions apply may be questioned, but it seems that dendritic release of neuropeptides is a widespread phenomenon; for example, in hippocampal neurones, BDNF and NT-3 are in vesicles targeted for regulated release from dendrites (Brigadski et al. 2005; Kolarow et al. 2007), and this release, like that of dendritic oxytocin and vasopressin, involves activation of intracellular Ca2+ stores. Differences in the mechanisms underlying regulation of large dense-core vesicles and small synaptic vesicles have been extensively reviewed elsewhere (Salio et al. 2006). The involvement of many proteins in synaptic vesicle exocytosis is now well understood, at least in outline, and similar molecular events appear to underlie exocytosis of large dense-core vesicles. However, while exocytosis of synaptic vesicles requires a rise of intracellular [Ca2+] in the proximity of the Ca2+ channels at synapses, peptide release is triggered by a small increase in intracellular [Ca2+]. Thus, while a focal increase in Ca2+ at the presynaptic membrane tends to trigger neurotransmitter release, a more diffuse rise in intracellular Ca2+ favours peptide release. These differences are now widely recognized; however, there is clearly a massive qualitative discrepancy between the rates of release of synaptic vesicles and of peptide-containing vesicles. A typical synapse contains several thousand small synaptic vesicles, each containing a few thousand molecules of amino acid transmitter; re-uptake mechanisms for the transmitters allow these to be re-used, allowing a relatively tight association between spike activity and synaptic transmitter release. By contrast, neurones contain relatively few large dense-core vesicles. Peptide-containing vesicles may contain more than 10 times as much cargo (in terms of the number of messenger molecules), and peptides comprise a more potent cargo in that they have nanomolar affinity for their receptors, compared to micromolar affinity of conventional transmitters. However, there are no known reuptake mechanisms for the peptides and the vesicles cannot be re-used. Thus release of a peptide-containing vesicle is a comparatively rare event for any neurone, but one with potentially widespread and profound consequences (cf. volume transmission Fuxe et al. 2007). As we have argued elsewhere (Leng & Ludwig, 2006) neurotransmitters pass whispered secrets from one particular cell to another, they carry a message that matters only at a particular time and a particular place. By contrast, peptides are public announcements, the messages endure, at least for a while; they are messages not from one cell to another, but from one population of neurones to another.
Acknowledgments
This work was supported by the BBSRC and the Wellcome Trust.
References
- Bandaranayake RC. Morphology of the accessory neurosecretory nuclei and of the retrochiasmatic part of the supraoptic nucleus of the rat. Acta Anat (Basel) 1971;80:14–22. doi: 10.1159/000143670. [DOI] [PubMed] [Google Scholar]
- Barg S, Eliasson L, Renström E, Rorsman P. A subset of 50 secretory granules in close contact with L-type Ca2+ channels accounts for first-phase insulin secretion in mouse β-cells. Diabetes. 2002;51:S74–S82. doi: 10.2337/diabetes.51.2007.s74. [DOI] [PubMed] [Google Scholar]
- Belin V, Moos F. Paired recordings from supraoptic and paraventricular oxytocin cells in suckled rats: recruitment and synchronization. J Physiol. 1986;377:369–390. doi: 10.1113/jphysiol.1986.sp016192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell RJ, Brown D, Chapman C, Hancock PD, Leng G. Reversible fatigue of stimulus–secretion coupling in the rat neurohypophysis. J Physiol. 1984;348:601–613. doi: 10.1113/jphysiol.1984.sp015128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell RJ, Leng G. Relative efficiency of neural firing patterns for vasopressin release in vitro. Neuroendocrinology. 1981;33:295–299. doi: 10.1159/000123248. [DOI] [PubMed] [Google Scholar]
- Brigadski T, Hartmann M, Lessmann V. Differential vesicular targeting and time course of synaptic secretion of the mammalian neurotrophins. J Neurosci. 2005;25:7601–7614. doi: 10.1523/JNEUROSCI.1776-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol Rev. 2003;83:581–632. doi: 10.1152/physrev.00031.2002. [DOI] [PubMed] [Google Scholar]
- Cazalis M, Dayanithi G, Nordmann JJ. The role of patterned burst and interburst interval on the excitation–coupling mechanism in the isolated rat neural lobe. J Physiol. 1985;369:45–60. doi: 10.1113/jphysiol.1985.sp015887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheramy A, Leviel V, Glowinski J. Dendritic release of dopamine in the substantia nigra. Nature. 1981;289:537–542. doi: 10.1038/289537a0. [DOI] [PubMed] [Google Scholar]
- Coombes JE, Robinson ICAF, Antoni FA, Russell JA. Release of oxytocin into blood and into cerebrospinal fluid induced by naloxone in anaesthetized morphine-dependent rats: The role of the paraventricular nucleus. J Neuroendocrinol. 1991;3:551–561. doi: 10.1111/j.1365-2826.1991.tb00316.x. [DOI] [PubMed] [Google Scholar]
- Czaczkes JW, Kleeman CR. The effect of various states of hydration and the plasma concentration on the turnover of antidiuretic hormone in mammals. J Clin Invest. 1964;43:1649–1658. doi: 10.1172/JCI105040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kock CP, Wierda KD, Bosman LW, Min R, Koksma JJ, Mansvelder HD, Verhage M, Brussaard AB. Somatodendritic secretion in oxytocin neurons is upregulated during the female reproductive cycle. J Neurosci. 2003;23:2726–2734. doi: 10.1523/JNEUROSCI.23-07-02726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrunz LE. Release probability is regulated by the size of the readily releasable vesicle pool at excitatory synapses in hippocampus. Int J Dev Neurosci. 2002;20:225–236. doi: 10.1016/s0736-5748(02)00015-1. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Dutton A, Dyball REJ. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol. 1979;290:433–440. doi: 10.1113/jphysiol.1979.sp012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Ebner K, Landgraf R. Behavioural impact of intraseptally released vasopressin and oxytocin in rats. Exp Physiol. 2000;85S:125S–130S. doi: 10.1111/j.1469-445x.2000.tb00015.x. [DOI] [PubMed] [Google Scholar]
- Ermisch A, Brust P, Kretschmar R, Rühle HJ. Peptides and blood–brain barrier transport. Physiol Rev. 1993;73:489–527. doi: 10.1152/physrev.1993.73.3.489. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons MD, Roberts MM, Sherman TG, Robinson AG. Models of neurohypophyseal homeostasis. Am J Physiol Regul Integr Comp Physiol. 1992;262:R1121–R1130. doi: 10.1152/ajpregu.1992.262.6.R1121. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Dahlström A, Höistad M, Marcellino D, Jansson A, Rivera A, Diaz-Cabiale Z, Jacobsen K, Tinner-Staines B, Hagman B, Leo G, Staines W, Guidolin D, Kehr J, Genedani S, Belluardo N, Agnati LF. From the Golgi-Cajal mapping to the transmitter-based characterization of the neuronal networks leading to two modes of brain communication: wiring and volume transmission. Brain Res Rev. 2007;55:17–54. doi: 10.1016/j.brainresrev.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Giovannucci DR, Stuenkel EL. Regulation of secretory granule recruitment and exocytosis at rat neurohypophysial nerve endings. J Physiol. 1997;498:735–751. doi: 10.1113/jphysiol.1997.sp021898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Wu LG. The debate on the kiss-and-run fusion at synapses. Trends Neurosci. 2007;30:447–455. doi: 10.1016/j.tins.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Höistad M, Samskog J, Jacobsen KX, Olsson A, Hansson HA, Brodin E, Fuxe K. Detection of β-endorphin in the cerebrospinal fluid after intrastriatal microinjection into the rat brain. Brain Res. 2005;1041:167–180. doi: 10.1016/j.brainres.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Huang LY, Neher E. Ca2+-dependent exocytosis in the somata of dorsal root ganglion neurons. Neuron. 1996;17:135–145. doi: 10.1016/s0896-6273(00)80287-1. [DOI] [PubMed] [Google Scholar]
- Ingram CD, Bicknell RJ, Brown D, Leng G. Rapid fatigue of neuropeptide secretion during continual electrical stimulation. Neuroendocrinology. 1982;35:424–428. doi: 10.1159/000123418. [DOI] [PubMed] [Google Scholar]
- Jones CW, Pickering BT. Intra-axonal transport and turnover of neurohypophysial hormones in the rat. J Physiol. 1972;227:553–564. doi: 10.1113/jphysiol.1972.sp010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PM, Robinson ICAF. Differential clearance of neurophysin and neurohypophysial peptides from the cerebrospinal fluid in conscious guinea pigs. Neuroendocrinology. 1982;34:297–302. doi: 10.1159/000123316. [DOI] [PubMed] [Google Scholar]
- Kimura T, Makino Y, Bathgate R, Ivell R, Nobunaga T, Kubota Y, Kumazawa I, Saji F, Murata Y, Nishihara T, Hashimoto M, Kinoshita M. The role of N-terminal glycosylation in the human oxytocin receptor. Mol Hum Reprod. 1997;3:957–963. doi: 10.1093/molehr/3.11.957. [DOI] [PubMed] [Google Scholar]
- Kolarow R, Brigadski T, Lessmann V. Postsynaptic secretion of BDNF and NT-3 from hippocampal neurons depends on calcium calmodulin kinase II signaling and proceeds via delayed fusion pore opening. J Neurosci. 2007;27:10350–10364. doi: 10.1523/JNEUROSCI.0692-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombian SB, Mouginot D, Pittman QJ. Dendritically released peptides act as retrograde modulators of afferent excitation in the supraoptic nucleus in vitro. Neuron. 1997;19:903–912. doi: 10.1016/s0896-6273(00)80971-x. [DOI] [PubMed] [Google Scholar]
- Krsulovic J, Peruzzo B, Alvial G, Yulis CR, Rodriguez EM. The destination of the aged, nonreleasable neurohypophyseal peptides stored in the neural lobe is associated to the remodeling of the neurosecretory axon. Microsc Res Tech. 2005;68:347–359. doi: 10.1002/jemt.20245. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Leng G, Bicknell RJ, Brown D, Bowden C, Chapman C, Russell JA. Stimulus-induced depletion of pro-enkephalins, oxytocin and vasopressin and pro-enkephalin interaction with posterior pituitary hormone release in vitro. Neuroendocrinology. 1994;60:559–566. doi: 10.1159/000126797. [DOI] [PubMed] [Google Scholar]
- Leng G, Brown CH, Russell JA. Physiological pathways regulating the activity of magnocellular neurosecretory cells. Prog Neurobiol. 1999;57:625–655. doi: 10.1016/s0301-0082(98)00072-0. [DOI] [PubMed] [Google Scholar]
- Leng G, Ludwig M. Information processing in the hypothalamus: Peptides and analogue computation. J Neuroendocrinol. 2006;18:379–392. doi: 10.1111/j.1365-2826.2006.01428.x. [DOI] [PubMed] [Google Scholar]
- Lincoln DW, Wakerley JB. Electrophysiological evidence for the activation of supraoptic neurones during the release of oxytocin. J Physiol. 1974;242:533–554. doi: 10.1113/jphysiol.1974.sp010722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau M, Stuenkel EL, Nordmann JJ. Depolarization, intracellular calcium and exocytosis in single vertebrate nerve endings. Biophys J. 1992;61:19–30. doi: 10.1016/S0006-3495(92)81812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Pittman QJ. Talking back: dendritic neurotransmitter release. Trends Neurosci. 2003;26:255–261. doi: 10.1016/S0166-2236(03)00072-9. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Sabatier N, Bull PM, Landgraf R, Dayanithi G, Leng G. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature. 2002;418:85–89. doi: 10.1038/nature00822. [DOI] [PubMed] [Google Scholar]
- Matthews G. A lie detector test for presynaptic capacitance measurements. Neuron. 1998;21:940–941. doi: 10.1016/s0896-6273(00)80611-x. [DOI] [PubMed] [Google Scholar]
- Mens WB, Witter A, van Wimersma Greidanus TB. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain Res. 1983;262:143–149. doi: 10.1016/0006-8993(83)90478-x. [DOI] [PubMed] [Google Scholar]
- Möhring J, Schoun J, Kintz J, Robinson IC, McNeill JR. Vasopressin and oxytocin content are decreased in the brain stems of spontaneously hypertensive rats. Neuroendocrinology. 1983;36:457–461. doi: 10.1159/000123498. [DOI] [PubMed] [Google Scholar]
- Morris JF. Hormone storage in individual neurosecretory granules of the pituitary gland: a quantitative ultrastructural approach to hormone storage in the neural lobe. J Endocrinol. 1976a;68:209–224. doi: 10.1677/joe.0.0680209. [DOI] [PubMed] [Google Scholar]
- Morris JF. Distribution of neurosecretory granules among the anatomical compartments of the neurosecretory processes of the pituitary gland: a quantitative ultrastructural approach to hormone storage in the neural lobe. J Endocrinol. 1976b;68:225–234. doi: 10.1677/joe.0.0680225. [DOI] [PubMed] [Google Scholar]
- Morris JF, Pow DV. Capturing and quantifying the exocytotic event. J Exp Biol. 1988;139:81–103. doi: 10.1242/jeb.139.1.81. [DOI] [PubMed] [Google Scholar]
- Morris JF, Pow DV. Widespread release of peptides in the central nervous system: quantitation of tannic acid-captured exocytoses. Anat Rec. 1991;231:437–445. doi: 10.1002/ar.1092310406. [DOI] [PubMed] [Google Scholar]
- Neumann I, Ludwig M, Engelmann M, Pittman QJ, Landgraf R. Simultaneous microdialysis in blood and brain: oxytocin and vasopressin release in response to central and peripheral osmotic stimulation and suckling in the rat. Neuroendocrinology. 1993a;58:637–645. doi: 10.1159/000126604. [DOI] [PubMed] [Google Scholar]
- Neumann I, Russell JA, Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: a microdialysis study. Neuroscience. 1993b;53:65–75. doi: 10.1016/0306-4522(93)90285-n. [DOI] [PubMed] [Google Scholar]
- Nordmann JJ. Ultrastructural morphometry of the rat neurohypophysis. J Anat. 1977;123:213–218. [PMC free article] [PubMed] [Google Scholar]
- Nordmann JJ, Morris JF. Method for quantitating the molecular content of a subcellular organelle: hormone and neurophysin content of newly formed and aged neurosecretory granules. Proc Natl Acad Sci U S A. 1984;81:180–184. doi: 10.1073/pnas.81.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32:435–439. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- Rhodes CH, Morrell JI, Pfaff DW. Immunohistochemical analysis of magnocellular elements in rat hypothalamus: distribution and numbers of cells containing neurophysin, oxytocin, and vasopressin. J Comp Neurol. 1981;198:45–64. doi: 10.1002/cne.901980106. [DOI] [PubMed] [Google Scholar]
- Salio C, Lossi L, Ferrini F, Merighi A. Neuropeptides as synaptic transmitters. Cell Tissue Res. 2006;326:583–598. doi: 10.1007/s00441-006-0268-3. [DOI] [PubMed] [Google Scholar]
- Shaw FD, Bicknell RJ, Dyball REJ. Facilitation of vasopressin release from the neurohypophysis by application of electrical stimuli in bursts. Relevant stimulation parameters. Neuroendocrinology. 1984;39:371–376. doi: 10.1159/000124007. [DOI] [PubMed] [Google Scholar]
- Soldo BL, Giovannucci DR, Stuenkel EL, Moises HC. Ca2+ and frequency dependence of exocytosis in isolated somata of magnocellular supraoptic neurones of the rat hypothalamus. J Physiol. 2004;555:699–711. doi: 10.1113/jphysiol.2003.051136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark H, Burbach JP, Van Der Kleij AA, De Wied D. In vivo conversion of vasopressin after microinjection into limbic brain areas of rats. Peptides. 1989;10:717–720. doi: 10.1016/0196-9781(89)90102-2. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Nijveldt F, Pool CW. Distribution of oxytocin and vasopressin in the rat supraoptic and paraventricular nucleus. J Endocrinol. 1975;67:461–462. doi: 10.1677/joe.0.0670461. [DOI] [PubMed] [Google Scholar]
- Zilberter Y, Harkany T, Holmgren CD. Dendritic release of retrograde messengers controls synaptic transmission in local neocortical networks. Neuroscientist. 2005;11:334–344. doi: 10.1177/1073858405275827. [DOI] [PubMed] [Google Scholar]